Abstract
Executive functions, or cognitive control, are higher-order cognitive functions needed for adaptive goal-directed behaviours and are significantly impaired in majority of neuropsychiatric disorders. Different models and approaches are proposed for describing how executive functions are functionally organised in the brain. One popular and recently proposed organising principle of executive functions is the distinction between hot (i.e. reward or affective-related) versus cold (i.e. purely cognitive) domains of executive functions. The prefrontal cortex is traditionally linked to executive functions, but on the other hand, anterior and posterior cingulate cortices are hugely involved in executive functions as well. In this review, we first define executive functions, their domains, and the appropriate methods for studying them. Second, we discuss how hot and cold executive functions are linked to different areas of the prefrontal cortex. Next, we discuss the association of hot versus cold executive functions with the cingulate cortex, focusing on the anterior and posterior compartments. Finally, we propose a functional model for hot and cold executive function organisation in the brain with a specific focus on the fronto-cingular network. We also discuss clinical implications of hot versus cold cognition in major neuropsychiatric disorders (depression, schizophrenia, anxiety disorders, substance use disorder, attention-deficit hyperactivity disorder, and autism) and attempt to characterise their profile according to the functional dominance or manifest of hot–cold cognition. Our model proposes that the lateral prefrontal cortex along with the dorsal anterior cingulate cortex are more relevant for cold executive functions, while the medial–orbital prefrontal cortex along with the ventral anterior cingulate cortex, and the posterior cingulate cortex are more closely involved in hot executive functions. This functional distinction, however, is not absolute and depends on several factors including task features, context, and the extent to which the measured function relies on cognition and emotion or both.
Keywords: Executive functions, hot–cold cognition, prefrontal cortex, anterior cingulate cortex, posterior cingulate cortex, non-invasive brain stimulation, tDCS, TMS, neuroimaging, fMRI
Introduction
Executive functions and their domains
Executive functions (EFs), also called cognitive control, refer to a family to top-down cognitive processes required for goal-directed behaviours (Diamond, 2013; Miller and Cohen, 2001). These higher-order cognitive functions involve active maintenance of goal presentations and the means to achieve these goals (Miller and Cohen, 2001). In this process, different types of information processing, different sensory modalities (e.g. visual and auditory), and different systems responsible for response execution, memory updating and retrieval, and emotional evaluation are involved. Accordingly, a wide range of functions and brain regions are involved in EFs. These higher-order cognitive functions are also required for adapting and regulating behaviour, mental and physical health, and cognitive, social, and psychological development (Diamond, 2013). Deficits of EFs or executive dysfunctions are commonly observed in patients with psychiatric and mental disorders (Elliott, 2003; Reimann et al., 2020). It is important to consider EFs as a meta-cognitive, supervisory, or controlling system rather than being tied to particular cognition domains (Ward, 2020). Nevertheless, EFs are commonly described in terms of specific types of information processing or cognitive functions.
Traditionally, the concept of EFs was closely related to the distinction between two types of information processing: automatic versus controlled processing (Shiffrin and Schneider, 1977). In this framework, EFs refer to those behaviours and processes that require intentional, online exert of control. Another popular model of EFs is to categorise them into separate modular-type processes and specific cognitive functions. There is a general agreement about three core EFs: response inhibition (e.g. inhibitory control), working memory, and cognitive flexibility (Miyake et al., 2000). Similar to this, early works attempted to describe EFs in terms of certain kinds of information processing associated with specific behavioural tasks. These processes can be summarised in (1) task-setting and problem-solving abilities, (2) response inhibition abilities, (3) task switching abilities, and (4) multitasking (Ward, 2020). In addition to these well-established accounts of EFs, results of neuroimaging studies suggest several organising principles of EFs. One of these organising principles is related to hemispheric differences of the neural substrates of EFs, which considers dissociated functional roles of the left and right hemispheres. In one such model, left lateral prefrontal cortex (PFC) is considered specialised for task-setting functions and the right lateral PFC is specialised for monitoring performance (Stuss and Alexander, 2000). Another proposed model organises the neural substrates of EFs anatomically from anterior to posterior parts of the brain. In one such model, a posterior to anterior gradient is considered for the lateral PFC with a differential functional specificity of the dorsal (linked to action planning) versus ventral (linked to language and objects) routes (Badre and D’Esposito, 2009). The updated version of this theory emphasizes on separate brain networks that interact via local and global hierarchical structure (Badre and Nee, 2018).
A recently emerging and perhaps the least controversial organising principle of EFs is to distinguish between EFs based on the extent they are related to emotion (e.g. hot EFs) or purely cognitive aspects (e.g. cold EFs) (Ward, 2020). Hot EFs, involve processing of information related to reward, emotion, and motivation, while cold EFs involve purely cognitive information processing. Examples of hot and cold EFs are ‘monetary delay discounting’ and ‘working memory letter’ tasks, respectively. The hot versus cold principle has several advantages for organising EFs. First, in this model both cognition and emotion are considered. Second, it presents EFs in a spectrum-like model, indicating that all domains of EFs can be hot or cold depending on contextual information, and third, broader regions of the brain are considered for EFs. A network approach, however, is needed to more accurately depicts functional organization of hot vs cold domains of EF. The current knowledge of neural substrates of hot versus cold EFs distinct between lateral (cold-related) and medial (hot-related) regions of the PFC. This, however, is not limited to the PFC regions and other cortical and subcortical areas appear to be involved as well. Major domains, tasks, and neural substrates of hot versus cold EFs based on the currently available studies are summarized in Figure 1.
Figure 1.
Current knowledge about domains and behavioural tasks of executive functions (a), involved brain structures (b) and underlying assumptions/features (c) of hot versus cold executive functions.
SST: stop signal task; AX-CPT: AX Continuous Performance Task; ERT: emotional regulation task.
Although the hot–cold organising principle of EFs has been most often linked to the PFC (as shown in Figure 1), here we attempt to broaden this principle to cingulate areas as well, due to their significant involvement in executive and cognitive control functions. It is, however, notable that EFs and especially hot EFs are closely related to subcortical areas involved in emotional processing, including the amygdala, insula, striatum (including putamen, caudate, and nucleus accumbens) hippocampus, and brainstem (Ardila, 2019; Heyder et al., 2004; Pessoa, 2009). As the scope of this review is focused on prefrontal and cingulate cortices, the specific contributions of these subcortical regions will not be covered in detail here, but will be mentioned where required. In the next section, we describe hot and cold EFs with a specific focus on majorly involved cortical regions, namely, the PFC and cingulate cortex. Before that, we briefly mention methods in cognitive neuroscience that provide important insights into the relevance of brain areas, and networks associated with EFs.
Studying EFs: neuroimaging versus non-invasive brain stimulation methods
Recent advances in the cognitive neurosciences have provided us with novel, non-invasive methods for studying human cognition. Neuroimaging, particularly functional magnetic resonance imaging (fMRI), has become a dominant tool in cognitive neuroscience research and especially human cognition (Dolan, 2008). The emergence of this method has revolutionized study of the living human brain and fMRI is the most widely used technique in cognitive neuroscience (Newman, 2019). fMRI relies on blood oxygenation level–dependent (BOLD) contrast, which arises due to the magnetic susceptibility of deoxyhaemoglobin (deoxy-Hb). To put it briefly, an increase of neural activity leads to an increase of blood volume and thus the proportion of oxygenated haemoglobin (oxy-Hb) in the region, resulting in an increased BOLD signal. This BOLD signal is indicative of brain activity. When it is time-locked to an event/stimulus, it can be used to reveal neural correlates of cognition. A region with enhanced activity refers to a local increase of brain metabolism during performance of an experimental task compared to the baseline. With fMRI, we can investigate which brain regions are activated during cognitive task performance, including EFs. Most of our knowledge about the brain regions involved in EFs comes from neuroimaging studies (Elliott, 2003; Yuan and Raz, 2014). However, they come with some limitations. Apart from a relatively poor temporal resolution, which is, however, not the case for electroencephalogram (EEG), the other well-known neuroimaging method, fMRI delivers correlational information about the involvement of brain areas and networks in human cognition. In other words, the evidence provided by brain imaging methods is purely correlative and does not allow us to infer causal relationships between brain and behaviour.
While such correlative information about brain–behaviour relations is valuable and informative, it does not allow to easily infer causality of brain–behaviour relationships. Here, tools that allow active manipulation of brain activity come into play. Non-invasive brain stimulation (NIBS) is a group of methods for modulating neural processes of the brain, enabling us to directly study how an experimentally altered neural activity affects behaviour (Polania et al., 2018). Transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (tES) are two commonly used and well-established NIBS techniques. TMS is based on principles of electromagnetism which ultimately leads to electrical stimulation of brain regions in a focal way, and transcranial direct current stimulation (tDCS), the most common used tES methods, uses a weak, painless electrical current applied to the scalp, thereby modulating brain excitability in a more non-focal way (Nitsche and Paulus, 2000). Depending on a specific frequency (for TMS) and stimulation polarity/intensity/duration (for tDCS), different TMS and tDCS protocols can result in excitatory or inhibitory after-effects that might last for several minutes and in this case are linked to long-term potentiation or long-term depression (Polania et al., 2018). Due to such effects on cortical excitability and neuroplasticity, which are physiological foundations of cognition, these techniques have great potential for experimental investigation of the physiological foundations behind human cognition.
As briefly mentioned, various cortical and subcortical regions are involved in EFs. While neuroimaging methods can show the functional and structural correlates of EFs in the brain, with NIBS (e.g. TMS and tDCS) we can further complement our knowledge of the brain regions/networks supporting EFs. In this review, we will mostly focus on the evidence coming from these methods (i.e. fMRI, TMS, and tDCS) in order to picture how hot versus cold EFs are organised in the brain. We focus mainly on studies conducted in healthy individuals in this review. However, due to high relevance of hot versus cold cognition in neuropsychiatric disorders, we discuss important clinical implications of this distinction at the end. A brief description of the research methods used in the studies of this review is summarised in Table 1.
Table 1.
Characteristics of commonly applied neuroimaging and non-invasive brain stimulation methods for studying human cognition.
| Method | Type | Delivered information | Invasiveness | Principle of action | Resolution/focality | |
|---|---|---|---|---|---|---|
| Neuroimaging | fMRI | Recording | Correlative | Non-invasive | Brain haemodynamic | High spatial |
| Low temporal | ||||||
| EEG | Recording | Correlative | Non-invasive | Brain electrical activity | Low spatial | |
| High temporal | ||||||
| Non-invasive brain stimulation | TMS | Stimulation | Causal | Non-invasive | Electromagnetic stimulation | Focal |
| tES (e.g. tDCS) | Stimulation | Causal | Non-invasive | Electrical stimulation | Non-focal |
fMRI: functional magnetic resonance imaging; EEG: electroencephalogram; TMS: transcranial magnetic stimulation; tES: transcranial electrical stimulation.
Hot versus cold EFs in the PFC
In traditional and contemporary conceptualisations of EFs, there is a consensus that the frontal lobe and especially the PFC have a critical role (Miller and Cohen, 2001). The PFC has extensive connections with almost all sensory systems, cortical regions, and subcortical structures involved in action, motor response, memory, emotion, and affect (Miller and Cohen, 2001). Our focus here is on how PFC structures are related to hot versus cold EFs. Broadly speaking, the most basic anatomical division within the PFC defines three cortical areas: the lateral PFC, the medial PFC, and the orbital PFC. The lateral PFC lies anterior to the premotor areas and the frontal eye fields and is situated close to the surface of the skull. It includes the dorsolateral prefrontal cortex (DLPFC) (Brodmann’s areas 46 and 9) and the ventrolateral prefrontal cortex (VLPFC) (Brodmann’s areas 44, 45, and 47) (Ward, 2020). The medial PFC lies between the two hemispheres and anterior to the corpus callosum and the anterior cingulate cortex (ACC) (Brodmann’s area 24 and adjacent regions). The orbitofrontal cortex (OFC) lies above the orbits of the eyes and the nasal cavity (Brodmann’s areas 11, 12, 13, and 14). It is of note that the OFC is functionally and anatomically related to the ventral part of the medial PFC and is sometimes referred to as the ventromedial prefrontal cortex (VMPFC) (Brodmann’s area 10, 14, 25, and 32 and parts of 11, 12, and 13) (Öngür and Price, 2000), but these areas are not identical at finer anatomical divisions (Ward, 2020).
The EF domains related to these areas can be classified in different ways. One popular classification is to functionally specify these areas based on the extent to which these are involved in hot (e.g. emotion and motivation-related) and/or cold EFs (e.g. purely cognitive). Hot EFs mainly involve the orbital and medial PFC, including the OFC and VMPFC, and cold EFs engage the lateral PFC, including the DLPFC and VLPFC (Öngür and Price, 2000; Stuss, 2011; Ward, 2020). Functionally speaking, hot EFs are top-down cognitive processes that operate in contexts with significant emotional and motivational salience, gratification, rewards and/or punishment (Zelazo and Carlson, 2012; Zelazo et al., 2005). Examples of hot EF are delay discounting, affective/risky decision-making, and interpersonal and social behaviour. Cold EFs are top-down cognitive processes that are logically based or mechanistic (Chan et al., 2008) and operate in affectively neutral contexts (Zelazo and Carlson, 2012). Examples of cold EFs include working memory, response inhibition, attentional control, and planning as far as these functions are not presented in an emotional context. In what follows, we provide evidence from neuroimaging (i.e. fMRI) and brain stimulation studies (i.e. TMS and tDCS) about the relation of hot versus cold EFs to different PFC areas.
Neuroimaging studies
PFC and cold EFs
A large body of evidence from neuroimaging studies show that the lateral PFC, including DLPFC and VLPFC, are involved in cold EFs. Response inhibition, the ability to suppress unrelated or inappropriate stimuli/responses, is a core cold component of EFs. It is well-established that a specific region of the PFC, the right inferior frontal gyrus (r-IFG), is critical for inhibitory control (Aron et al., 2004, 2014; Hampshire et al., 2010). The r-IFG is moreover connected with the ACC, involved in error detection, and the lateral OFC when conveying information from non-reward systems (Du et al., 2020). The left IFG is also involved in verbal fluency, another major cold EF domain (Costafreda et al., 2006). The lateral PFC, including the DLPFC, is another well-documented region actively involved in working memory updating and tasks requiring executive control (Lemire-Rodger et al., 2019; Wagner et al., 2001). The PFC, however, should be considered as a part of a larger brain network, the fronto–cingulo–parietal network, that supports cognitive control via interaction of different cortical (and also subcortical) structures.
A great example of a cold EF and its association with subregions of PFC is navigation behavior. Planning, decision-making, goal-coding, and adaptive behavior are those domains of EFs required for real-world navigation (Patai and Spiers, 2021), all of which are functionally cold. At anatomical level, navigation behavior involves interaction of subregions of PFC (e.g., DLPFC, VLPFC), cingulate cortex (dorsal ACC) as well subcortical regions such as hippocampus (Patai and Spiers, 2021). An fMRI meta-analytic study of 193 studies revealed a common pattern of activation in the lateral PFC, dorsal ACC, and parietal cortex across major cold EF domains (working memory, inhibition, flexibility, and planning) (Niendam et al., 2012), indicating that cold EFs are supported by this cognitive control network with the DLPFC as a key region (Figure 2). The connectivity between the lateral PFC and dorsal ACC indicates that these regions are rather involved in cold EFs which are discussed in more detail in the section dedicated to the cingulate cortex.
Figure 2.
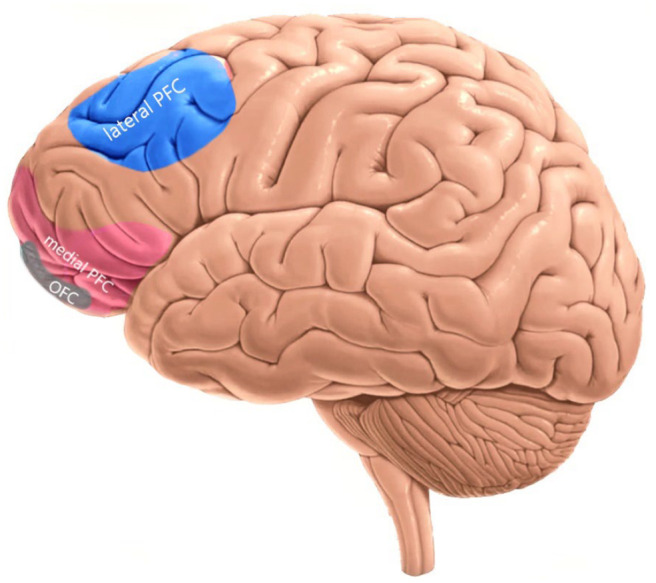
Lateral view of the prefrontal cortex (PFC) regions and association with hot and cold EFs. The lateral PFC includes dorsolateral prefrontal cortex (DLPFC) and ventrolateral prefrontal cortex (VLPFC) that are predominately involved in cold EFs (in blue). The medial PFC and orbitofrontal cortex (OFC) are predominantly involved in hot EFs. The hot PFC regions have extensive connections with several subcortical structures that process emotion and motivation will be discussed later (Figure 4).
Marked regions are close approximate to the intended regions. Also note that circuit nodes and connections are excluded in this and later figures for clarity.
PFC and hot EFs
A large and compelling body of evidence from neuroimaging studies shows that the medial and orbital PFC, specifically the VMPFC and OFC, are involved in cognitive functions related to reward, emotion, motivation, and social evaluation. During cognitive control of emotional stimuli, the medial PFC and OFC are usually activated (Ochsner and Gross, 2005). These regions, however, interact with the lateral PFC (e.g. VLPFC) and ACC during effortful control mechanisms when it comes to emotional and motivational stimuli (Ochsner and Gross, 2005; Pessoa, 2009). This indicates that hot EFs involve both brain regions involved in cold executive control (e.g. lateral PFC and ACC), and those involved in processing of emotion and motivation. One major hot EF is risky decision-making or decision-making under uncertainty. Neuroimaging studies have repeatedly shown that the VMPFC and OFC are involved in decision-making under uncertainty (Bechara et al., 2000; Clark et al., 2008; Fellows and Farah, 2007; Lipsman et al., 2014; Windmann et al., 2006). Delay discounting or temporal discounting is another classic example of hot EFs. Here again, studies show a prominent involvement of the VMPFC and OFC (Wang et al., 2016). What makes the medial–orbital PFC at least partially relevant for emotional and motivational processing is their connectivity with subcortical structures such as the limbic system, amygdala, and insula (Matyi and Spielberg, 2020; Sharpe and Schoenbaum, 2016). These regions are also connected with the posterior cingulate cortex (PCC), the counterpart region in the cingulate cortex which is discussed in the next section. Indeed, proposed models for delay-discounting behaviour in humans based on neuroimaging data assume that a unitary system encompassing the medial PFC, including VMPFC, and PCC are involved in immediate and delayed reward evaluation (Kable and Glimcher, 2007; Peters and Büchel, 2010). In this line, an fMRI study showed coactivation of the VMPFC and PCC during monetary reward encoding (Lin et al., 2011). Another fMRI study showed that when people consider themselves to experience a positive future, greater activity was observed in the VMPFC and PCC, indicating the connecting of these regions as well (Blair et al., 2013).
NIBS studies
Attentional control is a core component of cold EFs. NIBS studies have shown that both TMS and tDCS over the left, right, or bilateral DLPFC enhance selective attention (Gladwin et al., 2012; Pecchinenda et al., 2015; Vanderhasselt et al., 2010). Other NIBS studies have moreover shown a performance-enhancing effect of increasing activity of the lateral PFC, including DLPFC and r-IFG, on attentional control and sustained attention (Coffman et al., 2012; Hwang et al., 2010). Regarding inhibitory control, the DLPFC and the IFG, with a right hemispheric predominance, are involved in response inhibition by both tDCS and TMS studies (for a review, see Brevet-Aeby et al., 2016). Working memory is another major component of cold EFs which was widely studied by NIBS. Recent review and meta-analytic studies have confirmed an enhancing effect of increased activity of DLPFC on working memory task performance (Bagherzadeh et al., 2016; Brunoni and Vanderhasselt, 2014; Hill et al., 2016; Mancuso et al., 2016). Another recent relevant meta-analysis investigated the effects of prefrontal tDCS here on executive function and found that anodal tDCS over the DLPFC increases performance of updating tasks and global EF performance under specific stimulation parameters (Imburgio and Orr, 2018). A recent tDCS study that targeted the DLPFC, temporal cortex, and posterior parietal cortex showed that DLPFC activation contributes to EFs regardless of task modality (semantic, phonemic, and visuospatial) (Ghanavati et al., 2019). Other studies show also enhanced problem-solving, and cognitive flexibility as a result of increased activity of lateral PFC regions via NIBS (Cerruti and Schlaug, 2009; Lucchiari et al., 2018; Metuki et al., 2012; Nejati et al., 2018a). These studies clearly show that the DLPFC and lateral PFC regions are involved in working memory and other cold EFs, although these structures are involved in specific aspects of emotional processing too (Nejati et al., 2021; Lindquist et al., 2012).
Regarding hot EFs, numerous NIBS studies show involvement of medial and orbital PFC regions. The involvement of the medial–orbital PFC (e.g. VMPFC and OFC) in reward and emotion processing is well-documented by both tDCS (Abend et al., 2019; Manuel et al., 2019) and TMS studies (Konikkou et al., 2017). A recent tDCS–fMRI study showed a causal link between VMPFC activation and the experience and regulation of anger, a hot EF domain, in an anger-provoking game involving fair and unfair offers, supporting its role in anger regulation (Gilam et al., 2018). Activity of the VMPFC in this study was coupled with both ACC and PCC activation, depending on the specific offer with more PCC activation during unpleasant offers. Social variables that include evaluation, interaction, theory of mind, and empathy are also considered hot EFs and NIBS studies have shown that activation of the VMPFC modulates such social variables (Adenzato et al., 2017; Chib et al., 2013; Li et al., 2020; Salehinejad et al., 2020a). A recent tDCS study specifically investigated the interaction of the DLPFC and OFC in hot versus cold EFs by applying excitability-enhancing anodal and excitability-reducing cathodal stimulation (Nejati et al., 2018a). Participants conducted response inhibition and problem-solving tasks as measures of cold EFs and risky decision-making and delay-discounting tasks as measures of hot EFs while receiving combined left DLPFC-right OFC stimulation. Increased activity of the left DLPFC concurrent with decreased activity of the OFC prominently improved cold EFs while hot EFs were enhanced under both protocols, those that activated the left DLPFC and the right OFC. The results of this study suggest that hot and cold EFs are placed on a spectrum, with lateral and medial–orbital contributions to cold and hot EFs, respectively, and that no EFs are purely cold or hot. This depends to the extent that each EF domain involves emotion/reward or cognition processing which determines engagement of relevant brain region. The brain regions should be predominantly, but not purely, considered cold and hot as well and this is determined by task feature too.
Hot versus cold EFs in the cingulate cortex
The major anatomical divisions in the cingulate cortex include the anterior, mid, and posterior cingulate cortices, named ACC, MCC and PCC, respectively (Caruana et al., 2018; Vogt, 2005) although some classifications only include ACC and PCC. Here our focus is specifically on the ACC and PCC. Studying involvement of the cingulate cortex in EFs has been mostly limited to the anterior portion of the cingulate cortex or ACC (Brodmann’s area 24 and adjacent regions). The ACC is traditionally linked to the ability of error detection, a cognitive mechanism that monitors for errors and recalibrates task performance accordingly (Carter et al., 1998). As mentioned earlier, one fundamental domain of EFs is inhibitory control or response inhibition (Miyake et al., 2000) which is usually involved in situations with conflicting stimuli. The Stroop test and Go/No-Go tasks are well introduced behavioural examples with conflicting and competing stimuli. Conflict monitoring signals the need for increased cognitive control to resolve current conflicts, and here, the ACC can be linked to EFs (Cole et al., 2009; Shenhav et al., 2013). The involvement of the ACC in error detection abilities, which requires attentional control, suggests its predominant role in cold EFs. However, there is compelling evidence for an involvement of the ACC in emotion and reward–related processes as well. In fact, the ACC can be subdivided into areas differentially related to cognitive versus emotional functions. The dorsal ACC is linked to cognitive, whereas the ventral ACC is linked to emotional processing (Gasquoine, 2013; Lockwood and Wittmann, 2018). In addition to the ventral ACC, the PCC has been increasingly studied in recent years and linked to some domains of EFs related to hot cognition (Platt and Plassmann, 2014). In what follows, we present evidence from fMRI and brain stimulation studies about how hot and cold EFs are linked to the cingulate cortex with a primary focus on the ACC and PCC.
Neuroimaging studies
ACC and cold/hot EFs
The relation of the ACC to cold EFs is based on its primary role in conflict detection during information processing, which signals the need to engage top-down attentional control and performance monitoring (Petersen and Posner, 2012). In this line, an early fMRI study aimed to investigate which levels of processing are being monitored by the ACC during performance of a task with conflicting stimuli and responses. It was shown that the ACC has a highly specific contribution to EFs through detection of conflicts at response level which usually occurs late during information processing (van Veen et al., 2001). This suggests that the executive control exerted by ACC is different from the contribution of the DLPFC. This was confirmed in another fMRI study that specifically compared the roles of the DLPFC versus ACC in attentional control. Attentional control is a clear example of cold EFs as it requires exerting control over the goal, monitoring of the goal, and the processes needed to achieve the goal. It is a fundamental component of executive control that comes into play in almost all EF domains and is traditionally linked to the DLPFC (Miller and Cohen, 2001). In that fMRI study, it was, however, shown that not only the DLPFC takes a leading role in implementing top-down attentional control, but also that the ACC is involved in specific additional aspects of attentional control, such as response-related processes (Milham et al., 2003). Regardless of the type of attentional control, the contribution of the ACC to this effortful process indicates that it is relevantly involved in cold EFs.
Involvement of the ACC in cold EFs is more precisely linked to the dorsal ACC. This region is a key hub in a network of brain regions involved in domain-general EFs in humans (Petersen and Posner, 2012; Shenhav et al., 2013); however, this ‘domain-general’ region has also a domain-specific function related to stimuli valence. An fMRI study in healthy participants showed that while dorsal ACC activity is required for processing task-irrelevant information during the Stroop task performance, which is distracting due to its cognitive content, the ventral ACC is activated during presentation of task-irrelevant information which is distracting due to emotional content (Mohanty et al., 2007). This study is a good example of how cognitive versus emotional information in the context of conflicting stimuli is processed by dorsal versus ventral ACC, respectively. In other words, both dorsal and ventral ACC seem to be involved in effortful control over stimuli, but these areas differ with respect to the kind of stimuli processed, that is, cognitive or emotional. Another fMRI study in healthy subjects, as well as in patients with ACC lesions, found that the dorsal ACC is actively involved in effortful cognitive and motor behaviour in healthy individuals, but that these activities were blunted in patients with focal lesions of the ACC (Critchley et al., 2003). Together, these studies suggest that the dorsal ACC is involved in cold EFs. Another influential account, however, links dorsal ACC functions with motivation and reward–based decision-making (Wallis and Kennerley, 2011). One of the most recent accounts for the role of the dorsal ACC integrates these two perspectives and suggests that that the dorsal ACC plays a central role in decisions about the allocation of cognitive control based on a cost/benefit analysis that identifies the highest expected value of control (Shenhav et al., 2016). According to this theory, exerting effortful control (cold EF) is based on the analysed value of control (hot EFs), and the dorsal ACC has a central role in these processes.
In contrast to the controversy over the functions of the dorsal ACC, there is a general agreement that the ventral region of the ACC is rather linked to emotional, motivational, and social information processing (Gasquoine, 2013; Lockwood and Wittmann, 2018). An fMRI study that measured performance monitoring with a valence-based task showed that the ventral ACC along with the PCC is specifically sensitive to the valence of feedback (Mies et al., 2011), specifically the perigenual ACC and subgenual ACC. These are two subregions of the ventral ACC that are linked with hot EFs involving emotion, motivation, and social decision-making (Lockwood and Wittmann, 2018). In accordance, a recent fMRI study showed connectivity between both perigenual and subgenual ACC and OFC/VMPFC, which are involved in emotional and motivational processing (Du et al., 2020). This might partially explain why the ventral ACC is rather involved in hot EFs, as it is structurally connected and anatomically closer located to those regions of the PFC that are relevant for hot EFs (Figure 3).
Figure 3.
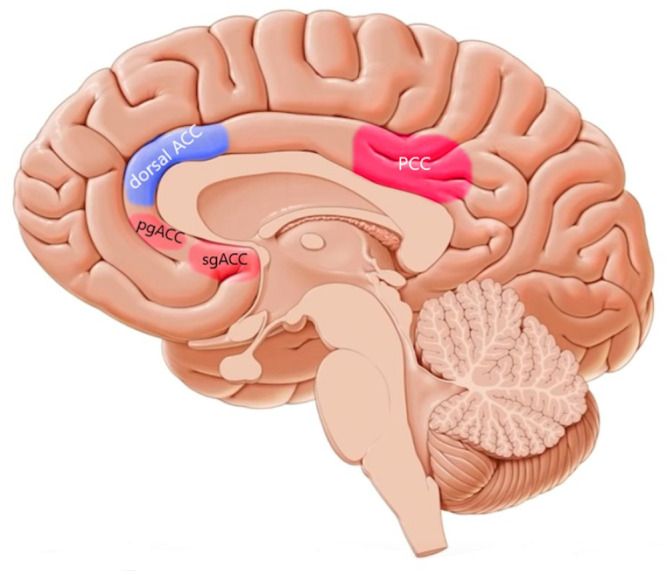
The cingulate cortex in the human brain and association with hot and cold EFs. The anterior cingulate cortex (ACC) includes dorsal ACC (dACC) that is predominately involved in cold (in blue) and ventral ACC, consisting of perigenual (pgACC) and subgenual (sgACC) that are predominately involved in hot EFs (in red), respectively. The posterior cingulate cortex (PCC) is predominantly involved in hot EFs (in red). Note that the anatomical borders of the cingulate cortex in this figure is based on the anatomical studies (see Caruana et al., 2018 and Vogt, 2005 for details). In some studies, the mid-cingulate cortex is part of the dACC.
Marked regions are close approximate to the intended regions. Also, note that most circuit nodes and connections (specially subcortical regions) are excluded for clarity.
PCC and hot EFs
In comparison to the ACC, a relatively limited number of studies explored functional organisation of the PCC in EFs. Previous fMRI studies mainly investigated PCC activation during memory processes, specifically episodic memory. However, functional imaging studies consistently found that emotionally salient stimuli activate the PCC (Maddock, 1999), and the involvement of PCC in episodic and autobiographical memory could be due to its role in the interaction between emotion and memory (Maddock et al., 2001). The involvement of the PCC in emotion, and thus hot EFs, is anatomically related to its connectivity and coactivation with the amygdala, insula, and OFC (Vogt et al., 2000). An fMRI study found that the PCC was activated bilaterally during both unpleasant and pleasant, as compared to neutral words in a memory task (Maddock et al., 2003). Recent studies have provided more convincing evidence for the involvement of the PCC in emotional stimuli processing. Le et al. (2019) investigated neural substrates underlying behavioural avoidance in alcohol drinkers using a valence-based Go/No-Go task. Their major finding was increased activity in the PCC during motivated avoidance and incentivised inhibition of action which was correlated with sensitivity to punishment. In another recent TMS–fMRI study, 1 Hz rTMS was applied to the medial PFC of healthy participants who immediately thereafter underwent fMRI while performing an emotional self-referential task (De Pisapia et al., 2019). Neuroimaging findings revealed that the PCC was the only region that was specifically activated by negative-valence stimuli and as a result of TMS (TMS–valence interaction). Another recent study showed elevated functional connectivity between the PCC and subgenual PFC (e.g. ventral–medial PFC) as a maker of rumination, in depressed individuals versus healthy controls (Benschop et al., 2021). Rumination is a cognitive risk factor resulting from deficient cognitive control over negative emotions and a maladaptive self-referential processing and thus related to hot EFs. Overall, findings of these studies indicate relevant connectivity between the medial PFC and PCC, which will be discussed later in our prefrontal-cingular network model for hot versus cold EFs (Figure 3).
NIBS studies
In contrast to neuroimaging studies that provide correlates of brain–behaviour relations, NIBS methods allow to infer the causality of these associations. The feasibility of NIBS to modulate ACC and PCC physiology is, however, limited in part due to the anatomical depth of these regions. Regarding cold EFs, some NIBS studies investigated the impact of the ACC stimulation on EFs and support the contribution of this region to these EFs.
In a recent tDCS study, using a high-definition (HD) stimulation protocol (i.e. 4 × 1 electrode montage), anodal and cathodal tDCS were applied over the dorsal ACC during performance of a cognitive and emotional cognitive Stroop task (To et al., 2018). Anodal stimulation over the dorsal ACC enhanced performance on the cognitive incongruent stimuli of the task, which requires effortful attentional control, while cathodal stimulation over the same region enhanced performance on the block including emotional incongruent stimuli. Furthermore, anodal stimulation significantly increased beta frequency band activity, which is associated with attentional control. A recent tDCS–fMRI study applied anodal tDCS over the ACC and measured behavioural performance in a colour-word Stroop task, and resting-state fMRI after stimulation (Khan et al., 2020). While behavioural findings showed enhanced Stroop task performance as a result of improved cognitive control, neuroimaging findings showed a significant decrease of functional connectivity of the cognitive control network, including ACC, which is associated with less effortful information processing. In a TMS study that targeted the ACC during a counting Stroop task performance (a cold EF), excitatory 10 Hz rTMS (Hayward et al., 2004) over both the dorsal and ventral ACC abolished Stroop interference. Together, NIBS studies show that stimulation of the dorsal ACC is associated with enhanced cold executive control.
Not many NIBS studies are conducted to modulate the activity of the PCC to explore its impact on cognitive functions. The TMS–fMRI study conducted by De Pisapia et al. (2019), which found a TMS–valance interaction for the activation of the PCC after applying TMS over the medial PFC, is, however, a relavant example. In this study, the neural basis of emotional content in self-referential processing, a hot EF, was investigated by stimulating the medial PFC with 1 Hz TMS. Participants then conducted a valence-based self-referential task. Stimulating the medial PFC activated a network of regions including the PCC which was specifically sensitive to emotionally negative aspects of the stimuli. In another study, the right DLPFC was stimulated with inhibitory TMS, and delay-discounting task performance was monitored during positron emission tomography (PET) scan. The PCC, and especially the posterior parietal lobule, which is part of the PCC, were activated during this task (Cho et al., 2012). Together, NIBS studies available so far show that activation of the PCC is observed during performing hot EF tasks (Table 2).
Table 2.
Summary of hot versus cold executive functions in the studies of this review based on the applied tasks, techniques, and involved regions.
| COLD executive functions – prefrontal regions | |||||
|---|---|---|---|---|---|
| Study | Domain | Used task | Technique | Region | Major finding |
| Aron et al. (2014) a | Inhibitory control | Stop signal, Go/No-Go | fMRI | r-IFG | Inhibition as a central component of executive control relies on activation of the r-IFG |
| Hampshire et al. (2010) | Inhibitory control | Stop signal | fMRI | r-IFG | r-IFG is recruited in detecting inhibition cues |
| Costafreda et al. (2006) | Verbal fluency | Phonemic fluency, semantic fluency | fMRI | l-IFG | Dorsal–ventral regions of l-IFG are recruited in phonologic and semantic fluency |
| Wagner et al. (2001) | Executive control | Executive control, working memory | fMRI | DLPFC, VLPFC | DLPFC relevant for monitoring working memory stimuli, VLPFC relevant for maintenance and monitoring of information |
| Le et al. (2019) | Executive control | Executive control, working memory, task switching | fMRI | DLPFC, medial PFC | DLPFC involved in working memory, inhibition engaged lateral and superior medial PFC, task switching engaged bilateral DLPFC |
| Niendam et al. (2012) b | Working memory, flexibility, inhibition, planning | n-back, PASAT, AX-CPT; task switching, WCST; Go/No-Go, flanker task; tower maze | fMRI | DLPFC, dACC | Common pattern of activation observed in the DLPFC, anterior cingulate and parietal cortices across executive function domains. Unique subcortical regions such as basal ganglia and cerebellum are involved |
| Gladwin et al. (2012) | Selective attention | Sternberg task | tDCS | DLPFC | tDCS over DLPFC improved reaction time of probes involving distracter stimuli |
| Pecchinenda et al. (2015) | Selective attention | Face-word interference task | tDCS | DLPFC | Anodal left DLPFC tDCS did not improve selective attention, cathodal left DLPFC tDCS reduced interference |
| Vanderhasselt et al. (2010) | Attentional control | Reaction-time attention task | HF-rTMS | DLPFC | HF-rTMS of the left DLPFC improved performance on the primary task, but not for the distracters |
| Coffman et al. (2012) | Attentional control | Attention network task | tDCS | Inferior frontal cortex (F10) | Alerting, but not orienting or executive attention, was significantly higher after 2 mA anodal tDCS |
| Hwang et al. (2010) | Attentional control | Continuous performance test | HF-rTMS | DLPFC | Fewer commission errors during trials after rTMS of left DLPFC as compared with sham stimulation |
| Brunoni and Vanderhasselt (2014) a | Working memory | n-back task | tDCS, rTMS | DLPFC | Active vs sham rTMS presented faster and more accurate responses. Active vs sham tDCS presented faster responses only |
| Bagherzadeh et al. (2016) | Working memory | Verbal digit span task, 2-back task | HF-rTMS | DLPFC | rTMS of left DLPFC enhanced working memory performance |
| Imburgio and Orr (2018) a | Global executive function | Inhibition, set-shifting, updating tasks | tDCS | DLPFC | Significant effect of anodal unilateral tDCS on updating but not on inhibition or set-shifting tasks, importance of stimulation parameters (electrode size, location) for observed effects |
| Ghanavati et al. (2019) | Global executive function | Verbal fluency task, semantic fluency task | tDCS | DLPFC | DLPFC activation contributes to EFs regardless of task modality (semantic, phonemic, and visuospatial) |
| Metuki et al. (2012) | Problem-solving | Verbal insight problem task | tDCS | DLPFC | Left DLPFC executive control enhances semantic processing of verbal insight problems |
| Cerruti and Schlaug (2009) | Cognitive flexibility | Remote associates test | tDCS | DLPFC | Anodal left DLPFC stimulation improves verbal problem-solving task which is dependent on significant executive function capacity |
| Nejati et al. (2018a) | Problem-solving, response inhibition | Tower of Hanoi, Go/No-Go test | tDCS | DLPFC | Response inhibition and problem-solving were prominently affected by anodal l-DLPFC–cathodal OFC stimulation |
| COLD executive functions – cingulate cortex | |||||
| Petersen and Posner (2012) a | Attentional control, performance monitoring | Attentional tasks | fMRI | ACC, dACC | Established role of the ACC in top-down control, conflict detection and performance monitoring |
| van Veen et al. (2001) | Conflict detection | Flanker interference task | fMRI | ACC | ACC is responsive to detection of response conflict |
| Milham et al. (2003) | Top-down attentional control | Stroop task | fMRI | ACC | ACC is involved in specific aspects of attentional control, such as response-related processes |
| COLD executive functions – cingulate cortex | |||||
| Study | Domain | Used task | Technique | Region | Major finding |
| Shenhav et al. (2013) a | Cognitive control | Control-demanding tasks (e.g. Stroop) | fMRI | dACC | dACC is involved in allocation of control based on an evaluation of the expected value of control |
| Mohanty et al. (2007) | Cognitive–emotional control | Emotion-word Stroop, colour-word Stroop | fMRI | dACC, rACC | Differential engagement dACC and rACC in cognitive and emotional processing, respectively |
| Critchley et al. (2003) | Motor control | Effortful motor task | fMRI/lesion | dACC | dACC is involved during effortful cognitive and motor behaviour |
| To et al. (2018) | Cognitive–attentional control | Emotional cognitive Stroop task | HD-tDCS | dACC | Anodal and cathodal tDCS over dACC enhanced performance on the cognitive and emotional incongruent stimuli, respectively |
| Khan et al. (2020) | Cognitive–attentional control | Colour-word Stroop task | tDCS–fMRI | ACC | Anodal tDCS improved behavioural performance, significant decrease of functional connectivity of the cognitive control network including ACC |
| Hayward et al. (2004) | Cognitive–attentional control | Stroop task | HF-rTMS | ACC | HF-rTMS dorsal and ventral ACC abolished Stroop interference (performance enhancement) |
| HOT executive functions – prefrontal regions | |||||
| Ochsner and Gross (2005) a | Cognitive control of emotion | N/A | fMRI | Medial PFC, OFC, ACC | Medial PFC, OFC, and ACC are involved in emotional appraisal systems; VLPFC, OFC, ACC, and medial–lateral PFC are involved in attentional control over emotions |
| Pessoa (2009) a | Cognitive control of emotion and motivation | N/A | fMRI/PET | ACC, amygdala, nucleus accumbens | ACC is engaged in integrating affective signals in the amygdala and nucleus accumbens with control signals in the PFC |
| Clark et al. (2008) | Risky decision-making | Iowa gambling task | Lesion study | VMPFC | VMPFC damage was associated with riskier decision-making |
| Fellows and Farah (2007) | Value-based decision-making | Preference judgement task | Lesion study | VMPFC | VMPFC damage, but not frontal lobe damage leads to impaired decision-making under certainty |
| Lipsman et al. (2014) | Affective decision-making | Emotion tracking task | EEG | VMPFC | 15–20 Hz coherent activity in VMPFC is a functional signature of a valuation process |
| Windmann et al. (2006) | Risky decision-making | Iowa gambling task | fMRI | OFC | Lateral OFC is involved in processing of unsteady (changing) rewards |
| Wang et al. (2016) | Risky decision-making | Delay-discounting and stop signal tasks | MRI/fMRI | VMPFC | Grey matter of middle frontal gyrus and connectivity between frontal pole and VMPFC predicted discounting rate but not impulsive choice |
| Kable and Glimcher (2007) | Risky decision-making | Monetary-discounting task | fMRI | Medial PFC, PCC, ventral striatum | Activity in the ventral striatum, medial PFC, and PCC tracks the subjective value of delayed monetary rewards |
| Peters and Büchel (2010) | Value-based decision-making | Monetary-discounting task | fMRI | PCC, VMPFC | Greater activity in PCC and right VMPFC in the monetary condition with subject-specific reward vs control (only monetary) |
| Lin et al. (2011) | Reward processing | Learning task with monetary or social rewards | fMRI | VMPFC, PCC | Coactivation of the VMPFC and PCC during monetary reward encoding |
| HOT executive functions – prefrontal regions | |||||
| Study | Domain | Used task | Technique | Region | Major finding |
| Blair et al. (2013) | Valenced bias estimation | Valenced bias estimation task | fMRI | VMPFC, PCC | Positive bias estimation was associated with greater activity within VMPFC and PCC |
| Manuel et al. (2019) | Emotional delay discounting | Valenced delay-discounting task | tDCS | VMPFC | Anodal VMPFC tDCS decreased impulsivity and cathodal tDCS increase impulsivity after positive emotions |
| Abend et al. (2019) | Emotion regulation | Emotion induction task | tDCS–fMRI | Medial PFC | Active tDCS reduced intensity of perceived negative emotions. sgACC activation correlated with reported emotion intensity |
| Gilam et al. (2018) | Emotion regulation | Anger-infused ultimatum game | tDCS–fMRI | VMPFC, PCC | Activity of the VMPFC was coupled with PCC activation during unpleasant offers |
| Li et al. (2020) | Social cognition, conformity | Social decision-making task | tDCS | VMPFC | Cathodal stimulation of VMPFC increased conformity tendency |
| Adenzato et al. (2017) | Theory of mind | Reading the Mind in the Eyes task, attribution of intentions task | tDCS | Medial PFC | Anodal tDCS over the medial PFC enhances ToM in females but not in males |
| Nejati et al. (2018a) | Risky decision-making | Risk-taking and delay-discounting tasks | tDCS | OFC, DLPFC | Activation of both left DLPFC and the right OFC with anodal tDCS improved performance on hot EFs tasks |
| Figner et al. (2010) | Risky decision-making | Monetary-discounting task | rTMS | Lateral PFC | Disruption of left lateral PFC, increased choices of immediate rewards over larger delayed rewards |
| HOT executive functions – cingulate cortex | |||||
| Lockwood and Wittmann (2018) a | Social decision-making | Social cognition tasks | fMRI | Ventral ACC, subgenual ACC, perigenual ACC | Subgenual ACC and perigenual ACC are activated during social decision-making and social prediction error |
| Mies et al. (2011) | Valence-based time-estimation | Time-estimation task | fMRI | rACC, PCC | The rACC and PCC were primarily sensitive to the valence of the feedback and more active after positive feedback |
| Maddock et al. (2003) | Emotional memory | Valenced memory task | fMRI | PCC, subgenual ACC | The PCC was significantly activated bilaterally during both unpleasant/pleasant vs neutral words with strongest activity in subgenual ACC |
| Le et al. (2019) | Incentivised inhibition | Reward Go/No-Go task | fMRI | PCC | Increased activity in the PCC during motivated avoidance and incentivised inhibition |
| De Pisapia et al. (2019) | Self-referential processing | Emotional self-referential task | TMS–fMRI | PCC | PCC was the only region that was specifically activated by negative-valence stimuli and as a result of TMS |
| Cho et al. (2012) | Risky decision-making | Delay-discounting task | LF-rTMS/PET | PCC | The PCC, and especially the posterior parietal lobule, were activated during task performance |
DLPFC: dorsolateral prefrontal cortex; VLPFC: ventrolateral prefrontal cortex; dACC: dorsal anterior cingulate cortex; sgACC: subgenual anterior cingulate cortex; vACC: ventral anterior cingulate cortex; rACC: rostral anterior cingulate cortex; OFC: orbitofrontal cortex; TBS: Theta Burst Transcranial Stimulation; HF-rTMS: high-frequency (excitatory) transcranial magnetic stimulation; LF-rTMS: low-frequency (inhibitory) transcranial magnetic stimulation; tDCS: transcranial direct current stimulation; PET: positron emission tomography; VMPFC: ventromedial prefrontal cortex; PASAT: Paced Auditory Serial Addition Test; AX-CPT: AX-Continuous Performance Test; WCST: Wisconsin Card Sorting Task; N/A: not applicable; r-IFG: right inferior frontal gyrus; l-IFG: left inferior frontal gyrus; ToM: theory of mind.
Review articles based on neuroimaging or brain stimulation studies.
Meta-analysis articles based on fMRI or NIBS studies.
The studies in this table include selective neuroimaging/NIBS studies.
A prefrontal-cingular network model for hot versus cold EFs
So far, we discussed how hot and cold EFs can be linked to different regions within the PFC and the cingulate cortex. Anatomical and functional connectivity between the cingulate and prefrontal cortices can explain functional organisations of hot–cold EFs in this network. Two major functional connectivity branches are considered in this proposed model: (1) the connectivity between the lateral PFC (e.g. DLPFC) and ACC (specifically the dorsal ACC) and (2) the connectivity between the orbital–medial PFC (e.g. VMPFC and OFC), ventral ACC, and PCC (Figure 4). In this section, we discuss a prefrontal-cingular network that may more comprehensively account for hot and cold EFs. As subcortical regions are highly involved in hot EFs, we also depicted major subcortical limbic structures involved in emotional and motivational processing which are connected to hot-related prefrontal-cingular structures.
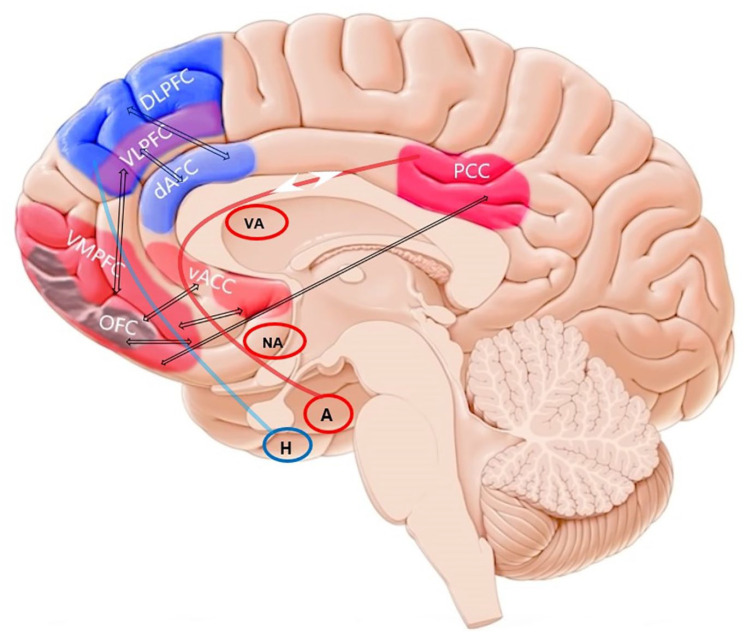
Figure 4.
The prefrontal-cingular network in the human brain and association with hot and cold EFs. The lateral PFC, including DLPFC and VLPFC, along with dorsal ACC are predominantly related to cold EFs and can be considered as the cold stream. The PCC, medial and orbital PFC (VMPFC and OFC), and ventral ACC constitute the hot stream and are predominantly related to hot EFs. The VLPFC is also connected to medial and orbital PFC. The hot EFs stream is closely connected with several limbic structures that are involved in emotional and motivational processing (red curve). The connectivity between the hippocampus and lateral prefrontal cortex subregions is also relevant for major cold EFs such as working memory and navigation behavior.
DLPFC: dorsolateral prefrontal cortex; VLPFC: ventrolateral prefrontal cortex; ACC: anterior cingulate cortex; dACC: dorsal anterior cingulate cortex; vACC: ventral anterior cingulate cortex; VMPFC: ventromedial prefrontal cortex; OFC: orbitofrontal cortex; PCC: posterior cingulate cortex; VA: ventral striatum; NA: nucleus accumbens; A: amygdala; H: hippocampus.
Marked regions are close approximate to the intended regions. Note that some circuit nodes and connections specially with subcortical areas are excluded for clarity and that some connections (shown by arrows) may be indirect.
The cingulate cortex in monkeys and humans has extensive connections with the PFC (Pandya et al., 1981). The dorsal ACC (Brodmann’s area 32) projects mostly to the lateral PFC, including DLPFC, and the mid-OFC (Pandya et al., 1981), and the ventral ACC (Brodmann’s area 25), which is part of the VMPFC, has connections with subcortical regions like amygdala and insula, and projects also to the VMPFC, OFC also lateral PFC. The PCC (Brodmann’s area 31), however, projects to the VMPFC, ventral ACC, and OFC (Leech and Sharp, 2014). Although there is a relative overlap between the ACC and PCC connectivity with PFC regions at the anatomical level, anterior and posterior parts of the cingulate cortex show a more differentiated functional specificity. As discussed in the previous section, both neuroimaging and brain stimulation studies show that the dorsal anterior part of the cingulate cortex is mainly involved in cognitive control functions (Critchley et al., 2003) such as response inhibition and conflict monitoring (Botvinick et al., 2004). From a functional perspective, these EF domains are mostly relevant for cold EFs and similar to those domains the lateral PFC is involved in, although a functional difference is observed for the timing of attentional control exerted by the DLPFC and dorsal ACC (Milham et al., 2003). The connectivity between the lateral PFC and ACC and their coactivation during cognitive control tasks supports this functional link (Tik et al., 2017). The ventral ACC is mainly involved in exerting control over emotional stimuli which indicates that the ACC is involved in both cold and hot EFs (Gasquoine, 2013; Lockwood and Wittmann, 2018). However, the cognitive functions specific to the posterior part of cingulate cortex (e.g. PCC) are shown to be involved in emotional processing, value-based decision-making, subjective valuation, and motivational states (Platt and Plassmann, 2014; Vogt et al., 2000). In this connection, neuroimaging studies have shown coactivations between the PCC and the medial–orbital PFC (e.g. VMPFC and OFC) (De Pisapia et al., 2019; Le et al., 2019; Lin et al., 2011; Vogt et al., 2000).
This relative functional specificity of the anterior versus posterior parts of the cingulate cortex seems similar to the ‘hot–cold’ organising principle of EFs in the PFC. In the PFC, the hot versus cold organisation is proposed based on functional differences between the lateral versus medial–orbital regions. Neuroimaging and brain stimulation studies have documented that cold EFs are rather supported by the lateral PFC, while hot EFs are related to the medial–orbital PFC (Nejati et al., 2018a; Ochsner and Gross, 2005; Öngür and Price, 2000; Peterson and Welsh, 2014; Ward, 2020). Integrating this functional differentiation in the PFC and cingulate cortex with respect to hot versus cold EF allows us to consider a broader network. According to the prefrontal-cingular network, the lateral PFC (e.g. DLPFC and VLPFC) is functionally more closely related to the dorsal ACC, while the medial–orbital PFC (e.g. VMPFC and OFC) is functionally and anatomically more closely related to the ventral ACC and PCC. This does not, however, exclude a contribution of the dorsal ACC, which is documented to be involved in emotion, affect, and pain, to hot EFs (Shackman et al., 2011). Considering a purely segregationist model for the ACC seems to be no longer appropriate (Shackman et al., 2011), however, our discussion here is limited to EFs and specifically hot versus cold domains, which seems to be functionally supported by different regions of the cingulate cortex.
It is important to consider the following additional aspects with regard to the proposed prefrontal-cingular network. First, the involvement of the lateral PFC and dorsal ACC in cold EFs, and the medial–orbital PFC, ventral ACC, and PCC in hot EFs does not imply that these regions are functionally limited to cold or hot cognition. They rather have a predominant functional specificity. As shown in previous studies, there is an interplay between these cold-related and hot-related regions (Le et al., 2019; Nejati et al., 2018a) and importantly these regions show coactivations depending on specific task features. Second, the hot versus cold EFs distinction should not likewise be considered as representing two separate and unrelated domains of EFs. Although some domains are most purely cognitive, such as inhibitory control or working memory, they might be enriched by emotional features depending on the task, stimuli, and the context used for measuring them. Finally, it should be taken into account that we narrowed our discussion to the prefrontal and cingulate cortices. The prefrontal-cingular network includes the cortical regions most closely involved in EFs based on previous studies. This is not meant to underestimate the engagement of other brain regions, especially subcortical limbic regions, the amygdala–hippocampal systems, and sensorimotor regions of the dorsal striatum (e.g. putamen and caudate nuclei) in EFs, which were, however, beyond the scope of this review.
Clinical implications of hot–cold EFs for neuropsychiatric disorders
The hot–cold distinction of cognition has important clinical implications for both characterising and applying appropriate treatment of neuropsychiatric disorders. In the majority of neuropsychiatric disorders, the core pathophysiology involves cortico-subcortical regions of the brain, and here the prefrontal-cingular network is highly involved (Heilbronner and Haber, 2014; Miller and Cummings, 2017). This is in line with the network approach in cognitive neuroscience, which assumes that a dynamically changing pattern of activity over several brain regions is critical for cognitive processes (Ward, 2020). Disturbances of these networks – structural and functional – are related to symptoms and pathophysiology of neuropsychiatric disorders. Accordingly, it is possible to identify symptom-relevant brain networks, and their disturbances, based on connectivity mapping of the human connectome which is one of the major approaches in current biological psychiatry. Abnormalities of the prefrontal-cingular and prefrontal-limbic networks are largely involved in the pathophysiology, symptom expression, and course of the major neuropsychiatric disorders, including but not limited to depression, schizophrenia, anxiety disorder, substance use, and impulse control disorders, as well as major neurodevelopmental disorders (attention-deficit hyperactivity disorder (ADHD) and autism). In what follows, we briefly discuss the respective pathophysiology of some of these disorders and outline whether their cognitive profiles (i.e. hot vs cold) are fundamental (central) for or rather manifest (relevant expression) in the psychopathology of each disease. A summary of the specific hot versus cold profile of each disorder is shown in Table 3.
Table 3.
Hot–cold cognitive profile in major neuropsychiatric disorders.
| Disorder | Cold cognition profile | Hot cognition profile | ||||
|---|---|---|---|---|---|---|
| Cognitive profile | Impaired domain | Brain region | Cognitive profile | Impaired domain | Brain region | |
| Depression | Deficient cold cognition (fundamentala) | Cognitive control (central) | DLPFC | Deficient hot cognition (manifestb) | Emotion regulation (rumination) | sgACC |
| Cold cognition turned hot | Memory (verbal, working) | ACC | Negative hot top-down expectation | Emotional bias/perception | Amygdala | |
| Attention (sustained) | Hippocampus | Deficient hot bottom-up processes | Reward/punishment processing | Striatum including nucleus accumbens | ||
| Time-estimation | Valenced cold cognition (e.g. affective Go/No-Go) | |||||
| Schizophrenia | Deficient cold cognition (fundamental, manifest) | Cognitive control | Lateral PFC | Deficient hot cognition | Social cognition (mind reading) | OFC |
| Memory (verbal, working) | ACC | Risky decision-making | Amygdala | |||
| Attention | Thalamus | Emotion recognition | ||||
| Reasoning | SensorimotorHippocampus | Emotional appraisal | ||||
| Processing speed | ||||||
| Time-estimation | ||||||
| Anxiety disorders | Deficient cold cognition | Inhibition | DLPFC | Deficient hot cognition (fundamental, manifest) | Emotion regulation | Medial PFC |
| Deficient attentional control | Set-shifting | ACC | Reward/punishment processing | VMPFC/OFC | ||
| Attention | amygdala | |||||
| Working memory | ||||||
| Substance use | Deficient cold cognition (fundamental) | Executive control | DLPFC | Deficient hot cognition (fundamental, manifest) | Reward/punishment processing | VMPFC/OFC |
| Task switching | ACC | Deficient hot bottom-up processes | Valenced cold cognition (e.g. cue-dependent attention) | PCC | ||
| Response inhibition | Basal ganglia | Insula, amygdala | ||||
| Nucleus accumbens | ||||||
| ADHD | Deficient cold cognition (fundamental, manifest) | Response inhibition | Inferior PFC (e.g. r-IFG) | Deficient hot cognition in few domains | Valenced cold cognition (e.g. executive reward processing) | Ventral striatum |
| Working memory | DLPFC | Emotion regulation | Medial PFC | |||
| Sustained attention | Parieto-temporal | Delay discounting | OFC | |||
| Basal ganglia | VMPFC | |||||
| Autism | Deficient cold cognition | Working memory | DLPFC | Deficient hot cognition (fundamental, manifest) | Emotion recognition | VMPFC |
| Response initiation | IFG | Social inference | Precuneus | |||
| Planning | Delay discounting | PCC | ||||
| Cognitive flexibility | Affective decision-making | Amygdala/insula | ||||
DLPFC: dorsolateral prefrontal cortex; ACC: anterior cingulate cortex; sgACC: subgenual anterior cingulate cortex; OFC: orbitofrontal cortex; PCC: posterior cingulate cortex.
Fundamental refers to the condition in which respective deficit or profile has influence over other domains/deficits.
Manifest refers to the deficit or profile that is more commonly observed, but not fundamental or core deficits.
Depression
Emotional dysregulation is the core phenotype in depression and in agreement with this, deficits of hot cognition are a common manifestation in depression. From a hot–cold perspective, however, dysfunctional cold EFs, especially cognitive control deficits, are central for the psychopathology of depression, in line with cognitive theories of depression (Gotlib and Joormann, 2010). In other words, cold cognition turns hot in depression (Roiser and Sahakian, 2013). Deficient cold EFs are observed in cognitive control, working memory, and attention (Nikolin et al., 2021; Salehinejad et al., 2017), while hot EF deficits involve those EF tasks (mainly cold) that utilise emotionally valenced stimuli, and reward and punishment processing (Roiser and Sahakian, 2013). In accordance, neuroimaging studies show abnormalities of frontal-limbic structures that account for both cold and hot cognitive deficits (Keren et al., 2018; Rive et al., 2013). A central functional abnormality of the left and right PFC is proposed in depression with a hypoactivated left and hyperactivated right DLPFC, supported by the results of neuroimaging studies (Grimm et al., 2008). Modulation of the activity of these regions is consequently one focus of NIBS treatment in depression (Chen et al., 2020; Razza et al., 2020; Rostami et al., 2017). Treatment approaches in depression should consider fundamental cold executive dysfunctions as the primary target more than before in line with the hot–cold pathology of the disease explained above.
Schizophrenia
In schizophrenia, deficient cold cognition has been more extensively studied with respect to the disease pathophysiology and symptoms (Sheffield et al., 2018). A deficient cold cognitive profile seems to be both fundamental to and manifest of the symptoms and underlying pathophysiology. This is also in agreement with the developmental aspect of schizophrenia, including onset in adolescents, where cold cognition deficits are central (James et al., 2016). A well-documented deficient network in schizophrenia that is involved in cold cognition is the thalamocortical circuitry, especially the thalamus-PFC pathway (Giraldo-Chica and Woodward, 2017) and prefrontal-hippocampal connectivity (Meyer-Lindenberg et al., 2005). Deficient cognitive control, processing speed, memory (verbal, working), and reasoning are commonly reported cold EF deficits in schizophrenia (Table 3). However, impaired hot EFs, including risky decision-making, theory of mind, and emotion recognition are also reported in schizophrenic patients, and associated with psychotic symptoms (MacKenzie et al., 2017; Ruiz-Castañeda et al., 2020). Regarding treatment approaches, therapeutic targeting of cold EF deficits aligns, however, best with the fundamentally involved cold cognitive profile and pathophysiological characteristics of the disease.
Anxiety disorders
Anxiety disorders are traditionally linked to emotional and threat-related difficulties, and thus, hot cognition deficits (e.g. emotion regulation, threat perception, reward–punishment processing) are central for the pathology of these disorders. These emotional difficulties are, however, strongly linked to deficits in several cold EFs that are stable over time (Nejati et al., 2018b; Zainal and Newman, 2018). Two well-known theories in this respect are the ‘Attentional Control Theory’ (Eysenck and Derakshan, 2011) and the ‘Cognitive Model’ of pathological worry (Hirsch and Mathews, 2012). In these theories, impaired cold EFs (specifically inhibition and set-shifting abilities), on one hand, and threat-related perceptual and attentional bias, on the other hand, are proposed to be responsible for the overwhelming experience of worry and anxiety. This effect is, however, dependent on the extent to which respective EF tasks include threatening stimuli or significant cognitive load (Leonard and Abramovitch, 2018). Neuroimaging and brain stimulation studies have shown functional and structural abnormalities of cortical regions related to both hot and cold EFs, with a specific focus on the prefrontal-amygdala network in anxiety disorders (Ironside et al., 2019). Here, a hyperactivation of the medial PFC (VMPFC and OFC) and ventral ACC (hot pathway), which is highly relevant for fear memory and extinction (Marcovic et al., 2021), and a hypoactivation of the DLPFC and dorsal ACC (cold pathway) are shown to be linked to hypersensitivity of the amygdala and other limbic structures (Vicario et al., 2019).
Substance use disorders
A common and core feature of substance use disorder (SUD) is impaired control over craving, or impulsive behaviour. In accordance, here again deficits of both cold and hot EFs are central to the psychopathology of SUD. According to the neurocognitive model of addiction, hot and cold executive deficits play a fundamental and manifest role in different stages of addiction. In the first two stages (binge/intoxication and withdrawal/negative affect), a deficient reward system is central (Koob and Volkow, 2016), which is related to a deficient hot cognition, and in the preoccupation/anticipation stage, where craving behaviour dominates, a deficient executive control system is relevantly involved (Koob and Volkow, 2016), which is related to cold EFs. Therefore, both hot and cold EFs deficits are involved in the psychopathology of SUD, although hot manifestations of symptoms are predominant. The dual-process model of addiction similarly emphasises on both a cold-related ‘controlled’ system (related to the lateral PFC) and a hot-related ‘impulsive’ system (including mesolimbic and nigrostriatal pathways) (Wise, 2009). Novel treatment approaches in SUD have shown the relevance of targeting the cold-related ‘controlled’ as well as the hot-related ‘impulsive’ system by modulating activity of brain structures including DLPFC and ACC which are connected to reward system (Alizadehgoradel et al., 2020; Zhao et al., 2021).
ADHD
ADHD is a major neurodevelopmental disorder, and executive dysfunctions are central for its psychopathology (Willcutt et al., 2005). Results of functional and structural neuroimaging studies, and behavioural studies exploring EFs show predominantly cold EF deficits in the psychopathology, and pathophysiology of ADHD (Antonini et al., 2015; Hobson et al., 2011; Molavi et al., 2020b; Rubia, 2018). Regarding hot EFs, results are mixed, with some studies reporting deficits in affective/motivational EF tasks (Nejati et al., 2020), while others report unimpaired hot EF functions (Antonini et al., 2015). However, neuroimaging, brain stimulation, and behavioural studies have recently shown an impairment of several hot-related cognitive processes and an involvement of medial PFC regions in hot EF task performance (Nejati et al., 2020; Rubia, 2018). It might be speculated that hot EF deficits in ADHD are caused by central cold executive deficits and do not exist independently (Van Cauwenberge et al., 2015). In accordance, the pathology of the functional activity profile of the brain in ADHD is more closely aligned with predominantly cold EF deficits with a fundamental involvement of the frontoparietal network (including IFG, DLPFC, ACC, and temporoparietal regions), the basal ganglia, and the cerebellum (Rubia, 2011). NIBS studies, in this line, have been mostly focused on improving cold EFs in ADHD (Salehinejad et al., 2019, 2020b).
Autism spectrum disorder
Core symptoms in autism spectrum disorder (ASD) include deficits in reciprocity behaviours (required for successful social interaction), and repetitive behaviours. However, ASD is rather known as a disorder of social abilities, although this depends also on the phenotype of the disease. In contrast to this prevailing view, the majority of studies about EFs in ASD investigated cold EFs. Recently, however, hot EFs are studied more extensively in ASD. Briefly, these studies show that ASD involves deficits of both cold and hot EFs (Kouklari et al., 2018; Zimmerman et al., 2016). However, deficits related to hot cognition (e.g. reciprocity abilities and theory of mind) seem to be predominant (Kouklari et al., 2017, 2018; Zimmerman et al., 2016), which aligns with the central role of the medial PFC and PCC in the pathophysiology of ASD (Li et al., 2017; Patriquin et al., 2016). Cold EFs are also relevantly impaired, but these deficits might be secondary and largely restricted to those cold domains needed for the performance of hot EFs (Zimmerman et al., 2016). This is in line with the development of EFs in ASD. Cold but not hot EFs improve significantly as a function of age (Kouklari et al., 2018), suggesting that hot deficits are more fundamental for the psychopathology of ASD. Considering the heterogeneity of the disease, more detailed research is required to determine the hot–cold profile in ASD, and its subtypes.
Other relevant disorders
Hot–cold executive dysfunctions and respective pathophysiology in the prefrontal-cingular network are prominent in psychopathology other neuropsychiatric disorders as well including but not limited obsessive–compulsive disorders (cold-deficits driven), borderline personality disorder (hot-deficits driven), and impulse control disorders (cold–hot deficits driven) (Gruner and Pittenger, 2017; Schulze et al., 2019). In this line, recent NIBS studies have also shown than modulating activity of the prefrontal-cingular and relevant subcortical regions are promising for the treatment of these disorders (Brevet-Aeby et al., 2016; Molavi et al., 2020a; Rostami et al., 2020).
Conclusion
This review was focused on how hot and cold EFs can be functionally organised in the prefrontal and cingulate cortices. Based on evidence from neuroimaging and NIBS studies, we propose a prefrontal-cingular network that can explain neuronal correlates of hot versus cold EFs more comprehensively and in line with the current network-driven approach. In this network, the lateral PFC and associated regions (e.g. DLPFC, VLPFC, and IFG) along with the ACC, specifically the dorsal ACC, are more closely involved in cold EFs (e.g. attentional control, inhibition, error detection, and working memory), whereas the medial and orbital PFC regions (e.g. VMPFC and OFC) and ventral ACC along with the PCC are more relevant for hot EFs that involve emotional, motivational, reward/punishment based, and social stimuli. The extent to which these regions are hot or cold EF-related does not exclude a role of these networks in the other EFs, but rather indicates a gradual dominance for the respective type of information processing. The hot–cold distinction in EFs, and broadly in cognition, provide a novel, network-based approach for studying underlying pathophysiology in major neuropsychiatric disorders that usually come with both cognitive and emotional disturbances. This can promote more effective therapeutic intervention congruent with cognitive profile of the diseases.
Footnotes
Author contributions: M.A.S. contributed to the conceptualisation, investigation, visualisation, validation, writing – original draft, and writing – review and editing. E.G. contributed to the investigation, visualisation, and writing – review and editing. M.H.A.R. contributed to the investigation. M.A.N. contributed to the supervision, and writing – review and editing.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.A.N. is a member of the Scientific Advisory Board of Neuroelectrics and NeuroDevic. All other authors declare no competing interests.
Funding: The publication of this article was funded by the Open Access Fund of the Leibniz Association.
ORCID iD: Mohammad Ali Salehinejad  https://orcid.org/0000-0003-1913-4677
https://orcid.org/0000-0003-1913-4677
References
- Abend R, Sar-el R, Gonen T, et al. (2019) Modulating emotional experience using electrical stimulation of the medial-prefrontal cortex: A preliminary tDCS-fMRI study. Neuromodulation: Technology at the Neural Interface 22(8): 884–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adenzato M, Brambilla M, Manenti R, et al. (2017) Gender differences in cognitive Theory of Mind revealed by transcranial direct current stimulation on medial prefrontal cortex. Scientific Reports 7(1): 41219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadehgoradel J, Sadeghi Nejati V, Movahed F, et al. (2020) Repeated stimulation of the dorsolateral-prefrontal cortex improves executive dysfunctions and craving in drug addiction: A randomized, double-blind, parallel-group study. Brain Stimulation 13(3): 582–593. [DOI] [PubMed] [Google Scholar]
- Antonini TN, Becker SP, Tamm L, et al. (2015) Hot and cool executive functions in children with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. Journal of the International Neuropsychological Society 21(8): 584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila A. (2019) Executive functions brain functional system. In: Ardila A, Fatima S, Rosselli M. (eds) Dysexecutive Syndromes: Clinical and Experimental Perspectives. Cham: Springer International Publishing, pp. 29–41.. [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. (2004) Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences 8(4):170–177. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. (2014) Inhibition and the right inferior frontal cortex: One decade on. Trends in Cognitive Sciences 18(4): 177–185. [DOI] [PubMed] [Google Scholar]
- Badre D, D’Esposito M. (2009) Is the rostro-caudal axis of the frontal lobe hierarchical? Nature Reviews Neuroscience 10(9): 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Nee DE. (2018) Frontal cortex and the hierarchical control of behavior. Trends in Cognitive Sciences 22(2): 170–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagherzadeh Y, Khorrami A, Zarrindast MR, et al. (2016) Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex enhances working memory. Experimental Brain Research 234(7): 1807–1818. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. (2000) Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex 10(3): 295–307. [DOI] [PubMed] [Google Scholar]
- Benschop L, Poppa T, Medani T, et al. (2021) Electrophysiological scarring in remitted depressed patients: Elevated EEG functional connectivity between the posterior cingulate cortex and the subgenual prefrontal cortex as a neural marker for rumination. Journal of Affective Disorders 281: 493–501. [DOI] [PubMed] [Google Scholar]
- Blair KS, Otero M, Teng C, et al. (2013) Dissociable roles of ventromedial prefrontal cortex (vmPFC) and rostral anterior cingulate cortex (rACC) in value representation and optimistic bias. NeuroImage 78: 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. (2004) Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences 8(12): 539–546. [DOI] [PubMed] [Google Scholar]
- Brevet-Aeby C, Brunelin J, Iceta S, et al. (2016) Prefrontal cortex and impulsivity: Interest of noninvasive brain stimulation. Neuroscience & Biobehavioral Reviews 71: 112–134. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Vanderhasselt M-A. (2014) Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: A systematic review and meta-analysis. Brain and Cognition 86: 1–9. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, et al. (1998) Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280(5364): 747–749. [DOI] [PubMed] [Google Scholar]
- Caruana F, Gerbella M, Avanzini P, et al. (2018) Motor and emotional behaviours elicited by electrical stimulation of the human cingulate cortex. Brain 141(10): 3035–3051. [DOI] [PubMed] [Google Scholar]
- Cerruti C, Schlaug G. (2009) Anodal transcranial direct current stimulation of the prefrontal cortex enhances complex verbal associative thought. Journal of Cognitive Neuroscience 21(10): 1980–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RCK, Shum D, Toulopoulou T, et al. (2008) Assessment of executive functions: Review of instruments and identification of critical issues. Archives of Clinical Neuropsychology 23(2): 201–216. [DOI] [PubMed] [Google Scholar]
- Chen L, Hudaib A-R, Hoy KE, et al. (2020) Efficacy, efficiency and safety of high-frequency repetitive transcranial magnetic stimulation applied more than once a day in depression: A systematic review. Journal of Affective Disorders 277: 986–996. [DOI] [PubMed] [Google Scholar]
- Chib VS, Yun K, Takahashi H, et al. (2013) Noninvasive remote activation of the ventral midbrain by transcranial direct current stimulation of prefrontal cortex. Translational Psychiatry 3(6): e268–e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SS, Pellecchia G, Ko JH, et al. (2012) Effect of continuous theta burst stimulation of the right dorsolateral prefrontal cortex on cerebral blood flow changes during decision making. Brain Stimulation 5(2): 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Bechara A, Damasio H, et al. (2008) Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain 131(5): 1311–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman BA, Trumbo MC, Clark VP. (2012) Enhancement of object detection with transcranial direct current stimulation is associated with increased attention. BMC Neuroscience 13(1): 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Yeung N, Freiwald WA, et al. (2009) Cingulate cortex: Diverging data from humans and monkeys. Trends in Neurosciences 32(11): 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Fu CHY, Lee L, et al. (2006) A systematic review and quantitative appraisal of fMRI studies of verbal fluency: Role of the left inferior frontal gyrus. Human Brain Mapping 27(10): 799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, et al. (2003) Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain 126(10): 2139–2152. [DOI] [PubMed] [Google Scholar]
- De Pisapia N, Barchiesi G, Jovicich J, et al. (2019) The role of medial prefrontal cortex in processing emotional self-referential information: A combined TMS/fMRI study. Brain Imaging and Behavior 13(3): 603–614. [DOI] [PubMed] [Google Scholar]
- Diamond A. (2013) Executive functions. Annual Review of Psychology 64(1): 135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ. (2008) Neuroimaging of cognition: Past, present, and future. Neuron 60(3): 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Rolls ET, Cheng W, et al. (2020) Functional connectivity of the orbitofrontal cortex, anterior cingulate cortex, and inferior frontal gyrus in humans. Cortex 123: 185–199. [DOI] [PubMed] [Google Scholar]
- Elliott R. (2003) Executive functions and their disorders: Imaging in clinical neuroscience. British Medical Bulletin 65(1): 49–59. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N. (2011) New perspectives in attentional control theory. Personality and Individual Differences 50(7): 955–960. [Google Scholar]
- Fellows LK, Farah MJ. (2007) The role of ventromedial prefrontal cortex in decision making: Judgment under uncertainty or judgment per se? Cerebral Cortex 17(11): 2669–2674. [DOI] [PubMed] [Google Scholar]
- Figner B, Knoch D, Johnson EJ, et al. (2010) Lateral prefrontal cortex and self-control in intertemporal choice. Nature Neuroscience 13(5): 538–539. [DOI] [PubMed] [Google Scholar]
- Gasquoine PG. (2013) Localization of function in anterior cingulate cortex: From psychosurgery to functional neuroimaging. Neuroscience & Biobehavioral Reviews 37(3): 340–348. [DOI] [PubMed] [Google Scholar]
- Ghanavati E, Salehinejad MA, Nejati V, et al. (2019) Differential role of prefrontal, temporal and parietal cortices in verbal and figural fluency: Implications for the supramodal contribution of executive functions. Scientific Reports 9(1): 3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilam G, Abend R, Gurevitch G, et al. (2018) Attenuating anger and aggression with neuromodulation of the vmPFC: A simultaneous tDCS-fMRI study. Cortex 109: 156–170. [DOI] [PubMed] [Google Scholar]
- Giraldo-Chica M, Woodward ND. (2017) Review of thalamocortical resting-state fMRI studies in schizophrenia. Schizophrenia Research 180: 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin TE, den Uyl TE, Fregni FF, et al. (2012) Enhancement of selective attention by tDCS: Interaction with interference in a Sternberg task. Neuroscience Letters 512(1): 33–37. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. (2010) Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology 6(1): 285–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Beck J, Schuepbach D, et al. (2008) Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: An fMRI study in severe major depressive disorder. Biological Psychiatry 63(4): 369–376. [DOI] [PubMed] [Google Scholar]
- Gruner P, Pittenger C. (2017) Cognitive inflexibility in obsessive-compulsive disorder. Neuroscience 345: 243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, et al. (2010) The role of the right inferior frontal gyrus: Inhibition and attentional control. NeuroImage 50(3): 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G, Goodwin GM, Harmer CJ. (2004) The role of the anterior cingulate cortex in the counting Stroop task. Experimental Brain Research 154(3): 355–358. [DOI] [PubMed] [Google Scholar]
- Heilbronner SR, Haber SN. (2014) Frontal cortical and subcortical projections provide a basis for segmenting the cingulum bundle: Implications for neuroimaging and psychiatric disorders. The Journal of Neuroscience 34(30): 10041–10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyder K, Suchan B, Daum I. (2004) Cortico-subcortical contributions to executive control. Acta Psychologica 115(2): 271–289. [DOI] [PubMed] [Google Scholar]
- Hill AT, Fitzgerald PB, Hoy KE. (2016) Effects of anodal transcranial direct current stimulation on working memory: A systematic review and meta-analysis of findings from healthy and neuropsychiatric populations. Brain Stimulation 9(2): 197–208. [DOI] [PubMed] [Google Scholar]
- Hirsch CR, Mathews A. (2012) A cognitive model of pathological worry. Behaviour Research and Therapy 50(10): 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson CW, Scott S, Rubia K. (2011) Investigation of cool and hot executive function in ODD/CD independently of ADHD. Journal of Child Psychology and Psychiatry 52(10): 1035–1043. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Kim SH, Park CS, et al. (2010) Acute high-frequency rTMS of the left dorsolateral prefrontal cortex and attentional control in healthy young men. Brain Research 1329: 152–158. [DOI] [PubMed] [Google Scholar]
- Imburgio MJ, Orr JM. (2018) Effects of prefrontal tDCS on executive function: Methodological considerations revealed by meta-analysis. Neuropsychologia 117: 156–166. [DOI] [PubMed] [Google Scholar]
- Ironside M, Browning M, Ansari TL, et al. (2019) Effect of prefrontal cortex stimulation on regulation of amygdala response to threat in individuals with trait anxiety: A randomized clinical trial. JAMA Psychiatry 76(1): 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A, Joyce E, Lunn D, et al. (2016) Abnormal frontostriatal connectivity in adolescent-onset schizophrenia and its relationship to cognitive functioning. European Psychiatry 35: 32–38. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. (2007) The neural correlates of subjective value during intertemporal choice. Nature Neuroscience 10(12): 1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren H, O’Callaghan G, Vidal-Ribas P, et al. (2018) Reward processing in depression: A conceptual and meta-analytic review across fMRI and EEG studies. American Journal of Psychiatry 175(11): 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Wang X, Ti CHE, et al. (2020) Anodal transcranial direct current stimulation of anterior cingulate cortex modulates subcortical brain regions resulting in cognitive enhancement. Frontiers in Human Neuroscience 14: 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konikkou K, Konstantinou N, Fanti K. (2017) Decision making and emotional processing following Theta Burst Transcranial Magnetic Stimulation on medial prefrontal cortex. Brain Stimulation 10(2): 350. [Google Scholar]
- Koob GF, Volkow ND. (2016) Neurobiology of addiction: A neurocircuitry analysis. The Lancet Psychiatry 3(8): 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouklari E-C, Thompson T, Monks CP, et al. (2017) Hot and cool executive function and its relation to theory of mind in children with and without autism spectrum disorder. Journal of Cognition and Development 18(4): 399–418. [Google Scholar]
- Kouklari E-C, Tsermentseli S, Monks CP. (2018) Hot and cool executive function in children and adolescents with autism spectrum disorder: Cross-sectional developmental trajectories. Child Neuropsychology 24(8): 1088–1114. [DOI] [PubMed] [Google Scholar]
- Le TM, Zhornitsky S, Wang W, et al. (2019) Posterior cingulate cortical response to active avoidance mediates the relationship between punishment sensitivity and problem drinking. The Journal of Neuroscience 39(32): 6354–6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ. (2014) The role of the posterior cingulate cortex in cognition and disease. Brain: A Journal of Neurology 137(Pt 1): 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire-Rodger S, Lam J, Viviano JD, et al. (2019) Inhibit, switch, and update: A within-subject fMRI investigation of executive control. Neuropsychologia 132: 107134. [DOI] [PubMed] [Google Scholar]
- Leonard K, Abramovitch A. (2018) Cognitive functions in young adults with generalized anxiety disorder. European Psychiatry 56(1): 1–7. [DOI] [PubMed] [Google Scholar]
- Li D, Karnath H-O, Xu X. (2017) Candidate biomarkers in children with autism spectrum disorder: A review of MRI studies. Neuroscience Bulletin 33(2): 219–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang J, Ye H, et al. (2020) Modulating the activity of vmPFC regulates informational social conformity: A tDCS study. Frontiers in Psychology 11: 566977–566977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Adolphs R, Rangel A. (2011) Social and monetary reward learning engage overlapping neural substrates. Social Cognitive and Affective Neuroscience 7(3): 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, et al. (2012) The brain basis of emotion: A meta-analytic review. The Behavioral and brain sciences 35(3): 121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsman N, Kaping D, Westendorff S, et al. (2014) Beta coherence within human ventromedial prefrontal cortex precedes affective value choices. NeuroImage 85: 769–778. [DOI] [PubMed] [Google Scholar]
- Lockwood PL, Wittmann MK. (2018) Ventral anterior cingulate cortex and social decision-making. Neuroscience & Biobehavioral Reviews 92: 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchiari C, Sala PM, Vanutelli ME. (2018) Promoting creativity through transcranial direct current stimulation (tDCS). A critical review. Frontiers in Behavioral Neuroscience 12: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie LE, Patterson VC, Zwicker A, et al. (2017) Hot and cold executive functions in youth with psychotic symptoms. Psychological Medicine 47(16): 2844–2853. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. (1999) The retrosplenial cortex and emotion: New insights from functional neuroimaging of the human brain. Trends in Neurosciences 22(7): 310–316. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. (2001) Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104(3): 667–676. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. (2003) Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Human Brain Mapping 18(1): 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso LE, Ilieva IP, Hamilton RH, et al. (2016) Does transcranial direct current stimulation improve healthy working memory? A meta-analytic review. Journal of Cognitive Neuroscience 28(8): 1063–1089. [DOI] [PubMed] [Google Scholar]
- Manuel AL, Murray NWG, Piguet O. (2019) Transcranial direct current stimulation (tDCS) over vmPFC modulates interactions between reward and emotion in delay discounting. Scientific Reports 9(1): 18735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcovic V, Vicario Carmelo M, Yavari F, et al. (2021) A systematic review on the effect of transcranial direct current and magnetic stimulation on fear memory and extinction. Frontiers in Human Neuroscience 15: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyi MA, Spielberg JM. (2020) Differential spatial patterns of structural connectivity of amygdala nuclei with orbitofrontal cortex. Human Brain Mapping 42: 1391–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metuki N, Sela T, Lavidor M. (2012) Enhancing cognitive control components of insight problems solving by anodal tDCS of the left dorsolateral prefrontal cortex. Brain Stimulation 5(2): 110–115. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, et al. (2005) Regionally specific disturbance of dorsolateral prefrontal–hippocampal functional connectivity in schizophrenia. Archives of General Psychiatry 62(4): 379–386. [DOI] [PubMed] [Google Scholar]
- Mies GW, van der Molen MW, Smits M, et al. (2011) The anterior cingulate cortex responds differently to the validity and valence of feedback in a time-estimation task. NeuroImage 56(4): 2321–2328. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Claus ED, et al. (2003) Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control☆☆This study was supported by the Beckman Institute for Advanced Science and Technology at the University of Illinois, Urbana-Champaign; Carle Clinic, Urbana, Illinois; and NIMH MD/PhD predoctoral National Research Service Award provided support to M.P.M. (MH12415-01). NeuroImage 18(2): 483–493. [DOI] [PubMed] [Google Scholar]
- Miller BL, Cummings JL. (2017) The Human Frontal Lobes: Functions and Disorders. New York: Guilford Publications. [Google Scholar]
- Miller EK, Cohen JD. (2001) An integrative theory of prefrontal cortex function. Annual Review of Neuroscience 24(1): 167–202. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, et al. (2000) The unity and diversity of executive functions and their contributions to complex ‘frontal lobe’ tasks: A latent variable analysis. Cognitive Psychology 41(1): 49–100. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Engels AS, Herrington JD, et al. (2007) Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology 44(3): 343–351. [DOI] [PubMed] [Google Scholar]
- Molavi P, Aziziaram S, Basharpoor S, et al. (2020. a) Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: A randomized, sham-controlled, parallel-group study. Journal of Affective Disorders 274: 93–102. [DOI] [PubMed] [Google Scholar]
- Molavi P, Salvat Nadermohammadi M, Ghojehbeiglou H, et al. (2020. b) ADHD subtype-specific cognitive correlates and association with self-esteem: A quantitative difference. BMC Psychiatry 20(1): 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejati V, Majdi R, Salehinejad MA, et al. (2021) The role of dorsolateral and ventromedial prefrontal cortex in the processing of emotional dimensions. Scientific Reports 11(1): 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejati V, Salehinejad MA, Nitsche MA. (2018. a) Interaction of the left dorsolateral prefrontal cortex (l-DLPFC) and right orbitofrontal cortex (OFC) in hot and cold executive functions: Evidence from transcranial direct current stimulation; (tDCS). Neuroscience 369(Suppl. C): 109–123. [DOI] [PubMed] [Google Scholar]
- Nejati V, Salehinejad MA, Sabayee A. (2018. b) Impaired working memory updating affects memory for emotional and non-emotional materials the same way: Evidence from post-traumatic stress disorder (PTSD). Cognitive Processing 19(1): 53–62. [DOI] [PubMed] [Google Scholar]
- Nejati V, Sarraj Khorrami A, Nitsche MA. (2020) Transcranial direct current stimulation improves reward processing in children with ADHD. Journal of Attention Disorders. Epub ahead of print 29 May. DOI: 10.1177/1087054720923094. [DOI] [PubMed] [Google Scholar]
- Newman A. (2019) Research Methods for Cognitive Neuroscience. Thousand Oaks, CA: SAGE. [Google Scholar]
- Niendam TA, Laird AR, Ray KL, et al. (2012) Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective, & Behavioral Neuroscience 12(2): 241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolin S, Tan YY, Schwaab A, et al. (2021) An investigation of working memory deficits in depression using the n-back task: A systematic review and meta-analysis. Journal of Affective Disorders 284: 1–8. [DOI] [PubMed] [Google Scholar]
- Nitsche M, Paulus W. (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of Physiology 527(3): 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. (2005) The cognitive control of emotion. Trends in Cognitive Sciences 9(5): 242–249. [DOI] [PubMed] [Google Scholar]
- Öngür D, Price JL. (2000) The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex 10(3): 206–219. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Van Hoesen GW, Mesulam M-M. (1981) Efferent connections of the cingulate gyrus in the rhesus monkey. Experimental Brain Research 42(3): 319–330. [DOI] [PubMed] [Google Scholar]
- Patai EZ, Spiers HJ. (2021) The Versatile Wayfinder: Prefrontal Contributions to Spatial Navigation. Trends in Cognitive Sciences. Epub ahead of print 19 March 2021. DOI: 10.1016/j.tics.2021.02.010. [DOI] [PubMed] [Google Scholar]
- Patriquin MA, DeRamus T, Libero LE, et al. (2016) Neuroanatomical and neurofunctional markers of social cognition in autism spectrum disorder. Human Brain Mapping 37(11): 3957–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecchinenda A, Ferlazzo F, Lavidor M. (2015) Modulation of selective attention by polarity-specific tDCS effects. Neuropsychologia 68: 1–7. [DOI] [PubMed] [Google Scholar]
- Pessoa L. (2009) How do emotion and motivation direct executive control? Trends in Cognitive Sciences 13(4): 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Büchel C. (2010) Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron 66(1): 138–148. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. (2012) The attention system of the human brain: 20 years after. Annual Review of Neuroscience 35: 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E, Welsh MC. (2014) The development of hot and cool executive functions in childhood and adolescence: Are we getting warmer? In: Goldstein S, Naglieri JA. (eds) Handbook of Executive Functioning. New York: Springer, pp. 45–65. [Google Scholar]
- Platt ML, Plassmann H. (2014) Multistage valuation signals and common neural currencies. In: Glimcher PW, Fehr E. (eds) Neuroeconomics (2nd edn). San Diego, CA: Academic Press, pp. 237–258. [Google Scholar]
- Polania R, Nitsche MA, Ruff CC. (2018) Studying and modifying brain function with non-invasive brain stimulation. Nature Neuroscience 21(2): 174–187. [DOI] [PubMed] [Google Scholar]
- Razza LB, Palumbo P, Moffa AH, et al. (2020) A systematic review and meta-analysis on the effects of transcranial direct current stimulation in depressive episodes. Depression and Anxiety 37(7): 594–608. [DOI] [PubMed] [Google Scholar]
- Reimann Z, Miller JR, Dahle KM, et al. (2020) Executive functions and health behaviors associated with the leading causes of death in the United States: A systematic review. Journal of Health Psychology 25(2): 186–196. [DOI] [PubMed] [Google Scholar]
- Rive MM, van Rooijen G, Veltman DJ, et al. (2013) Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neuroscience & Biobehavioral Reviews 37(10 Pt 2): 2529–2553. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Sahakian BJ. (2013) Hot and cold cognition in depression. CNS Spectrums 18(3): 139–149. [DOI] [PubMed] [Google Scholar]
- Rostami R, Kazemi R, Jabbari A, et al. (2020) Efficacy and clinical predictors of response to rTMS treatment in pharmacoresistant obsessive-compulsive disorder (OCD): A retrospective study. BMC Psychiatry 20(1): 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami R, Kazemi R, Nitsche MA, et al. (2017) Clinical and demographic predictors of response to rTMS treatment in unipolar and bipolar depressive disorders. Clinical Neurophysiology 128(10): 1961–1970. [DOI] [PubMed] [Google Scholar]
- Rubia K. (2011) ‘Cool’ inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus ‘hot’ ventromedial orbitofrontal-limbic dysfunction in conduct disorder: A review. Biological Psychiatry 69(12): e69–e87. [DOI] [PubMed] [Google Scholar]
- Rubia K. (2018) Cognitive neuroscience of attention deficit hyperactivity disorder (ADHD) and its clinical translation. Frontiers in Human Neuroscience 12: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Castañeda P, Santiago-Molina E, Aguirre-Loaiza H, et al. (2020) ‘Cool’ and ‘hot’ executive functions in patients with a predominance of negative schizophrenic symptoms. Frontiers in Psychology 11: 2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehinejad MA, Ghanavai E, Rostami R, et al. (2017) Cognitive control dysfunction in emotion dysregulation and psychopathology of major depression (MD): Evidence from transcranial brain stimulation of the dorsolateral prefrontal cortex (DLPFC). Journal of Affective Disorders 210: 241–248. [DOI] [PubMed] [Google Scholar]
- Salehinejad MA, Nejati V, Nitsche MA. (2020. a) Neurocognitive correlates of self-esteem: From self-related attentional bias to involvement of the ventromedial prefrontal cortex. Neuroscience Research 161: 33–43. [DOI] [PubMed] [Google Scholar]
- Salehinejad MA, Nejati V, Mosayebi-Samani M, et al. (2020. b) Transcranial direct current stimulation in ADHD: A systematic review of efficacy, safety, and protocol-induced electrical field modeling results. Neuroscience Bulletin 36(10): 1191–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehinejad MA, Wischnewski M, Nejati V, et al. (2019) Transcranial direct current stimulation in attention-deficit hyperactivity disorder: A meta-analysis of neuropsychological deficits. PLoS ONE 14(4): e0215095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze L, Schulze A, Renneberg B, et al. (2019) Neural correlates of affective disturbances: A comparative meta-analysis of negative affect processing in borderline personality disorder, major depressive disorder, and posttraumatic stress disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 4(3): 220–232. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, et al. (2011) The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience 12(3): 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe MJ, Schoenbaum G. (2016) Back to basics: Making predictions in the orbitofrontal–amygdala circuit. Neurobiology of Learning and Memory 131: 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JM, Karcher NR, Barch DM. (2018) Cognitive deficits in psychotic disorders: A lifespan perspective. Neuropsychology Review 28(4): 509–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick Matthew M, Cohen Jonathan D. (2013) The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron 79(2): 217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Cohen JD, Botvinick MM. (2016) Dorsal anterior cingulate cortex and the value of control. Nature Neuroscience 19(10): 1286–1291. [DOI] [PubMed] [Google Scholar]
- Shiffrin RM, Schneider W. (1977) Controlled and automatic human information processing: II. Perceptual learning, automatic attending and a general theory. Psychological Review 84(2): 127–190. [Google Scholar]
- Stuss DT. (2011) Functions of the frontal lobes: Relation to executive functions. Journal of the International Neuropsychological Society 17(5): 759. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. (2000) Executive functions and the frontal lobes: A conceptual view. Psychological Research 63(3): 289–298. [DOI] [PubMed] [Google Scholar]
- Tik M, Hoffmann A, Sladky R, et al. (2017) Towards understanding rTMS mechanism of action: Stimulation of the DLPFC causes network-specific increase in functional connectivity. NeuroImage 162: 289–296. [DOI] [PubMed] [Google Scholar]
- To WT, Eroh J, Hart J, et al. (2018) Exploring the effects of anodal and cathodal high definition transcranial direct current stimulation targeting the dorsal anterior cingulate cortex. Scientific Reports 8(1): 4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauwenberge V, Sonuga-Barke EJS, Hoppenbrouwers K, et al. (2015) ‘Turning down the heat’: Is poor performance of children with ADHD on tasks tapping ‘hot’ emotional regulation caused by deficits in ‘cool’ executive functions? Research in Developmental Disabilities 47: 199–207. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, et al. (2001) Anterior cingulate cortex, conflict monitoring, and levels of processing. NeuroImage 14(6): 1302–1308. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt MA, De Raedt R, Leyman L, et al. (2010) Role of the left DLPFC in endogenous task preparation: Experimental repetitive transcranial magnetic stimulation study. Neuropsychobiology 61(3): 162–168. [DOI] [PubMed] [Google Scholar]
- Vicario CM, Salehinejad MA, Felmingham K, et al. (2019) A systematic review on the therapeutic effectiveness of non-invasive brain stimulation for the treatment of anxiety disorders. Neuroscience & Biobehavioral Reviews 96: 219–231. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Absher JR, Bush G. (2000) Human retrosplenial cortex: Where is it and is it involved in emotion? Trends in Neurosciences 23(5): 195–196. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Bjork RA, et al. (2001) Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral Prefrontal cortex. NeuroImage 14(6): 1337–1347. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Kennerley SW. (2011) Contrasting reward signals in the orbitofrontal cortex and anterior cingulate cortex. Annals of the New York Academy of Sciences 1239(1): 33–42. [DOI] [PubMed] [Google Scholar]
- Wang Q, Chen C, Cai Y, et al. (2016) Dissociated neural substrates underlying impulsive choice and impulsive action. NeuroImage 134: 540–549. [DOI] [PubMed] [Google Scholar]
- Ward J. (2020) The Student’s Guide to Cognitive Neuroscience. London: Taylor & Francis. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, et al. (2005) Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry 57(11): 1336–1346. [DOI] [PubMed] [Google Scholar]
- Windmann S, Kirsch P, Mier D, et al. (2006) On framing effects in decision making: Linking lateral versus medial orbitofrontal cortex activation to choice outcome processing. Journal of Cognitive Neuroscience 18(7): 1198–1211. [DOI] [PubMed] [Google Scholar]
- Wise RA. (2009) Roles for nigrostriatal – Not just mesocorticolimbic – Dopamine in reward and addiction. Trends in Neurosciences 32(10): 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Raz N. (2014) Prefrontal cortex and executive functions in healthy adults: A meta-analysis of structural neuroimaging studies. Neuroscience & Biobehavioral Reviews 42: 180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainal NH, Newman MG. (2018) Executive function and other cognitive deficits are distal risk factors of generalized anxiety disorder 9 years later. Psychological Medicine 48(12): 2045–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD, Carlson SM. (2012) Hot and cool executive function in childhood and adolescence: Development and plasticity. Child Development Perspectives 6(4): 354–360. [Google Scholar]
- Zelazo PD, Qu L, Müller U. (2005) Hot and cool aspects of executive function: Relations in early development. In: Schneider W, Schumann-Hengsteler R, Sodian B. (eds) Young Children’s Cognitive Development: Interrelationships among Executive Functioning, Working Memory, Verbal Ability, and Theory of Mind. Mahwah, NJ: Lawrence Erlbaum Associates Publishers, pp. 71–93. [Google Scholar]
- Zhao Y, Sallie SN, Cui H, et al. (2021) Anterior Cingulate Cortex in Addiction: New Insights for Neuromodulation. Neuromodulation: Technology at the Neural Interface 24(2): 187–196. [DOI] [PubMed] [Google Scholar]
- Zimmerman DL, Ownsworth T, O’Donovan A, et al. (2016) Independence of hot and cold executive function deficits in high-functioning adults with autism spectrum disorder. Frontiers in Human Neuroscience 10: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]