Graphical abstract
Keywords: SARS-CoV-2, Vaccines, Platforms, Reverse genetics system, mRNA
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the coronavirus disease 2019 (COVID-19) pandemic that emerged in December 2019 in Wuhan city, China. An effective vaccine is urgently needed to protect humans and to mitigate the economic and societal impacts of the pandemic. Despite standard vaccine development usually requiring an extensive process and taking several years to complete all clinical phases, there are currently 184 vaccine candidates in pre-clinical testing and another 88 vaccine candidates in clinical phases based on different vaccine platforms as of April 13, 2021. Moreover, three vaccine candidates have recently been granted an Emergency Use Authorization by the United States Food and Drug Administration (for Pfizer/BioNtech, Moderna mRNA vaccines, and Johnson and Johnson viral vector vaccine) and by the UK government (for University of Oxford/AstraZeneca viral vector vaccine). Here we aim to briefly address the current advances in reverse genetics system of SARS-CoV-2 and the use of this in development of SARS-CoV-2 vaccines. Additionally, we cover the essential points concerning the different platforms of current SARS-CoV-2 vaccine candidates and the advantages and drawbacks of these platforms. We also assess recommendations for controlling the COVID-19 pandemic and future pandemics using the benefits of genetic engineering technology to design effective vaccines against emerging and re-emerging viral diseases with zoonotic and/or pandemic potential.
1. Introduction1
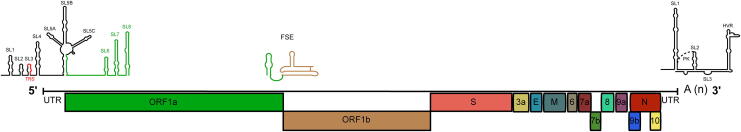
In late December 2019, China reported cases of idiopathic pneumonia in the city of Wuhan. One month later, the causative agent was identified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. SARS-CoV-2 caused the epidemic of coronavirus disease 2019 (COVID-19) in different cities in China, which then spread globally and was later classified as a pandemic by the World Health Organization (WHO) [1], [2]. SARS-CoV-2 belongs to the genus Betacoronavirus in the family Coronaviridae. SARS-CoV-2 is an enveloped virus that contains a single-stranded positive-sense RNA genome of 29,903 nucleotides in length with 11 open reading frames (ORFs), which encode 27 viral proteins. ORF1a/b is 21,290 nucleotides length and encodes 16 nonstructural proteins (nsp1–nsp16). The last part of the genome is 8613 nucleotides that encodes four structural and six accessory proteins. The structural proteins are the spike (S, virus attachment and major antigenic protein), envelope (E), matrix (M), and nucleocapsid (N) proteins while accessory proteins include ORF3a, ORF6, ORF7a, ORF7b, ORF8, and ORF10 as shown in Fig. 1. [3], [4].
Fig. 1.
Genome Organization of SARS-CoV-2. The SARS-CoV-2 RNA genome is ~ 30 kb in length and is organized into at least 11 open reading frames (ORFs). The viral genome is capped at the 5′ end and polyadenylated at the 3′ ends. ORF1a and ORF1b, which occupy the two-thirds of the viral genome, encode the nonstructural proteins (nsp1 to nsp16), whereas the four structural proteins, which include the spike (S), envelope (E), membrane (M), and nucleocapsid (N), are encoded by the structural genes. In addition, accessory proteins are also encoded by the structural genes. Identified cis-acting regulatory elements are also shown at the 5′ end (SL1 to SL8), at the ORF1a/b frameshifting region (FSE), and at the 3′ untranslated region. SL: stem-loop; TRS: transcriptional regulatory sequence; FSE: frameshifting element; PK: pseudoknot; HVR: hypervariable region.
SARS-CoV-2 is a closely related to SARS-CoV that circulated from 2002 to 2004 [1]. SARS-CoV together with the Middle Eastern respiratory syndrome coronavirus (MERS-CoV) have caused epidemics with high case fatality rates in humans [5]. Despite this, there was no current licensed vaccine against coronaviruses in humans, and the reasons for this may be related to the low priority given to these viruses by vaccine developers due to few sporadic human deaths caused by these viruses and their limited geographic distribution. Moreover, a broad variety of viruses cause human colds and so, development of multivalent coronavirus vaccine would only prevent a minor proportion of these [6]. Several vaccines against SARS-CoV were developed pre-clinically with two candidates tested in Phase I trials [7], [8]. However, these clinical phases did not proceed further because these epidemics stopped and these viral infections have not re-emerged in humans since 2004, which might indicate that the control measurements were effective and/or that these viral infections were self-limiting. However, vaccines against MERS-CoV have been actively developed, and through all these pre-clinical studies, the spike protein has been identified as the antigenic target for coronavirus vaccines [9], [10].
Fortunately, lessons learned from pre-clinical and clinical data of SARS-CoV and MERS-CoV vaccine trials provided sufficient experience to design promising SARS-CoV-2 vaccine candidates in just a few weeks after the emergence of this new virus and to begin the first clinical trial in March 2020 (trial number NCT04283461) with an overlapping schedule, such as using Phase I/II trials followed by rapid start of Phase III trials [6]. Currently, 184 vaccine candidates are in pre-clinical development, and 88 vaccine candidates are in clinical development stages [11] with three vaccines recently granted an Emergency Use Authorization (EUA) by the Food and Drug Administration (FDA). Here, we briefly summarize the advantages and disadvantages of the current vaccine candidates/platforms used in Phase III trials against SARS-CoV-2 and their efficacy against the new variants. We also investigate why using recent advances in genetic engineering offers promising solutions for rapid design of effective vaccines against emerging and re-emerging viral diseases that have zoonotic and/or pandemic potential.
2. SARS-CoV-2 reverse genetics system
Reverse genetics (RG) is a powerful tool that is widely used for the genetic manipulation of RNA viruses from their full-length cloned DNA (cDNA) and can lead to the development of successful countermeasures. [12]. A number of RG systems were previously developed for various coronaviruses, including SARS-CoV, MERS-CoV, murine hepatitis virus, and human coronavirus 229E (HCoV-229) [13]. However, difficulties in rescuing infectious viral RNA from cDNA have always been challenging in coronaviruses because of the large size of their genomes and the instability of some regions within the viral genome [13]. To overcome these challenges, a robust RG system for SARS-CoV-2 and other RNA viruses was rapidly developed during the early events of the COVID-19 pandemic [14]. In this yeast-based system, viral overlapping subgenomic cDNA fragments are produced and reassembled in Saccharomyces cerevisiae using transformation-associated recombination cloning [14]. Viral RNA is in vitro transcribed using T7 polymerase and then transfected together with an mRNA that expresses the N protein of the SARS-CoV-2 into BHK-21 cells or BHK cells that express the SARS-CoV N protein (BHK-SARS-N) to recover viable SARS-CoV-2. A similar robust RG system was also developed for SARS-CoV-2 and is based on an in vitro ligation method that resulted in viral replication kinetics similar to the original clinical isolate [15]. In this RG system, the full-length SARS-CoV-2 genome is initially assembled from seven cDNA fragments, one of which contains the T7 promoter, using an in vitro ligation approach followed by in vitro transcription of the cDNA to produce the viral RNA [15]. The recovery of recombinant virus can then be obtained by electroporating the in vitro transcribed viral RNA into Vero E6 cells. A third RG method has been established using bacterial artificial chromosomes (BAC) [16]; and a similar system has been previously used for other coronaviruses such as SARS-CoV [17]. Full-length SARS-CoV-2 cDNA is first assembled by sequentially cloning the five fragments of the viral genome into a BAC plasmid under the control of the cytomegalovirus (CMV) promoter. The SARS-CoV-2 BAC plasmid can then be transfected into Vero E6 or other susceptible cell lines to rescue the infectious recombinant SARS-CoV-2 [16]. Moreover, an additional RG method that relies on the use of the infectious-subgenomic amplicons (ISA) approach has been developed [18]. This involves polymerase chain reaction amplification of eight overlapping synthetic subgenomic cDNA fragments with the insertion of the CMV promoter upstream the first cDNA fragment to allow transcription initiation; the hepatitis delta ribozyme is inserted downstream the last cDNA fragment to terminate the transcription [18]. Rescue of infectious viral particles is achieved by transfecting the full-length cDNA in permissive cells. The main advantage of the ISA approach is that it does not require cloning [19]. Similarly, another approach has also been developed by assembling 10 SARS-CoV-2 cDNA fragments using circular polymerase extension reaction followed by the transfection of the circular genome into a susceptible cell line [19].
Hence, the fast development of these RG systems may answer several questions regarding the SARS-CoV-2 biology and pathogenesis. For example, a recent study used the RG system to generate reporter viruses to gain insight into SARS-CoV-2 pathogenesis and tropism [20]. Furthermore, the availability of these systems could also lead to the development of a live-attenuated vaccine and/or antiviral therapeutics. One way to achieve this is by using these RG systems to characterize and generate mutant viruses via mutating an essential part of the SARS-CoV-2 genome, such as the cis-acting RNA elements within the 5′ and 3′ ends of the viral RNA genome [21], [22]. In addition, RG also enables the study and characterization of the different aspects of viral the life cycle, including replication and pathogenesis [23].
3. Current Phase III vaccine candidates/platforms
Currently, there are 16 vaccine candidates in Phase III trials as of April 13, 2021 [11], with encouraging efficacy data from testing in nonhuman primates and Phase I and II trials. Most current vaccines in Phase III are administered intramuscularly (few are administered using different routes such as skin, e.g., AG0302-COVID19). Intramuscular vaccination induces strong IgG responses that protect the lower respiratory tract but does not induce sufficient secretory IgA to protect the upper respiratory tract, as in the case of natural infection. These Phase III vaccine candidates have been developed using different platforms (Table 1). Several of these platforms have already produced licensed vaccines, whereas other have not, such as the mRNA platform [6]. Here we summarize the major vaccine platforms that have progressed to Phase III trials.
Table 1.
Summary of the current clinical Phase III and IV SARS-CoV-2 vaccine candidates.
| Vaccine/Commercial Name | Developer | Platform | Seroprevalence of Vector Used | Needs Freezing | Need for Booster | Immunogenicity in Humans | Licensed Vaccines From Platform | Phase III Registration | Emergency Use Authorization (EUA) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Weakened adenovirus (ChAdOx1-S; AZD1222)a | University of Oxford/ AstraZeneca | Deficient chimpanzeeadenovirus | Very low | Stable for at least 6 months at 2–8 °C | Yes | High (90%) | No | NCTa04516746 | Yes | 6, 11, 46 |
| Inactivated + alum (CoronaVac; formerly PiCoVacc) | Sinovac | Inactivated whole virus | Very low | No, needs refrigeration | Yes | Unknown | Yes | NCT04456595 | Yes, in UAE and China | 6, 11, 30 |
| Inactivated SARS-CoV-2 | Inactivated Wuhan Institute of Biological Products/ Sinopharm | Inactivated whole virus | Very low | No, needs refrigeration | Yes | Unknown | Yes | ChiCTR-2000034780 | Limited use China and UAE | 6, 11 |
| Inactivated (BBIBP-CorV) | Beijing Institute of Biological Products/ Sinopharm | Inactivated whole virus | Very low | No, needs refrigeration | Yes | Very high (86%) | Yes | ChiCTR-2000034780 | Limited use in China, approved in UAE and Bahrain | 6, 11 |
| Adenovirus Type 5 Vector (Ad5-nCoV) | CanSino Biological Inc./ Beijing Institute of Biotechnology | Deficient adenovirus-5 | High | No, needs refrigeration | Single dose | High | No | NCT04526990NCT04540419 | Limited use in China | 6, 11 |
| Bharat Biotech, India(BBV152) | Covaxin | Inactivated whole virus | Very low | No, needs refrigeration | Yes | Unknown | Yes | CTRI/2020/11/028976 | Yes, in India | 11 |
| Adenovirus-based (Gam-COVID-Vac) | Gamaleya Research InstituteSputnik V | Deficient adenovirus-5 | High | No, needs refrigeration | Yes | Very High (91.4%) | No | NCT04530396 | Early use in Russia | 6, 11 |
| Ad26.COV2.S e | Janssen Pharmaceutical CompaniesAd26.COV2.S | Deficient adenovirus-26 | Very Low but high in sub-Saharan African populations | Stable for 2 years at − 20 °C and 3 months at 2–8 °C | No | Unknown | Yes, Ad26 prime MVA boost-based ebolavirusvaccine was licensed in Europe | NCT04505722 | Yes | 6, 11 |
| Recombinant glycoprotein nanoparticle (NVX-CoV2373) | Novavax | Recombinant protein | N/Ad | Stableat 2–8 °C | Yes | High (89.3%) | Yes, such as FluBlok | 2020–004123-16 | No | 6, 11 |
| 3 LNP-mRNAs (BNT162) Comirnaty | BioNTech/ Fosun Pharma/ Pfizerb | RNA-based vaccine | N/A | Yes (−70 °C) | Yes | Very high (95%) | No | NCT04537949 | Yes | 6, 11 |
| LNP-encapsulated mRNA (mRNA-1273) | Moderna/ NIAIDc | RNA-based vaccine | N/A | Yes (−20 °C) | Yes | Very high (94.5%) | No | NCT04470427 | Yes | 6, 11 |
aThis vaccine has been granted EUA by the UK government, India, Brazil, and the European Union (recent). bThis mRNA vaccine candidate from Pfizer/BioNTech was fully approved in Canada, Bahrain, and Saudi Arabia and was approved for limited or emergency use in USA, UK, Panama, Ecuador, Chile, Costa Rica, Singapore, Mexico, Kuwait, UAE and the European Union (recent). c Moderna’s mRNA vaccine was recently granted FDA EUA and was also recently approved in the UK. d N/A: Not applicable. e This vaccine has been recently granted EUA by the FDA but has been put on hold in USA due to safety concerns.
3.1. Live-attenuated virus vaccines
Currently, there are only two live-attenuated SARS-CoV-2 vaccine candidates in clinical Phase I as of April 13, 2021. The first vaccine candidate, called COVI-VAC (trial number NCT04619628), was developed by Codagenix in cooperation with the Serum Institute of India. The trial for this candidate was started on December 11, 2020. The second vaccine candidate, called MV-014–212 (trial number NCT04798001), was developed by Meissa Vaccines, Inc, with an estimated trial initiation date of March 31, 2021 [11]. Generally, live-attenuated virus vaccines (Fig. 2A) are usually produced by either using an avirulent strain of the virus and/or by constructing a genetically weakened form of the virus, whose limited rounds of replication are insufficient to cause disease but can elicit immune responses similar to that induced by a natural infection. Virus attenuation can be achieved by exposing and adapting the virus to unfavorable conditions such as low temperature growth in a non-susceptible host or cells. Genetic modification can also be used via the RG system for codon de-optimization or deletion of genes that are essential for stimulating innate immune recognition [24], [25].
Fig. 2.
Summary of SARS-CoV-2 Vaccine platforms. A) Live-attenuated vaccine platform in which SARS-CoV-2 is engineered by RG system to produce modified vaccine seed that is used for vaccine production in susceptible cells such as Vero E6 cells. B) Inactivated virus vaccine platform whereby SARS-CoV-2 prepared vaccine seed is propagated (scaled-up) in Vero E6 cells and is then chemically inactivated and finally formulated with a specific adjuvant. C) Protein subunit platform in which whole or part of spike protein, such as the receptor-binding domain, is expressed in mammalian or insect cells and/or yeast, purified, and finally mixed with a specific adjuvant. D) Viral vector platform (replication-deficient adenovirus) in which the adenovirus genome is modified by RG, the open reading frame (ORF) of the spike protein is cloned into adenovirus genome, and finally infectious recombinant virus is rescued in complementing cells. The final rescued virus is adenovirus expressing SARS-CoV-2 spike protein. E) Genetic vaccine (plasmid DNA vaccine) in which SARS-CoV-2 spike ORF is cloned into a plasmid DNA under a strong promoter such as that of human cytomegalovirus, and then, the plasmid is scaled-up in bacteria and finally purified. The final purified plasmid is inoculated into humans using an electroporation gun. F) Genetic vaccine (mRNA vaccine) in which SARS-CoV-2 spike mRNA is chemically synthesized and enclosed with lipid nanoparticles for efficient delivery into human cells. This figure was created with BioRender (https://bioRender.com/).
The advantages of the use of live-attenuated SARS-CoV-2 vaccine candidates include the targeting and stimulation of robust mucosal and cellular immunity, which is essential for protection without the need for adjuvants [26]. However, this type of vaccine has some drawbacks: (1) SARS-CoV-2 has been shown to be excreted in the feces of infected patients [27], [28], and this generates a major safety concern that the live-attenuated SARS-CoV-2 vaccine maybe excreted in the feces of vaccinees, thereby leading to potential virus transmission to unvaccinated individuals. (2) The use of live-attenuated SARS-CoV-2 vaccine may increase the risk of recombination between the vaccine strain and the circulating wildtype virus, generating new viral variants. (3) Production and formulation processes are labor-intensive and require stringent quality control, which makes large-scale vaccine production a slow response to the pandemic [26].
3.2. Inactivated virus vaccine
There are six inactivated vaccine candidates currently in Phase III trials as of April 13, 2021 [11]. The first vaccine candidate, with the trial numbers ChiCTR2000034780 and ChiCTR2000039000, was developed by Sinopharm in collaboration with China National Biotec Group co and Wuhan Institute of Biological Products. The second candidate, with the trial number NCT04659239, was developed by the Chinese Institute of Medical Biology and Academy of Medical Sciences. The third candidate, called Qaz-Covid-in with the trial number NCT04691908, was developed by Research Institute for Biological safety problems, Republic of Kazakhstan. The fourth candidate, called BBV152 with the trial number NCT04641481; CTRI/2020/11/028976, was developed by the Indian company Bharat Biotech International Limited Hyderabad, India [11]. The fifth candidate, with trial numbers NCT04560881 and NCT04510207, was developed by Sinopharm (Shanghai, China) in collaboration with China National Biotec Group Co and the Beijing Institute of Biological Products. The sixth candidate, with trial numbers IRCT20201202049567N1 and IRCT20201202049567N2, was developed by Shifa Pharmed Industrial company (Tehran, Iran) and is currently in Phase II/III trials. An example of this kind of SARS-CoV-2-developed vaccine is CoronaVac, which has been developed by Sinovac Biotech Ltd, China, and has recently been granted limited use in both China and the United Arab Emirates (Table 1). Briefly, inactivated virus vaccines, as shown in Fig. 2B, are usually produced by growing a working virus seed of SARS-CoV-2 in a susceptible cell line, such as Vero E6, followed by chemical inactivation [29], [30]. Additionally, inactivated vaccines are usually adjuvanted with alum or specific oils and then administered intramuscularly; this tends to produce broad immune responses as the vaccine induces targeting of a range of different virus proteins such as S, M, and N [6]. The targeted immune response in this kind of vaccine is usually humoral and cellular, with little reactogenicity, resulting in a high safety profile of the vaccine in the vaccinee. There has been over 70 years of research on the development of such vaccines [6], [26]. However, the production and formulation of this type of vaccine currently face three challenges. (1) Cultivation of the live infectious SARS-CoV-2 to high titers (scale-up) in biosafety level (BSL) 3 facilities, which has major safety implications. (2) Inadequate chemical inactivation of the cultivated virus would pose a potential risk to production facility workers, causing disease outbreaks and/or harmful immune responses. (3) It is challenging to manufacture billions of doses in short time, making response to the pandemic particularly slow [26].
3.3. Protein subunit vaccines
There are seven protein subunit vaccine candidates currently in Phase III trials as of April 13, 2021 [11]. The first candidate was developed by Novavax, Inc. (Gaithersburg, Maryland) (Table 1), with the trial number NCT04611802. The second candidate was developed by Anhui Zhifei Longcom Biopharmaceutical (China) and the Institute of Microbiology, Chinese Academy of Sciences, with the trial number NCT04646590. The third candidate was developed by Sanofi Pasteur (Lyon, France) and GlaxoSmithKline (GSK; Brentford, UK), with the trial number PACTR202011523101903. The fourth candidate was developed by Clover Biopharmaceuticals Inc. (Chengdu, China) with GSK and Dynavax (Emeryville, CA) and is currently in Phase II/III trial, with the trial number NCT04672395. The fourth candidate (called FINLAY-FR-2 anti-SARS-CoV-2 vaccine) was developed by Instituto Finlay de Vacunas (Havana, Cuba), with the trial number RPCEC00000354. The fifth candidate (called EpiVacCorona) was developed by the Federal Budgetary Research Institution State Research Center of Virology and Biotechnology “Vector,” with the trial number NCT04780035. The sixth candidate (called UB-612) was developed by Vaxxinity (Dallas, Texas) and is currently in Phase II/III trial, with the trial number NCT04683224. The seventh candidate (called CIGB-66) was developed by the Center for Genetic Engineering and Biotechnology (CIGB; Trieste, Italy), with the trial number RPCEC00000359 [11].
Protein subunit vaccine candidates (Fig. 2C) depend on expressing S protein or simply a region of it, such as the receptor-binding domain (RBD), or other virus genes in different expression systems, including insect, mammalian, and/or yeast cells [31], [32]. Therefore, this type of vaccine lacks many drawbacks, such as pre-existing antivector immunity, reversion to virulence, and safety concerns of incomplete virus inactivation; hence, they are considered very safe after administration to vaccinees [33]. The target immune response is mainly humoral, which is usually boosted by conjugation with an adjuvant [26].
Moreover, this platform has already produced several licensed vaccines, such as FluBlok for influenza [6]. However, there some drawbacks in this platform, particularly for SARS-COV-2, wherein the S protein is difficult to express, making production process a major challenge. However, this could be solved by expressing only the RBD, although expression of RBD alone may be sub-optimum as this does not include all the antigenic epitopes present in full-length S protein [6]. Another drawback is related to the formulation process, which is labor-intensive and requires development and establishment of new production protocols and stability assays for each new antigen [26].
3.4. Replication-deficient vectors
There are four vaccine candidates currently in Phase III trials using deficient/non-replicating viral vector platform as of April 13, 2021[11]. The first vaccine candidate was developed by CanSino Biological Inc./Beijing Institute of Biotechnology, with the trial numbers NCT04526990 and NCT04540419. The second candidate was developed by Gamaleya Research Institute, Health Ministry of the Russian Federation, with the trial number NCT04530396. The third vaccine candidate was developed by Janssen Pharmaceutical (Beerse, Belgium), with the trial numbers NCT04505722 and NCT04614948. The latter vaccine candidate has recently been granted EUA by the FDA. The fourth candidate (called GRAd-COV2) was developed by ReiThera, Leukocare (Munich, Germany,) and Univercells (Nivelles, Belgium) and is currently in Phase II/III trial, with the trial number NCT04791423 [11].
Replication-deficient vaccines depend on using a weakened virus vector generated by the RG system as shown in Fig. 2D. These vaccines have advantages because of the feasibility of their production process and their broad targeted immune responses that include both humoral and cellular responses without the use of adjuvant [6]. Further, these advantages and many years of experience have recently resulted in some licensed vaccines from this platform, such as Ad-26 prime-modified vaccinia Ankara (MVA) boost-based ebolavirus vaccine [6]. However, there are some drawbacks that are currently associated with their use: (1) Pre-existing immunity against the used viral vector would render the developed vaccine candidate ineffective [34]; however, this could be minimized by priming with another type of vaccine, such as a DNA vaccine [35], or by changing the route of administration between the prime and booster doses [36]. (2) The viral vector itself induces an immune response that subsequently interferes with future vaccines using the same vector [34]. (3) Strong reactivity in vaccinees indicate genetic toxicity induced by the use of some viral vectors; however, this can be minimized by using hybrid vectors [37]. (4) Chance for virus recombination during the production process [26].
3.5. Genetic vaccines (DNA and RNA vaccines)
Currently, there are three DNA-based vaccine candidates in Phase III, whereas there is only one RNA-based vaccine candidate currently in Phase III as of April 13, 2021 [11]. The first DNA-based vaccine candidate (called INO-4800) was developed by Inovio Pharmaceuticals (Plymouth Meeting, PA) with the International Vaccine Institute and Advaccine (Suzhou, China) Biopharmaceutical Co., Ltd., and is currently in Phase II/III, with the trial number NCT04642638. The second candidate (called AG0301-COVID19) was developed by AnGes, Takara Bio (Kusatsu, Shiga, Japan) in collaboration with Osaka University, and is currently in Phase II/III, with the trial number NCT04655625. The third vaccine candidate (called nCov vaccine) was developed by the Zydus Cadila (Ahmedabad, India,), with the trial number CTRI/2020/07/026352 [11]. The RNA vaccine candidate is called CVnCoV vaccine and was developed by CureVac AG (Tübingen, Germany), with the trial number NCT04652102 [11].
DNA vaccines (Fig. 2E) depend on cloning the SARS-CoV-2 S gene into bacterial plasmids that contain a strong mammalian promoter, such as CMV and/or SV40, followed by large plasmid production in competent bacteria. The first proof-of-concept DNA vaccine was tested in 1990 by injecting DNA vectors expressing chloramphenicol acetyltransferase, luciferase, and beta-galactosidase into mouse skeletal muscle [38]. Different possible mechanisms of antigen presentation by DNA vaccines were proposed: (1) Phagocytosis of plasmid-transfected somatic cells such as myocytes by antigen presenting cells (APCs) such as dendritic cells, resulting in antigen presentation to both CD4 and CD8 T-cells. (2) Attraction of APCs to the injection site with subsequent transfection with the injected plasmid DNA; the expressed antigens are presented to T-cells through major histocompatibility (MHC) class I and II complexes [39].
Plasmid DNA vaccines have many advantages, such as targeting and stimulating both humoral and cellular immune responses; flexible and simple large-scale production and formulation processes over short timescales, making them ideal for rapid responses during pandemics; flexibility for multivalency; and room-temperature storage of the final vaccine. However, there are some crucial drawbacks for this type of vaccines: (1) Low immunogenicity in humans, which requires several doses of the vaccine to achieve optimum protection. (2) Risk of carcinogenesis due to potential integration in cellular chromosomes [26].
In contrast, RNA vaccines have shown very promising results in many pre-clinical studies with a significant success recently against COVID-19 with protection percentages of 95% and 94.5% for both US FDA EUA approved vaccine candidates from Pfizer and Moderna, respectively. Generally, the development of an mRNA vaccine is a straightforward process. Once the target antigen from the emerging pathogen is identified, the gene is sequenced, chemically synthesized with some modifications, such as the addition of specific signal peptides and transmembrane domains (Fig. 2F) to target certain cellular locations, and finally cloned into plasmid DNA. The plasmid DNA is then subjected to in vitro transcription, following which the vaccine candidate is ready to be tested in pre-clinical trials. In vivo, mRNA vaccine candidates use the host cell machinery to translate the mRNA to the corresponding antigen, thereby stimulating both humoral and cellular immune responses [40]. Currently, there are two types of mRNA vaccines: (1) Traditional or conventional mRNA encoding the gene of interest (GOI) flanked by 5′ and 3′ untranslated regions (UTRs). (2) Self-amplifying mRNA mainly derived from the genome of positive-stranded RNA viruses, such as alphaviruses and flaviviruses. These are similar to conventional mRNA, but encode the viral replication machinery necessary for intracellular RNA amplification, stimulating high levels of expression of the GOI. Both traditional and self-amplifying mRNA vaccines have components similar to those in eukaryotic mRNAs, such as cap structures, a 5′ UTR followed by the ORF of the GOI, a 3′ UTR, and finally a poly A tail [41]. Manufacturing of mRNA vaccines against targets from different viruses only requires the replacement of the sequence encoding the GOI, without affecting the final physicochemical characteristics of the RNA molecule [40]. This kind of vaccine has several advantages, as it targets both humoral and cellular immune responses,
has a high safety profile, does not result in human genome integration, has no antivector immunity, and has no chance of infectious virus. However, there are some drawbacks with this platform: (1) Storage at very low temperatures (Table 1) is essential to preserve vaccine stability. (2) The high cost of the production process makes this kind of vaccine not ideal for low-income countries [26]. (3) A lack of systematic approaches to the identification of the main mechanisms of physicochemical degradation of the final formulated mRNA vaccine [42]. (4) This platform is relatively new and knowledge about its scale-up is currently lacking [6].
4. Concerns about Adenovirus-5 (Ad-5) vector use
Previous studies, such as the Phambili Phase IIb that assessed the Ad5-HIV vaccine candidate inoculated via three vaccinations, revealed an increased risk of HIV-1 acquisition among vaccinated men [43], [44]. Moreover, the step trial showed that men who had high serum titers for Ad-5 on entry into the trial and were uncircumcised had an elevated risk of HIV-1 acquisition during the first 18 months of follow-up [45]. Moreover, men who were uncircumcised with high serum titers for Ad-5 and who reported unprotected insertive anal sex with a HIV-1-positive partner with HIV-1 antibody titers had the highest risk rate, suggesting the potential for an increased risk of penile acquisition of HIV-1. Additionally, a similar increased risk of HIV infection was also observed in heterosexual men who were enrolled in the Phambili study [43]. On the basis of Ad-5-HIV vaccine candidates, there is concern that the use of SARS-CoV-2 Ad-5-based vaccine may similarly be associated with similar problems among vaccinated men. However, this hypothesis needs further investigation.
In regards to SARS-CoV-2 Ad-5-based vaccines, CanSino reported in their Phase II trial that 266 out of 508 volunteers had high pre-existing antibody titers to the Ad-5 vector. Moreover, the older volunteers exhibited a significantly lower immune response post vaccination, indicating that the SARS-CoV-2 Ad-5 vaccine may not work well in the older population [5], [6]. Another concern is that antibody titers to Ad-5 vector, which may be long-lasting, vary between different ages of the population, making use of Ad-5 vector less promising. However, Janssen Pharmaceuticals uses Ad-26, a rarer serotype with a very low seroprevalence among different human populations, which makes this a more promising vector compared with Ad-5 [6].
A chimpanzee adenovirus vector was developed by the University of Oxford [46]. There is very low seroprevalence in humans against this vector; however, using this vector in humans will also generate immunity against it, which may lower the efficacy of any other future vaccines developed using the same vector. Another drawback of vectored-vaccines in general is that their reactogenicity/safety profile is not high. This may have bigger impacts for vaccinating younger people as they may experience more strong postvaccination reactions that include local symptoms such as redness, swelling and pain at the injection site, as well as systemic symptoms, such as fever and/or headache. These reactions could be a drawback in countries with large young populations such as India and many Middle Eastern countries [6].
5. Promising virus vectors requiring further exploration
5.1. Measles virus vector
The measles virus (MV) belongs to the genus Morbillivirus, family Paramyxoviridae, and ranges from 150 to 350 nm in size. MV is an enveloped negative-sense virus with a non-segmented, single-stranded RNA genome that is between 15,894 to 16,000 kb in length and encodes six structural proteins, including nucleoprotein (N), phosphoprotein (P), matrix (M), fusion (F), hemagglutinin (H), polymerase (L), and two nonstructural proteins, V and C (Fig. 3A) [47]. MV replicates in the cytoplasm of the infected cell without viral integration into host chromosomes and/or reversion to virulence [48]. MV vaccine strains have shown a high profile of genetic stability, which facilitated scale-up and distribution at low-cost to several countries [48]. Former pre-clinical trials against MERS-CoV [49], [50] and SARS-CoV [51], [52] using an attenuated MV, the Schwartz strain, showed very promising results. Interestingly, the pre-existing immunity to measles vector acquired by earlier infection in the elderly or vaccination in young people did not dampen responses to a Chikungunya-MV-based vaccine [53], making the Schwartz measles vector a very promising platform comparable with adenovirus vectors. A trial in France and Belgium was started in August 2020 to test a SARS-CoV-2 vaccine candidate based on a replicating measles vaccine sponsored by Themis company that was acquired by Merck later (trial number NCT04497298). Moreover, this vaccine [called V591-001- Measles-vector based (TMV-o38)] is currently in Phase I/II trials, with trial number NCT04498247 [11].
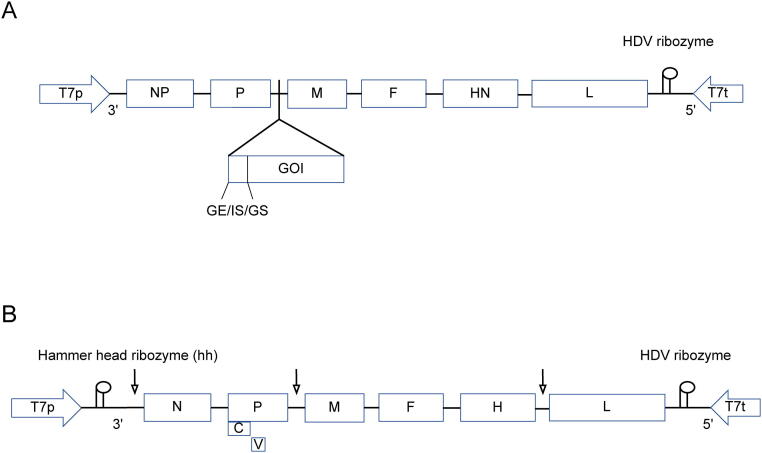
Fig. 3.
Schematic design of recombinant NDV and MV constructs. A) The gene of interest (GOI) is designed according to the rule of six with consideration of sequences of gene end (GE), intergenic sequence (IS), and gene start (GS) of next gene and is cloned into P and M junction of NDV LaSota strain antigenomic cDNA that is under the T7 RNA polymerase promoter (T7p) and the T7 RNA polymerase terminator (T7t) sequence to obtain highest gene expression. B) Schematic design of the recombinant measles virus (MV) vector construct. The GOI is designed according to the rule of six with consideration of sequences as above and is cloned into the full-length viral antigenomic cDNA of the measles such as Schwarz vaccine and cloned at various positions to obtain either high or low protein expression dependent on the insertion site (arrows indication). MV genes: N (nucleoprotein), PVC (phosphoprotein and V/C proteins), M (matrix), F (fusion), H (hemagglutinin), L (polymerase), T7p (T7 RNA polymerase promoter), hh (hammerhead ribozyme), T7t (T7 RNA polymerase terminator), and (hepatitis delta virus ribozyme).
5.2. Newcastle disease virus vector
Recombinant Newcastle disease virus (NDV) vectors developed with the RG system have displayed highly promising results as safe and potent vectored-vaccines against several avian and human pathogens [54]. NDV belongs to subfamily Avulavirinae, genus Orthoavulavirus, species Avian orthoavulavirus [55]. NDV is a negative-sense single-stranded RNA with a genome length ranging from 15,186 to 15,192 bp that encodes six structural polypeptides in the order of (3′-NP-P-MF- HN-L-′5) and two nonstructural proteins (V and W) synthesized by P-gene mRNA editing [56], [57] (Fig. 3B). NDV could be a very promising vector for human vaccine development because of the lack of pre-existing human immunity. Moreover, the NDV vector has potential as a promising vector against several emerging pathogens, particularly those affecting the human respiratory tract, such us SARS-COV. An early study reported that African green monkeys immunized intranasally with two doses of SARS-COV-NDV-based vaccine candidates (NDV-BC/S or NDV-VF/S) developed significant SARS-CoV-neutralizing antibody titers comparable with the robust secondary response observed in animals immunized with a different experimental SARS-CoV vaccine that was based on a recombinant attenuated parainfluenza virus type 3 vector expressing the full-length SARS-CoV S protein, which was developed by the Laboratory of Infectious Diseases, National Institutes of Health, USA, and were then challenged with SARS-CoV [58], [59]. A recent study reported an inactivated SARS-COV-2-NDV-based vaccine candidate elicited high levels of neutralizing antibody titers in both mice and hamsters and significantly protected vaccinated animals from viral challenge [60]. A major advantage of the NDV-based vaccine platform is that the egg-based production of recombinant NDV vaccines can produce millions of doses at low-cost and under BSL2 laboratory conditions, thereby facilitating easy distribution to middle- and low-income countries.
5.3. Adeno-associated virus vector
Adeno-associated virus vector (AAV) is the leading platform for gene delivery with three current licensed products (Glybera, Luxturna, and Zolgensma). AAV vectors potentially have significant number of advantages compared with other vaccine vectors, particularly those based on Ad-5 and poxviruses: 1) AAV vectors are replication-defective viruses [61]; 2) AAV vectors can effectively transduce dividing and nondividing cells and several tissues in vivo; and 3) the presence of different AAV serotypes and variants offers flexibility to develop prime/boost regiments by shifting the nature of the AAV capsids, which avoids an anti-capsid-neutralizing immune response being elicited post-priming. AAV is a small non-enveloped virus in the genus Dependovirus within the family Parvoviridae [62]. AAV is 4.6-kb single-stranded DNA genome that contains two viral genes: rep and cap. These genes can be removed and replaced with a cassette expressing a therapeutic GOI along with the necessary rep and cap genes in trans [63]. The first study that documented the capacity of AAV to induce a strong humoral and cellular response against the herpes simplex virus type 1 glycoprotein B was reported in 1997 [64] followed by an increasing number of uses of AAV for genetic vaccinations.
A recent study showed that recombinant AAV (rAAV) vectors that expressed influenza virus hemagglutinin (HA) or chimeric HA protected mice against homologous and heterologous virus challenges. Unexpectedly, immunization even with wild‐type HA-induced antibodies recognizing the HA‐stalk and activated FcγR‐dependent responses, indicating that AAV‐vectored expression balances HA head (hypervariable part of virus HA glycoprotein among different virus strains) and HA stalk (most conserved part of virus HA glycoprotein among different virus strains) specific humoral responses [65]. A recent study reported that a thermostable SARS-CoV-2 AAV-based (AAVrh32.33 capsid of the AAV-COVID) vaccine candidate demonstrated potent immunogenicity in mice and nonhuman primates after a single injection, with the peak neutralizing antibody titers remained sustained for five months [66].
6. Efficacy of COVID-19 vaccines against emergent variants of SARS-CoV-2
In late 2020, several SARS-CoV-2 variants emerged as a result of mutations that occurred within the RBD of the viral S protein. The first variant was reported in UK and was termed SARS-CoV-2B.1.1.7 (UK variant 20I/501Y.V1) or variant of concern, and has subsequently spread to other countries [67], [68], [69]. Two more variants were reported, known as B.1.351 (501Y.V2) and variant P1 (501Y.V3), also known as the South African variant and the Brazilian variant, respectively [70], [71]. These new SARS-CoV-2 lineages have been suggested to be more transmissible than the original virus, and a recent study has revealed that the B.1.351 and P1 variants are able to escape neutralizing antibodies that are induced after infection or vaccination, which has raised concerns about the efficacy of the currently licensed COVID-19 vaccines [72].
An in vitro study showed that the ChAdOx1 nCoV-19 (AZD1222) vaccine provided sufficient protection against the new B.1.1.7 variant, with 70.4% (95% CI 43.6–84.5) protection in symptomatic cases of the B.1.1.7 variant, compared with 81.5% protection in symptomatic cases of non-B.1.17 variants [73]. Another study has shown that the two-dose regimen of the BNT162b2 vaccine from Pfizer/BioNTech elicited neutralizing antibodies against both the UK and South African variants [74]. However, two doses of the ChAdOx1 nCoV-19 (AZD1222) vaccine had no efficacy against mild-to-moderate COVID-19 symptoms caused by the South African B.1.351 variant [75]. The efficacy of mRNA vaccines, including the Pfizer/BioNTech (BNT162b2) and the Moderna (mRNA-1273) vaccines was significantly reduced against the UK and South African variants [76]. Therefore, evaluation of the currently approved vaccines should be continued and the inevitable ongoing emergence of new SARS-CoV-2 variants should be monitored.
7. Summary and outlook
The main challenge of responding to emerging and re-emerging viral diseases is always in low- and middle-income countries where at least half the global population live. Therefore, to combat any emerging viral disease, scale-up manufacture and globally distribution of developed vaccines should be simple and as straightforward as possible, which is an advantage of live-attenuated vaccines such as measles vaccine. However, the situation is different for SARS-CoV-2 vaccine candidates that have been granted EUA or are currently in Phase III trials as many of these candidates are unlikely to be affordable by low-income countries and will not be cost-effective. This is very important as it is necessary to vaccinate this large human population to establish herd immunity in a short time before emergence of any virus mutant variants. SARS-CoV-2 inactivated vaccine may be of more value than live-attenuated vaccine because of more favorable safety profile, in particular for vulnerable persons. However, scale-up of this vaccine will require using large volumes of the propagated virus under BSL3 conditions and few of these laboratories and facilities are located in low- and middle-income countries. Genetic vaccines (DNA- and RNA-based vaccines) have a considerable promise; however, they have only recently been developed and their long-term safety and efficacy performance in humans requires further investigation. Moreover, scale-up of recently EAU-approved mRNA SARS-CoV-2 vaccines is expensive and needs specific logistics for shipment to low- and middle-income countries, which may not be the best option for those countries. There are many viral vectors that showed highly promising results in clinical trials, but pre-existing immunity is the main challenge for most of these. Therefore, having viral vectors that can evade the pre-existing immunity challenge and can be scaled-up at low-cost, such as measles, AAV, certain serotypes of adenovirus (chimpanzee or Ad-26), and NDV vectors, would be ideal platforms for rapid production and distribution of effective vaccines.
Author contributions
AN and BA conceived the project, collected data from the literature, and wrote and edited the manuscript. All authors interpreted and discussed the data and read and approved the final version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
adeno-associated virus vector; APC, antigen presenting cell; Ad-5, adenovirus-5; BAC, bacterial artificial chromosome; CIGB, Center for Genetic Engineering and Biotechnology; COVID-19, coronavirus disease 2019; CMV, cytomegalovirus; EUA, emergency use authorization; FDA, Food and Drug Administration; GE, gene end; GOI, gene of interest; GS, gene start; HSV, Herpes simplex virus; IS, intergenic sequence; ISA, infectious-subgenomic amplicons; MERS-CoV, Middle Eastern respiratory syndrome coronavirus; MHV, murine hepatitis virus; MV, measles virus; NDV Newcastle disease virus; ORF, open reading frame; RBD, receptor binding domain; RG, reverse genetics; SAR-CoV-2, severe acute respiratory syndrome coronavirus 2.
References
- 1.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu F., Zhao S., Yu B., Chen Y.-M., Wang W. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan J.-F.-W., Kok K.-H., Zhu Z., Chu H., To K.-K.-W. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microb Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helmy Y.A., Fawzy M., Elaswad A., Sobieh A., Kenney S.P. The COVID-19 pandemic: A comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J Clin Med. 2020;9(4):1225. doi: 10.3390/jcm9041225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu L., Zhong W., Bian Z., Li Z., Zhang K. A comparison of mortality-related risk factors of COVID-19, SARS, and MERS: A systematic review and meta-analysis. J Infect. 2020;81(4):e18–e25. doi: 10.1016/j.jinf.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 7.Lin J.-T., Zhang J.-S., Su N., Xu J.-G., Wang N. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Anti Vir Ther. 2007;12:1107. [PubMed] [Google Scholar]
- 8.Martin J.E., Louder M.K., Holman L.A., Gordon I.J., Enama M.E. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine. 2008;26:6338–6343. doi: 10.1016/j.vaccine.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham R.L., Donaldson E.F., Baric R.S. A decade after SARS: Strategies for controlling emerging coronaviruses. Nat Rev Microbiol. 2013;11:836–848. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yong C.Y., Ong H.K., Yeap S.K., Ho K.L., Tan W.S. Recent advances in the vaccine development against Middle East respiratory syndrome-coronavirus. Front Microbiol. 2019;10:1781. doi: 10.3389/fmicb.2019.01781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 12.Bridgen A (2013) Reverse Genetics of RNA Viruses: Applications and Perspectives. Wiley-Blackwell, NJ, USA (1–23 p).
- 13.Armesto M, Bentley K, Bickerton E, Keep S, Britton P (2013) Coronavirus Reverse Genetics. In: Reverse Genetics of RNA Viruses: Applications and Perspectives. Wiley-Blackwell, NJ, USA (25–63 p).
- 14.Thao T., Labroussaa F., Ebert N., V’kovski P., Stalder H. Rapid reconstruction of SARS-CoV-2 using a synthetic genomics platform. Nature. 2020;582:561–565. doi: 10.1038/s41586-020-2294-9. [DOI] [PubMed] [Google Scholar]
- 15.Xie X., Muruato A., Lokugamage K.G., Narayanan K., Zhang X. An infectious cDNA clone of SARS-CoV-2. Cell Host Microbe. 2020;27(5):841–848. doi: 10.1016/j.chom.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye C., Chiem K., Park J.G., Oladunni F., Platt R.N. Rescue of SARS-CoV-2 from a single bacterial artificial chromosome. MBio. 2020;11(5):e02168–e2220. doi: 10.1128/mBio.02168-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almazán F., DeDiego M.L., Galán C., Escors D., Álvarez E. Construction of a severe acute respiratory syndrome coronavirus infectious cDNA clone and a replicon to study coronavirus RNA synthesis. J Virol. 2006;80(21):10900–10906. doi: 10.1128/JVI.00385-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mélade J, de Lamballerie X. Infectious subgenomic amplicons method to expedite reverse genetics of SARS-CoV-2 and other coronaviruses. Available from: Doi: 10.21203/rs.3.rs-59766/v1
- 19.Torii S, Ono C, Suzuki R, Morioka Y, Anzai I, et al. (2020) Establishment of a reverse genetics system for SARS-CoV-2 using circular polymerase extension reaction. Cell Rep: 109014. Available from: https://www.biorxiv.org/content/10.1101/2020.09.23.309849v13. [DOI] [PMC free article] [PubMed]
- 20.Hou Y.J., Okuda K., Edwards C.E., Martinez D.R., Asakura T. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182(2):429–446. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alhatlani B.Y. In silico identification of conserved cis-acting RNA elements in the SARS-CoV-2 genome. Future Virol. 2020;15(7):409–417. doi: 10.2217/fvl-2020-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wacker A., Weigand J.E., Akabayov S.R., Altincekic N., Bains J.K. Secondary structure determination of conserved SARS-CoV-2 RNA elements by NMR spectroscopy. Nucleic Acids Res. 2020;48(22):12415–12435. doi: 10.1093/nar/gkaa1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Da Silva S.J.R., Mendes R.P.G., Da Silva C.T.A., Lorusso A., Kohl A. Insights into SARS-COV-2, the coronavirus underlying COVID-19: Recent genomic data and the development of reverse genetics systems. J Gen Virol. 2020;101(10):1021–1024. doi: 10.1099/jgv.0.001458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talon J., Salvatore M., O'Neill R.E., Nakaya Y., Zheng H. Influenza A and B viruses expressing altered NS1 proteins: A vaccine approach. Proc Natl Acad Sci U S A. 2000;97:4309–4314. doi: 10.1073/pnas.070525997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broadbent A.J., Santos C.P., Anafu A., Wimmer E., Mueller S. Evaluation of the attenuation, immunogenicity, and efficacy of a live virus vaccine generated by codon-pair bias de-optimization of the 2009 pandemic H1N1 influenza virus, in ferrets. Vaccine. 2016;34:563–570. doi: 10.1016/j.vaccine.2015.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frederiksen L.S.F., Yibang Zhang, Foged C., Thakur A. The long road toward COVID-19 herd immunity: Vaccine platform technologies and mass immunization strategies. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y., Li L. SARS-CoV-2: Virus dynamics and host response. Lancet Infect Dis. 2020;20:515–516. doi: 10.1016/S1473-3099(20)30235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xing Y.H., Ni W., Wu Q., Li W.J., Li G.J. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect. 2020;53:473–480. doi: 10.1016/j.jmii.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H., Zhang Y., Huang B., Deng Y., Wang W. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020;182:713–721.e719. doi: 10.1016/j.cell.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Q., Bao L., Mao H., Wang L., Xu K. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26:1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen W.-H., Tao X., Agrawai A.S., Peng Algaissi A, B-H, Yeast-expressed SARS-CoV recombinant receptor-binding domain (RBD219-N1) formulated with alum induces protective immunity and reduces immune enhancement. Vaccine. 2020;38(47):7533–7541. doi: 10.1016/j.vaccine.2020.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang N., Zheng B.J., Lu L., Zhou Y., Jiang S., Du L. Advancements in the development of subunit influenza vaccines. Microbes Infect. 2015;17:123–134. doi: 10.1016/j.micinf.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nayak S., Herzog R.W. Progress and prospects: Immune responses to viral vectors. Gene Ther. 2010;17:295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Z.Y., Wyatt L.S., Kong W.P., Moodie Z., Moss B., Nabel G.J. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J Virol. 2003;77:799–803. doi: 10.1128/JVI.77.1.799-803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandey A., Singh N., Vemula S.V., Couetil L., Katz J.M. Impact of preexisting adenovirus vector immunity on immunogenicity and protection conferred with an adenovirus-based H5N1 influenza vaccine. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0033428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang S., Kamihira M. Development of hybrid viral vectors for gene therapy. Biotechnol Adv. 2013;31:208–223. doi: 10.1016/j.biotechadv.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Wolff J.A., Malone R.W., Williams P., Chong W., Acsadi G. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(1465–1468):1690918. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 39.Li L., Petrovsky N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev Vaccines. 2016;15:313–329. doi: 10.1586/14760584.2016.1124762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maruggi G., Zhang C., Li J., Ulmer J.B., Yu D. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol Ther. 2019;27:757–772. doi: 10.1016/j.ymthe.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geall A.J., Mandl C.W., Ulmer J.B. RNA: The new revolution in nucleic acid vaccines. Semin Immunol. 2013;25:152–159. doi: 10.1016/j.smim.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 42.DaanJA Crommelin, Anchordoquy T.J., Volkin D.B., Jiskoot W., Mastrobattista E. Addressing the cold reality of mRNA vaccine stability. J Pharm Sci. 2021;110:997–1001. doi: 10.1016/j.xphs.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchbinder S.P., Mehrotra D.V., Duerr A., Fitzgerald D.W., Mogg R. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moodie Z., Metch B., Bekker L.G., Churchyard G., Nchabeleng M. Continued follow-up of Phambili phase 2b randomized HIV-1 vaccine trial participants supports increased HIV-1 acquisition among vaccinated men. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0137666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duerr A., Huang Y., Buchbinder S., Coombs R.W., Sanchez J. Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step Study) J Infect Dis. 2012;206:258–266. doi: 10.1093/infdis/jis342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dicks M.D., Spencer A.J., Edwards N.J., Wadell G., Bojang K. A novel chimpanzee adenovirus vector with low human seroprevalence: Improved systems for vector derivation and comparative immunogenicity. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griffin D. (2001) Measles virus. In D. Knipe and P. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa. v.1 (1401–1441).
- 48.Baldo A., Galanis E., Tangy F., Herman P. Biosafety considerations for attenuated measles virus vectors used in virotherapy and vaccination. Hum Vaccin Immunother. 2016;12(5):1102–1116. doi: 10.1080/21645515.2015.1122146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malczyk A.H., Kupke A., Prüfer S., Scheuplein V.A., Hutzler S. A highly immunogenic and protective Middle East respiratory syndrome coronavirus vaccine based on a recombinant measles virus vaccine platform. J Virol. 2015;89(22):11654–11667. doi: 10.1128/JVI.01815-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bodmer B.S., Fiedler A.H., Hanauer J.R.H., Prüfer S., Mühlebach M.D. Live-attenuated bivalent measles virus-derived vaccines targeting Middle East respiratory syndrome coronavirus induce robust and multifunctional T cell responses against both viruses in an appropriate mouse model. Virology. 2018;11(521):99–107. doi: 10.1016/j.virol.2018.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Escriou N., Callendret B., Lorin V., Combredet C., Marianneau P. Protection from SARS coronavirus conferred by live measles vaccine expressing the spike glycoprotein. Virology. 2014;452–453:32–41. doi: 10.1016/j.virol.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liniger M., Zuniga A., Tamin A., Azzouz-Morin T.N., Knuchel M. Induction of neutralising antibodies and cellular immune responses against SARS coronavirus by recombinant measles viruses. Vaccine. 2008;26(17):2164–2174. doi: 10.1016/j.vaccine.2008.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerke C., Frantz P.N., Ramsauer K., Tangy F. Measles-vectored vaccine approaches against viral infections: A focus on Chikungunya. Expert Rev Vaccines. 2019;4:393–403. doi: 10.1080/14760584.2019.1562908. [DOI] [PubMed] [Google Scholar]
- 54.Molouki A., Nagy A. Rescue of recombinant Newcastle disease virus: A promising vector with two decades of intensive vaccine research. Future Virol. 2019;14(9):617–628. doi: 10.2217/fvl-2019-0063. [DOI] [Google Scholar]
- 55.Dimitrov K.M., Abolnik C., Afonso C.L., Albina E., Bahl J. Updated unified phylogenetic classification system and revised nomenclature for Newcastle disease virus. Infect Genet Evol. 2019;74 doi: 10.1016/j.meegid.2019.103917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Samson A.C.R., Levesley I., Russell P.H. The 36K polypeptide synthesized in Newcastle disease virus-infected cells possesses properties predicted for the hypothesized 'V' protein. J Gen Virol. 1991;72(7):1709–1713. doi: 10.1099/0022-1317-72-7-1709. [DOI] [PubMed] [Google Scholar]
- 57.Steward M., Vipond B., Millar N.S., Emmerson P. RNA editing in Newcastle disease virus. J Gen Virol. 1993;74(12):2539–2547. doi: 10.1099/0022-1317-74-12-2539. [DOI] [PubMed] [Google Scholar]
- 58.Bukreyev A., Lamirande E.W., Buchholz U.J., Vogel L.N., Elkins W.R. Mucosal immunisation of African green monkeys (Cercopithecus aethiops) with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet. 2004;363(9427):2122–2127. doi: 10.1016/S0140-6736(04)16501-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DiNapoli J.M., Kotelkin A., Yang L., Elankumaran S., Murphy B.R. Newcastle disease virus, a host range-restricted virus, as a vaccine vector for intranasal immunization against emerging pathogens. Proc Natl Acad Sci U S A. 2007;104:9788–9793. doi: 10.1073/pnas.0703584104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun W., Leist S.R., McCroskery S., Liu Y., Slamanig S. Newcastle disease virus (NDV) expressing the spike protein of SARS-CoV-2 as vaccine candidate. Vaccines. 2020;8(4):771. doi: 10.1016/j.ebiom.2020.103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flotte T.R., Berns K.I. Adeno-associated virus: A ubiquitous commensal of mammals. Hum Gene Ther. 2005;16:401–407. doi: 10.1089/hum.2005.16.401. [DOI] [PubMed] [Google Scholar]
- 62.Melnick J.L., Mayor H.D., Smith K.O., Rapp F. Association of 20-millimicron particles with adenoviruses. J Bacteriol. 1965;90:271–274. doi: 10.1128/jb.90.1.271-274.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Becerra S.P., Koczot F., Fabisch P., Rose J.A. Synthesis of adeno-associated virus structural proteins requires both alternative mRNA splicing and alternative initiations from a single transcript. J Virol. 1988;62:2745–2754. doi: 10.1128/jvi.62.8.2745-2754.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manning W.C., Paliard X., Zhou S., Bland M.P., Lee A.Y. Genetic immunization with adeno-associated virus vectors expressing herpes simplex virus type 2 glycoprotein B and D. J Virol. 1997;71:7960–7962. doi: 10.1128/jvi.71.10.7960-7962.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Demminger DE, Walz L, Dietert K, Hoffmann H, Planz O, et al. (2020) Adeno-associated virus‐vectored influenza vaccine elicits neutralizing and Fcγ receptor‐activating antibodies. EMBO Mol Med 12: e10938. 10.15252/emmm.201910938. [DOI] [PMC free article] [PubMed]
- 66.Zabaleta N, Dai W, Bhatt U, Chichester JA, Estelien R, et al. (2021) Immunogenicity of an AAV-based, room-temperature stable, single dose COVID-19 vaccine in mice and non-human primates. bioRxiv doi: https://doi.org/10.1101/2021.01.05.422952. [DOI] [PMC free article] [PubMed]
- 67.Kidd M, Richter A, Best A, Cumley N, Mirza J, et al. (2021) S-variant SARS-CoV-2 lineage B1.1.7 is associated with significantly higher viral loads in samples tested by ThermoFisher TaqPath RT-qPCR. J Infect Dis. 10.1093/infdis/jiab082. [DOI] [PMC free article] [PubMed]
- 68.Galloway S.E., Paul P., Maccannell D.R., Johansson M.A., Brooks J.T. Emergence of SARS-CoV-2 B.1.1.7 lineage - United States, December 29, 2020–January 12, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:95–99. doi: 10.15585/mmwr.mm7003e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Claro I.M., da Silva Sales F.C., Ramundo M.S., Candido D.S., Silva C.A.M. Local transmission of SARS-CoV-2 lineage B.1.1.7, Brazil, December 2020. Emerg Infect Dis. 2021;27:970–972. doi: 10.3201/eid2703.210038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mwenda M., Saasa N., Sinyange N., Busby G., Chipimo P.J. Detection of B.1.351 SARS-CoV-2 Variant Strain - Zambia, December 2020. MMWR Morb Mortal Wkly Rep. 2021;70:280–282. doi: 10.15585/mmwr.mm7008e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Francisco R.D.S., Jr., Benites L.F., Lamarca A.P., de Almeida L.G.P., Hansen A.W. Pervasive transmission of E484K and emergence of VUI-NP13L with evidence of SARS-CoV-2 co-infection events by two different lineages in Rio Grande do Sul, Brazil. Virus Res. 2021;296 doi: 10.1016/j.virusres.2021.198345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoffmann M., Arora P., Grob R., Seidel A., Hörnich B.F. ISSN 0092–8674 SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021 doi: 10.1016/j.cell.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Emary K.R.W., Golubchik T., Aley P.K., Ariani C.V., Angus B. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): An exploratory analysis of a randomised controlled trial. Lancet. 2021;397:1351–1362. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xie X., Liu Y., Liu J., Zhang X., Zou J. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera, E484K and N501Y. Nat Med. 2021;27:620–621. doi: 10.1038/s41591-021-01270-4. [DOI] [PubMed] [Google Scholar]
- 75.Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 Variant. N Engl J Med. 2021 doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Z., Schmidt F., Weisblum Y., Muecksch F., Barnes C.O. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021 doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]