Abstract
Objective
To evaluate the impact of surgical simulation training using a three-dimensional (3D)-printed model of tetralogy of Fallot (TOF) on surgical skill development.
Materials and Methods
A life-size congenital heart disease model was printed using a Stratasys Object500 Connex2 printer from preoperative electrocardiography-gated CT scans of a 6-month-old patient with TOF with complex pulmonary stenosis. Eleven cardiothoracic surgeons independently evaluated the suitability of four 3D-printed models using composite Tango 27, 40, 50, and 60 in terms of palpation, resistance, extensibility, gap, cut-through ability, and reusability of. Among these, Tango 27 was selected as the final model. Six attendees (two junior cardiothoracic surgery residents, two senior residents, and two clinical fellows) independently performed simulation surgeries three times each. Surgical proficiency was evaluated by an experienced cardiothoracic surgeon on a 1–10 scale for each of the 10 surgical procedures. The times required for each surgical procedure were also measured.
Results
In the simulation surgeries, six surgeons required a median of 34.4 (range 32.5–43.5) and 21.4 (17.9–192.7) minutes to apply the ventricular septal defect (VSD) and right ventricular outflow tract (RVOT) patches, respectively, on their first simulation surgery. These times had significantly reduced to 17.3 (16.2–29.5) and 13.6 (10.3–30.0) minutes, respectively, in the third simulation surgery (p = 0.03 and p = 0.01, respectively). The decreases in the median patch appliance time among the six surgeons were 16.2 (range 13.6–17.7) and 8.0 (1.8–170.3) minutes for the VSD and RVOT patches, respectively. Summing the scores for the 10 procedures showed that the attendees scored an average of 28.58 ± 7.89 points on the first simulation surgery and improved their average score to 67.33 ± 15.10 on the third simulation surgery (p = 0.008).
Conclusion
Inexperienced cardiothoracic surgeons improved their performance in terms of surgical proficiency and operation time during the experience of three simulation surgeries using a 3D-printed TOF model using Tango 27 composite.
Keywords: 3D printing, Congenital heart disease, Simulation surgery, Cardiothoracic surgery
INTRODUCTION
Proper training of surgeons, especially cardiothoracic surgeons, is a costly process worldwide, requiring a large amount of time. In particular, owing to the rarity of individual diseases [1], the limited number of institutions performing curative surgeries, difficult surgical procedures requiring an accurate understanding of complex three-dimensional (3D) anatomy, and risk of training in the operating room, adequate training of congenital heart disease surgery is extremely challenging. Nonetheless, congenital heart disease remains the most common significant birth defect in live births, with a prevalence of 4–50/1000 [2,3,4].
In this context, simulation surgery utilizing 3D-printed models may help enhance the surgical skills of inexperienced cardiothoracic surgeons [5]. Recently, owing to the high resolution of CT and the development of 3D printing technology, various attempts have been made to simulate sophisticated surgeries beforehand using 3D-printed models [6,7,8,9]. In congenital heart disease, various 3D models have been reported since the initial description by Binder et al. [10,11,12,13] in 2000.
Simulation surgeries using 3D-printed models have been used for congenital heart disease [14]. Some researchers have reported the usefulness of preoperative simulation before surgeries or interventional therapies [15,16,17,18]. Hussein et al. [19,20] reported increased surgeon performance after simulation surgeries for arterial switch operation and Yoo et al. [21] reported on a hands-on trial for trainees. However, few studies have compared the composite hardness of various 3D-printed models and analyzed their effectiveness in inexperienced surgeons.
This study compared various composite hardnesses to identify the most suitable congenital heart disease model for simulation surgeries and evaluated the effectiveness of the simulation surgeries for inexperienced cardiothoracic surgeons. We aimed to evaluate the impact of surgical simulation training in surgical skill development using a 3D-printed model of tetralogy of Fallot (TOF), the third most common surgical congenital heart disease, after atrial septal defect and ventricular septal defect (VSD) [1].
MATERIALS AND METHODS
Case Selection
The case used for the simulation surgeries was based on CT images [22] of a 6-month-old male patient with TOF with complex pulmonary stenosis. Electrocardiography-gated CT was performed in retrospective gating and reconstructed in the diastolic phase using a 64-slice dual-source CT scanner (SOMATOM Definition, Siemens Healthineers). The scanning parameters were as follows: tube voltage, 80 kVp; slice thickness, 0.75 mm; and reconstruction interval, 0.75 mm. The Institutional Review Board of Seoul National University Hospital approved the use of the patient's CT images and waived the requirement for informed consent (IRB No. 1502-045-647).
Image Post-Processing and Composite Selection
Postprocessing and Model Printing
The postprocessing procedure included segmentation, conversion of the Digital Imaging and Communication in Medicine (DICOM) file to stereolithography or Standard Tessellation Language (STL) formats, and computer-aided design. During segmentation, the pooled blood was segmented using the thresholding technique with a 1.5 mm offset in Mimics Innovation Suite (Materialise NV, Belgium) and then converted to the 3D object. The computer-aided design software 3-matic (Materialise NV, Belgium) was then used for further image modification. The “hollow” function in the outside direction was applied in the software, followed by manual adjustment using the “trim (cut)” function. Insignificant structures were excluded to reveal significant structures and reduce the cost. Surface smoothing was performed using “local smoothing” and the software was used under optimal parameters (diameter and strength). Additionally, a plate-like structure was added to support the model during the simulation surgery (Fig. 1C). After segmentation, the 3D data were converted to the STL format for 3D printing.
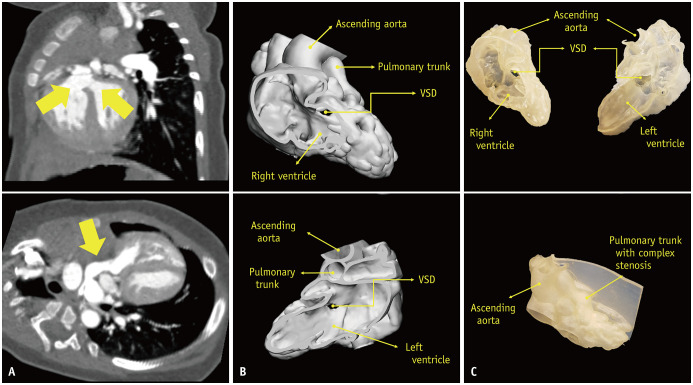
Fig. 1. A case of a 6-month-old male patient was selected for our simulation model.
A. On the preoperative CT scan, VSD and overriding aorta (arrows above) with complex pulmonary stenosis (arrow below) are visualized. B. After segmentation, the Digital Imaging and Communication in Medicine data were converted to Stereolithography or Standard Tessellation Language format for three-dimensionall printing using 3-matic computer-aided design software. C. The printed model using Tango 27 composite successfully demonstrated major abnormalities including VSD, overriding ascending aorta, and complex pulmonary stenosis. VSD = ventricular septal defect
The construction of the model, including segmentation and printing, required about 15 hours: 3 hours for segmentation, 2 hours for engineering design for rapid prototyping preparation, and 10 hours for unsupervised automated printing. The supporting material was removed mechanically by water jet blasting and a dissolving solvent (0.4 kg NaOH and 0.2 kg Na2SiO3 dissolved in 20 L of water) was then applied [23]. No additional coating was performed as the model was deemed appropriate for the surgical procedures. The printing cost was approximately $100 for each model, including printing material, the actual printing process, and supporting material removal using a dissolving solvent. The VSD, overriding aorta, and complex pulmonary stenosis were successfully visualized in the model (Fig. 1).
Preliminary Evaluation of Composite Hardness
The final format was printed on a life-size scale using a Stratasys Object500 Connex2 printer (Stratasys). The preliminary models were printed with seven different composite hardness values of composite Tango (Stratasys) with shore values of 27, 40, 50, 60, 70, 85, and 95, where a higher number indicated a harder texture. Two board-certified cardiothoracic surgeons with 24 and 6 years of experience, respectively, evaluated the suitability of the models for the simulation surgery and selected four candidates (Tango 27, 40, 50, and 60) for simulation surgery.
Four models were printed using the four different composite hardness candidates selected in the preliminary evaluation. Eleven cardiothoracic surgeons (one professor, two board-certified clinical fellows, five senior residents, and three junior residents) independently performed simple surgical procedures, including suture and dissection, and evaluated the suitability of the models for simulation surgeries in terms of palpation (softness of the model when manually handled), resistance (resistance when needling the model with 5-0 Prolen), extensibility (ability to extend when pulling a suture needle), gap (dehiscence of the material when retracting the model with a suture needle), cut-through ability (ability to cut the model using a surgical scissor), and reusability (ability to reuse the model), on a five-point scale in which higher values indicated more human tissue-like texture (except for reusability), indicating a more suitable model for simulation surgeries, while higher reusability indicated more durable material after sutures [24]. The Tango 27 composite ranked the highest in the pooled score of palpation, resistance, extensibility, and gap categories, achieving the highest average score of 3.92 (Supplementary Table 1). Thus, Tango 27 was selected as the final composite hardness.
Simulation Surgery
For the printed model using selected composite hardness, six attendees (two junior, two senior cardiothoracic surgery residents, and two clinical fellows) each conducted simulation surgeries three times. The simulation surgeries were performed in an actual operating room with an assisting surgical nurse, under the same setting as actual cardiac surgery (Fig. 2, Supplementary Movie 1). The simulation surgery comprised the following nine procedures: patch design for VSD closure (procedure 1), patch closure of VSD with bovine pericardium-inferior margin (procedure 2), posteroinferior margin (procedure 3), anterosuperior margin (procedure 4), superior margin (procedure 5), saline leakage test of the VSD, main pulmonary arteriotomy (procedure 6), right ventriculotomy (procedure 7), patch design for transannular right ventricular outflow tract (RVOT) widening (procedure 8), patch angioplasty of the main pulmonary artery (procedure 9), and RVOT with bovine pericardium (procedure 10), pass test with number 10 Hegar dilator (CooperSurgical Inc.), and saline leakage test for the RVOT. To evaluate the surgical skillfulness of the attendees, the time required for the two main procedures, the appliance of the VSD (procedures 2–5) and RVOT (procedures 9–10) patches, were measured individually. None of the participants knew about this evaluation method of surgical skillfulness. Each surgeon performed three simulation surgeries at approximately 1-week intervals. Every simulation surgery was video-recorded and observed by one cardiothoracic surgery professor. The skillfulness of each procedure was evaluated by the professor reviewing the recorded videos on a 1–10 scale, in which higher scores indicated a more skilled performance.
Fig. 2. Example of a simulation surgery performed by six cardiothoracic surgeons.
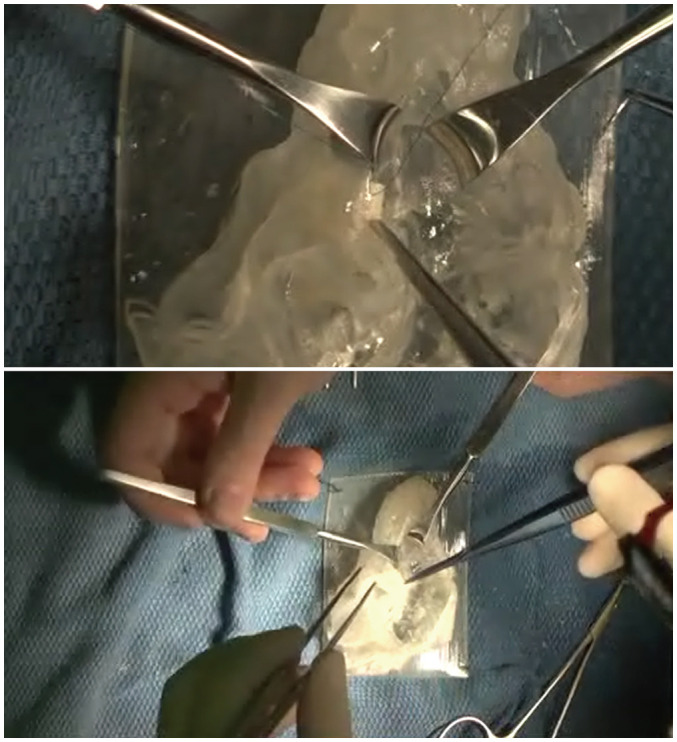
Each simulation surgery was conducted in an operating room with an assisting surgical nurse. All surgical procedures were video-recorded (Supplementary Movie 1).
Statistical Analysis
The times required for each simulation surgery and subjective evaluation of surgical proficiency were compared by either Wilcoxon signed-rank or paired t tests after normality tests, as appropriate. The statistical analyses were performed using MedCalc software version 15.8 (MedCalc). For all tests, p < 0.05 were considered to indicate statistical significance.
RESULTS
Surgical Time Reduction for VSD and RVOT Patch Appliance
Six attendees conducted three simulation surgeries independently. The times required by each surgeon to apply the VSD (procedures 2–5) and RVOT (procedures 9–10) patches are shown in Table 1. For the VSD patch appliance, six surgeons required a median of 34.4 minutes (range, 32.5–43.5 minutes) on the first simulation surgery; this time decreased to 24.3 (16.5–31.9) and 17.3 (16.2–29.5) minutes in the second and third simulation surgeries, respectively. Regarding RVOT patch application, the six surgeons required 21.4 (17.9–192.7) minutes in the first simulation surgery, which decreased to 16.1 (4.2–27.6) and 13.6 (10.3–30.0) minutes in the second and third simulation surgeries, respectively. All six attendees had reduced surgery times from the first to third simulation surgeries. Both VSD and RVOT patch appliance times decreased significantly from the first to third simulation surgeries (p = 0.03 and p = 0.01, respectively). The median decreases in patch appliance time among the six surgeons were 16.2 (13.6–17.7) minutes and 8.0 (1.8–170.3) minutes for the VSD and RVOT patches, respectively (Table 2). The amount of time decrease was not correlated with attendee experience (p > 0.05).
Table 1. Time Consumed by Each Cardiothoracic Surgeon for Each Surgical Procedure during Three Simulation Surgeries.
| Surgeon* (Years) | Simulation 1 | Simulation 2 | Simulation 3 | Time Reduced between Simulation 1 and 3† | ||||
|---|---|---|---|---|---|---|---|---|
| VSD Patch | RVOT Patch | VSD Patch | RVOT Patch | VSD Patch | RVOT Patch | VSD Patch (%) | RVOT Patch (%) | |
| Surgeon 1, 2 | 37′ 28″ | 192′ 35″ | 29′ 53″ | 27′ 34″ | 23′ 54″ | 22′ 20″ | 13′ 34″ (36.2) | 170′ 15″ (88.4) |
| Surgeon 2, 2 | 43′ 30″ | 36′ 50″ | 29′ 30″ | 23′ 54″ | 29′ 29″ | 33′ 01″ | 14′ 01″ (32.2) | 6′ 49″ (10.4) |
| Surgeon 3, 3 | 35′ 07″ | 17′ 56″ | 20′ 30″ | 4′ 11″ | 17′ 23″ | 10′ 19″ | 17′ 44″ (50.5) | 7′ 37″ (42.5) |
| Surgeon 4, 4 | 33′ 08″ | 24′ 02″ | 16′ 27″ | 36′ 29″ | 16′ 19″ | 22′ 24″ | 16′ 59″ (50.8) | 13′ 45″ (6.8) |
| Surgeon 5, 5 | 33′ 38″ | 18′ 40″ | 17′ 32″ | 13′ 12″ | 16′ 09″ | 10′ 17″ | 17′ 29″ (52.0) | 8′ 23″ (44.9) |
| Surgeon 6, 5 | 32′ 28″ | 18′ 38″ | 31′ 55″ | 18′ 57″ | 17′ 10″ | 16′ 53″ | 15′ 18″ (47.1) | 1′ 45″ (9.4) |
| Average | 35′ 53″ | 51′ 27″ | 24′ 18″ | 16′ 50″ | 20′ 02″ | 16′ 41″ | 15′ 51″ (44.2) | 34′ 46″ (67.6) |
| Median | 34′ 23″ | 21′ 21″ | 25′ 00″ | 16′ 05″ | 17′ 17″ | 13′ 36″ | 16′ 09″ (49.7) | 8′ 00″ (36.3) |
Data are presented as minutes′ seconds′′ consumed by each surgeon on each surgical procedure. *Experience of each surgeon on cardiothoracic surgery training is provided, †Reduced time presented as minutes′ seconds′′ (% decrease). RVOT = right ventricular outflow tract, VSD = ventricular septal defect
Table 2. Comparison of Time Consumed by Pooled Cardiothoracic Surgeons during Each Simulation Surgery.
| VSD Patch Appliance | RVOT Patch Appliance | |
|---|---|---|
| Time consumed by 6 cardiothoracic surgeons* | ||
| 1st simulation | 35.9 ± 4.1 | 51.5 ± 69.5 |
| 34.4 (32.5–43.5) | 21.4 (17.9–192.3) | |
| 2nd simulation | 24.3 ± 6.9 | 16.8 ± 8.4 |
| 25.0 (16.5–31.9) | 16.1 (4.2–27.6) | |
| 3rd simulation | 20.0 ± 5.5 | 16.7 ± 8.2 |
| 17.3 (16.2–29.5) | 13.6 (10.3–30.0) | |
| p values from comparison tests between simulation surgeries† | ||
| 1st vs. 2nd simulation | 0.03 | 0.06 |
| 2nd vs. 3rd simulation | 0.03 | 1.00 |
| 1st vs. 3rd simulation | 0.03 | 0.01 |
*Data provided with mean ± SD followed by median (range) minutes of time consumed by six cardiothoracic surgeons, †p values calculated from Wilcoxon signed-rank test or paired t test was used after normality test. RVOT = right ventricular outflow tract, VSD = ventricular septal defect
Subjective Evaluation of Surgical Proficiency
Subjective evaluation of attendee skillfulness showed significant improvement for all 10 surgical procedures from the first to third simulation surgeries (Table 3). The attendees showed difficulties in suturing the anterosuperior margin of the VSD patch (procedure 4; average score 2.00 ± 0.85) and patch angioplasty of the RVOT (procedure 10; average score 2.17 ± 0.84) but showed improved performance for both procedures in the third simulation surgery (average scores 5.67 ± 1.41 and 5.56 ± 1.67; p = 0.007 for both). The summed scores for 10 procedures increased from 28.58 ± 7.89 to 67.33 ± 15.10 (p = 0.008) (Table 3).
Table 3. Performance Evaluation Results of 10 Surgical Procedures during the Three Simulation Surgeries.
| Procedures* | Simulation 1 | Simulation 2 | Simulation 3 | P† | ||
|---|---|---|---|---|---|---|
| 1 vs. 2 | 2 vs. 3 | 1 vs. 3 | ||||
| Procedure 1 | 3.42 ± 0.79 | 5.58 ± 1.00 | 7.78 ± 1.72 | 0.001 | 0.007 | 0.007 |
| Procedure 2 | 3.17 ± 0.72 | 5.33 ± 1.07 | 7.33 ± 1.58 | 0.002 | 0.006 | 0.007 |
| Procedure 3 | 2.83 ± 0.84 | 4.83 ± 0.94 | 6.67 ± 1.32 | 0.001 | 0.006 | 0.007 |
| Procedure 4 | 2.00 ± 0.85 | 4.08 ± 1.00 | 5.67 ± 1.41 | 0.001 | 0.006 | 0.007 |
| Procedure 5 | 3.08 ± 0.79 | 5.08 ± 1.08 | 6.78 ± 1.56 | 0.002 | 0.006 | 0.007 |
| Procedure 6 | 3.08 ± 0.90 | 5.25 ± 1.14 | 7.11 ± 1.54 | 0.001 | 0.006 | 0.007 |
| Procedure 7 | 2.50 ± 1.17 | 4.42 ± 1.08 | 6.33 ± 1.50 | 0.001 | 0.006 | 0.007 |
| Procedure 8 | 3.25 ± 0.75 | 5.25 ± 1.22 | 7.56 ± 1.74 | 0.002 | 0.006 | 0.007 |
| Procedure 9 | 3.08 ± 0.90 | 5.00 ± 1.13 | 6.56 ± 1.67 | 0.002 | 0.006 | 0.007 |
| Procedure 10 | 2.17 ± 0.84 | 4.00 ± 1.13 | 5.56 ± 1.67 | 0.002 | 0.006 | 0.007 |
| Sum | 28.58 ± 7.89 | 48.83 ± 10.18 | 67.33 ± 15.10 | 0.002 | 0.008 | 0.008 |
Data provided with mean ± SD scores of six attendees evaluated by one cardiothoracic surgery professor in 1–10 scale, higher score indicating more skillful procedure. *Procedures of each simulation surgery was divided into 10 procedures: patch design for VSD closure (procedure 1), patch closure of VSD with bovine pericardium-inferior margin (procedure 2), posteroinferior margin (procedure 3), anterosuperior margin (procedure 4), and superior margin (procedure 5), saline leakage test for VSD, main pulmonary arteriotomy (procedure 6), right ventriculotomy (procedure 7), patch design for the transannular right ventricular outflow track widening (procedure 8), patch angioplasty of main pulmonary artery (procedure 9) and right ventricular outflow tract with bovine pericardium (procedure 10), pass test with number 10 Hegar dilator (CooperSurgical Inc.), and saline leakage test for the right ventricular outflow tract, †p values calculated from Wilcoxon signed-rank test or paired t test was used after normality test. VSD = ventricular septal defect
DISCUSSION
The results of this study demonstrated that, among various composite hardnesses, Tango 27 showed the best scores in terms of palpation, resistance, extensibility, and gap. The final product successfully visualized all patient anomalies and enabled all surgical procedures for cardiothoracic surgeons. Six inexperienced cardiothoracic surgeons successfully conducted a series of three simulation surgeries individually and showed significantly improved surgical proficiency and reduced VSD and RVOT patch appliance times from the first to third simulation surgeries.
Although various studies have reported successful 3D printing for congenital heart disease models, few have assessed the effectiveness of simulation surgeries on surgeon performance [10,11,12,13,25]. In this study, 11 thoracic surgeons first evaluated the most appropriate composite and six cardiothoracic surgeon attendees performed three simulation surgeries in actual operating rooms to observe the effect on surgical proficiency. The median timed required for VSD and RVOT patch appliance reduced by approximately 15 minutes and 8 minutes, respectively, corresponding to time reductions of approximately 47% and 38% from the first simulation surgery. Additionally, subjectively evaluated surgical proficiency substantially increased during the experience of simulation surgeries. Unlike other reports on the effectiveness of simulation surgeries [26,27], our study has a strength in that we calculated objective and subjective measures representing the skillfulness in surgical procedures rather than a subjective evaluation of the experience. This result corresponds to that of a previous study reporting increased surgical proficiency and reduced operation time for arterial switch operation between two sequential simulation surgeries [19,20]. Furthermore, our study results demonstrated further improvement in surgical skills from the second to third simulations, implying the additional effects of repeated experience of simulation surgeries.
While our 3D-printed model of TOF visualized the major anomalies of the patient it was not the same as an actual human heart. Real open-heart surgery is more complex than our simulation surgeries and requires additional unique procedures, including the approach to the heart, insertion of catheters for cardiopulmonary bypass pump, and opening of the heart. In our model, we partly opened the right atrium and the right ventricle to allow easy access to the VSD. Additionally, tricuspid valve structures and pulmonary valves were not visualized. We simplified the model to focus on the teaching and training of the most important part of cardiac surgery and the pathologic anatomy of the disease. While the model itself could be designed to be more complex, we aimed to show the possibility of surgical training using a 3D-printed model.
Other reasons contributed to the simplification of the model. To train various doctors, a large number and variety of models are needed. To reduce the cost, the total mass and profile of the models must be reduced. We focused on the most important structures and tried to minimize the efforts to visualize relatively less important structures such as the right ventricle anterior wall and left ventricular posterior wall. Another reason for simplifying the model was technical reasons. The valve structures are indeed important for surgical training; however, the 3D printing process requires the construction of a supporting structure or material followed by postprocessing to remove them. The valves could be a major obstacle to the entire process; thus, we eliminated complex valve structures from the model.
The printing materials available for 3D printers are limited [28,29]. We did not have many options and chose Tango 27 for the surgical training model because it enabled proper surgical procedures, including stitches, patch closure of the VSD, and incision along the right ventricular outflow to the pulmonary artery followed by patch widening. While the texture of the model was not exactly that of human heart tissue, we concluded that it would be sufficient for surgical training. With the increasing application of 3D printing for surgical training, diversification of printing materials is warranted to provide improved surgical simulation.
Our study has some limitations. First, we performed three simulation surgeries using the same case. Even for the same disease entity, patients with congenital heart disease tend to have various anatomic and hemodynamic differences. As it is rarely possible to meet the same patient case, the preparation of various models with the same disease entity might be needed. Further application of the technique to various cases would be beneficial. Second, owing to the nature of 3D printing, model preparation has high costs and requires intensive labor. The advancement of 3D printing technology is expected to solve these problems. Third, the categories we used to compare various composites might be controversial and the results did not differ significantly among the different composites. In addition, as there are various printing materials depending on the printers and a new material, Agilus, was released for the specific printer we used, our results evaluating Tango may lack clinical significance. However, the evaluation criteria we proposed could be referenced and further modified by other researchers. Finally, the time required to perform the surgical procedure is not a definite parameter for evaluating surgical proficiency. Other objective measures, which may predict the clinical outcome of the surgery, would be beneficial.
In conclusion, inexperienced cardiothoracic surgeons showed improved performance in terms of surgical proficiency and operation time during the experience of three simulation surgeries using a 3D-printed TOF model using Tango 27 composite. Further improvements in 3D printing technology and the design of simulation surgery are warranted.
Footnotes
This work was supported by Research Resettlement Fund for the new faculty of Seoul National University and grant no 03-2015-0020 from the SNUH Research Fund.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Whal Lee.
- Data curation: Whal Lee, Baren Jeong.
- Formal analysis: Ju Gang Nam.
- Funding acquisition: Whal Lee.
- Investigation: Yujin Kwak, Hong-Gook Lim.
- Methodology: Ju Gang Nam, Whal Lee, Hong-Gook Lim.
- Project administration: Whal Lee.
- Resources: Whal Lee.
- Software: Baren Jeong.
- Supervision: Whal Lee.
- Validation: Yujin Kwak, Hong-Gook Lim.
- Visualization: Ju Gang Nam.
- Writing—original draft: Ju Gang Nam.
- Writing—review & editing: Whal Lee, Baren Jeong, Eun-Ah Park, Ji Yeon Lim.
Supplementary Materials
The Data Supplement is available with this article at https://doi.org/10.3348/kjr.2020.0682.
Results of Suitability Evaluation for Surgical Simulation Model among Different Composite-Hardness
Edited video clip sample of a simulation surgery performed by an attendee.
References
- 1.van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241–2247. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2017 Congenital Heart Disease Collaborators. Global, regional, and national burden of congenital heart disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc Health. 2020;4:185–200. doi: 10.1016/S2352-4642(19)30402-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 4.Dolk H, Loane M, Garne E European Surveillance of Congenital Anomalies (EUROCAT) Working Group. Congenital heart defects in Europe: prevalence and perinatal mortality, 2000 to 2005. Circulation. 2011;123:841–849. doi: 10.1161/CIRCULATIONAHA.110.958405. [DOI] [PubMed] [Google Scholar]
- 5.Wilson HK, Feins RH. Simulation in cardiothoracic surgery. In: Stefanidis D, Korndorffer Jr J, Sweet R, editors. Comprehensive healthcare simulation: surgery and surgical subspecialties. Cham: Springer; 2019. pp. 263–274. [Google Scholar]
- 6.Rose AS, Webster CE, Harrysson OL, Formeister EJ, Rawal RB, Iseli CE. Pre-operative simulation of pediatric mastoid surgery with 3D-printed temporal bone models. Int J Pediatr Otorhinolaryngol. 2015;79:740–744. doi: 10.1016/j.ijporl.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Burdall OC, Makin E, Davenport M, Ade-Ajayi N. 3D printing to simulate laparoscopic choledochal surgery. J Pediatr Surg. 2016;51:828–831. doi: 10.1016/j.jpedsurg.2016.02.093. [DOI] [PubMed] [Google Scholar]
- 8.Ryan JR, Almefty KK, Nakaji P, Frakes DH. Cerebral aneurysm clipping surgery simulation using patient-specific 3D printing and silicone casting. World Neurosurg. 2016;88:175–181. doi: 10.1016/j.wneu.2015.12.102. [DOI] [PubMed] [Google Scholar]
- 9.Jastifer JR, Gustafson PA. Three-dimensional printing and surgical simulation for preoperative planning of deformity correction in foot and ankle surgery. J Foot Ankle Surg. 2017;56:191–195. doi: 10.1053/j.jfas.2016.01.052. [DOI] [PubMed] [Google Scholar]
- 10.Binder TM, Moertl D, Mundigler G, Rehak G, Franke M, Delle-Karth G, et al. Stereolithographic biomodeling to create tangible hard copies of cardiac structures from echocardiographic data: in vitro and in vivo validation. J Am Coll Cardiol. 2000;35:230–237. doi: 10.1016/s0735-1097(99)00498-2. [DOI] [PubMed] [Google Scholar]
- 11.Yoo SJ, Thabit O, Kim EK, Ide H, Yim D, Dragulescu A, et al. 3D printing in medicine of congenital heart diseases. 3D Print Med. 2015;2:3. doi: 10.1186/s41205-016-0004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs S, Grunert R, Mohr FW, Falk V. 3D-imaging of cardiac structures using 3D heart models for planning in heart surgery: a preliminary study. Interact Cardiovasc Thorac Surg. 2008;7:6–9. doi: 10.1510/icvts.2007.156588. [DOI] [PubMed] [Google Scholar]
- 13.Ma XJ, Tao L, Chen X, Li W, Peng ZY, Chen Y, et al. Clinical application of three-dimensional reconstruction and rapid prototyping technology of multislice spiral computed tomography angiography for the repair of ventricular septal defect of tetralogy of Fallot. Genet Mol Res. 2015;14:1301–1309. doi: 10.4238/2015.February.13.9. [DOI] [PubMed] [Google Scholar]
- 14.Hands-on surgical simulation in congenital heart surgery: literature review and future perspective. Semin Thorac Cardiovasc Surg. 2020;32:98–105. doi: 10.1053/j.semtcvs.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Chen SA, Ong CS, Malguria N, Vricella LA, Garcia JR, Hibino N. Digital design and 3D printing of aortic arch reconstruction in HLHS for surgical simulation and training. World J Pediatr Congenit Heart Surg. 2018;9:454–458. doi: 10.1177/2150135118771323. [DOI] [PubMed] [Google Scholar]
- 16.Jivanji SGM, Qureshi SA, Rosenthal E. Novel use of a 3D printed heart model to guide simultaneous percutaneous repair of severe pulmonary regurgitation and right ventricular outflow tract aneurysm. Cardiol Young. 2019;29:534–537. doi: 10.1017/S1047951119000106. [DOI] [PubMed] [Google Scholar]
- 17.Meyer-Szary J, Woźniak-Mielczarek L, Sabiniewicz D, Sabiniewicz R. Feasibility of in-house rapid prototyping of cardiovascular three-dimensional models for planning and training non-standard interventional procedures. Cardiol J. 2019;26:790–792. doi: 10.5603/CJ.2019.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velasco Forte MN, Byrne N, Valverde I, Gomez Ciriza G, Hermuzi A, Prachasilchai P, et al. Interventional correction of sinus venosus atrial septal defect and partial anomalous pulmonary venous drainage: procedural planning using 3D printed models. JACC Cardiovasc Imaging. 2018;11:275–278. doi: 10.1016/j.jcmg.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Hussein N, Honjo O, Haller C, Coles JG, Hua Z, Van Arsdell G, et al. Quantitative assessment of technical performance during hands-on surgical training of the arterial switch operation using 3-dimensional printed heart models. J Thorac Cardiovasc Surg. 2020;160:1035–1042. doi: 10.1016/j.jtcvs.2019.11.123. [DOI] [PubMed] [Google Scholar]
- 20.Hussein N, Lim A, Honjo O, Haller C, Coles JG, Van Arsdell G, et al. Development and validation of a procedure-specific assessment tool for hands-on surgical training in congenital heart surgery. J Thorac Cardiovasc Surg. 2020;160:229–240.e1. doi: 10.1016/j.jtcvs.2019.11.130. [DOI] [PubMed] [Google Scholar]
- 21.Yoo SJ, Spray T, Austin EH, 3rd, Yun TJ, van Arsdell GS. Hands-on surgical training of congenital heart surgery using 3-dimensional print models. J Thorac Cardiovasc Surg. 2017;153:1530–1540. doi: 10.1016/j.jtcvs.2016.12.054. [DOI] [PubMed] [Google Scholar]
- 22.Cantinotti M, Valverde I, Kutty S. Three-dimensional printed models in congenital heart disease. Int J Cardiovasc Imaging. 2017;33:137–144. doi: 10.1007/s10554-016-0981-2. [DOI] [PubMed] [Google Scholar]
- 23.Yang DH, Park S-H, Lee K, Kim T, Kim JB, Yun TJ, et al. Applications of three-dimensional printing in cardiovascular surgery: a case-based review. Cardiovasc Imaging Asia. 2018;2:166–175. [Google Scholar]
- 24.Yoo JS, Kim YJ, Kim SH, Choi SH. Study on genipin: a new alternative natural crosslinking agent for fixing heterograft tissue. Korean J Thorac Cardiovasc Surg. 2011;44:197–207. doi: 10.5090/kjtcs.2011.44.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valverde I, Gomez G, Gonzalez A, Suarez-Mejias C, Adsuar A, Coserria JF, et al. Three-dimensional patient-specific cardiac model for surgical planning in Nikaidoh procedure. Cardiol Young. 2015;25:698–704. doi: 10.1017/S1047951114000742. [DOI] [PubMed] [Google Scholar]
- 26.Fann JI, Calhoon JH, Carpenter AJ, Merrill WH, Brown JW, Poston RS, et al. Simulation in coronary artery anastomosis early in cardiothoracic surgical residency training: the Boot Camp experience. J Thorac Cardiovasc Surg. 2010;139:1275–1281. doi: 10.1016/j.jtcvs.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fann JI, Feins RH, Hicks GL, Jr, Nesbitt JC, Hammon JW, Crawford FA. Evaluation of simulation training in cardiothoracic surgery: the Senior Tour perspective. J Thorac Cardiovasc Surg. 2012;143:264–272. doi: 10.1016/j.jtcvs.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Oropallo W, Piegl LA. Ten challenges in 3d printing. Eng Comput. 2016;32:135–148. [Google Scholar]
- 29.Leigh SJ, Bradley RJ, Purssell CP, Billson DR, Hutchins DA. A simple, low-cost conductive composite material for 3D printing of electronic sensors. PLoS One. 2012;7:e49365. doi: 10.1371/journal.pone.0049365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of Suitability Evaluation for Surgical Simulation Model among Different Composite-Hardness
Edited video clip sample of a simulation surgery performed by an attendee.