Abstract
Objective
To compare the lumen parameters measured by the location-adaptive threshold method (LATM), in which the inter- and intra-scan attenuation variabilities of coronary computed tomographic angiography (CCTA) were corrected, and the scan-adaptive threshold method (SATM), in which only the inter-scan variability was corrected, with the reference standard measurement by intravascular ultrasonography (IVUS).
Materials and Methods
The Hounsfield unit (HU) values of whole voxels and the centerline in each of the cross-sections of the 22 target coronary artery segments were obtained from 15 patients between March 2009 and June 2010, in addition to the corresponding voxel size. Lumen volume was calculated mathematically as the voxel volume multiplied by the number of voxels with HU within a given range, defined as the lumen for each method, and compared with the IVUS-derived reference standard. Subgroup analysis of the lumen area was performed to investigate the effect of lumen size on the studied methods. Bland-Altman plots were used to evaluate the agreement between the measurements.
Results
Lumen volumes measured by SATM was significantly smaller than that measured by IVUS (mean difference, 14.6 mm3; 95% confidence interval [CI], 4.9–24.3 mm3); the lumen volumes measured by LATM and IVUS were not significantly different (mean difference, −0.7 mm3; 95% CI, −9.1–7.7 mm3). The lumen area measured by SATM was significantly smaller than that measured by LATM in the smaller lumen area group (mean of difference, 1.07 mm2; 95% CI, 0.89–1.25 mm2) but not in the larger lumen area group (mean of difference, −0.07 mm2; 95% CI, −0.22–0.08 mm2). In the smaller lumen group, the mean difference was lower in the Bland-Altman plot of IVUS and LATM (0.46 mm2; 95% CI, 0.27–0.65 mm2) than in that of IVUS and SATM (1.53 mm2; 95% CI, 1.27–1.79 mm2).
Conclusion
SATM underestimated the lumen parameters for computed lumen segmentation in CCTA, and this may be overcome by using LATM.
Keywords: Coronary artery, Coronary computed tomographic angiography, Full width at half maximum, Lumen segmentation
INTRODUCTION
Automated measurement of coronary artery lumen volume using coronary computed tomographic angiography (CCTA) has shown moderate to strong correlations with coefficients of 0.65–0.84, which indicate the need to improve automated methods for lumen segmentation (1,2,3). An explanation for this may be that the threshold-based methods used for lumen segmentation are applied by most vendors, and the Hounsfield unit (HU) values are fixed for the threshold values without consideration of the individual variations of the intraluminal attenuation of the coronary artery. New software, such as QAngioCT (3) or AUTOPLAQ (4), introduced a different thresholding per scan for lumen volume quantification, which is intended to overcome issues with inter-scan variations.
A recent phantom study indicated that the intraluminal attenuation of vessels gradually decreased with a decrease in diameter (5); therefore, lumen volume measurement may be incorrect for cases involving stenosis within the target segment if the same threshold value was applied to both stenotic and nonstenotic locations, even though the inter-scan variability of intraluminal attenuation was adjusted. Consequently, lumen volume would be underestimated if the threshold was set to a nonstenotic location.
Therefore, a location-adaptive threshold method (LATM), involving the application of different thresholds at every location within a target segment, has been hypothesized as more accurate for determining the inner lumen boundary on CCTA than a scan-adaptive threshold method (SATM) with which threshold levels within the target segment does not change. This proof-of-concept study was designed to compare LATM and SATM, used for assessing the lumen parameters of the coronary artery, to the reference standard of invasive intravascular ultrasonography (IVUS).
MATERIALS AND METHODS
This retrospective study was approved by our Institutional Review Board and the requirement for informed consent was waived (IRB No. 1512-138-730).
Subjects
This study included 30 target segments from 25 consecutive patients with suspected or known coronary artery disease who underwent CCTA and clinically indicated invasive coronary angiography with IVUS from March 1, 2009, to June 30, 2010, at Seoul National University Hospital. The exclusion criteria were suboptimal image quality due to motion artifacts in the target segment on CCTA (n = 6) and failure of segmentation by dedicated software (n = 2). Finally, 22 target segments in 15 patients (mean age, 64.6 years; male, 80.0%) were enrolled in this proof-of-concept study. Seven target segments contained only noncalcified plaques; however, other segments contained partially calcified plaques.
CCTA Image Acquisition and Reconstruction
CCTA was performed in compliance with guidelines for the Society of Cardiovascular Computed Tomography (6). CCTA data were acquired with a dual-source CT scanner (Somatom Definition, Siemens Healthineers) (n = 12), a 16-slice CT scanner (Sensation 16, Siemens Healthineers) (n = 7), and a 256-slice CT scanner (iCT, Philips Healthcare) (n = 2). The scan parameters were: collimation of 32 × 0.6 mm/16 × 0.75 mm/128 × 0.625 mm, a tube voltage of 100 kVp or 120 kVp depending on body habitus, tube currents of 104–620 mA depending on body habitus, and rotation times of 270–370 ms. The images were reconstructed with a retrospective electrocardiographic-gated technique using a mono-segment reconstruction algorithm. Motion-free data sets, typically in mid-diastole, were collected. Reconstruction parameters included a slice thickness of 0.8–1 mm, increments of 0.5–0.7 mm, and a medium soft convolution kernel.
IVUS Imaging Protocol and Analysis
A commercially available 2.9-F 40 MHz catheter (Boston Scientific/SCIMED) with an axial resolution of ± 80 µm and a lateral resolution of ± 200 µm was used to perform IVUS imaging throughout the length of the segment of interest after intracoronary nitroglycerine. The images were acquired using a standard automated motorized pullback system that permitted a cross-sectional area measurement at 0.5 mm/s with 30 frames per second.
A cardiologist analyzed the IVUS images of the lesion-containing target segments using commercially available computerized planimetry software (echoPlaque, INDEC Medical Systems Inc.). The external elastic membrane and the lumen-intima interface were manually traced on each cross-sectional image for the measurement of the lumen area. Volume parameters per lesion were calculated from the cross-sectional areas according to Simpson's rule.
The locations of the target segments of the coronary artery containing plaques were recorded using an initial landmark of side branches and lesion lengths along with the documentation of the downstream or upstream direction. This location information was also annotated on a three-dimensional volume-rendered image of the CCTA that provided references to the exact extent of the target segment.
CCTA Voxel Data Extraction
In this study, the lumen parameters were experimentally calculated using the voxel area or volume multiplied by the number of voxels within a selected range of HUs, estimated as a lumen. For this work, the HU and the size of all the voxels corresponding to the target vessel segment were extracted using a dedicated workstation (Xelis Cardiac research version, Infinitt Healthcare) installed with a customized solution for extracting voxel data from CCTA.
After the thin-section CCTA data were transferred to a dedicated workstation, contouring of the external vessel wall and centerline extraction were automatically performed for all coronary arteries. The target segment was selected by an independent researcher ensuring that the length and location of each analyzed segment were identical to those of IVUS, based on the indicator generated from the IVUS analysis. For carefully selected target segments, the HU values of all the voxels within the external vessel boundary on each cross-section perpendicular to the centerline were exported to a Microsoft Excel sheet. The HU values of the vessel center at each cross-section were also output, which referred to the center of gravity in the outer vessel contour regarded as the maximal attenuation value on the attenuation profile of that vessel. Also, the three-dimensional size of a voxel on each cross-section was recorded.
Threshold Definition for Calculating Lumen Parameters
The diameter of the imaged object, such as vessels, was often characterized by the full width of the profile at 50% of its maximum value (full width at half maximum, FWHM) calculated from the attenuation profile through the center of the vessel. In terms of an attenuation profile of a segmented vessel on a perpendicular plane in this study, the minimum value was regarded as zero because the initial profile of the outer vessel boundary was generated with 0 HU by the dedicated software. The threshold values for selecting experimental lumen were therefore determined by 50% of the HU value for the estimated maximum value within the lumen. To discriminate the lumen from the calcified plaques for target segments with partly calcified plaques, two threshold values were required and defined as follows.
For the SATM, the average HU value of two lumens in each target segment was set as a scan-specific HU, which was applied at every cross-section through a target segment. Two lumens were selected at the proximal and distal stenosis-free points of each target segment, and HU was measured at the center of the lumen with a round region of interest of a diameter approximately 50% of the lumen. For the determination of the threshold between the noncalcified plaque and inner lumen, 50% of the scan-specific HU was set according to the FWHM. The threshold for separating the inner vessel lumen and the calcified plaque was 110% of the scan-specific HU because the standard deviation of the scan-specific HU was usually less than 10%; therefore, voxels with over 110% of the scan-specific HU were believed to be part of the calcified plaques rather than the lumen.
For the LATM, threshold values were differently applied at each cross-section. To determine the threshold between the noncalcified plaque and the inner lumen for a given cross-section, 50% of the corresponding center HU was applied. To discriminate the inner lumen from the calcified plaque, voxels with more than 110% of the center HU were regarded as calcified plaques.
Comparison of Lumen Volume
The lumen volume was a summation of the cross-sectional lumen volumes calculated as a voxel volume multiplying the number of voxels for which the HU satisfied the corresponding range. The lumen volumes calculated from each method were compared to those derived from IVUS.
Subgroup Analysis according to the Lumen Size
According to a previous study (5), lumen volume measured by SATM and LATM should differ more for smaller lumen sizes because attenuation decreased with lumen size. Therefore, subgroup analysis ascertained if the lumen size affected the difference between the lumen volumes of SATM and LATM. For this subgroup analysis, only noncalcified plaque segments were included, because a trace of misclassified calcified plaques and lumen could cause significant errors in the lumen parameter measurements, especially for small lumens.
Since a frame obtained by IVUS was 1/60 mm and different from the CCTA section thickness, which varied from 0.26 mm to 0.32 mm according to the scan, IVUS frames were grouped according to the section thickness of the corresponding CCTA for subgroup analysis; lumen area values measured by IVUS on every 15.6 to 19.2 frames (with rounding to the nearest integer) were averaged.
Based on the modified lumen areas measured by IVUS and equalized to CCTA section thickness, the total cross-sections of noncalcified plaque segments (n = 362) were divided into three subgroups of lumen sizes of < 3 mm2 (approximately 2 mm in lumen diameter, n = 48), 3–7mm2 (n = 202), and ≥ 7 mm2 (approximately 3 mm in lumen diameter, n = 112). The subgroup with lumen sizes of < 3 mm2 was excluded because the lumen areas were believed to be too small and prone to errors despite the low mismatch between the IVUS and CCTA cross-sections. For subgroups with smaller (3–7 mm2) and larger (≥ 7 mm2) lumen areas, the lumen areas measured by LATM and SATM were compared to those derived by IVUS.
Statistical Analysis
Continuous variables were presented as means; a 95% confidence interval (CI) was compared using repeated measures ANOVA or paired t test. The Friedman test was used for the volume analysis of the noncalcified plaque segments instead of repeated measures ANOVA due to the few cases. Bonferroni correction was applied to multiple comparisons. For agreement evaluation, the Bland-Altman analysis was performed by plotting the mean against the difference in measurements (7). The limits of agreement were defined as the mean difference plus and minus 1.96 times the standard deviation of the differences for the upper and lower limits, respectively. Intraclass correlation coefficients (ICCs) were used to evaluate the reliability of the CCTA-derived methods by applying the two-way model, single measures, and absolute agreement type (8). A p value of 0.05 or less indicated a statistically significant difference. All analyses and graphs were performed using MedCalc® software Version 17.1 (MedCalc Software).
RESULTS
Comparison of Lumen Volume Measured by Two Different Threshold Methods and IVUS
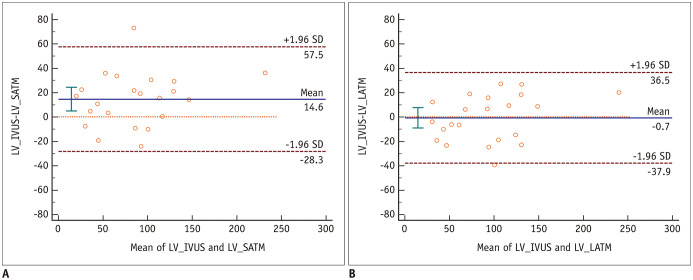
Table 1 shows the mean lumen volumes and 95% CIs measured by IVUS, SATM, and LATM, in addition to the ICCs between these methods. The mean lumen volume measured by SATM (78.4 mm3) was significantly lower than that measured by IVUS (96.0 mm3) (p = 0.015), and there was no significant difference between the lumen volumes measured by IVUS and LATM (93.7 mm3) (p = 0.999). On the Bland-Altman plots for lumen volumes, there was a systemic bias between IVUS and SATM, but not between IVUS and LATM (Fig. 1).
Table 1. Comparison of Lumen Volume Measured by IVUS, SATM, and LATM.
| IVUS | SATM | LATM | P | ICC | |
|---|---|---|---|---|---|
| Lumen volume (mm3) | 93.0 (70.0–116.3) | 78.4 (57.2–99.6) | 93.7 (73.0–114.5) | 0.005 | |
| Pairwise comparisons* | IVUS vs. SATM | 0.015 | 0.872 (0.610–0.952) | ||
| IVUS vs. LATM | 0.999 | 0.930 (0.840–0.971) | |||
| SATM vs. LATM | < 0.001 | 0.916 (0.330–0.977) |
Data are means. Data in parentheses are 95% confidence interval. *Bonferroni corrected. ICC = intraclass correlation coefficient, IVUS = intravascular ultrasonography, LATM = location-adaptive threshold method, SATM = scan-adaptive threshold method
Fig. 1. Bland-Altman plots showing the differences between the CCTA-derived and IVUS LVs.
A. Bland-Altman plot of the difference between the LVs measured by IVUS and SATM (mean difference = 14.6 mm3; 95% CI of mean difference, 4.9 to 24.3 mm3). B. Bland-Altman plot of the difference between the LVs measured by IVUS and LATM (mean difference = −0.7 mm3; 95% CI of mean difference, −9.1 to 7.7 mm3). CCTA = coronary computed tomographic angiography, CI = confidence interval, IVUS = intravascular ultrasonography, LATM = location-adaptive threshold method, LV = lumen volume, SATM = scan-adaptive threshold method, SD = standard deviation
Comparison of Lumen Volume in Noncalcified Plaque Segments
For noncalcified plaque segments (n = 7), the mean lumen volume measured by SATM was significantly lower than that measured by IVUS (p < 0.05), while there was no significant difference between the lumen volumes measured by IVUS and LATM. The ICC for the lumen volumes measured by SATM and IVUS was 0.817 (95% CI, 0.053–0.969) and that of LATM and IVUS was 0.945 (95% CI, 0.634–0.991).
Results of Subgroup Analysis according to the Lumen Size
Table 2 presents the mean values and the 95% CIs for the calculated lumen areas using the lumen sizes based on the reference standard, in addition to the ICCs of the methods. In the smaller lumen group, the mean lumen area was significantly larger when measured by LATM (4.92 mm2; 95% CI, 4.75–5.09 mm2) than by SATM (3.85 mm2; 95% CI, 3.54–4.15 mm2) (p < 0.001). However, in the larger lumen group, the mean lumen areas measured by SATM (7.09 mm2; 95% CI, 6.67–7.50 mm2) and LATM (7.01 mm2; 95% CI, 6.69–7.34 mm2) were not significantly different (p = 0.999). The mean lumen area values measured by CCTA-derived methods and IVUS were significantly different in all the lumen-size groups (p < 0.001).
Table 2. Subgroup Analysis of Lumen Area Measured by SATM and LATM according to Lumen Area Based on the Reference Standard.
| Lumen Area on IVUS | IVUS | SATM | LATM | P | ICC |
|---|---|---|---|---|---|
| 3–7 mm2 (n = 202) | |||||
| Lumen area (mm2) | 5.38 (5.21–5.55) | 3.85 (3.54–4.15) | 4.92 (4.75–5.09) | < 0.001 | |
| Pairwise comparisons* | < 0.001 | 0.334 (0.027–0.550) | |||
| IVUS vs. LATM | < 0.001 | 0.348 (0.211–0.469) | |||
| < 0.001 | 0.633 (0.192–0.812) | ||||
| ≥ 7 mm2 (n = 112) | |||||
| Lumen area (mm2) | 8.43 (8.17–8.69) | 7.09 (6.67–7.50) | 7.01 (6.69–7.34) | < 0.001 | |
| Pairwise comparisons* | IVUS vs. SATM | < 0.001 | 0.555 (0.068–0.775) | ||
| IVUS vs. LATM | < 0.001 | 0.544 (-0.071–0.805) | |||
| SATM vs. LATM | 0.999 | 0.920 (0.886–0.944) | |||
| Total (n = 314) | |||||
| Lumen area (mm2) | 6.47 (6.25–6.68) | 5.00 (4.70–5.30) | 5.67 (5.47–5.86) | < 0.001 | |
| Pairwise comparisons* | IVUS vs. SATM | < 0.001 | 0.614 (0.173–0.798) | ||
| IVUS vs. LATM | < 0.001 | 0.668 (0.432–0.791) | |||
| SATM vs. LATM | < 0.001 | 0.815 (0.669–0.885) |
Data are means. Data in parentheses are 95% confidence interval. *Bonferroni corrected.
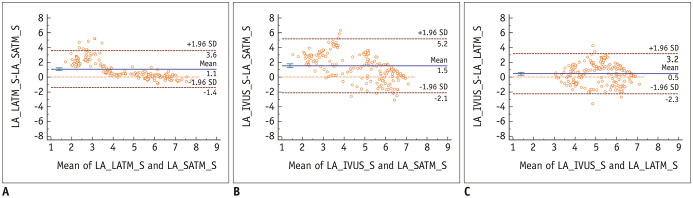
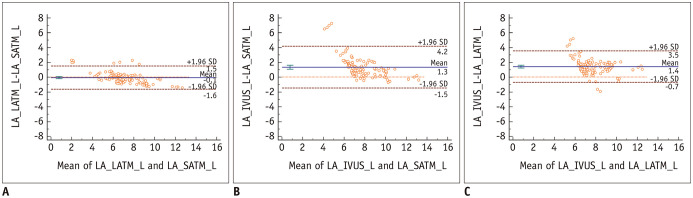
The Bland-Altman plots of the lumen areas measured by the SATM and LATM in the smaller lumen group showed a positive systemic bias for lumen area values measured by LATM (mean of difference, 1.07 mm2; 95% CI, 0.89–1.25 mm2, p < 0.001) (Fig. 2). However, in the larger lumen group, no systemic bias was observed (mean of difference, −0.07 mm2; 95% CI, −0.22–0.08 mm2, p = 0.336), and the limit of agreement was narrower than that of the smaller lumen group (Figs. 3, 4).
Fig. 2. Bland-Altman plots of LA measured by SATM versus LATM and IVUS in the smaller lumen group.
A. Bland-Altman plot for LATM and SATM (mean difference = 1.07 mm2; 95% CI of mean difference, 0.89 to 1.25 mm2). B. Bland-Altman plot for SATM and IVUS (mean difference = 1.53 mm2; 95% CI of mean difference, 1.27 to 1.79 mm2). C. Bland-Altman plot for LATM and IVUS (mean difference = 0.46 mm2; 95% CI of mean difference, 0.27 to 0.65 mm2). LA = lumen area, S = smaller group
Fig. 3. Bland-Altman plots of LA measured by SATM versus LATM and IVUS in the larger lumen group.
A. Bland-Altman plot for LATM and SATM (mean difference = −0.07 mm2; 95% CI of mean difference, −0.22 to 0.08 mm2). B. Bland-Altman plot for SATM and IVUS (mean difference = 1.34 mm2; 95% CI of mean difference, 1.07 to 1.61 mm2). C. Bland-Altman plot for LATM and IVUS (mean difference = 1.41 mm2; 95% CI of mean difference, 1.21 to 1.62 mm2). L = larger group
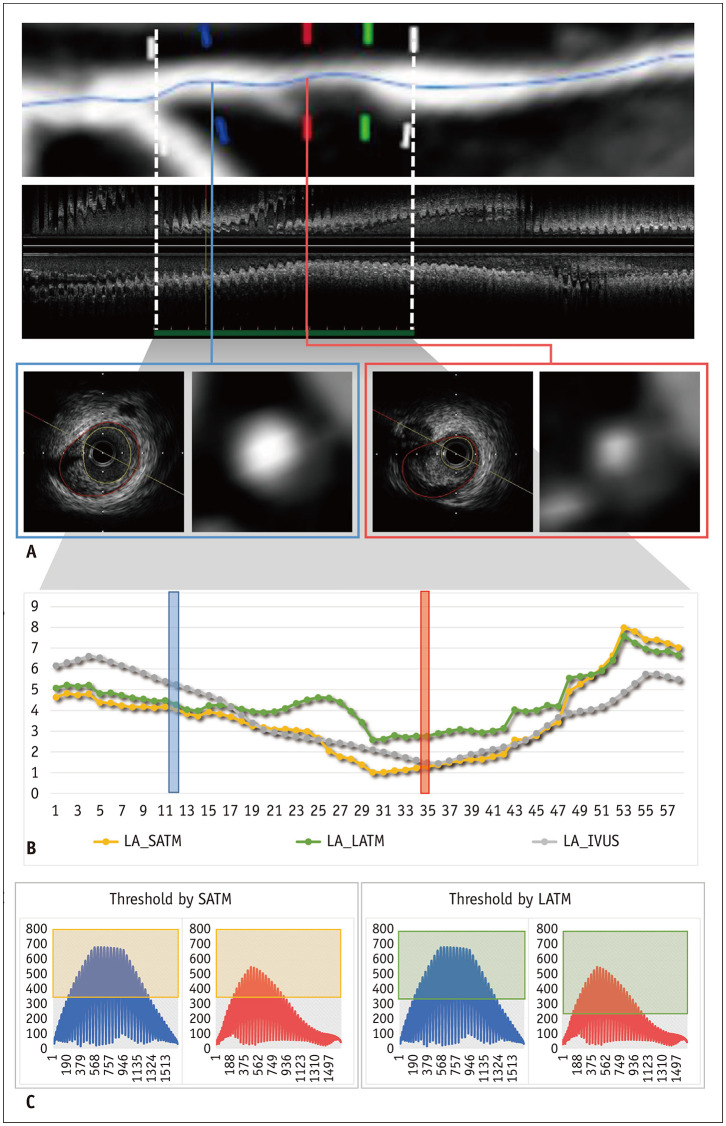
Fig. 4. A representative case for the comparison of the LATM and SATM for measuring LA.
A. Images show CCTA and IVUS measurements of the left anterior descending artery in a 64-year-old male. The target segment with noncalcified plaque and stenosis was 15 mm. The LAs measured by IVUS were 5.25 mm2 and 2.77 mm2 at the two representative cross-sections for the large (blue) and small (red) lumens, respectively. B. Graph plots of the LAs measured by IVUS (gray) and calculated by scan-SATM (yellow) and LATM (green). In the mid portion of the target segment with stenosis, the LA calculated by the SATM was smaller than that calculated by the LATM; however, those measured by both methods were similar for both sides of the target segments without stenosis. C. In the graph, dots indicate HU values of all pixels composing a vessel on the corresponding cross-section. For the SATM, the threshold was the same as 328 HU for both cross-sections; however, a different threshold was applied in the LATM for the sections with large (320 HU) and small (214 HU) lumens. The pixel values within the light green and yellow boxes were multiplied by the LAs measured by the SATM and LATM, respectively. Note that the LAs calculated by both methods were similar in the section with the large lumen, but different in the section with the small lumen. HU = Hounsfield unit
In the smaller lumen group, the mean difference was lower in the Bland-Altman plot of the IVUS and LATM (mean of difference = 0.46 mm2; 95% CI, 0.27–0.65 mm2) than in that of the IVUS and SATM (mean of difference = 1.53 mm2; 95% CI, 1.27–1.79 mm2) (Fig. 2). In the larger lumen group, the mean difference between the values measured by IVUS and LATM as well as IVUS and SATM was 1.41 mm2 (95% CI, 1.21–1.62 mm2) and 1.34 mm2 (95% CI, 1.07–1.61 mm2), respectively (Fig. 3).
DISCUSSION
This proof-of-concept study demonstrated that the lumen volume measured by the SATM was significantly lower than the reference volume, and this underestimation was corrected by the LATM. The subgroup analysis also indicated that this underestimation occurred in the smaller lumen area group. It is worthwhile that the concept of the LATM can be applied to all the coronary artery stenosis evaluations, including fractional flow reserve based on CCTA and lumen volume quantification as part of the coronary plaque volume quantification.
CT images of real objects have blurred edges at the boundary, and they can be presented by a point-spread function of the imaging system. The boundary of the object was generally defined by the FWHM. Consequently, an error could occur if an inadequate threshold was applied instead of the half maximum. For the automated quantification of atherosclerosis using CCTA, the concept of the SATM was introduced, which would correct the inter-individual variation of the contrast enhancement degree. However, to our knowledge, there has been no interest in the intra-individual variation of the intraluminal attenuation, and the current study should be the first to prove this concept.
The lumen volume agreement between the LATM and IVUS was excellent, with an ICC value of 0.930. Few studies have quantified lumen volume or area using CCTA and compared them with the gold standard measurements by IVUS. Most of these studies also conducted a correlation analysis instead of an agreement analysis, which would be a more appropriate analytic method for evaluating the performance of a new measurement system. A direct comparison of the performance of the LATM with those of previous studies has been difficult; however, the LATM indicated better agreement given that the reported correlation coefficients (r) in the previous studies ranged from 0.65 to 0.90 (1,3,9,10,11).
The threshold was set to the half value of the presumed maximum intraluminal attenuation for its application to lumen segmentation on CCTA. The attenuation profile of the vessel on cross-section shows a three-dimensional bell shape, and the lowest attenuation along the vessel boundary may not be zero but variable within a certain range depending on the plaque geometry and composition, whereas the highest attenuation was static. Consequently, the half-maximum value, which serves as the threshold for segmentation, may also be variable along the vessel boundary, and it may not be a single value on a cross-section set to half of the maximum intraluminal attenuation value in the present study. This error may account for the lower agreement between the lumen areas (ICC, 0.668) measured by IVUS and LATM.
Although LATM may correct the underestimation of the lumen parameters by SATM, especially in the stenotic segment, it may overestimate them. LATM works by lowering the threshold at the stenotic portion during lumen segmentation. Therefore, lumen parameters can be overestimated if the threshold level is lower than the correct value; for example, an incorrect centerline extraction in the stenotic portion may result in erroneous lower thresholds.
Some limitations should be considered in this study. First, the sample size was relatively small. However, subgroup analysis per cross-section was performed with a sufficient number that provided more detailed information. Second, there may have been a mismatch between the target segments of IVUS and CCTA; however, the degree of mismatch was believed to be minimal since it was double-checked with text and image references. There may have also been a mismatch because a given value of z-spacing in CCTA may have hindered the synchronization of the cross-section with IVUS under the condition of matching cross-sections that cannot be calculated by multiplying the 1/60 mm of the fixed frame thickness of IVUS. However, this mismatch is a minor issue because of the inherent nature of imaging modalities. Efforts were made to minimize the degree of mismatch of cross-sections. Also, the subgroup with lumen sizes of < 3 mm2 was excluded from the subsegmental analysis because of concerns about location mismatch. This subgroup, however, may be clinically significant for the severe luminal stenosis, therefore further investigations including this subgroup are warranted. Fourth, since these components were estimated based on attenuation values without location information, there may have been an error, and the relatively low attenuating calcification could have been incorrectly identified as lumen.
In conclusion, the lumen parameters of coronary arteries with stenosis were underestimated by the SATM, which applied the static threshold value through a target segment. However, this was overcome by LATM, which used a different threshold value at each location within the target segment.
Footnotes
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HI20C2092).
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Voros S, Rinehart S, Qian Z, Vazquez G, Anderson H, Murrieta L, et al. Prospective validation of standardized, 3-dimensional, quantitative coronary computed tomographic plaque measurements using radiofrequency backscatter intravascular ultrasound as reference standard in intermediate coronary arterial lesions: results from the ATLANTA (assessment of tissue characteristics, lesion morphology, and hemodynamics by angiography with fractional flow reserve, intravascular ultrasound and virtual histology, and noninvasive computed tomography in atherosclerotic plaques) I study. JACC Cardiovasc Interv. 2011;4:198–208. doi: 10.1016/j.jcin.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Sun J, Zhang Z, Lu B, Yu W, Yang Y, Zhou Y, et al. Identification and quantification of coronary atherosclerotic plaques: a comparison of 64-MDCT and intravascular ultrasound. AJR Am J Roentgenol. 2008;190:748–754. doi: 10.2214/AJR.07.2763. [DOI] [PubMed] [Google Scholar]
- 3.Boogers MJ, Broersen A, van Velzen JE, de Graaf FR, El-Naggar HM, Kitslaar PH, et al. Automated quantification of coronary plaque with computed tomography: comparison with intravascular ultrasound using a dedicated registration algorithm for fusion-based quantification. Eur Heart J. 2012;33:1007–1016. doi: 10.1093/eurheartj/ehr465. [DOI] [PubMed] [Google Scholar]
- 4.Dey D, Schepis T, Marwan M, Slomka PJ, Berman DS, Achenbach S. Automated three-dimensional quantification of noncalcified coronary plaque from coronary CT angiography: comparison with intravascular US. Radiology. 2010;257:516–522. doi: 10.1148/radiol.10100681. [DOI] [PubMed] [Google Scholar]
- 5.Park EA, Lee W, Park SJ, Kim YK, Hwang HY. Influence of coronary artery diameter on intracoronary transluminal attenuation gradient during CT angiography. JACC Cardiovasc Imaging. 2016;9:1074–1083. doi: 10.1016/j.jcmg.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 6.Abbara S, Arbab-Zadeh A, Callister TQ, Desai MY, Mamuya W, Thomson L, et al. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2009;3:190–204. doi: 10.1016/j.jcct.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 8.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 9.Feuchtner G, Loureiro R, Bezerra H, Rocha-Filho JA, Sarwar A, Pflederer T, et al. Quantification of coronary stenosis by dual source computed tomography in patients: a comparative study with intravascular ultrasound and invasive angiography. Eur J Radiol. 2012;81:83–88. doi: 10.1016/j.ejrad.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Sun Z, Xu L. Coronary CT angiography in the quantitative assessment of coronary plaques. Biomed Res Int. 2014;2014:346380. doi: 10.1155/2014/346380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akutagawa O, Kijima Y, Kume K, Sakai T, Okura A, Ide K, et al. Feasibility and limitation of coronary plaque volumetry by contrast-enhanced 64-row multi-detector computed tomography. Int J Cardiol. 2011;150:118–120. doi: 10.1016/j.ijcard.2011.04.033. [DOI] [PubMed] [Google Scholar]