Abstract
Objective
To assess the safety and clinical efficacy of percutaneous transhepatic enteral stent placement for recurrent malignant obstruction in patients with surgically altered bowel anatomy.
Materials and Methods
Between July 2009 and May 2019, 36 patients (27 men and 9 women; mean age, 62.7 ± 12.0 years) underwent percutaneous transhepatic stent placement for recurrent malignant bowel obstruction after surgery. In all patients, conventional endoscopic peroral stent placement failed due to altered bowel anatomy. The stent was placed with a transhepatic approach for an afferent loop obstruction (n = 27) with a combined transhepatic and peroral approach for simultaneous stent placement in afferent and efferent loop obstruction (n = 9). Technical and clinical success, complications, stent patency, and patient survival were retrospectively evaluated.
Results
The stent placement was technically successful in all patients. Clinical success was achieved in 30 patients (83.3%). Three patients required re-intervention (balloon dilatation [n = 1] and additional stent placement [n = 2] for insufficient stent expansion). Major complications included transhepatic access-related perihepatic biloma (n = 2), hepatic artery bleeding (n = 2), bowel perforation (n = 1), and sepsis (n = 1). The 3- and 12-months stent patency and patient survival rates were 91.2%, 66.5% and 78.9%, 47.9%, respectively.
Conclusion
Percutaneous enteral stent placement using transhepatic access for recurrent malignant obstruction in patients with surgically altered bowel anatomy is safe and clinically efficacious. Transhepatic access is a good alternative route for afferent loop obstruction and can be combined with a peroral approach for simultaneous afferent and efferent loop obstruction.
Keywords: Percutaneous transhepatic enteral stent; Surgically altered bowel anatomy, Recurrent malignant obstruction; Self-expandable metal stent; Afferent loop syndrome, Pancreaticoduodenectomy
INTRODUCTION
Self-expandable metal stent placement has been widely utilized for the treatment of malignant gastrointestinal obstruction in patients not eligible for surgery [1]. Endoscopy [2,3] or fluoroscopy [4,5] guided peroral stent placement is technically feasible in most patients with native bowel anatomy. However, in patients with recurrent bowel obstruction after surgery, it is frequently difficult to address obstructions with the conventional peroral approach due to their surgically-altered bowel anatomy.
In such patients, the percutaneous transhepatic approach has been used as an alternative technique for successful stent placement [6,7,8]. However, clinical experiences with transhepatic enteral stent placement have been limited to small case series including less than 10 patients [6,7]. In addition, prior studies [6,7,8] exclusively addressed afferent loop obstruction after Billroth II reconstruction. However, the transhepatic approach has the potential to address more complicated lesions such as concurrent afferent and efferent loop obstruction of gastrojejunostomy (GJ-stomy) and jejunojejunostomy (JJ-stomy). The purpose of this study was to evaluate the safety and clinical efficacy of transhepatic enteral stent placement in patients with recurrent malignant obstructions after various surgical bowel reconstructions.
MATERIALS AND METHODS
Patients
Our Institutional Review Board approved this retrospective study, and the requirement for informed consent was waived (IRB No. B-2006-618-110). From July 2009 to May 2019, we found 39 patients who underwent percutaneous transhepatic enteral stent placement using an electronic medical record search. We excluded three patients who underwent stent placement for benign obstruction. All 36 patients (27 men and 9 women) included in this study had recurrent bowel obstructions that occurred after surgical resection of a primary malignancy with bowel reconstruction. First, conventional endoscopic peroral stent placement was attempted but failed due to surgically altered bowel anatomy. All patients had obstructive symptoms, and the recurrent obstructions were confirmed with contrast-enhanced CT. Surgical treatment was not available due to the patients' poor general condition.
Procedure
The potential risks and benefits of the procedure were explained to the patients and/or their family members, and informed consent was obtained in all cases. One of the three interventional radiologists performed the procedures. All procedures were performed under local anesthesia. Conscious sedation was administered with continuous monitoring of the patients' heart rate, blood pressure, and oxygen saturation.
Percutaneous transhepatic bile drainage (PTBD) was performed under ultrasonography and fluoroscopic guidance. The laterality of PTBD was chosen based on the pre-procedure CT considering intrahepatic bile duct (IHD) dilatation and synchronous biliary obstruction. An 8.5-French multi-side hole drainage catheter (Cook Medical) was placed with the distal end of the catheter positioned in the distended afferent loop for bowel decompression.
Stent placement was attempted in the same PTBD session for two patients. In 34 patients, a delayed procedure was performed within 3 weeks for the treatment of cholangitis (n = 32) or within 2 months of chemotherapy (n = 2). In patients with A-loop obstruction, stent placement was performed using transhepatic access. The PTBD catheter was exchanged with an 8-French curved vascular sheath (Balkin; Cook Medical) positioned in the afferent loop. A 0.035-inch hydrophilic guidewire and a 5-French angiographic catheter (Cook Medical) were advanced into the A-loop across the ampulla of Vater and manipulated to cross the target obstruction. The location and length of the obstruction were confirmed using a small amount of contrast media. The guidewire was exchanged with a 260 cm super-stiff guidewire (Amplatz; Boston Scientific). Transhepatic access was serially dilated up to 10 French, and a self-expandable uncovered stent (Hercules and EGIS, S&G BioTech Inc.) was introduced along the guidewire using a sheathless technique and deployed to cover the obstruction.
In case of concurrent afferent and efferent loop obstruction of the GJ-stomy and JJ-stomy, a combined transhepatic and peroral approach was used for the simultaneous stent placement of both obstructions. First, the afferent loop obstruction was crossed using transhepatic access, as described above. The angiographic catheter and guidewire were then advanced into the stomach. A snare catheter (Amplatz Goose Neck Snare Kit, ev3 Inc.) was introduced via the peroral approach, and the guidewire transhepatically introduced was captured and pulled out of the mouth. A 4.9 mm, 60 cm guiding sheath (G.I. sheath; S&G BioTech Inc.) [9] was introduced over the guidewire with the peroral approach, and the distal end of the guiding sheath was positioned close to the obstruction. Another assembly of a 5-French angiographic catheter and 0.035 in guidewire was introduced into the guiding sheath and manipulated to cross the efferent loop obstruction, which resulted in two parallel guidewires crossing the afferent and efferent loop obstruction. The guiding sheath positioned close to the obstruction facilitated the negotiation of the efferent loop obstruction. The guidewire in the efferent loop was exchanged with 260 cm super-stiff guidewires. The guiding sheath was removed, and two self-expandable stents were perorally introduced along each guidewire and deployed to cover both afferent and efferent loop obstructions in a parallel fashion (Fig. 1). Post-stent balloon dilatation (10–16 mm, Mustang and XXL; Boston Scientific) was performed if the stent expanded to less than 25% of its nominal diameter with a disturbance of contrast passage. The stent length was chosen to cover at least an extra 2 cm on each side of the obstruction (8–12 cm). The stent diameter was 14–24 mm for the afferent and 18–24 mm for the efferent loop obstruction. The adequate position and function of the stent were confirmed with contrast media. In patients with synchronous biliary or esophagojejunostomy (EJ-stomy) obstruction, stent placement was performed in the same session.
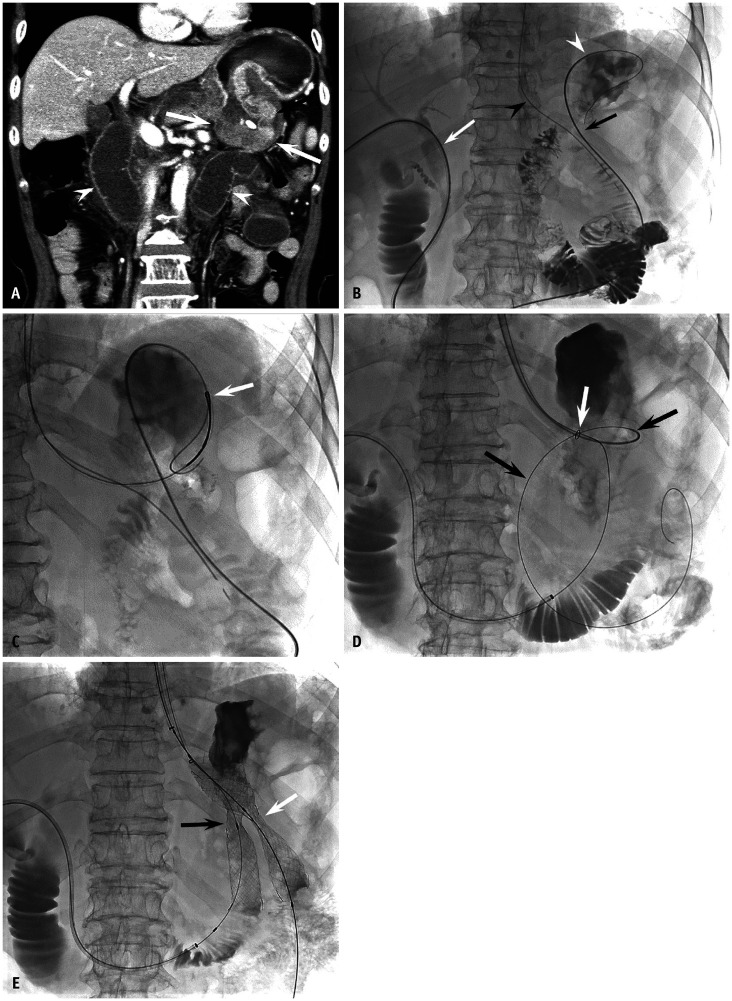
Fig. 1. A 67-year-old man with recurrent obstruction after distal gastrectomy with Billroth II reconstruction.
A. A contrast-enhanced CT shows recurrent tumor at gastrojejunostomy (arrows) with a distended afferent loop (arrowheads). B–E. Photographs obtained during stent placement with a combined transhepatic and peroral approach. B. A 5-French angiographic catheter (Cook Medical) and a 0.035″ guidewire were introduced using transhepatic access (white arrow), manipulated to retrogradely cross the afferent loop obstruction (black arrow), and positioned into the gastric fundus (white arrowhead). The black arrowhead indicates the nasogastric tube. C. The guidewire was captured with a snare catheter (arrow) (ev3 Inc.) and pulled out of the mouth to establish a wire loop. D. A guiding sheath was introduced from the mouth along the wire loop and positioned close to the obstruction. The white arrow indicates the radiopaque marker at the distal end of the guiding sheath. Another angiographic catheter and guidewire assembly was introduced into the guiding sheath and manipulated to cross the efferent loop obstruction (black arrows). E. Two self-expandable stents (S&G BioTech Inc.) were perorally introduced along each guidewire and deployed to cover the efferent (white arrow, 22 mm × 9 cm) and afferent (black arrow, 18 mm × 9 cm) loop obstruction with parallel fashion. Note focal waists at the mid-portion of the stents.
Follow-Up
After stent placement, patients were observed for symptomatic improvement. Daily abdominal radiographs were taken to monitor bowel decompression and to exclude stent migration and a bowel perforation. The gastric outlet obstruction (GOO) score [10] of all patients was evaluated before and after stent placement. The GOO scoring system assigns a point score depending on the patient's level of oral intake: 0, no/inadequate oral intake; 1, liquids or thickened liquids; 2, semi-solids/low-residue diet; and 3, unmodified diet. When the obstructive symptoms did not improve 3 days after stent placement, contrast-enhanced CT was performed to reveal the cause of persistent symptoms. If the cause of the persistent symptoms was assumed to be insufficient stent expansion, re-intervention (balloon dilatation or additional stent placement) was attempted. After the resolution of obstructive symptoms and normalization of laboratory findings, including liver function tests and complete blood count, a PTBD tubogram was obtained to confirm stent patency and bowel decompression. After that, the PTBD catheter was capped for at least 3 days before removal.
After discharge, the patients were followed-up in the outpatient clinic every 3 months. Contrast-enhanced CT was performed at 3-month intervals for 1 year, and every 6 months thereafter. Patients with recurrent obstruction during follow-up underwent additional stent placement.
Definition and Analysis
success was defined as the accurate positioning of the stent to cover the target obstruction and patent contrast passage through the stent. For patients with afferent loop obstruction, clinical success was defined as PTBD catheter removal or capping without any symptoms related to obstruction. For patients with afferent and efferent loop obstruction, clinical success was defined as PTBD catheter removal or capping without obstructive symptoms and improvement of the GOO score of more than one point. Stent patency was defined as the period between stent placement and stent malfunction. Complications were classified into major and minor complications according to the Society of Interventional Radiology classification system for complications by outcome [11]. Kaplan-Meier estimates were used for stent patency and patient survival. Data were considered censored if stents were patent until death or were lost to follow-up. Data were analyzed using Stata 14 (Stata Corporation).
RESULTS
Patient Characteristics
Baseline patient characteristics are summarized in Table 1. The mean age of the 36 patients was 62.7 ± 12.0 years (range 42–86 years). Pancreatic cancer (n = 14) and gastric cancer (n = 14) were the most common primary malignancies, followed by bile duct cancer (n = 4), ampulla of Vater cancer (n = 3), and duodenal cancer (n = 1). Pylorus-preserving pancreaticoduodenectomy (PPPD) was the most commonly performed surgery (n = 11). Bowel reconstruction including Billroth II GJ-stomy (n = 14) was the most common. Recurrent obstruction most frequently involved the afferent loop of GJ-stomy (n = 20), followed by the afferent loop of JJ-stomy (n = 7), and concurrent afferent and efferent loops of GJ-stomy (n = 6) and JJ-stomy (n = 3). The median interval between surgery and stent placement was 16.3 months (range: 0.5–60.8 months).
Table 1. Baseline Characteristics of 36 Patients with Postoperative Recurrent Bowel Obstruction.
| Parameter | Patients |
|---|---|
| Age (years, mean ± SD) | 62.7 ± 12.0 |
| Sex | |
| Men | 27 |
| Women | 9 |
| Primary malignancy | |
| Pancreas cancer | 14 |
| Gastric cancer | 14 |
| Ampulla of Vater cancer | 3 |
| Bile duct cancer | 4 |
| Duodenal cancer | 1 |
| Surgery | |
| PPPD | 11 |
| Partial gastrectomy | 9 |
| Total gastrectomy | 7 |
| Whipple's operation | 5 |
| Extended left hemi-hepatectomy | 2 |
| Palliative GJ-stomy | 1 |
| Palliative CBD resection with HJ-stomy | 1 |
| Bowel reconstruction | |
| Billroth II GJ-stomy | 24 |
| EJ-stomy with Roux-en-Y reconstruction | 7 |
| HJ-stomy with Roux-en-Y reconstruction | 3 |
| GJ-stomy with uncut Roux-en-Y reconstruction | 2 |
| Recurrent obstruction | |
| Afferent loop of GJ-stomy | 20 |
| Afferent and efferent loop of GJ-stomy | 6 |
| Afferent loop of JJ-stomy | 7 |
| Afferent and efferent loop of JJ-stomy | 3 |
CBD = common bile duct, EJ-stomy = esophagojejunostomy, GJ-stomy = gastrojejunostomy, HJ-stomy = hepaticojejunostomy, JJ-stomy = jejunojejunostomy, PPPD = pylorus-preserving pancreaticoduodenectomy
Procedural Outcomes
Percutaneous transhepatic access was made either on the right side (S5 or S6 IHD) (n = 27) or on the left side (S3 IHD) (n = 8). One patient underwent bilateral PTBD for palliation of synchronous hepaticojejunostomy (HJ-stomy) obstruction. Transhepatic stent placement was performed for the obstruction at the afferent loop of the GJ-stomy (n = 20) or JJ-stomy (n = 7). The location of the obstruction was proximal (n = 11), mid (n = 3), and distal (n = 10), classified according to the specifications of a previous study [8]. Three patients had two obstructions: proximal and mid (n = 2), and proximal and distal (n = 1). Nine patients with concurrent afferent and efferent loop obstruction of GJ-stomy (n = 6) or JJ-stomy (n = 3) underwent stent placement with a combined transhepatic and peroral approach for simultaneous stent placement. Twenty-three patients underwent post-stent balloon dilatation with 10–16 mm balloon catheters. Eleven patients had synchronous malignant obstructions in HJ-stomy (n = 5), common bile duct (n = 4), and EJ-stomy (n = 1), which were treated with stent placement in the same session. The procedural outcomes are summarized in Table 2.
Table 2. Procedural Outcome of 36 Patients.
| Parameter | Values |
|---|---|
| A-loop stent | |
| Total number of stent | 42 |
| Size (mm)* | 18 (12–24) |
| Length (cm)* | 8 (4–12) |
| E-loop stent | |
| Total number of stent | 9 |
| Size (mm)* | 20 (14–24) |
| Length (cm)* | 10 (8–12) |
| Post-ballooning | |
| Total number of patient | 23 |
| Size (mm)* | 12 (10–18) |
| Simultaneous procedure | |
| HJ-stomy stent placement | 5 |
| EJ-stomy stent placement | 4 |
| CBD stent placement | 2 |
| PTBD in patients with A-loop obstruction | |
| Duration (days)* | 4 (2–22) |
| Removal (%)† | 22 (81.5) |
| PTBD in patients with A-and E-loop obstruction | |
| Duration (days)* | 6 (3–7) |
| Removal (%)‡ | 4 (44.4) |
*Values are presented as median (range), †Values are available for 27 patients, ‡Values are available for 9 patients. A-loop = afferent loop, CBD = common bile duct, E-loop = efferent loop, EJ-stomy = esophagojejunostomy, HJ-stomy = hepaticojejunostomy, PTBD = percutaneous transhepatic biliary drainage
Technical and Clinical Outcomes
In all patients, stent placement was technically successful. Three of 27 patients with afferent loop obstruction complained of persistent obstructive symptoms. In these patients, insufficient stent expansion with upstream bowel distention was revealed on follow-up CT. Additional stent placement (n = 2) and balloon dilatation (n = 1) were performed in these three patients, 6, 7, and 12 days after initial stent placement. After these re-interventions, one patient with additional stent placement achieved resolution of obstructive symptoms. After that, the PTBD catheter was capped in 25 patients without obstructive symptoms. None of the 25 patients showed aggravation of clinical symptoms and laboratory findings following the cessation of PTBD drainage. Among them, the PTBD catheter was removed in 22 patients after 2–22 days (median 4 days) of stent placement. The PTBD catheter was capped; however, it was not removed in three patients because of the clinical need for safe chemotherapy. In the remaining two patients, obstructive symptoms were persistent and the PTBD catheter was kept. In this context, clinical success was achieved in 25 patients.
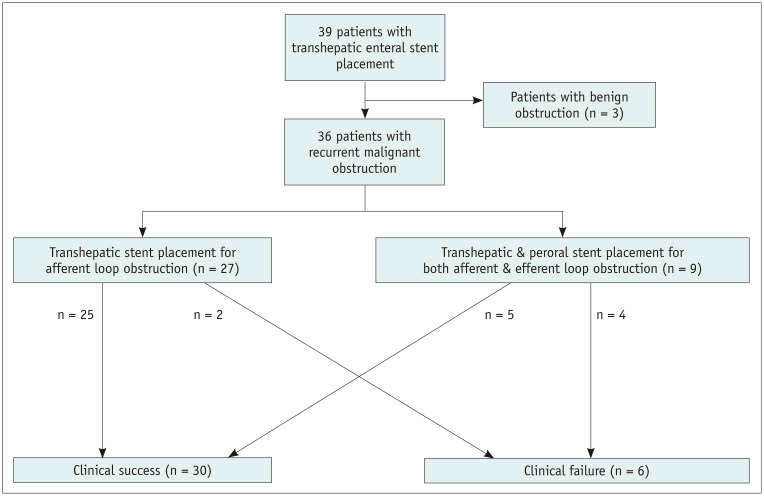
Three of nine patients with afferent and efferent loop obstruction showed persistent obstructive symptoms and no improvement in GOO score. The PTBD catheter was kept in these three patients. In the remaining six patients, obstructive symptoms improved after stent placement, and the GOO score increased two points in four patients, one point in one patient, and no improvement in one patient. Among the five patients with improved obstructive symptoms and GOO score, the PTBD catheter was removed in four patients after 3–7 days (median 6 days) of stent placement. The PTBD catheter was capped; however, it was not removed in one patient because of the clinical need for safe chemotherapy. In this context, clinical success was achieved in all patients. Finally, clinical success of all patients, including those with afferent loop obstruction and patients with afferent and efferent loop obstruction was achieved in 30 out of 36 patients (83.3%). The clinical outcome of all patients was demonstrated as a flow chart (Fig. 2).
Fig. 2. Flow charts of procedure and clinical outcomes.
Major complications were identified in 5 out of 36 patients (13.9%). Four complications were associated with transhepatic access: hepatic artery bleeding (n = 2) and perihepatic biloma (n = 2); these were successfully treated with transarterial embolization and percutaneous catheter drainage, respectively. Hepatic artery bleeding (5.4%) occurred after PTBD placement at the non-dilated S3 IHD. Bowel perforation (n = 1) occurred at the distal end of the stented afferent loop 22 days after stent placement, possibly associated with systemic chemotherapy with paclitaxel and oxaliplatin. The patient was treated with bypass surgery. One patient died 3 days after stent placement due to sepsis of unknown origin.
Two minor complications were found in two patients. Stent fracture was found on a follow-up CT 22 months after stent placement, but it did not cause any clinical symptoms. Stent migration occurred with decreased tumor burden following chemotherapy (156 days after stent placement) and did not cause any specific symptoms. The migrated stents were spontaneously removed through the anus.
Stent Patency and Patients' Survival
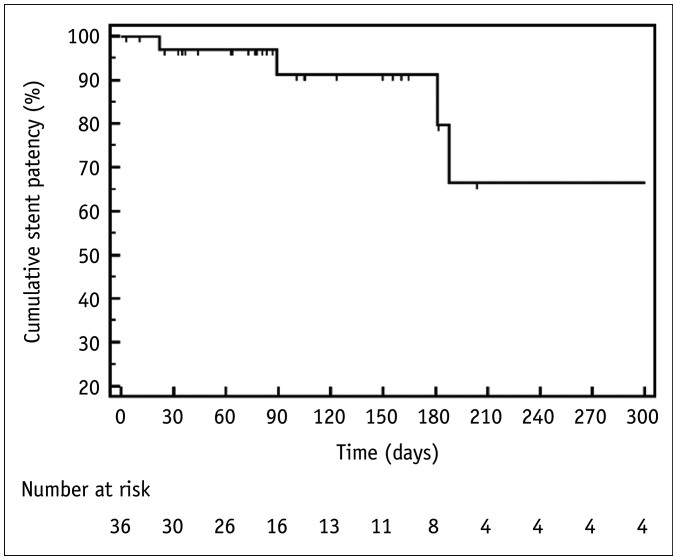
The median follow-up duration was 98.5 days (range: 3–2721 days). Stent failure was identified in six patients. The cause of stent failure was tumor ingrowth (n = 4), perforation (n = 1), and stent fracture (n = 1). Tumor ingrowth occurred in afferent loop stents 89, 188, 372, and 372 days after the initial stent placement, and was treated with peroral (n = 1) or transhepatic (n = 3) coaxial placement of an additional stent. The third, sixth, and twelfth-month primary stent patency rates were 91.2%, 79.8%, and 66.5%, respectively (median 372 days) (Fig. 3).
Fig. 3. Kaplan-Meier Curve of stent patency.
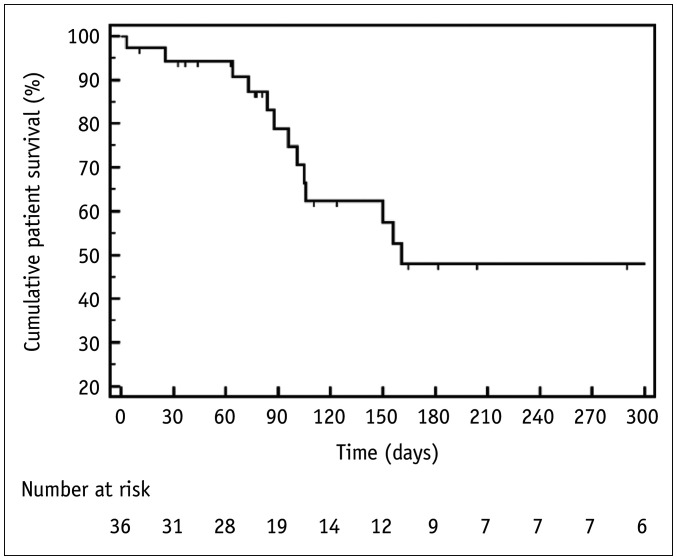
Fourteen patients died 3–1023 days (median, 98.5 days) after stent placement. The cause of death was disease progression (n = 13) and sepsis of unknown origin (n = 1). Fifteen patients were lost to follow-up (two patients within 1 month, seven patients between 1 and 3 months, and six patients between 3 months and 2 years), and seven patients were alive at present. The 3-, 6-, and 12-month patient survival rates were 78.9%, 47.9%, and 47.9%, respectively (median 161 days) (Fig. 4).
Fig. 4. Kaplan-Meier Curve of patient survival.
DISCUSSION
This study demonstrated that transhepatic stent placement is an effective treatment for recurrent malignant obstruction in patients with altered bowel anatomy. The technical feasibility of this procedure has been suggested in a few prior studies, including 10 or fewer cases [6,7,8]. However, to date, its application has been limited to afferent loop obstruction after Billroth II reconstruction. This study showed that the transhepatic approach is a useful option not only for afferent loop obstruction but also for more complex lesions such as concurrent afferent and efferent loop obstruction after Billroth II and Rouxen-Y reconstruction (n = 9). The procedure was technically successful in all patients despite a prior failed peroral approach, and the obstructive symptoms resolved in most patients (83.3%).
Transhepatic stent placement has an advantage over the peroral approach when the obstruction is far from the mouth and/or is tortuous (e.g., obstruction in the proximal part of the afferent loop or obstruction of JJ-stomy after Roux-en-Y reconstruction). In addition, if the patient has severe cholangitis or biliary sepsis due to afferent loop obstruction, the transhepatic approach can be used for bile drainage and enteral stent placement.
There were nine patients with concurrent afferent and efferent loop obstruction in the current study: six patients with GJ-stomy and three patients with JJ-stomy. In these cases, simultaneous stent placement for afferent and efferent loop obstruction is required [12]. However, positioning two guidewires across each obstruction with the peroral approach could be difficult, time-consuming, and even impossible [13]. Therefore, the transhepatic antegrade approach to afferent loop obstruction was technically favorable because it was easier to pass a guidewire from the narrower small bowel to the wider stomach than vice versa. Afterwards, a large lumen guiding sheath was introduced retrogradely and positioned close to the afferent loop obstruction and adjacent efferent loop obstruction. The guiding sheath prevented the guidewire from wandering in the redundant stomach lumen, and provided solid support for the negotiation of efferent loop obstruction.
There were four transhepatic access-related complications: hepatic artery bleeding (n = 2) and perihepatic biloma (n = 2). Hepatic artery bleeding is a rare complication of PTBD. The left side PTBD and non-dilated IHD were revealed as risk factors for hepatic artery injury [14,15]. Two patients (5.4%) with hepatic artery injury in the present study had these two risk factors. Considering serial dilatation of transhepatic access up to 10-French for stent delivery, the right side PTBD must avoid this complication. One bowel perforation in this study was presumed to be associated with chemotherapy due to the use of chemotherapeutic agents known to be associated with perforation [16].
Three patients complained of persistent obstructive symptoms after stent placement despite successful recanalization of the obstruction. The cause of clinical failures was assumed to be decreased bowel movement related to peritoneal carcinomatosis. Stent patency rates at 3-, 6-, and 12-months were 91.2%, 79.8%, and 66.5%, respectively. These results are similar to peroral stent placement in postoperative recurrent gastrointestinal obstruction [17,18]. Also, similar to previous studies [18,19], the most common cause of stent failure was tumor ingrowth, which is the major weakness of an uncovered stent. A covered stent could be a solution to prevent tumor ingrowth. However, the increased profile and stiffness of currently available covered stents need to be improved for transhepatic delivery. This study had several limitations. First, the inherent limitations of a retrospective observational study may have been present, including the possibility of selection bias. Second, a small study population from a single center precluded generalizing the results of our study. This study included one or two focal obstructions. However, postoperative recurrent bowel obstruction tends to be diffuse or multifocal, and stent placement would be less beneficial in such patients. Third, the follow-up duration was short because of advanced disease, which led to longer stent patency than patient survival. Thus, stent patency might be overestimated in this study due to censored data.
In conclusion, percutaneous enteral stent placement using transhepatic access may offer successful palliation of postoperative recurrent malignant obstruction. Transhepatic access is a good alternative route for afferent loop obstruction and can be combined with a peroral approach for simultaneous afferent and efferent loop obstruction.
Footnotes
This research was supported by grant no 14-2019-026 from the Seoul National University Bundang Hospital Research Fund.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: all authors.
- Data curation: all authors.
- Formal analysis: Won Seok Choi, Chang Jin Yoon.
- Funding acquisition: Chang Jin Yoon.
- Investigation: all authors.
- Methodology: all authors.
- Project administration: Chang Jin Yoon.
- Resources: all authors.
- Software: Won Seok Choi.
- Supervision: Chang Jin Yoon.
- Validation: Chang Jin Yoon, Jae Hwan Lee.
- Visualization: Won Seok Choi, Chang Jin Yoon.
- Writing—original draft: Won Seok Choi.
- Writing—review & editing: all authors.
References
- 1.Morikawa S, Suzuki A, Nakase K, Yasuda K. Palliation of malignant upper gastrointestinal obstruction with self-expandable metal stent. Korean J Radiol. 2012;13 Suppl 1:S98–S103. doi: 10.3348/kjr.2012.13.S1.S98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pais-Cunha I, Castro R, Libânio D, Pita I, Bastos RP, Silva R, et al. Endoscopic stenting for palliation of intra-abdominal gastrointestinal malignant obstruction: predictive factors for clinical success. Eur J Gastroenterol Hepatol. 2018;30:1033–1040. doi: 10.1097/MEG.0000000000001178. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, Choi IJ, Kim CG, Lee JY, Cho SJ, Park SR, et al. Self-expandable metallic stent placement for malignant obstruction in patients with locally recurrent gastric cancer. Surg Endosc. 2011;25:1505–1513. doi: 10.1007/s00464-010-1426-y. [DOI] [PubMed] [Google Scholar]
- 4.Bekheet N, Kim MT, Park JH, Kim KY, Tsauo J, Zhe W, et al. Fluoroscopic gastroduodenal stent placement in 55 patients with endoscopic stent placement failure. Cardiovasc Intervent Radiol. 2018;41:1233–1240. doi: 10.1007/s00270-018-1933-0. [DOI] [PubMed] [Google Scholar]
- 5.Bakheet N, Tsauo J, Song HY, Kim KY, Park JH, Wang Z, et al. Fluoroscopic self-expandable metallic stent placement for treating post-operative nonanastomotic strictures in the proximal small bowel: a 15-year single institution experience. Br J Radiol. 2019;92:20180957. doi: 10.1259/bjr.20180957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jinno N, Naitoh I, Nagura Y, Fujioka K, Mizuno Y, Momose J, et al. Percutaneous transhepatic self-expanding metallic stent placement for the treatment of malignant afferent loop obstruction. Intern Med. 2018;57:333–337. doi: 10.2169/internalmedicine.9382-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gwon DI. Percutaneous transhepatic placement of covered, self-expandable nitinol stent for the relief of afferent loop syndrome: report of two cases. J Vasc Interv Radiol. 2007;18:157–163. doi: 10.1016/j.jvir.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Han K, Song HY, Kim JH, Park JH, Nam DH, Ryu MH, et al. Afferent loop syndrome: treatment by means of the placement of dual stents. AJR Am J Roentgenol. 2012;199:W761–W766. doi: 10.2214/AJR.12.8575. [DOI] [PubMed] [Google Scholar]
- 9.Park JH, Song HY, Kim MS, Chung R, Kim JH, Na HK, et al. Usefulness of a guiding sheath for fluoroscopic stent placement in patients with malignant gastroduodenal obstruction. Acta Radiol. 2013;54:267–271. doi: 10.1258/ar.2012.120621. [DOI] [PubMed] [Google Scholar]
- 10.Holt AP, Patel M, Ahmed MM. Palliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: the treatment of choice. Gastrointest Endosc. 2004;60:1010–1017. doi: 10.1016/s0016-5107(04)02276-x. [DOI] [PubMed] [Google Scholar]
- 11.Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:S199–S202. doi: 10.1097/01.rvi.0000094584.83406.3e. [DOI] [PubMed] [Google Scholar]
- 12.Song HY, Kim TH, Choi EK, Kim JH, Kim KR, Shin JH, et al. Metallic stent placement in patients with recurrent cancer after gastrojejunostomy. J Vasc Interv Radiol. 2007;18:1538–1546. doi: 10.1016/j.jvir.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 13.Park JH, Song HY, Kim SH, Shin JH, Kim JH, Kim BS, et al. Metallic stent placement in patients with recurrent malignant obstruction in the surgically altered stomach. Ann Surg Oncol. 2014;21:2036–2043. doi: 10.1245/s10434-014-3566-0. [DOI] [PubMed] [Google Scholar]
- 14.Shiau EL, Liang HL, Lin YH, Li MF, Chiang CL, Chen MC, et al. The complication of hepatic artery injuries of 1,304 percutaneous transhepatic biliary drainage in a single institute. J Vasc Interv Radiol. 2017;28:1025–1032. doi: 10.1016/j.jvir.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Choi SH, Gwon DI, Ko GY, Sung KB, Yoon HK, Shin JH, et al. Hepatic arterial injuries in 3110 patients following percutaneous transhepatic biliary drainage. Radiology. 2011;261:969–975. doi: 10.1148/radiol.11110254. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Wu S, Kundra A, Aja Onu I, Gotlieb V, Wang JC. Gastric perforation in a patient receiving neoadjuvant chemoradiotherapy. World J Oncol. 2015;6:383–386. doi: 10.14740/wjon924w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye BW, Chou CK, Hsieh YC, Li CP, Chao Y, Hou MC, et al. Metallic stent expansion rate at day one predicts stent patency in patients with gastric outlet obstruction. Dig Dis Sci. 2017;62:1286–1294. doi: 10.1007/s10620-017-4534-x. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki R, Sakai Y, Tsuyuguchi T, Nishikawa T, Fujimoto T, Mikami S, et al. Endoscopic management of unresectable malignant gastroduodenal obstruction with a nitinol uncovered metal stent: a prospective Japanese multicenter study. World J Gastroenterol. 2016;22:3837–3844. doi: 10.3748/wjg.v22.i14.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamao K, Kitano M, Kayahara T, Ishida E, Yamamoto H, Minaga K, et al. Factors predicting through-the-scope gastroduodenal stenting outcomes in patients with gastric outlet obstruction: a large multicenter retrospective study in West Japan. Gastrointest Endosc. 2016;84:757–763.e6. doi: 10.1016/j.gie.2016.03.1498. [DOI] [PubMed] [Google Scholar]