Abstract
Background
The temporal changes in the Staphylococcus aureus genotypes causing S. aureus bacteremia (SAB) and the corresponding clinical changes over the last decade in South Korea are rarely investigated.
Methods
A longitudinal study of adult SAB patients was conducted in a large referral hospital in Seoul, South Korea. Adult monomicrobial SAB patients were enrolled between August 2008 and December 2018. Genotyping was performed by multilocus sequence typing (MLST) and staphylococcal protein A (spa) typing. Trends in changes were identified by linear regression analysis.
Results
Of 1782 adult SAB patients, the blood isolates of 1,778 (99.8%) and 1,634 (91.7%) were determined to be MLST and spa type, respectively. ST5 (–2.626%/year) and ST239 (–0.354%/year) decreased during the study period (P < 0.001 for both), but ST72 (2.009%/yr)-and ST8 (0.567%/yr) increased (P < 0.001 for both). The most common genotype was changed from ST5 in 2008 (44.9%) to ST72 in 2018 (36.3%). Panton-Valentine leukocidin-positive spa-t008-MRSA (USA300) was found in 28.6%. Central venous catheter (CVC)-related SAB (–2.440%/yr) and persistent SAB (–1.016%/yr) decreased, but mortality and recurrence rates were unchanged.
Conclusion
Over the last decade, the hospital clones ST5 and ST239 have been replaced by community genotype ST72. This was associated with decreased CVC-related and persistent SAB. Increased USA300 was observed in community and hospital settings. Further research is required to identify the reasons for the ST72 epidemic and predict the impending epidemic of ST8 strains, including USA300.
Keywords: Staphylococcus aureus, Bacteremia, Genotype, Multilocus Sequence Typing
Graphical Abstract
INTRODUCTION
Staphylococcus aureus is a leading cause of community and hospital-acquired (HA) infections worldwide.1 Although 70 years have passed since the introduction of penicillin, this microorganism still remains a major threat to public health due to its ability to adapt and evolve.2 Methicillin-resistant S. aureus (MRSA) appeared more than 60 years ago in hospitals.3,4 In the late 1990s, MRSA emerged outside hospitals in North America and Europe, which was called as community-associated MRSA (CA-MRSA).2 In the US, USA300, one of the predominant clones, became endemic in the community. Before long, USA300 clone was introduced into hospitals and has become a significant cause of HA infections.5
Since the mid-2000s, CA-MRSA has also been reported in South Korea,6 where multilocus sequence typing (MLST) sequence type 72 (ST72) is the predominant CA-MRSA genotype, not USA300.7 Since the late 2000s, ST72 MRSA has been reported to be a major genotype not only in the community but also in hospitals.8,9 It appears that clonal changes related to CA-MRSA are now taking place in South Korea. Despite this, most South Korean S. aureus genotypic studies cover strains discovered from the late 2000s to early 2010s and are thus not representative of current conditions.7,8,9
To elucidate recent changes in S. aureus genotypes causing SAB and the corresponding changes in clinical features of SAB over the last decade, we conducted a longitudinal study of adult monomicrobial S. aureus bacteremia (SAB) patients over 11 years.
METHODS
Study design and patients
This study was conducted between August 2008 and December 2018 at Asan Medical Center, a 2,700 bed university-affiliated teaching hospital in Seoul, Republic of Korea. In the study hospital, infectious diseases consultations were mandated for all adult (age ≥ 18 years) patients who had a positive blood culture test. Of these patients, those who had SAB were prospectively enrolled after the informed consent. Patients with SAB were excluded if they had polymicrobial bacteremia or clinically insignificant SAB, which means that there were no clinical findings consistent with bacteremia and no subsequent antimicrobial therapy. Clinical study nurses registered the patient information into the case report form. Infectious disease physicians involved in this study reviewed the data once every two weeks.
Data collection
The following information was obtained from all patients included in the study: age, gender, place of acquisition, previous underlying diseases or conditions, site of infection, initial clinical severity, and clinical outcomes. All SAB cases were categorized as HA or community-acquired. Community-acquired cases were further categorized into community-acquired healthcare-associated (C-HCA) and community-acquired non-healthcare-associated (C-NHCA) cases, according to previously defined criteria.10 Persistent bacteremia was defined as ≥ 7 days (persistent SAB-I) or ≥ 6 days (persistent SAB-II) of positive blood cultures, even after appropriate antimicrobial therapy. The infection site was determined by experienced infectious diseases physicians based on clinical, radiological, and bacteriological investigations. Central venous catheter (CVC)-related infection was considered to be the infection site in patients with an intravascular device according to the Infectious Diseases Society of America guidelines.11 As differentiating entry portals from sites of metastatic infection was difficult and sometimes inaccurate, this study did not classify the two separately. The Charlson comorbidity index produced a composite score of comorbid conditions.12 The Pitt bacteremia and Acute Physiology and Chronic Health Evaluation (APACHE) II scores were used to determine the severity of acute illness.13
Microbiological data
S. aureus isolates were identified using standard methods. Antimicrobial susceptibilities were determined using the MicroScan system (Dade Behring, West Sacramento, CA, USA) and the standard criteria of the CLSI document M100.14 Vancomycin minimum inhibitory concentrations (MICs) were determined through the standard broth microdilution (BMD) test for all isolates. MLST was performed as previously described.15 MLST alleles and STs were derived from the MLST database (http://www.mlst.net). spa typing was performed according to a previously described protocol.16 spa types were assigned via Ridom StaphType software version 2.2.1 (Ridom GmbH, Műnster, Germany) and SpaServer (http://www.spaserver.ridom.de). The staphylococcal cassette chromosome mec (SCCmec) types were identified as previously described.17 Presence of genes encoding PVL was assessed by polymerase chain reaction (PCR).18
Statistical analyses
The frequency and proportion of each calendar year were calculated for all variables. Linear regression analysis identified whether variables increased or decreased over time. Univariate analyses and multivariate logistic regression models assessed the associations of clinical characteristics and clonal types with CVC-related infection, persistent bacteremia, and 30-day mortality. Statistical analyses were performed using the SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA). All significance tests were two-tailed, and P < 0.05 was considered statistically significant.
Ethics statement
The present study protocol was reviewed and approved by the Institutional Review Board of Asan Medical Center (approval No. 2013-0234). Informed consent was submitted by all subjects when they were enrolled.
RESULTS
Baseline characteristics and their changes over time
The study included 1,782 adult monomicrobial SAB patients. The median age of the studied patients was 63 years (interquartile range, 52–71), and 62.1% (1,113) patients were male. The most common underlying disease was solid tumor (37.9%), followed by diabetes mellitus (31.1%), liver cirrhosis (15.5%), and neurological disease (11.9%). Over a third of the patients had CVCs (39.5%). SAB occurred during intensive care unit (ICU) admission (13.1%) in over a tenth of the patients. An increasing trend was observed for female gender and heart failure, whereas a decreasing trend was observed for hematologic malignancy, hematopoietic cell transplantation, ICU care, mechanical ventilation, CVC, and indwelling urinary catheter (Table 1).
Table 1. Annual changes in demographics, underlying diseases, and underlying conditions of adult Staphylococcus aureus bacteremia.
| Characteristics | No. (%) of patients (n = 1,782) | Annual change (%) | P value | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Median age, yr (IQR) | 63 (52–71) | 0.125 | 0.266 | ||
| Male sex | 1,106 (62.1) | −0.877 | 0.019 | ||
| Underlying disease | |||||
| Solid tumor | 676 (37.9) | −0.004 | 0.993 | ||
| Diabetes | 555 (31.1) | 0.224 | 0.697 | ||
| Liver cirrhosis | 276 (15.5) | −0.039 | 0.910 | ||
| Neurologic disease | 212 (11.9) | 0.761 | 0.077 | ||
| End stage renal disease | 200 (11.2) | 0.082 | 0.602 | ||
| Hematologic malignancy | 153 (8.6) | −0.669 | 0.018 | ||
| Biliary disease | 119 (6.7) | −0.567 | 0.095 | ||
| Solid organ transplant | 105 (5.9) | 0.241 | 0.228 | ||
| Heart failure | 84 (4.7) | 0.675 | 0.003 | ||
| Chronic renal failure | 68 (3.8) | −0.386 | 0.060 | ||
| Multiple trauma | 43 (2.4) | −0.224 | 0.206 | ||
| Hematopoietic cell transplantation | 37 (2.1) | −0.328 | 0.011 | ||
| Underlying condition | |||||
| Immunosuppressant use | 504 (28.3) | 1.015 | 0.248 | ||
| Recent surgery | 337 (18.9) | −0.787 | 0.079 | ||
| Recent chemotherapy | 273 (15.3) | 0.252 | 0.551 | ||
| Neutropenia | 102 (5.7) | −0.135 | 0.417 | ||
| Charlson comorbidity index, median score (IQR) | 3 (2–5) | −0.024 | 0.193 | ||
| ICU care | 234 (13.1) | −1.422 | 0.003 | ||
| Mechanical ventilation | 158 (8.9) | −0.619 | 0.021 | ||
| Central venous catheter | 704 (39.5) | −2.133 | 0.001 | ||
| Short-term | 386 (21.7) | −1.984 | < 0.001 | ||
| Indwelling urinary catheter | 425 (23.8) | −1.974 | 0.005 | ||
| Non-catheter foreign body | 303 (17.0) | 0.597 | 0.311 | ||
IQR = interquartile range, ICU = intensive care unit.
Clinical features of SAB and their changes over time
The median APACHE II score (interquartile range, IQR) was 15 (11–20) on the day of bacteremia development. The median Pitt bacteremia score (IQR) was 1 (0–2). Both of these decreased over the 11-year period (annual decrease 0.163 points for APACHE II score, 0.039 points for Pitt bacteremia score; P = 0.003 and 0.007, respectively). Most diagnostic evaluations, such as transthoracic echocardiogram, spine magnetic resonance imaging (MRI), and ophthalmologic examination, increased over time. The most common site of infection was CVC (26.7%), followed by lungs (14.0%), skin/soft tissue (13.3%), bone/joint (12.7%), surgical site (5.6%), and heart valves (5.1%). CVC-related SAB showed a decreasing trend (annual decrease 2.440%; P < 0.001). The bacteremia duration tended to decrease over the study period (annual decrease 0.128 day per year; P = 0.051), as did the frequency of persistent SAB-II (annual decrease 1.016%; P = 0.021). There were no significant changes in mortality and recurrence rate (Table 2). Univariate and multivariate analyses were performed to investigate risk factors for persistent SAB-II, CVC-related SAB, and 30-day mortality. Their independent risk factors are presented in Supplementary Tables 1 and 2. ST5 was independently associated with all three clinical variables (for persistent SAB-II, adjusted odds ratio [aOR], 1.696; 95% confidence interval [CI], 1.051–2.740; P = 0.031; for CVC-related SAB, aOR, 1.715; 95% CI, 1.135–2.591; P = 0.010; for 30-day mortality, aOR, 1.821; 95% CI, 1.260–2.632; P = 0.001).
Table 2. Annual changes in initial severity, diagnostic evaluation, site of infection, antibiotic treatment, clinical course, and outcomes of adult SAB.
| Characteristics | No. (%) of patients (n = 1,782) | Annual change (%) | P value | |
|---|---|---|---|---|
| Initial severity of SAB | ||||
| Pitt bacteremia median score (IQR) | 1 (0–2) | −0.039 | 0.007 | |
| APACHE score (day of bacteremia), median (IQR) | 15 (11–20) | −0.163 | 0.003 | |
| Diagnostic evaluation for site of infection | ||||
| Transthoracic echocardiogram | 1,465 (82.2) | 1.222 | 0.007 | |
| Transesophageal echocardiogram | 263 (14.8) | 0.053 | 0.845 | |
| Spine MRI | 264 (14.8) | 2.282 | 0.004 | |
| Ophthalmologic evaluation | 1,179 (66.2) | 4.774 | < 0.001 | |
| Site of infection | ||||
| Central venous catheter infection | 475 (26.7) | −2.440 | < 0.001 | |
| Pneumonia | 250 (14.0) | 0.604 | 0.120 | |
| Skin-soft tissue infection | 237 (13.3) | −0.410 | 0.118 | |
| Bone-joint infection | 227 (12.7) | 0.309 | 0.386 | |
| Surgical site infection | 100 (5.6) | −0.111 | 0.629 | |
| Infective endocarditis | 90 (5.1) | 0.020 | 0.917 | |
| Central nervous system infection | 58 (3.3) | 0.110 | 0.566 | |
| Urinary tract infection | 44 (2.5) | −0.020 | 0.898 | |
| Primary bacteremia | 280 (15.7) | 0.239 | 0.534 | |
| Any metastatic infection | 319 (17.9) | −0.352 | 0.517 | |
| Courses and outcomes of SAB | ||||
| Persistent bacteremia I (SAB duration ≥ 7 days) | 245/1,760 (13.9) | −0.654 | 0.120 | |
| Persistent bacteremia II (SAB duration ≥ 6 days) | 304/1,760 (17.3) | −1.016 | 0.021 | |
| Duration of SAB, mean day (SD) (n = 1,760) | 3.53 (5.89) | −0.128 | 0.051 | |
| 30-day mortality | 283 (15.9) | −0.024 | 0.227 | |
| 60-day mortality | 402 (22.6) | −0.018 | 0.388 | |
| 90-day mortality | 488 (27.4) | −0.042 | 0.137 | |
| Recurrence within 3 mon | 90 (5.1) | −0.446 | 0.167 | |
SAB = Staphylococcus aureus bacteremia, IQR = interquartile range, APACHE = Acute Physiology and Chronic Health Evaluation, MRI = magnetic resonance imaging, SD = standard deviation.
Methicillin resistance, site of acquisition, MLST, and spa types, and their changes over time
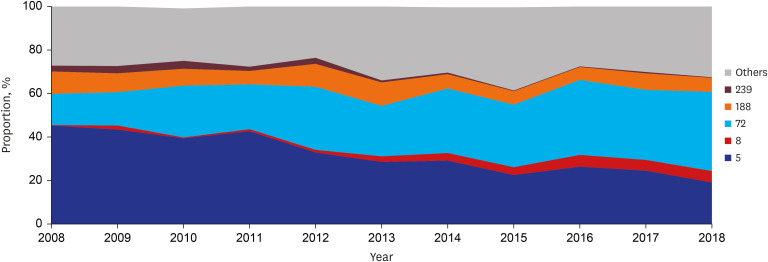
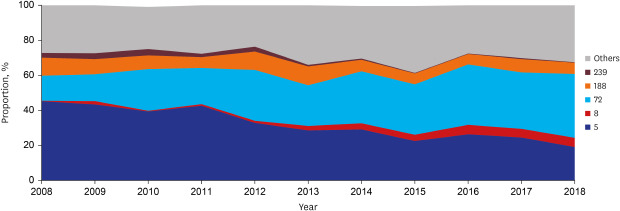
MRSA was observed in half of the patients (51.9%, 924). More than half of the patients (55.6%, 991 of 1782) had HA-SAB, followed by C-HCA-SAB (30.3%, 540) and C-NHCA-SAB (14.1%, 251). Over the study period, MRSA (annual decrease 1.598%, P = 0.017) and HA-SAB (annual decrease 2.366%, P = 0.002) decreased, whereas C-NHCA-SAB increased (annual increase 1.315%, P = 0.010) (Table 3). MLST STs and spa types were identified for 1778 (99.8% of 1782) and 1634 (91.7%) blood isolates, respectively. A total of 59 STs and 224 spa types were found. ST5 (annual decrease 2.626%, P < 0.001) and ST239 (annual decrease 0.354%, P < 0.001) decreased and ST72 (annual increase 2.009%, P < 0.001) and ST8 (annual increase 0.567%, P < 0.001) increased during the study period. In 2008, ST5 was the most common clone (44.9%), followed by ST72 (15.4%). However, ST72 was the most common clone (36.3%) in 2018, followed by ST5 (18.5%) (Fig. 1). Annual changes in MLST STs according to place of acquisition are presented in Supplementary Table 3.
Table 3. Annual changes in methicillin resistance and MLST sequence type among adult SAB patients.
| Characteristics | No. (%) of patients (n = 1,782) | Annual change (%) | P value | |||
|---|---|---|---|---|---|---|
| Methicillin resistance | ||||||
| MRSA | 924 (51.9) | −1.598 | 0.017 | |||
| Place of acquisition | ||||||
| Community-acquired nonhealthcare-associated | 251 (14.1) | 1.315 | 0.010 | |||
| Community-healthcare-associated | 540 (30.3) | 1.051 | 0.134 | |||
| Hospital-acquired | 991 (55.6) | −2.366 | 0.002 | |||
| Major MLST type (major corresponding spa types) according to methicillin resistance (n = 1,778) | ||||||
| MRSA (n = 921) | ||||||
| ST5 (t2460 [n = 298], t9353 [n = 56], t002 [n = 51]) | 538 (58.4) | −3.206 | < 0.001 | |||
| ST5/spa-t2460 | 298 (32.4) | −3.468 | 0.002 | |||
| ST5/spa-t9353 | 56 (6.1) | −0.501 | 0.093 | |||
| ST5/spa-t002 | 51 (5.5) | 0.276 | 0.273 | |||
| ST72 (t324 [n = 142], t664 [n = 44], t148 [n = 33]) | 299 (32.5) | 2.798 | < 0.001 | |||
| ST72/spa-t324 | 142 (15.4) | 0.773 | 0.074 | |||
| ST72/spa-t664 | 44 (4.8) | 0.249 | 0.363 | |||
| ST72/spa-t148 | 33 (3.6) | 0.232 | 0.182 | |||
| ST239 (t037 [n = 19]) | 26 (2.8) | −0.597 | < 0.001 | |||
| ST8 (t008 [n = 14]) | 18 (2.0) | 0.647 | 0.011 | |||
| ST8/spa-t008 | 14 (1.5) | 0.518 | 0.013 | |||
| MSSA (n = 857) | ||||||
| ST72 (t126 [n = 106]) | 161 (18.8) | 1.717 | 0.007 | |||
| ST72/spa-t126 | 106 (12.4) | 1.267 | 0.015 | |||
| ST188 (t189 [n = 116]) | 134 (15.6) | −1.188 | < 0.001 | |||
| ST188/spa-t189 | 116 (13.5) | −1.030 | 0.016 | |||
| ST30 (t338 [n = 15], t012 [n = 11]) | 74 (8.6) | −0.696 | 0.013 | |||
| ST15 (t084 [n = 16], t085 [n = 15]) | 71 (8.3) | 0.566 | 0.080 | |||
| ST1 (t127 [n = 46]) | 59 (6.9) | −0.585 | 0.146 | |||
| ST6 (t304 [n = 27]) | 55 (6.4) | −0.514 | 0.151 | |||
| ST5 (t688 [n = 11], t179 [n = 8]) | 41 (4.8) | −0.325 | 0.506 | |||
| ST8 (t008 [n = 18]) | 31 (3.6) | 0.454 | 0.027 | |||
| ST8/spa-t008 | 18 (2.1) | 0.395 | 0.021 | |||
MLST = multilocus sequence typing, SAB = Staphylococcus aureus bacteremia, MRSA = methicillin-resistant Staphylococcus aureus, MSSA = methicillin-susceptible Staphylococcus aureus.
Fig. 1. Longitudinal change of multilocus sequence typing ST proportion in adult patients with Staphylococcus aureus bacteremia.
Annual changes of MLST and spa types of MRSA isolates
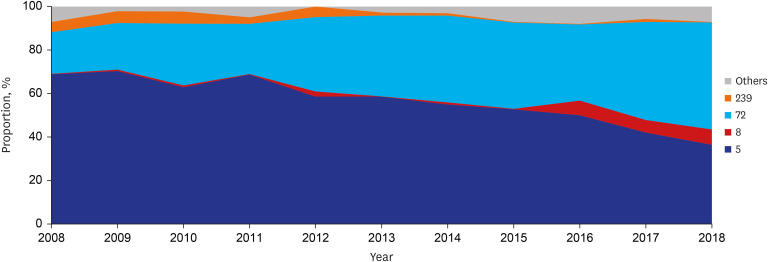
Among MRSA blood isolates, a decreasing trend was observed in ST5 (annual decrease 3.206%, P < 0.001), ST5-spa-t2460 (annual decrease 3.468%, P = 0.002), ST5-spa-t9353 (annual decrease 0.501%, P = 0.093), and ST239 (annual decrease 0.597%, P < 0.001), whereas an increasing trend was observed in ST72 (annual increase 2.798%, P < 0.001), ST72-spa-t324 (annual increase 0.773%, P = 0.074), ST8 (annual increase 0.647%, P = 0.011), and ST8-spa-t008 (annual increase 0.518%, P = 0.013) (Table 3 and Fig. 2). Reductions in ST5 (annual decrease 3.096%, P < 0.001) and ST239 (annual decrease 0.402%, P = 0.019) were statistically significant in the HA-MRSA group, and the decrease in ST239 (annual decrease 1.039%, P = 0.003) was also significant in the C-HCA-MRSA group (Supplementary Table 2). The increase in ST72 was significant in the HA-MRSA group (annual increase 3.141%, P < 0.001), while ST8 increased in the C-NHCA (annual increase 1.970%, P = 0.054), C-HCA (annual increase 0.777%, P = 0.023), and HA-MRSA groups (annual increase 0.432%, P = 0.055) (Supplementary Table 4). Of 49 SAB patients whose blood isolates were identified as ST8, 32 had spa-t008 (69.6% of 46 patients who had their spa types identified), 18 (36.7%) had MRSA, and 14 had spa-t008-MRSA (28.6%). All 14 isolates had the PVL gene, and 13 had SCCmec type IV. Of the 14 patients, 7 (50.0%), 4 (28.6%), and 3 (21.4%) had C-HCA, HA, and C-NHCA infections, respectively.
Fig. 2. Longitudinal change of multilocus sequence typing ST proportion in adult patients with methicillin-resistant Staphylococcus aureus bacteremia.
Annual changes of MLST and spa types of methicillin-susceptible S. aureus (MSSA) isolates
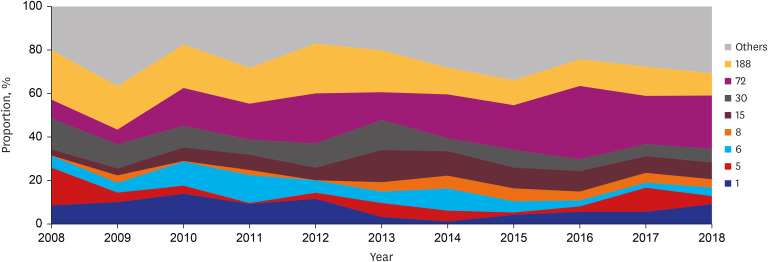
Among MSSA blood isolates, ST72 (annual increase 1.717%, P = 0.007), ST72-spa-t126 (annual increase 1.267%, P = 0.015), ST15 (annual increase 0.566%, P = 0.080), ST8 (annual increase 0.454%, P = 0.027), and ST8-spa-t008 (annual increase 0.395%, P = 0.021) increased, whereas ST188 (annual decrease 1.188%, P < 0.001), ST188-spa-t189 (annual decrease 1.030%, P = 0.016), and ST30 decreased (annual decrease 0.696%, P = 0.013) (Table 3 and Fig. 3). ST72 increased in all C-NHCA, C-HCA, and HA-MSSA groups, but was only statistically significant in the HA-MSSA group (annual increase 1.898%, P = 0.011). ST8 increased in all three groups, but only significantly increased in the C-NHCA-MSSA group (annual increase 0.562%, P = 0.068). ST188 decreased in the HA and C-HCA-MSSA groups, but was only statistically significant in the C-HCA-MSSA group (annual decrease 2.291%, P < 0.001) (Supplementary Table 4). Common spa types and their changes are presented in Supplementary Table 5.
Fig. 3. Longitudinal change of multilocus sequence typing ST proportion in adult patients with methicillin-susceptible Staphylococcus aureus bacteremia.
Antimicrobial susceptibilities according to methicillin resistance, MLST, and spa type
Antimicrobial susceptibilities according to methicillin resistance/MLST/spa type are presented in Supplementary Tables 6 and 7. Among MRSA isolates, ST5 and ST239 were more resistant to non-ß-lactam antimicrobial agents than ST72 and ST8. However, the rifampin resistance rate was low overall (2.0–10.6%), and the trimethoprim-sulfamethoxazole resistance rate was similar (~2.2%) except for ST239 (96.2%). Among CA-MRSA genotypes, ST8 was more resistant to levofloxacin (83.3% vs. 11.4%) and erythromycin (72.2% vs. 27.8%) than ST72. MSSA isolates were generally susceptible to non-ß-lactam antimicrobial agents. However, the fusidic acid resistance rate was high in ST72 (83.2%) and ST1 (62.7%), and ST30 was relatively more resistant to clindamycin (50.0%) and erythromycin (70.3%) than other MSSA STs. Unlike ST72-MRSA (major spa type, t324), ST72-MSSA (major spa type, t126) had high fusidic acid resistance (2.7% vs. 83.2%) (Supplementary Table 6). Of the ST5-MRSA strains, spa-t002 had lower fusidic acid resistance (37.3%) than other spa types (98.7–100%). Of the ST72-MRSA strains, spa-t148 had higher levofloxacin resistance (39.4%) than other spa types (4.5–8.5%) (Supplementary Table 7).
Vancomycin MICs
The mean vancomycin MICs (standard deviation) of all MRSA and MSSA isolates were 1.00 (0.27) and 0.81 (1.30), respectively. Although vancomycin use was not reduced over the 11 years, mean vancomycin MIC decreased for MRSA (annual change 0.018) and MSSA (annual change 0.001) but not statistically significantly (P = 0.27 in MRSA, and P = 0.73 in MSSA).
DISCUSSION
The proportion of SAB caused by ST5 or ST239 has declined over the last decade. Conversely, ST72 has increased and replaced ST5 as a cause of SAB, and ST8 has emerged as a cause of SAB in C-NHCA, C-HCA, and HA settings.
Developing MRSA typing methods led to the observation that certain MRSA genotypes increased in certain regions, peaked, decreased, and were replaced with new genotypes. This is the phenomenon of clonal replacement. However, the reason for the dynamic nature of S. aureus populations is not understood.2 In this study, ST5 and ST239 decreased significantly over time. Of 579 ST5 blood isolates, 92.9% (538) were ST5-MRSA-SCCmec type II, and 25 ST239 isolates (96.2%) were ST239-MRSA-SCCmec type III. These are major pandemic hospital strains in South Korea.19 As their decrease was accompanied by decreased ICU care, mechanical ventilation, CVC, indwelling urinary catheters, and CVC-related SAB, the reduction of CVC-related SAB in critically ill patients may be one of major factors resulting in the reduction of these genotypes. Furthermore, ST5 was the only genotype associated with CVC-related SAB in multivariate analysis. Various infection control measures to prevent CVC-related bacteremia were implemented in the study center (Supplementary Table 8). Infection control measures, especially related to CVC, are suggested to be one of reasons for decreased ST36 MRSA strain incidence in the UK.20 Efforts to prevent CVC-related infection may therefore be a means of reducing certain hospital genotypes among adult SAB patients, although further studies would be needed. Despite this decline in ST5, SAB occurrence has not decreased (annual decrease in SAB cases, 0.509 case, P = 0.947; annual decrease in proportion of SAB cases among all admitted patients, 0.005%, P = 0.431), as ST72 appears instead of hospital strains.
Several South Korean studies showed the increase of ST72 as a hospital genotype.7,8,9,21 While ST5 and ST239 decreased in the present study, the proportion of ST72 gradually increased, with ST72 becoming the most common genotype in 2018. Of the 6 subgroups classified based on location of acquisition and methicillin resistance, ST72 was the most frequently observed ST except in the HA-MRSA group. In the HA-MRSA group, the annual rate of decrease in the ST5 proportion (3.096%) and the annual rate of increase in the ST72 proportion (3.141%) were similar (Supplementary Table 4). ST72 is therefore widespread in both community and hospital settings, replacing ST5 in the latter. The fact that ST72 replaces ST5 and ST239 in a hospital setting was first discovered in this study. The reasons for the success of ST72 in the community and hospitals are to be determined. Joo et al.22 emphasized agr functionality and tolerance to hypotonic or desiccating environments of ST72 and suggested the resultant in-hospital persistence of ST72 as a factor. Easy transmissibility of the strain may be another reason, as is the case with the USA300 strain,23,24 but this has not been studied in ST72. The spa typing of ST72 and different antimicrobial susceptibilities between different ST72 spa types shows that the temporal expansion of ST72 results from simultaneous increases in multiple genetically similar strains (ST72-MRSA-spa-t324, ST72-MRSA-spa-t664, ST72-MRSA-spa-t148, and ST72-MSSA-spa-t126) rather than the expansion of a single clone. This suggests that several ST72 spa types may have unknown common characteristics, enabling effective adaptation in community and hospital settings, rather than easy transmissibility. The reason for USA300 strain dominance is being revealed via high-resolution bacterial genomics and various surveillance sample collections,25,26 and in-depth study of the remarkable increase in ST72 is needed to control the outbreak of ST72.
In this study, clonal replacement was associated with changes in the clinical features of SAB, as shown in a study of SAB patients over 21 years at a large US hospital.27 Though the increase of USA300 was accompanied by increased metastatic infection, decreased ST5 and decreased ST72 were accompanied by decreased CVC-related and persistent SAB. However, there was no significant change in clinical outcomes in either study. Despite a decrease in ST5-infected patients in this study, which were at higher risk of 30-day mortality than other ST-infected patients, the mortality rate did not decrease. This may be due to more standardized and advanced SAB treatment, such as thorough diagnostic evaluation, early source control, and early effective antimicrobial agent administration through mandatory infectious disease consultation.
Increased ST8-spa-t008 was observed regardless of methicillin resistance. Simultaneous MRSA-spa-t008 and MSSA-spa-t008 increases have already been reported in the US.28 The spread of the genotype is likely related more to the characteristics of the genotype than to methicillin resistance.29 The increase has occurred regardless of the place of acquisition. This demonstrates that the genotype has characteristics that can adapt well to both hospital and community settings. More than one fourth (n = 14, 28.6%) of ST8 strains were PVL-positive spa-t008-MRSA (USA300). It is therefore suggested that SAB caused by USA300 or its genetically related ST8 genotypes is increasing in Korea. This is consistent with recent findings from a large-scale collection of invasive S. aureus infectious strains in Korea,30 our study group,31 and a report from the military veterans' hospital in Seoul.32 The first South Korean USA300 case in 2008 occurred in a patient who had traveled to Hawaii.33 As subsequent cases had no overseas travel history, the possibility of autochthonous acquisition was suggested.34 To find the origin of USA300 strains in Korea and predict future epidemiology, comparing the genetic information of USA300 strains found in Korea and that of strains found in the US is required. Given the enormous impact of the USA300 strain in the US, continuous monitoring of the ST8 genotype is required in Korea.
Antimicrobial resistance patterns varied according to MLST and spa types. The fusidic acid resistance rates of MRSA and MSSA differed among the same ST72 strains. This is potentially related to the different spa types in the same ST72 strain. Similarly, among the ST5-MRSA and ST72-MRSA strains, resistance patterns were different according to spa type. This may be due to the fact that certain genotypes that can easily acquire resistance to specific antibiotics have a survival advantage over others. The relationship between specific genotypes and antimicrobial resistance requires further research.
This study has a few important limitations. The study was conducted at a representative referral hospital visited by patients nationwide. However, as HA-SAB strains must reflect the situation of the hospital and not that of the community, our data cannot be generalized to other hospitals with different characteristics. For example, ST5 or ST239 may still be the most common STs in hospitals with insufficient infection control activities for CVC-related infection. Nevertheless, the increase in ST72 and ST8 is remarkable and sufficiently demonstrates recent changes in SAB genotypes in Korean hospitals. The prospective collection of more than 1,700 SAB patients and their S. aureus blood isolates over the 11-year period provides important data on changing trends. Second, ST8 strains were not all molecularly characterized further. As aforementioned, whole genome sequencing of these strains and their comparative analysis with a reference USA300 strain is required to identify the origin of these strains and predict the current dynamics of ST8 in Korea.
In conclusion, over the past decade, hospital clones ST5 and ST239 have been replaced by the community genotype ST72. This has been accompanied by changes in the clinical picture of SAB, such as decreased persistent and CVC-related SAB. Although small, an increase in ST8-spa-t008 in the community and hospital settings has been observed and requires additional monitoring.
Footnotes
Funding: This work was supported by a grant from Korea Health Technology R & D Project through Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C2918).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Choi SH, Lee J, Kim YS.
- Data curation: Choi SH, Lee J, Jung J, Kim ES.
- Formal analysis: Choi SH, Lee J, Kim ES, Chong YP, Kim SH, Kim YS.
- Funding acquisition: Kim YS.
- Investigation: Choi SH, Lee J, Jung J, Chong YP.
- Methodology: Choi SH, Lee J, Jung J, Kim ES, Chong YP, Kim SH, Kim YS.
- Project administration: Choi SH, Kim ES, Chong YP, Kim YS.
- Resources: Choi SH, Lee J, Jung J, Kim ES, Kim YS.
- Software: Choi SH, Lee J.
- Supervision: Kim MJ, Chong YP, Kim SH, Lee SO, Choi SH, Woo JH, Kim YS.
- Validation: Choi SH, Lee J, Jung J, Kim ES, Kim MJ, Chong YP, Kim SH, Lee SO, Choi SH, Woo JH, Kim YS.
- Visualization: Choi SH, Lee J, Kim MJ, Kim YS.
- Writing - original draft: Choi SH, Lee J, Jung J, Kim YS.
- Writing - review & editing: Choi SH, Kim MJ, Chong YP, Kim SH, Lee SO, Choi SH, Woo JH, Kim YS.
SUPPLEMENTARY MATERIALS
Independent risk factors for persistent bacteremia or central venous catheter-related infection among adult SAB patients
Risk factors for 30-day mortality among adult SAB patients
Annual change of MLST sequence type and their corresponding spa types according to site of acquisition among adult patients with SAB
Annual change of MLST sequence type based on the site of acquisition and methicillin resistance among adult patients with SAB
Most common spa types of Staphylococcus aureus blood isolates and their corresponding MLST sequence types
Antimicrobial susceptibilities of Staphylococcus aureus blood isolates according to methicillin resistance and MLST sequence type
Antimicrobial susceptibilities of Staphylococcus aureus blood isolates according to methicillin resistance and spa type
Introduction of various infection control measures to prevent central venous catheter-related infection in the study center during the last decade
References
- 1.Holland TL, Fowler VG., Jr Epidemiology of Staphylococcus aureus bacteremia in adults. [Updated 2019]. [Accessed November 24, 2019]. https://www.uptodate.com.
- 2.Lakhundi S, Zhang K. Methicillin-resistant Staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin Microbiol Rev. 2018;31(4):e00020–18. doi: 10.1128/CMR.00020-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirby WM. Extraction of a highly potent penicillin inactivator from penicillin resistant staphylococci. Science. 1944;99(2579):452–453. doi: 10.1126/science.99.2579.452. [DOI] [PubMed] [Google Scholar]
- 4.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339(8):520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 5.Seybold U, Kourbatova EV, Johnson JG, Halvosa SJ, Wang YF, King MD, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis. 2006;42(5):647–656. doi: 10.1086/499815. [DOI] [PubMed] [Google Scholar]
- 6.Kim ES, Song JS, Lee HJ, Choe PG, Park KH, Cho JH, et al. A survey of community-associated methicillin-resistant Staphylococcus aureus in Korea. J Antimicrob Chemother. 2007;60(5):1108–1114. doi: 10.1093/jac/dkm309. [DOI] [PubMed] [Google Scholar]
- 7.Park KH, Chong YP, Kim SH, Lee SO, Choi SH, Lee MS, et al. Community-associated MRSA strain ST72-SCCmecIV causing bloodstream infections: clinical outcomes and bacterial virulence factors. J Antimicrob Chemother. 2015;70(4):1185–1192. doi: 10.1093/jac/dku475. [DOI] [PubMed] [Google Scholar]
- 8.Park SY, Chung DR, Yoo JR, Baek JY, Kim SH, Ha YE, et al. Sequence type 72 community-associated meticillin-resistant Staphylococcus aureus emerged as a predominant clone of nasal colonization in newly admitted patients. J Hosp Infect. 2016;93(4):386–389. doi: 10.1016/j.jhin.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Joo EJ, Chung DR, Kim SH, Baek JY, Lee NY, Cho SY, et al. Emergence of community-genotype methicillin-resistant Staphylococcus aureus in Korean hospitals: clinical characteristics of nosocomial infections by community-genotype strain. Infect Chemother. 2017;49(2):109–116. doi: 10.3947/ic.2017.49.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Health care--associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137(10):791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 11.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med. 2004;140(1):26–32. doi: 10.7326/0003-4819-140-1-200401060-00008. [DOI] [PubMed] [Google Scholar]
- 14.CLSI. Performance Standards for Antimicrobial Susceptibility testing. CLSI Supplement M100-S25. 25th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2015. [Google Scholar]
- 15.Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38(3):1008–1015. doi: 10.1128/jcm.38.3.1008-1015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shopsin B, Gomez M, Montgomery SO, Smith DH, Waddington M, Dodge DE, et al. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J Clin Microbiol. 1999;37(11):3556–3563. doi: 10.1128/jcm.37.11.3556-3563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliveira DC, de Lencastre H. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus . Antimicrob Agents Chemother. 2002;46(7):2155–2161. doi: 10.1128/AAC.46.7.2155-2161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lina G, Piémont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29(5):1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 19.Song JH, Hsueh PR, Chung DR, Ko KS, Kang CI, Peck KR, et al. Spread of methicillin-resistant Staphylococcus aureus between the community and the hospitals in Asian countries: an ANSORP study. J Antimicrob Chemother. 2011;66(5):1061–1069. doi: 10.1093/jac/dkr024. [DOI] [PubMed] [Google Scholar]
- 20.Wyllie D, Paul J, Crook D. Waves of trouble: MRSA strain dynamics and assessment of the impact of infection control. J Antimicrob Chemother. 2011;66(12):2685–2688. doi: 10.1093/jac/dkr392. [DOI] [PubMed] [Google Scholar]
- 21.Joo EJ, Chung DR, Ha YE, Park SY, Kang SJ, Kim SH, et al. Community-associated Panton-Valentine leukocidin-negative meticillin-resistant Staphylococcus aureus clone (ST72-MRSA-IV) causing healthcare-associated pneumonia and surgical site infection in Korea. J Hosp Infect. 2012;81(3):149–155. doi: 10.1016/j.jhin.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Joo EJ, Choi JY, Chung DR, Song JH, Ko KS. Characteristics of the community-genotype sequence type 72 methicillin-resistant Staphylococcus aureus isolates that underlie their persistence in hospitals. J Microbiol. 2016;54(6):445–450. doi: 10.1007/s12275-016-6157-x. [DOI] [PubMed] [Google Scholar]
- 23.Yang ES, Tan J, Eells S, Rieg G, Tagudar G, Miller LG. Body site colonization in patients with community-associated methicillin-resistant Staphylococcus aureus and other types of S. aureus skin infections. Clin Microbiol Infect. 2010;16(5):425–431. doi: 10.1111/j.1469-0691.2009.02836.x. [DOI] [PubMed] [Google Scholar]
- 24.Desai R, Pannaraj PS, Agopian J, Sugar CA, Liu GY, Miller LG. Survival and transmission of community-associated methicillin-resistant Staphylococcus aureus from fomites. Am J Infect Control. 2011;39(3):219–225. doi: 10.1016/j.ajic.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Uhlemann AC, Dordel J, Knox JR, Raven KE, Parkhill J, Holden MT, et al. Molecular tracing of the emergence, diversification, and transmission of S. aureus sequence type 8 in a New York community. Proc Natl Acad Sci U S A. 2014;111(18):6738–6743. doi: 10.1073/pnas.1401006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Copin R, Sause WE, Fulmer Y, Balasubramanian D, Dyzenhaus S, Ahmed JM, et al. Sequential evolution of virulence and resistance during clonal spread of community-acquired methicillin-resistant Staphylococcus aureus . Proc Natl Acad Sci U S A. 2019;116(5):1745–1754. doi: 10.1073/pnas.1814265116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Souli M, Ruffin F, Choi SH, Park LP, Gao S, Lent NC, et al. Changing characteristics of Staphylococcus aureus bacteremia: results from a 21-year, prospective longitudinal study. Clin Infect Dis. 2019;69(11):1868–1877. doi: 10.1093/cid/ciz112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miko BA, Hafer CA, Lee CJ, Sullivan SB, Hackel MA, Johnson BM, et al. Molecular characterization of methicillin-susceptible Staphylococcus aureus clinical isolates in the United States, 2004 to 2010. J Clin Microbiol. 2013;51(3):874–879. doi: 10.1128/JCM.00923-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orscheln RC, Hunstad DA, Fritz SA, Loughman JA, Mitchell K, Storch EK, et al. Genetically restricted, methicillin-susceptible strains contribute to ongoing epidemic of community-acquired Staphylococcus aureus infections. Clin Infect Dis. 2009;49:536–542. doi: 10.1086/600881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song KH, Kim ES, Park KH, Choi HJ, Kim KH, Lee S, et al. Clinical and molecular characterization of Panton-Valentine leukocidin-positive invasive Staphylococcus aureus infections in Korea. Microb Drug Resist. 2019;25(3):450–456. doi: 10.1089/mdr.2018.0238. [DOI] [PubMed] [Google Scholar]
- 31.Jung J, Song EH, Park SY, Lee SR, Park SJ, Sung H, et al. Emergence of Panton-Valentine leucocidin-positive ST8-methicillin-resistant Staphylococcus aureus (USA300 clone) in Korea causing healthcare-associated and hospital-acquired bacteraemia. Eur J Clin Microbiol Infect Dis. 2016;35(8):1323–1329. doi: 10.1007/s10096-016-2668-y. [DOI] [PubMed] [Google Scholar]
- 32.Bae E, Kim CK, Jang JH, Sung H, Choi YM, Kim MN. Impact of community-onset methicillin-resistant Staphylococcus aureus on S. aureus in a central Korea veterans health service hospital. Ann Lab Med. 2019;39:158–166. doi: 10.3343/alm.2019.39.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park CM, Lee DG, Choi SM, Park SH, Choi JH, Yoo JH, et al. A case of perianal abscess due to Panton-Valentine leukocidin positive community-associated methicillin-resistant Staphylococcus aureus: report in Korea and literature review from the Far East. Infect Chemother. 2008;40(2):121–126. [Google Scholar]
- 34.Lim S, Chung DR, Baek JY, Kim SH, Peck KR, Lee NY, et al. A third case of USA300 community-associated methicillin-resistant Staphylococcus aureus infection in Korea. Korean J Intern Med. 2013;28(2):258–260. doi: 10.3904/kjim.2013.28.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Independent risk factors for persistent bacteremia or central venous catheter-related infection among adult SAB patients
Risk factors for 30-day mortality among adult SAB patients
Annual change of MLST sequence type and their corresponding spa types according to site of acquisition among adult patients with SAB
Annual change of MLST sequence type based on the site of acquisition and methicillin resistance among adult patients with SAB
Most common spa types of Staphylococcus aureus blood isolates and their corresponding MLST sequence types
Antimicrobial susceptibilities of Staphylococcus aureus blood isolates according to methicillin resistance and MLST sequence type
Antimicrobial susceptibilities of Staphylococcus aureus blood isolates according to methicillin resistance and spa type
Introduction of various infection control measures to prevent central venous catheter-related infection in the study center during the last decade