Abstract
Background
Intended subtotal resection (STR) followed by adjuvant gamma knife radiosurgery (GKRS) has emerged as an effective treatment option for facial nerve (FN) preservation in vestibular schwannomas (VSs). This study aimed to identify the optimal cut-off volume of residual VS to predict favorable outcomes in terms of both tumor control and FN preservation.
Methods
This retrospective study assessed the patients who underwent adjuvant GKRS for residual VS after microsurgery. A total of 68 patients who had been followed up for ≥ 24 months after GKRS were included. Tumor progression was defined as an increase in tumor volume (TV) of ≥ 20%. House-Brackmann grades I and II were considered to indicate good FN function.
Results
The median residual TV was 2.5 cm3 (range: 0.3–27.4). The median follow-up period after the first adjuvant GKRS was 64 months (range: 25.7–152.4). Eight (12%) patients showed tumor progression. In multivariate analyses, residual TV was associated with tumor progression (P = 0.003; hazard ratio [HR], 1.229; 95% confidence interval [CI], 1.075–1.405). A residual TV of 6.4 cm3 was identified as the cut-off volume for showing the greatest difference in progression-free survival (PFS). The 5-year PFS rates in the group with residual TVs of < 6.4 cm3 (54 patients) and that with residual TVs of ≥ 6.4 cm3 (14 patients) were 93.3% and 69.3%, respectively (P = 0.014). A good FN outcome was achieved in 57 (84%) patients. Residual TV was not associated with good FN function during the immediate postoperative period (P = 0.695; odds ratio [OR], 1.024; 95% CI, 0.908–1.156) or at the last follow-up (P = 0.755; OR, 0.980; 95% CI, 0.866–1.110).
Conclusion
In this study, residual TV was associated with tumor progression in VS after adjuvant GKRS following STR. As preservation of FN function is not correlated with the extent of resection, optimal volume reduction is imperative to achieve long-term tumor control. Our findings will help surgeons predict the prognosis of residual VS after FN-preserving surgery.
Keywords: Gamma Knife Radiosurgery, Vestibular Schwannoma, Facial Nerve, Tumor Volume
Graphical Abstract
INTRODUCTION
Vestibular schwannomas (VSs) are benign tumors arising from the vestibular component of the eighth cranial nerve.1,2 Surgical resection is the mainstay of VS treatment, especially for large tumors.3,4 Although gross total resection (GTR) of VS is associated with long-term tumor control,5,6 it is not always achievable, and considerable risk of facial nerve (FN) dysfunction remains a concern.7,8,9 In this situation, the concept of intended subtotal resection (STR) followed by adjuvant stereotactic radiosurgery such as gamma knife radiosurgery (GKRS) has been introduced as a good treatment option for FN preservation with favorable tumor control.10,11,12,13,14,15 The strategy of intended STR in VS surgery is based on the concept that aggressive resection to achieve GTR poses a higher risk of damage to the FN.8,16 A previous review article reported 85.7–100% good FN outcomes in VS patients who underwent adjuvant GKRS following intended STR.12 However, the definition of STR is not standardized and varies with each study.3,4,8,17 As residual tumor volume (TV) has been associated with tumor recurrence after adjuvant GKRS,13,18,19 the optimal extent of STR not only for functional FN preservation but also for long-term tumor control has been discussed in many studies.6,19,20,21 Still, no standard evidence-based definition of STR has been established.22 If there is a standard cut-off value for residual TV that would predict favorable outcomes in both tumor control and FN preservation, it will provide useful information regarding the optimal extent of intended STR and help physicians predict the behaviors of residual tumors after adjuvant GKRS.
Therefore, this study retrospectively analyzed data from patients who underwent adjuvant GKRS for residual VS after microsurgery. Volumetry of the residual tumor was conducted, and its serial changes were investigated to identify the optimal cut-off volume associated with long-term tumor control and favorable clinical outcomes.
METHODS
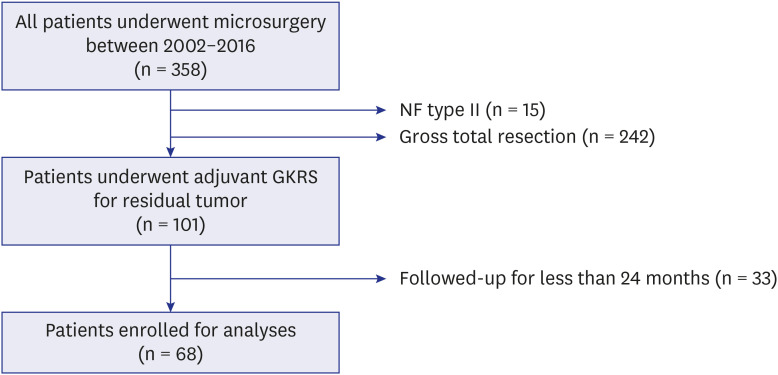
Between January 2002 and December 2016, 358 patients with VS underwent microsurgery at a single institute (shown in Fig. 1). This retrospective study assessed the patients who underwent adjuvant GKRS for residual VS after microsurgery. Fifteen patients with neurofibromatosis type 2 were excluded due to the aggressive nature of the disease. Except for the 242 patients with complete resection, 101 patients underwent adjuvant GKRS for residual tumors. All patients were intraoperatively and histopathologically confirmed to have VS. Patients who had been followed up for ≥ 24 months after GKRS were included, and 68 patients were finally enrolled.
Fig. 1. Flow chart showing the numbers of included and excluded patients.
A total of 68 patients were enrolled for the analyses.
NF = neurofibromatosis, GKRS = gamma knife radiosurgery.
Concerning tumor extent, intraoperative neuromonitoring (IOM) systems including motor-evoked potentials, somatosensory-evoked potentials, and brain stem auditory-evoked potentials were used, as well as other related cranial nerve electromyographies (EMGs). We generally pursued maximal safe resection of tumors, which is especially focused on the anatomical and functional preservation of the FN. Identification of the FN and tumor resection were performed under free-running EMG tracing and direct FN electrical stimulation. The decision of incomplete resection was made intraoperatively.23,24 When surgeons felt that further tumor resection would jeopardize FN function, incomplete resection was determined. Two different surgical approaches (retrosigmoid and translabyrinthine approach) were used as described previously.25 The majority of patients underwent surgery via the retrosigmoid (RS) approach. Except for cases involving a high jugular bulb and/or superficial location of the posterior semicircular canal, unroofing of the internal auditory canal (IAC) was performed to facilitate maximal resection.26,27,28,29 The translabyrinthine (TL) approach was preferred when a substantial cerebellar retraction was expected, such as when tumors were deeply invaginated in the cerebellar peduncle or brain stem.
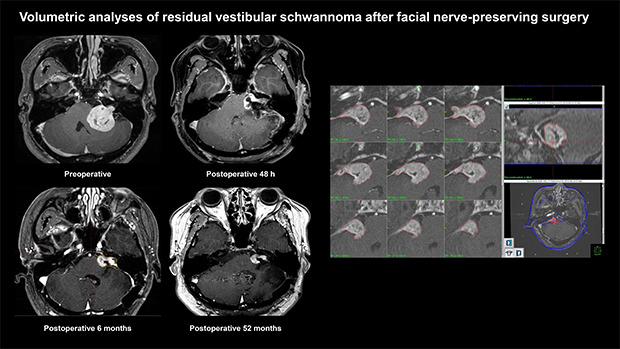
After microsurgery, patients generally underwent immediate postoperative IAC magnetic resonance imaging (MRI) within 48 hours for the evaluation of their baseline postoperative status. Considering the postoperative changes in residual VS, follow-up MRI was performed 3–6 months after surgery.14,20,23 Subsequently, upfront GKRS was scheduled if there was definite evidence of a residual tumor (Fig. 2).30 If it was difficult to determine or clarify any residual tumor on serial postoperative MRI, a follow-up MRI was conducted 1 year later.
Fig. 2. Examples of postoperative changes in the residual tumor on serial MRIs. Preoperative (A), immediate postoperative (B), and 6-month postoperative (C) T-1 weighted contrast-enhanced MRI of right VS. The thin tumor capsule covering the facial nerve was left during the surgery (white arrowhead). Postoperative image taken at 6 months showing progressive closure of the tumorectomy cavity, and the residual tumor was changed to a shape more suitable for gamma knife radiosurgery (white arrow). Preoperative (D), immediate postoperative (E), and 6-month postoperative (F) MRI of left VS. The residual tumor (black arrow) left around the porus acusticus was observed on 6-month postoperative images, but it was not visualized on immediate postoperative imaging.
MRI = magnetic resonance imaging, VS = vestibular schwannoma.
Stereotactic radiosurgery was performed using the Leksell Gamma Knife type B, type C, Perfection, and Icon (Elekta AB, Stockholm, Sweden). Treatment planning was conducted by expert neurosurgeons with specialized experience in radiosurgery using the Leksell Gammaplan® planning software. Patients were followed up in the outpatient clinic at 6 months, 1 year, and 2 years after GKRS using serial IAC MRI scans. Afterwards, follow-up was continued at 2- or 3-year intervals. Tumor progression was defined as a 20% or greater increase in TV as compared to that in the previous serial MRIs during the follow-up period.19,31 Transient swelling within 6 months after GKRS due to radiation-induced tumor necrosis was considered as pseudoprogression.32,33 Additional treatment for tumor progression was determined based on multiple factors: the size of the tumor, previous surgical findings, the patient's symptoms, and other medical conditions. Generally, repeat GKRS was conducted for small-to-medium-sized recurrent tumors. For large tumors, surgical treatment was recommended.
TV was measured using the Leksell Gammaplan® planning software version 11.1.1 (Leksell Gammma Plan; Elekta AB) on a series of contrast-enhanced T1-weighted IAC MRI scans. T2-weighted images were also used to clarify the tumor margin and surrounding tissues. Residual TV was measured at the time of the first adjuvant GKRS using MRI with a GKRS protocol of 1-mm slice thickness with no gap.
FN function was categorized according to the House-Brackmann (H-B) grading system.34 If there were any discrepancies in grading, the worst grade was considered. H-B grades I and II were considered to indicate good FN function.6,19 Hearing function was categorized using the Gardner-Robertson (G-R) scale.35 According to the audiometry result, G-R grades I and II were considered as serviceable and those higher than II as non-serviceable. Immediate postoperative function was defined as the status measured during the 7–14 days of hospital stay after microsurgery. Functional preservation was defined as the maintenance of good and serviceable status in each FN and cochlear nerve function at the last follow-up.
Statistical analyses
Data are presented as medians or means (with ranges) for continuous variables and as frequencies and percentages for categorical variables. Univariate statistical analyses (Cox and logistic regression analyses) were performed to assess categorical and continuous variables. Variables with P < 0.20 in the univariate analysis were selected for multivariate models using multiple Cox regression analyses. The sensitivity and specificity of the cut-off values were analyzed using the receiver operating characteristic (ROC) curve. MaxStat package of R (MaxStat Software, Jever, Germany) was used to identify the optimal cut-off values of the variables. A Kaplan-Meier curve was plotted for progression-free survival (PFS) from the time of GKRS to the last follow-up. Mean and frequency comparisons were performed using the Student's t-test, χ2 test, Mann-Whitney U test, or Fisher's exact test, as appropriate. Statistical analyses were performed using the IBM SPSS Statistics version 25 software (IBM Corporation, Armonk, NY, USA). A P value < 0.05 was considered significant.
Ethics statement
This study was approved by the hospital Institutional Review Board (IRB No. 2019-08-023-001). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Samsung Medical Center Institutional Review Board) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
RESULTS
Characteristics of the 68 patients enrolled in this study are described in Table 1. One patient received fractionated GKRS (20 Gy in 4 fractions) because the large surface of the residual tumor touched the brain stem. Four patients had a previous history of multiple microsurgeries before the first adjuvant GKRS. One patient underwent 2 surgeries using the TL approach at another hospital. This patient was referred to our institute, and we operated on the patient using the RS approach. The others underwent 2 surgeries using the RS approach. No patient had a previous history of radiosurgery. Three (4%) patients underwent ventriculo-peritoneal shunt (VPS) surgery before adjuvant GKRS due to postoperative obstructive hydrocephalus (HCP). The median preoperative TV in the available 65 patients was 15.4 cm3 (range: 3.2–40.9). The median residual TV in all patients was 2.5 cm3 (range: 0.3–27.4). The median follow-up period after the first adjuvant GKRS was 64 months (range: 25.7–152.4).
Table 1. Characteristics of the study population.
| Characteristics | Values | |
|---|---|---|
| No. of patients (%) | 68 (100.0) | |
| Median age, yr | 42.5 (14–83) | |
| Sex (%) | ||
| Female | 42 (61.8) | |
| Male | 26 (38.2) | |
| Approach (%) | ||
| Retrosigmoid | 66 (97.1) | |
| Translabyrinthine | 2 (2.9) | |
| Location of residual tumors (%) | ||
| IAC | 7 (10.3) | |
| CPA | 6 (8.8) | |
| IAC and CPA | 55 (80.9) | |
| Multiple microsurgeries before GKRS (%) | 4 (5.9) | |
| Obstructive HCP after surgery (%) | 3 (4.4) | |
| Median preoperative tumor volumea, cm3 | 15.4 (3.2–40.9) | |
| GKRS treatment features | ||
| Median residual TV, cm3 | 2.5 (0.3–27.4) | |
| Median time to GKRS after surgery, mon | 4.2 (0.7–16.2) | |
| Median prescription marginal dose, Gy | 12.5 (10.0–20.0) | |
| Median prescription isodose, % | 50 (20.0–50.0) | |
| Median follow-up period after GKRS, mon | 64 (25.7–152.4) | |
Values are presented as number of patients (%) or median (range).
IAC = internal auditory canal, CPA = cerebellopontine angle, GKRS = gamma knife radiosurgery, HCP = hydrocephalus, TV = tumor volume.
aDue to the loss of old imaging data, preoperative tumor volume was measured in 65 patients.
Tumor control
Tumor progression was observed in 8 (12%) patients, all of whom received additional treatments (Table 2). The median residual TV of these 8 patients was 7.0 cm3 (range: 0.5–27.4), and the median time to progression after the first GKRS was 15.8 months (range: 3.2–66.0). Although 3 patients (cases 5, 6, and 7) showed decreased TV after the first GKRS, additional treatment was determined as necessary because continuous regrowth of the residual tumors was detected on serial imaging.
Table 2. Summary of 8 patients who underwent additional treatments due to tumor progression.
| Case No. | Age | Sex | Residual TV, cm3 | Time to progression, mon | TV at progression, cm3 | Treatment | |
|---|---|---|---|---|---|---|---|
| 2nd | 3rd | ||||||
| 1 | 33 | F | 0.7 | 14.3 | 3.6 | GKRS | |
| 2 | 45 | F | 2.4 | 52.2 | 4.2 | GKRS | |
| 3 | 71 | M | 4.5 | 3.2 | 13.8 | Surgery | |
| 4 | 35 | F | 6.5 | 19.7 | 12.6 | Surgery | GKRS |
| 5 | 52 | M | 7.5 | 52.1 | 5.8 | GKRS | |
| 6 | 28 | F | 12.5 | 17.0 | 9.9 | GKRS | Surgery |
| 7 | 60 | F | 19.8 | 14.6 | 14.6 | Surgery | GKRS |
| 8 | 33 | M | 27.4 | 5.2 | 35.2 | Surgery | |
| Median (range) | 7.0 (0.7–27.4) | 15.8 (3.2–52.2) | |||||
TV = tumor volume, GKRS = gamma knife radiosurgery.
Four of the 8 patients underwent a second GKRS. In 1 patient (case 6), additional surgery was performed 12 months after the second GKRS owing to further tumor progression. No regrowth of the tumor was observed in the other 3 patients during the 74.6-month (range: 48.0–126.6) median follow-up period after the second GKRS. The remaining 4 patients underwent subsequent surgery as a second line treatment. In the second surgery, a more conservative approach was taken to preserve FN function. One patient (case 3) showed acute deterioration due to tumor bleeding 3.2 months after the first GKRS. The measured TV (13.8 cm3) at progression included the volume from a peritumoral hematoma. GTR of the residual tumor was achieved in only 1 patient (case 3). Additional adjuvant GKRS was performed in another 2 patients (cases 4 and 7). The other patient (case 8) received regular follow-up for the residual lesion after the second surgery.
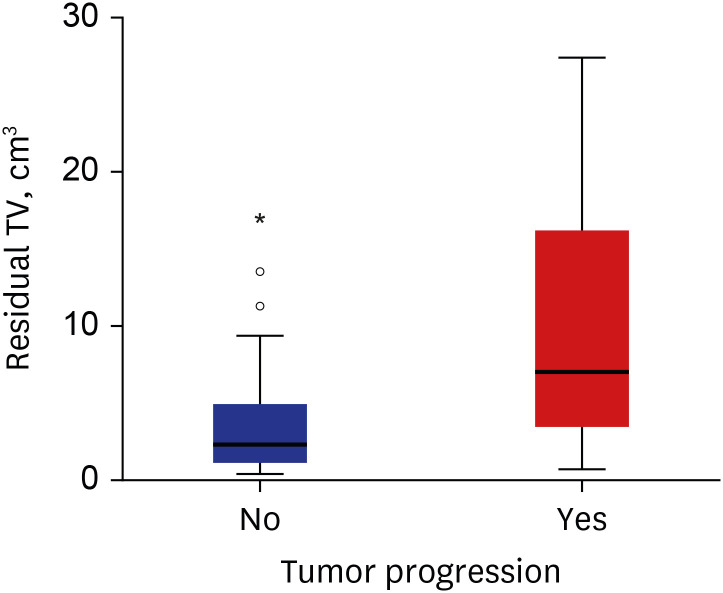
In the 60 patients without tumor progression, the median residual TV was 2.3 cm3 (range: 0.3–17.0). The distribution of residual TV according to the status of tumor progression is shown in Fig. 3. The median time interval between the surgery and first adjuvant GKRS in patients with tumor progression was 4.6 months (range: 1.8–7.2) and in patients without tumor progression was 4.2 months (range: 0.7–16.2) (P = 0.338).
Fig. 3. Box and whisker plots of residual TV distribution according to tumor progression The boxes indicate the 25th and 75th percentiles. The whiskers indicate the minimum and maximum values, dots indicate the outliers, asterisk indicates extreme values, and thick horizontal lines indicate the median value.
TV = tumor volume.
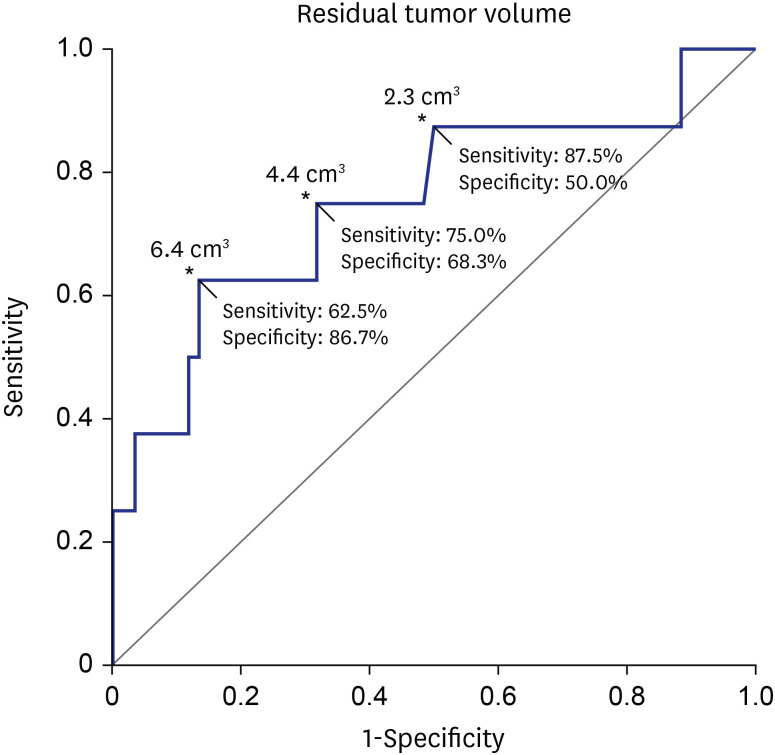
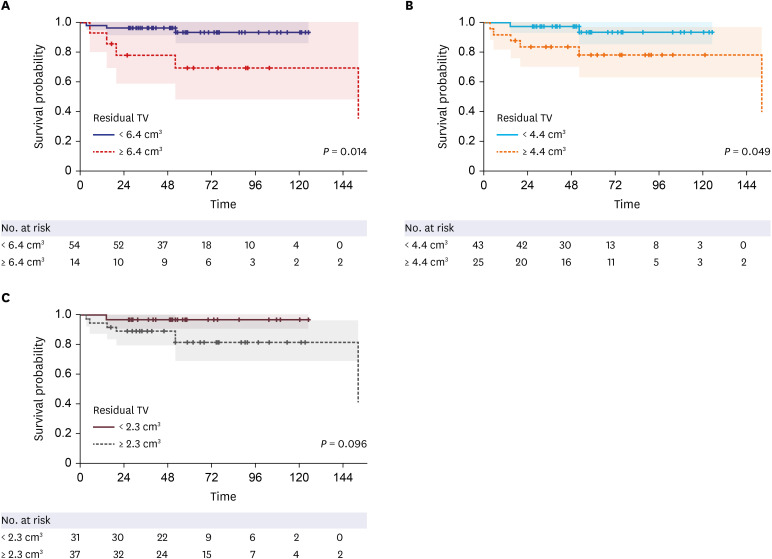
Residual TV was associated with tumor progression in both uni- (P = 0.002; hazard ratio [HR], 1.173; 95% confidence interval [CI], 1.062–1.296) and multivariate analyses (P = 0.003; HR, 1.229; 95% CI, 1.075–1.405) (Table 3). The ROC curve showed that the residual TV can be used as the cut-off variable to predict tumor progression, and several cut-off values of residual TV were proposed (P = 0.021; 95% CI, 0.546–0.960; area under the curve, 0.753) (Fig. 4). MaxStat package of R identified that 6.4 cm3 was the optimal cut-off of residual TV showing the greatest difference in PFS. The Kaplan-Meier plot showed the PFS in the 2 groups stratified according to several cut-off values of the residual TV (Fig. 5). The 5-year PFS rates in the group with residual TVs of < 6.4 cm3 (54 patients) and that with residual TVs of ≥ 6.4 cm3 (14 patients) were 93.3% and 69.3%, respectively (P = 0.014). Patients with a lower residual TV showed better 5-year PFS (93.6% in patients with a residual TV of < 4.4 cm3 and 96.8% in patients with a residual TV of < 2.3 cm3).
Table 3. Factors affecting tumor progression based on uni- and multivariate analyses.
| Factors | Univariate analyses | Multivariate analyses | |
|---|---|---|---|
| Age | 0.982 | ||
| Sex | 0.984 | ||
| Preoperative TV, cm3 | 0.415 | ||
| Location of residual tumors | |||
| IAC (reference) | |||
| CPA | 0.772 | ||
| IAC and CPA | 0.585 | ||
| Time to GKRS after surgery, mon | 0.711 | ||
| Residual TV, cm3 | 0.002 (HR, 1.173; 95% CI, 1.062–1.296) | 0.003 (HR, 1.229; 95% CI, 1.075–1.405) | |
| Prescription marginal dose, Gy | 0.190 | 0.520 | |
TV = tumor volume, IAC = internal auditory canal, CPA = cerebellopontine angle, GKRS = gamma knife radiosurgery, HR = hazard ratio, CI = confidence interval.
Fig. 4. Sensitivity and specificity analysis. Analyses were performed using the cut-off value of residual TV to predict tumor progression.
TV = tumor volume, CI = confidence interval.
*P = 0.021; 95% CI, 0.546–0.960; area under the curve, 0.753.
Fig. 5. Kaplan-Meier plot of PFS according to residual TV cut-offs. (A) The 5-year PFS rates in the group with residual TVs < 6.4 cm3 (54 patients) and that with residual TVs ≥ 6.4 cm3 (14 patients) were 93.3% and 69.3%, respectively (P = 0.014). (B) The 5-year PFS rates in the group with residual TVs < 4.4 cm3 (43 patients) and that with residual TVs ≥ 4.4 cm3 (25 patients) were 93.8% and 78.6%, respectively (P = 0.049). (C) The 5-year PFS rates in the group with residual TVs < 2.3 cm3 (31 patients) and that with residual TVs ≥ 2.3 cm3 (37 patients) were 96.8% and 81.7%, respectively (P = 0.096).
PFS = progression-free survival, TV = tumor volume.
Functional outcomes
Sixty-seven (99%) patients had a preoperative good FN function (Table 4). A good FN function was observed in 50 (74%) patients in the immediate postoperative period and 54 (81%) patients at the time of the first GKRS. Two patients experienced worsening of the FN function after GKRS. One patient (case 3) showed worsened FN function (H-B grade III → IV) due to the tumor bleeding after the first GKRS. Another patient (case 4) showed worsened FN function (H-B grade III → V) after the second GKRS after the additional surgery. At the last follow-up, 57 (84%) patients maintained a good FN function.
Table 4. Functional outcomes.
| Grades | No. of patients (n = 68) | ||||
|---|---|---|---|---|---|
| Preoperative | Immediate postoperative | At first GKRS | At last F/U | ||
| H-B grade | |||||
| 1 | 57 (83.8) | 33 (48.5) | 39 (57.4) | 44 (64.7) | |
| 2 | 10 (14.7) | 17 (25.0) | 15 (22.1) | 13 (19.1) | |
| 3 | 1 (1.5) | 7 (10.3) | 6 (8.8) | 2 (2.9) | |
| 4 | - | 9 (13.2) | 7 (10.3) | 7 (10.3) | |
| 5 | - | 2 (2.9) | 1 (1.5) | 2 (2.9) | |
| G-R scale | |||||
| Serviceable | 27 | 6 (22.2) | 6 (22.2) | 6 (22.2) | |
H-B = House-Brackman, GKRS = gamma knife radiosurgery, F/U = follow-up, G-R = Gardner-Robinson.
In the 8 patients with tumor progression, 6 patients maintained good FN function. The functional preservation rates of the FN in the group with a residual TV of < 6.4 cm3 and ≥ 6.4 cm3 were 85% and 79%, respectively (P = 0.684). Residual TV was not associated with functional preservation of the FN during the immediate postoperative period (P = 0.695; odds ratio [OR], 1.024; 95% CI, 0.908–1.156) or at the last follow-up (P = 0.755; OR, 0.980; 95% CI, 0.866–1.110).
Serviceable hearing was observed in 27 patients before surgery. Postoperative hearing preservation was achieved only in 6 (22%) patients. These findings did not change over the follow-up period. None of the patients maintained serviceable hearing in the tumor progression group.
Complications of microsurgery
After initial surgery, 1 (1%) patient underwent wound revision due to cerebrospinal fluid leakage (Table 5). One (1%) patient with diffuse cerebellar hematoma had to receive long-term rehabilitation. One (1%) patient underwent another craniotomy due to an epidural hematoma associated with a perioperative external ventricular drain. After emergent hematoma evacuation, the patient recovered without permanent neurologic sequelae. Two (3%) patients underwent swallowing rehabilitation due to transient dysphagia caused by lower cranial nerve palsy. A surgical site infection was observed in 1 (1%) patient after a second surgery.
Table 5. Complications.
| Complications | Values | |
|---|---|---|
| Microsurgery | ||
| Cerebrospinal fluid leakage | 1 (1.5) | |
| Cerebellar dysfunction | 1 (1.5) | |
| Epidural hematomaa | 1 (1.5) | |
| Lower cranial nerve palsy | 2 (2.9) | |
| Surgical site infection | 1 (1.5) | |
| Gamma knife radiosurgery | ||
| Hemifacial spasm | 3 (4.4) | |
| Tinnitus | 5 (7.4) | |
| Trigeminal neuralgia | 1 (1.5) | |
| Hydrocephalusb | 1 (1.5) | |
| Facial nerve palsy | 2 (2.9) | |
aDue to prophylactic external ventricular drain insertion; bDue to radiation-induced necrosis of residual tumor.
Complications of GKRS
After the first adjuvant GKRS, 3 (4%) patients complained of hemifacial spasm and 5 (7%) patients had transient tinnitus (Table 5). One (1%) patient underwent a second GKRS due to newly developed trigeminal neuralgia. One (1%) patient received a VPS due to HCP caused by radiation-induced necrosis of a residual tumor 8 months after the second GKRS. However, no further treatment was required as the tumor regressed gradually. Two (3%) patients showed aggravated FN palsy, as described above.
DISCUSSION
Functional preservation of the FN is one of the main goals of VS surgery. Many studies have revealed that intended STR of VS had superiority in terms of good FN outcomes as compared to GTR.4,11,36 However, the concerns related to regrowth of residual tumors remain.5,37 Several previous studies demonstrated that postoperative residual TV was associated with tumor regrowth.19,24,38 Adjuvant GKRS following intended STR of VS has been discussed as an effective treatment option in terms of FN preservation and long-term tumor control.9,10,11,14,15,17 In our previous study of VS, the tumor control rate in the GTR group was 91.9%, whereas the rates in the NTR and STR groups were 84.3% and 75.2%, respectively. However, 92.1% tumor control was achieved in the NTR and STR groups when NTR and STR were followed by adjuvant radiotherapy or radiosurgery.39 A recent meta-analysis also revealed favorable tumor control and functional outcomes from this “nerve-centered” treatment strategy.15 However, the volume of residual tumor after STR was also associated with tumor recurrence after adjuvant GKRS.13,18,19 The risk of tumor progression increases with the size of the residual tumor.14 In the present study, residual TV was the only factor associated with tumor progression after GKRS. When surgeons plan to do intended STR of VS for functional preservation of the FN, the optimal extent of the residual tumor associated with long-term tumor control using adjuvant GKRS needs to be discussed.4,8,12,23
This study aimed to identify the optimal cut-off volume of residual VS that can predict a favorable outcome in terms of both tumor control and FN preservation. A recent multicenter study demonstrated that a good FN outcome immediately post-operation was associated with a larger residual TV.18 Radwan et al.21 revealed that a postoperative residual TV of > 3 cm3 was associated with good FN outcomes after adjuvant GKRS. However, some authors assert that FN preservation is not correlated with the extent of resection if the dissection is performed with a priority given to FN preservation.4,40 In the present study, residual TV was not associated with either early or late good FN outcomes. However, this result can be affected by the technical bias related to individual surgeons and the surgical strategy. There are 2 ways of performing intended STR: planned STR and unplanned STR.22 In planned STR, the decision to pursue STR is made preoperatively. The plane between the tumor capsule and cranial nerves is not dissected.14 Our treatment strategy for VS was unplanned STR, as described above. We tried to maximize the extent of tumor resection to minimize the residual tumor load. If we had tried to dissect the tumor more conservatively, different outcomes may have been obtained. Resection guided by intraoperative FN monitoring may play a significant role in FN preservation during VS surgery.8,22,41 Regarding tumor control, better outcomes were obtained in patients with smaller residual TVs (Fig. 5). If we use a smaller TV as a cut-off value for STR, more favorable long-term outcomes would be achieved. Moreover, the amount of residual tumor as measured by the surgeon's subjective judgment during microsurgery can be different from postoperative MRI-based volumetric measurements. Second salvage operations have been frequently required for large recurrent tumors after radiosurgery.42,43 Functional preservation of the FN in revision surgery of recurrent VS is more challenging.40,44,45,46 Therefore, optimal volume reduction is an important factor in VS surgery, even if the intended STR was planned. Based on our result, favorable outcomes of FN preservation can be achieved without leaving significant amounts of residual tumor.
Both revision surgery and repeat GKRS can be used for patients with recurrent tumors after adjuvant GKRS. Repeat GKRS can be a reasonable treatment option for small-to-medium-sized recurrent tumors after adjuvant GKRS.47 A recent review article demonstrated that radiosurgery showed better FN outcomes than a repeat surgical resection for recurrent VS after primary surgery.48 Iorio-Morin et al.49 reported a 10-year tumor control rate of 92.2% in 76 patients who underwent repeat GKRS for recurrent VS. In the present study, favorable long-term tumor control was achieved in patients with small- to medium-sized recurrent VS using repeat GKRS. Further investigation through larger population studies will be needed to establish the role of repeat GKRS in the prevention of recurrent VS.
There are several limitations of this study. First, this was a retrospective study. Second, the small patient population limited the statistical power. The area under the ROC curve was only 0.753. While it supports the results of our study that larger residual TV are associated with a higher risk of tumor progression, it also shows that residual TV is a relatively weak predictor of the treatment failure. The sensitivity and specificity of the suggested cut-off volume (6.4 cm3) were also insufficient to make a clinical decision. Third, sufficiently longer follow-up periods will be required, as tumor progression can occur at various times and have diverse clinical courses.10,12,19 Finally, other factors that can affect the FN outcomes such as aberrant pathways of the FN, consistency of the tumor, surgical techniques of individual surgeons, and the loss of anatomical continuity of the FN during surgery were not evaluated in this study.50
In this study, residual TV was associated with tumor progression in VS after adjuvant GKRS following STR. As preservation of FN function was not correlated with the extent of resection, optimal volume reduction is imperative to achieve long-term tumor control. Our findings will help surgeons predict the prognosis of residual VS after FN-preserving surgery.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Seol HJ, Lee JI, Lee WJ.
- Data curation: Choi JW, Kong DS, Nam DH.
- Formal analysis: Lee WJ, Seol HJ. Kong DS.
- Methodology: Seol HJ, Lee JI. Cho YS, Shin HJ.
- Writing - original draft: Lee WJ, Lee JI.
- Writing - review & editing: Seol HJ, Choi JW, Nam DH, Kong DS, Cho YS, Shin HJ.
References
- 1.Stangerup SE, Caye-Thomasen P. Epidemiology and natural history of vestibular schwannomas. Otolaryngol Clin North Am. 2012;45(2):257–268. doi: 10.1016/j.otc.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Betchen SA, Walsh J, Post KD. Self-assessed quality of life after acoustic neuroma surgery. J Neurosurg. 2003;99(5):818–823. doi: 10.3171/jns.2003.99.5.0818. [DOI] [PubMed] [Google Scholar]
- 3.Bloch DC, Oghalai JS, Jackler RK, Osofsky M, Pitts LH. The fate of the tumor remnant after less-than-complete acoustic neuroma resection. Otolaryngol Head Neck Surg. 2004;130(1):104–112. doi: 10.1016/S0194-5998(03)01598-5. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Prasad SC, Di Lella F, Medina M, Piccirillo E, Taibah A, et al. The behavior of residual tumors and facial nerve outcomes after incomplete excision of vestibular schwannomas. J Neurosurg. 2014;120(6):1278–1287. doi: 10.3171/2014.2.JNS131497. [DOI] [PubMed] [Google Scholar]
- 5.Nakatomi H, Jacob JT, Carlson ML, Tanaka S, Tanaka M, Saito N, et al. Long-term risk of recurrence and regrowth after gross-total and subtotal resection of sporadic vestibular schwannoma. J Neurosurg. 2017 doi: 10.3171/2016.11.JNS16498. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 6.Seol HJ, Kim CH, Park CK, Kim CH, Kim DG, Chung YS, et al. Optimal extent of resection in vestibular schwannoma surgery: relationship to recurrence and facial nerve preservation. Neurol Med Chir (Tokyo) 2006;46(4):176–180. doi: 10.2176/nmc.46.176. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz MS, Kari E, Strickland BM, Berliner K, Brackmann DE, House JW, et al. Evaluation of the increased use of partial resection of large vestibular schwanommas: facial nerve outcomes and recurrence/regrowth rates. Otol Neurotol. 2013;34(8):1456–1464. doi: 10.1097/MAO.0b013e3182976552. [DOI] [PubMed] [Google Scholar]
- 8.Bernardeschi D, Pyatigorskaya N, Vanier A, Bielle F, Smail M, Lamas G, et al. Role of electrophysiology in guiding near-total resection for preservation of facial nerve function in the surgical treatment of large vestibular schwannomas. J Neurosurg. 2018;128(3):903–910. doi: 10.3171/2016.11.JNS161737. [DOI] [PubMed] [Google Scholar]
- 9.van de Langenberg R, Hanssens PE, van Overbeeke JJ, Verheul JB, Nelemans PJ, de Bondt BJ, et al. Management of large vestibular schwannoma. Part I. Planned subtotal resection followed by gamma knife surgery: radiological and clinical aspects. J Neurosurg. 2011;115(5):875–884. doi: 10.3171/2011.6.JNS101958. [DOI] [PubMed] [Google Scholar]
- 10.Bailo M, Boari N, Gagliardi F, Franzin A, Piloni M, Spina A, et al. Gamma knife radiosurgery for residual and recurrent vestibular schwannomas after previous surgery: clinical results in a series of 90 patients and review of the literature. World Neurosurg. 2017;98:60–72. doi: 10.1016/j.wneu.2016.10.066. [DOI] [PubMed] [Google Scholar]
- 11.Zumofen DW, Guffi T, Epple C, Westermann B, Krähenbühl AK, Zabka S, et al. Intended near-total removal of Koos grade IV vestibular schwannomas: reconsidering the treatment paradigm. Neurosurgery. 2018;82(2):202–210. doi: 10.1093/neuros/nyx143. [DOI] [PubMed] [Google Scholar]
- 12.Brokinkel B, Sauerland C, Holling M, Ewelt C, Horstmann G, van Eck AT, et al. Gamma knife radiosurgery following subtotal resection of vestibular schwannoma. J Clin Neurosci. 2014;21(12):2077–2082. doi: 10.1016/j.jocn.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 13.Iwai Y, Ishibashi K, Watanabe Y, Uemura G, Yamanaka K. Functional Preservation after planned partial resection followed by gamma knife radiosurgery for large vestibular schwannomas. World Neurosurg. 2015;84(2):292–300. doi: 10.1016/j.wneu.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Daniel RT, Tuleasca C, George M, Pralong E, Schiappacasse L, Zeverino M, et al. Preserving normal facial nerve function and improving hearing outcome in large vestibular schwannomas with a combined approach: planned subtotal resection followed by gamma knife radiosurgery. Acta Neurochir (Wien) 2017;159(7):1197–1211. doi: 10.1007/s00701-017-3194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starnoni D, Daniel RT, Tuleasca C, George M, Levivier M, Messerer M. Systematic review and meta-analysis of the technique of subtotal resection and stereotactic radiosurgery for large vestibular schwannomas: a “nerve-centered” approach. Neurosurg Focus. 2018;44(3):E4. doi: 10.3171/2017.12.FOCUS17669. [DOI] [PubMed] [Google Scholar]
- 16.Bretonnier M, Bernard F, Tinois J, Troude L, Cebula H, Godey B, et al. Functional sparing surgery policy for giant vestibular schwannomas. Clin Otolaryngol. 2020;45(5):762–767. doi: 10.1111/coa.13588. [DOI] [PubMed] [Google Scholar]
- 17.Haque R, Wojtasiewicz TJ, Gigante PR, Attiah MA, Huang B, Isaacson SR, et al. Efficacy of facial nerve-sparing approach in patients with vestibular schwannomas. J Neurosurg. 2011;115(5):917–923. doi: 10.3171/2011.7.JNS101921. [DOI] [PubMed] [Google Scholar]
- 18.Monfared A, Corrales CE, Theodosopoulos PV, Blevins NH, Oghalai JS, Selesnick SH, et al. Facial nerve outcome and tumor control rate as a function of degree of resection in treatment of large acoustic neuromas: preliminary report of the Acoustic Neuroma Subtotal Resection Study (ANSRS) Neurosurgery. 2016;79(2):194–203. doi: 10.1227/NEU.0000000000001162. [DOI] [PubMed] [Google Scholar]
- 19.Suero Molina E, van Eck AT, Sauerland C, Schipmann S, Horstmann G, Stummer W, et al. Local tumor control and clinical symptoms after gamma knife radiosurgery for residual and recurrent vestibular schwannomas. World Neurosurg. 2019;122:e1240–6. doi: 10.1016/j.wneu.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Iwai Y, Yamanaka K, Ishiguro T. Surgery combined with radiosurgery of large acoustic neuromas. Surg Neurol. 2003;59(4):283–289. doi: 10.1016/s0090-3019(03)00025-9. [DOI] [PubMed] [Google Scholar]
- 21.Radwan H, Eisenberg MB, Sandberg Knisely JP, Ghaly MM, Schulder M. Outcomes in patients with vestibular schwannoma after subtotal resection and adjuvant radiosurgery. Stereotact Funct Neurosurg. 2016;94(4):216–224. doi: 10.1159/000447520. [DOI] [PubMed] [Google Scholar]
- 22.Starnoni D, Giammattei L, Cossu G, Link MJ, Roche PH, Chacko AG, et al. Surgical management for large vestibular schwannomas: a systematic review, meta-analysis, and consensus statement on behalf of the EANS skull base section. Acta Neurochir (Wien) 2020;162(11):2595–2617. doi: 10.1007/s00701-020-04491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang SY, Kim DG, Chung HT, Park SH, Paek SH, Jung HW. Evaluation of tumour response after gamma knife radiosurgery for residual vestibular schwannomas based on MRI morphological features. J Neurol Neurosurg Psychiatry. 2008;79(4):431–436. doi: 10.1136/jnnp.2007.119602. [DOI] [PubMed] [Google Scholar]
- 24.Breshears JD, Morshed RA, Molinaro AM, McDermott MW, Cheung SW, Theodosopoulos PV. Residual tumor volume and location predict progression after primary subtotal resection of sporadic vestibular schwannomas: a retrospective volumetric study. Neurosurgery. 2020;86(3):410–416. doi: 10.1093/neuros/nyz200. [DOI] [PubMed] [Google Scholar]
- 25.Kim KH, Cho YS, Seol HJ, Cho KR, Choi JW, Kong DS, et al. Comparison between retrosigmoid and translabyrinthine approaches for large vestibular schwannoma: focus on cerebellar injury and morbidities. Neurosurg Rev. 2021;44(1):351–361. doi: 10.1007/s10143-019-01213-1. [DOI] [PubMed] [Google Scholar]
- 26.Shao KN, Tatagiba M, Samii M. Surgical management of high jugular bulb in acoustic neurinoma via retrosigmoid approach. Neurosurgery. 1993;32(1):32–36. doi: 10.1227/00006123-199301000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Tatagiba M, Roser F, Schuhmann MU, Ebner FH. Vestibular schwannoma surgery via the retrosigmoid transmeatal approach. Acta Neurochir (Wien) 2014;156(2):421–425. doi: 10.1007/s00701-013-1915-6. [DOI] [PubMed] [Google Scholar]
- 28.Koval J, Molcan M, Bowdler AD, Sterkers JM. Retrosigmoid transmeatal approach: an anatomic study of an approach used for preservation of hearing in acoustic neuroma surgery and vestibular neurotomy. Skull Base Surg. 1993;3(1):16–21. doi: 10.1055/s-2008-1060560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Day JD, Kellogg JX, Fukushima T, Giannotta SL. Microsurgical anatomy of the inner surface of the petrous bone: neuroradiological and morphometric analysis as an adjunct to the retrosigmoid transmeatal approach. Neurosurgery. 1994;34(6):1003–1008. doi: 10.1227/00006123-199406000-00008. [DOI] [PubMed] [Google Scholar]
- 30.González-Darder JM, Capilla-Guasch P, Escartín FP. Magnetic resonance imaging surveillance for vestibular schwannoma after microsurgical resection using a retrosigmoid transmeatal approach. World Neurosurg. 2020;139:e585–91. doi: 10.1016/j.wneu.2020.04.073. [DOI] [PubMed] [Google Scholar]
- 31.van de Langenberg R, de Bondt BJ, Nelemans PJ, Baumert BG, Stokroos RJ. Follow-up assessment of vestibular schwannomas: volume quantification versus two-dimensional measurements. Neuroradiology. 2009;51(8):517–524. doi: 10.1007/s00234-009-0529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayhurst C, Zadeh G. Tumor pseudoprogression following radiosurgery for vestibular schwannoma. Neuro-oncol. 2012;14(1):87–92. doi: 10.1093/neuonc/nor171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meijer OW, Weijmans EJ, Knol DL, Slotman BJ, Barkhof F, Vandertop WP, et al. Tumor-volume changes after radiosurgery for vestibular schwannoma: implications for follow-up MR imaging protocol. AJNR Am J Neuroradiol. 2008;29(5):906–910. doi: 10.3174/ajnr.A0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146–147. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- 35.Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988;97(1):55–66. doi: 10.1177/000348948809700110. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Liu W, Hui X, You C. Surgical treatment of giant vestibular schwannomas: facial nerve outcome and tumor control. World Neurosurg. 2016;94:137–144. doi: 10.1016/j.wneu.2016.06.119. [DOI] [PubMed] [Google Scholar]
- 37.Goldbrunner R, Weller M, Regis J, Lund-Johansen M, Stavrinou P, Reuss D, et al. EANO guideline on the diagnosis and treatment of vestibular schwannoma. Neuro-oncol. 2020;22(1):31–45. doi: 10.1093/neuonc/noz153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vakilian S, Souhami L, Melançon D, Zeitouni A. Volumetric measurement of vestibular schwannoma tumour growth following partial resection: predictors for recurrence. J Neurol Surg B Skull Base. 2012;73(2):117–120. doi: 10.1055/s-0032-1301395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheehan J, Lee CC, Bodach ME, Tumialan LM, Oyesiku NM, Patil CG, et al. Congress of neurological surgeons systematic review and evidence-based guideline for the management of patients with residual or recurrent nonfunctioning pituitary adenomas. Neurosurgery. 2016;79(4):E539–40. doi: 10.1227/NEU.0000000000001385. [DOI] [PubMed] [Google Scholar]
- 40.Troude L, Boucekine M, Montava M, Lavieille JP, Régis JM, Roche PH. Predictive factors of early postoperative and long-term facial nerve function after large vestibular schwannoma surgery. World Neurosurg. 2019;127:e599–608. doi: 10.1016/j.wneu.2019.03.218. [DOI] [PubMed] [Google Scholar]
- 41.Daoudi H, Lahlou G, Degos V, Sterkers O, Nguyen Y, Kalamarides M. Improving facial nerve outcome and hearing preservation by different degrees of vestibular schwannoma resection guided by intraoperative facial nerve electromyography. Acta Neurochir (Wien) 2020;162(8):1983–1993. doi: 10.1007/s00701-020-04397-4. [DOI] [PubMed] [Google Scholar]
- 42.Lefranc M, Da Roz LM, Balossier A, Thomassin JM, Roche PH, Regis J. Place of gamma knife stereotactic radiosurgery in grade 4 vestibular schwannoma based on case series of 86 patients with long-term follow-up. World Neurosurg. 2018;114:e1192–8. doi: 10.1016/j.wneu.2018.03.175. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe S, Yamamoto M, Kawabe T, Koiso T, Aiyama H, Kasuya H, et al. Long-term follow-up results of stereotactic radiosurgery for vestibular schwannomas larger than 8 cc. Acta Neurochir (Wien) 2019;161(7):1457–1465. doi: 10.1007/s00701-019-03951-z. [DOI] [PubMed] [Google Scholar]
- 44.Peng KA, Chen BS, Lorenz MB, Lekovic GP, Schwartz MS, Slattery WH, et al. Revision surgery for vestibular schwannomas. J Neurol Surg B Skull Base. 2018;79(6):528–532. doi: 10.1055/s-0038-1635256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aboukaïs R, Bonne NX, Touzet G, Vincent C, Reyns N, Lejeune JP. Progression of vestibular schawnnoma after GammaKnife radiosurgery: a challenge for microsurgical resection. Clin Neurol Neurosurg. 2018;168:77–82. doi: 10.1016/j.clineuro.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Wise SC, Carlson ML, Tveiten OV, Driscoll CL, Myrseth E, Lund-Johansen M, et al. Surgical salvage of recurrent vestibular schwannoma following prior stereotactic radiosurgery. Laryngoscope. 2016;126(11):2580–2586. doi: 10.1002/lary.25943. [DOI] [PubMed] [Google Scholar]
- 47.Yomo S, Arkha Y, Delsanti C, Roche PH, Thomassin JM, Régis J. Repeat gamma knife surgery for regrowth of vestibular schwannomas. Neurosurgery. 2009;64(1):48–54. doi: 10.1227/01.NEU.0000327692.74477.D5. [DOI] [PubMed] [Google Scholar]
- 48.Romiyo P, Ng E, Dejam D, Ding K, Sheppard JP, Duong C, et al. Radiosurgery treatment is associated with improved facial nerve preservation versus repeat resection in recurrent vestibular schwannomas. Acta Neurochir (Wien) 2019;161(7):1449–1456. doi: 10.1007/s00701-019-03940-2. [DOI] [PubMed] [Google Scholar]
- 49.Iorio-Morin C, Liscak R, Vladyka V, Kano H, Jacobs RC, Lunsford LD, et al. Repeat stereotactic radiosurgery for progressive or recurrent vestibular schwannomas. Neurosurgery. 2019;85(4):535–542. doi: 10.1093/neuros/nyy416. [DOI] [PubMed] [Google Scholar]
- 50.Prasad GL. Facial nerve localization and functional preservation in vestibular schwannomas. World Neurosurg. 2017;97:732–733. doi: 10.1016/j.wneu.2016.10.009. [DOI] [PubMed] [Google Scholar]