Abstract
Background
Despite some evidence of improved survival with intraoperative cholangiography during cholecystectomy, debate has raged about its benefit, in part because of its questionable benefit, time, and resources required to complete.
Methods
An International Prospective Register of Systematic Reviews–registered (ID CRD42018102154) meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines using PubMed, Scopus, Web of Science, and Cochrane library from 2003 to 2018 was undertaken including search strategy “intraoperative AND cholangiogra* AND cholecystectomy.” Articles scoring ≥ 16 for comparative and ≥ 10 for noncomparative using the Methodological Index for Non-Randomized Studies criteria were included. A dichotomous random effects meta-analysis using the Mantel-Haenszel method performed on Review Manager Version 5.3 was carried out.
Results
Of 2,059 articles reviewed, 62 met criteria for final analysis. The mean rate of intraoperative cholangiography was 38.8% (range 1.6%–96.4%).There was greater detection of bile duct stones during cholecystectomy with routine intraoperative cholangiography compared with selective intraoperative cholangiography (odds ratio = 3.28, confidence interval = 2.80–3.86, P value < .001). While bile duct injury during cholecystectomy was less with intraoperative cholangiography (0.39%) than without intraoperative cholangiography (0.43%), it was not statistically significant (odds ratio = 0.88, confidence interval = 0.65–1.19, P value = .41). Readmission following cholecystectomy with intraoperative cholangiography was 3.0% compared to 3.5% without intraoperative cholangiography (odds ratio = 0.91, confidence interval = 0.78–1.06, P value = .23).
Conclusion
The use of intraoperative cholangiography still has its place in cholecystectomy based on the detection of choledocholithiasis and the potential reduction of unfavorable outcomes associated with common bile duct stones. This meta-analysis, the first to review intraoperative cholangiography use, identified a marked variation in cholangiography use. Retrospective studies limit the ability to critically define association between intraoperative cholangiography use and bile duct injury.
Highlights
-
•
Marked variation exists in the use intraoperative cholangiography (IOC) during cholecystectomy internationally.
-
•
Routine IOC detects more than 3-fold the number of common bile duct stones as selective IOC, potentially reducing unfavorable outcomes associated with common bile duct stones.
-
•
The success rate of IOC is higher when a routine policy is adopted as opposed to a selective policy.
-
•
Bile duct injury and readmission rate are are not significantly reduced when comparing cholecystectomy with and without IOC.
-
•
Large prospective studies and surgical registries are required for the analysis of outcomes, particularly bile duct injury.
INTRODUCTION
There have been many paradigm shifts in cholecystectomy techniques since Carl Langenbuch reported the first cholecystectomy in 1882 and Mirizzi subsequently described cholangiography in 1932 [1,2]. Coupled with this have been significant changes in the management of choledocholithiasis, suggesting an increased trend toward bile duct clearance intraoperatively [3,4]. In general, 3%–12% of patients undergoing cholecystectomy have associated common bile duct (CBD) stone [5,6], and this is increased in those undergoing emergency surgery [7]. The impact of CBD stones is not clearly understood, confounded by variable rates of stone passage and adverse sequelae [8,9]. It has been suggested that failure to remove CBD stones has an unfavorable outcome in 25%, which is halved by clearance of the CBD stone [8].
Elderly patients with untreated CBS stone have a higher incidence of gallstone-related complications [10]. Historically, surgeons have striven to detect CBD stone and anatomical abnormalities during cholecystectomy by using intraoperative cholangiography (IOC) as part of a perceived better surgical practice. Its use is decreasing [11], performed in a variable fashion from routinely to never. The reason for this variance probably relates to the time required; difficulty of the procedure, especially in acute cholecystitis; and having a clear algorithm for detected CBD stones. The value of IOC is certainly in question, spurred by improved preoperative magnetic resonance cholangiopancreatography (MRCP) and widespread access to endoscopic ultrasound, endoscopic retrograde cholangiopancreatography (ERCP), and fluorescence cholangiography [12].
The aim of the current meta-analysis was to evaluate the variability in performance and potential impact of IOC.
MATERIALS AND METHODS
Search Strategy and Study Eligibility
A meta-analysis of all published articles was conducted at Letterkenny University Hospital Ireland in June 2018 using the electronic databases Pub Med, Scopus, Web of Science, and the Cochrane Library for a 15-year period from January 2003 to June 2018. Additionally, a manual troll of trial registries and reference lists for gray literature was undertaken. The reproducible search strategy “intraoperative AND cholangiogra* AND cholecystectomy” was used across all 4 databases to include all relevant articles.
Eligibility Assessment and Data Extraction
The primary outcome was to assess the variability and potential impact on surgical outcomes following the use of IOC during cholecystectomy. Secondary outcomes were to identify factors that contributed to any variability.
The methods of analysis and inclusion criteria were specified in advance to avoid selection bias and documented in a protocol, registered with the International Prospective Register of Systematic Reviews (CRD42018102154) on July 23, 2018. This meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [13].
Studies were included in the meta-analysis if the following criteria were met: either open or laparoscopic cholecystectomy, elective or emergency, where the use and findings of IOC were reported and full articles were available in English. Studies based on pediatric or pregnant patients were not included. Reviews, meta-analyses, case reports, errata, letters, protocols, surveys, studies that did not report key outcomes, and those whose data were inadequate for interpretation via meta-analysis were not included in this meta-analysis.
Eligibility assessment was performed independently in a blinded standardized manner by 2 reviewers, and disagreements between reviewers were resolved by discussion (ED, CM).
The descriptive and quantitative data from the screened studies were extracted by 2 reviewers (ED, MC) and compared to ensure that data extraction was complete. Data were collected using a data extraction sheet with prespecified criteria, which were further refined after pilot testing of randomly chosen studies.
Studies reporting the total number of cholecystectomies carried out with and without attempted IOC were analyzed to assess the variability in IOC use across different studies. The mean rate of IOC was defined as the total number of successful cholangiographies completed as a percentage of the number of cholecystectomies carried out. As the use of IOC depends on the policy of a surgeon or hospital, randomized trials where participants were randomly allocated to treatment groups were not used in analysis of the rate of IOC use during cholecystectomy but were included for analysis of other outcomes. Studies that did not report the total number of cholecystectomies performed with IOC and without a planned IOC during the study period were also not used for the analysis of rate.
Analysis of the rate under a selective and routine policy of IOC use was also carried out. An additional analysis of multicenter studies (representing more than 2 institutions) only was performed to analyze the variation in the use of IOC across different countries, with studies from a same country grouped together.
Data were extracted from studies that reported a routine or selective policy of IOC to evaluate the detection of CBD stones, incidence of bile duct injury, conversion rates, and intraoperative complication rates under each policy. The rates of each outcome were calculated as a percentage of the total cholecystectomies carried out.
The impact of IOC on biliary injury and readmission rate was investigated by analysis of studies reporting outcomes with and without the use of IOC.
Quality Assessment
The Methodological Index for Non-Randomized Studies (MINORS) criteria [14] were used for quality assessment of comparative and noncomparative surgical studies using a 3-point scale (0 = not reported, 1 = reported but inadequate, 2 = reported and adequate) on 8 items for noncomparative studies and 12 items for comparative studies. The ideal global score for noncomparative and comparative studies was chosen as 16 and 24, respectively. All collated studies including randomized controlled trials were marked against the MINORS criteria to assess the studies with the best methodologies to include in the final analysis. Although the criteria were designed for nonrandomized studies, randomized control trials were also marked using the criteria because they are the criterion standard of original published research and were used in validating the MINORS criteria. Three reviewers performed quality assessment independently in a blinded standardized manner, and disagreements between reviewers were resolved by discussion between the review authors (ED, MC, JC) and, if an agreement could not be reached, then by a fourth reviewer (LF). The studies with a MINORS score of ≥ 16 out of 24 for comparative and ≥ 10 out of 16 for noncomparative were included in the final analysis.
Statistical Analysis
A dichotomous meta-analysis using the Mantel-Haenszel method was used to analyze the data [15]. The results were presented as pooled odds ratios (ORs) with 95% confidence interval (CI) in a forest plot performed on Review Manager (RevMan) Version 5.3. Statistical heterogeneity was measured using I2 scores calculated using Review Manager. A random-effects model was used when the I2 statistic reached more than 50%; otherwise, a fixed-effects model would be used. Any levels of substantial heterogeneity were explored in conjunction with the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, with an I2 statistic of 0%–40% representing little heterogeneity between studies, 30%–60% moderate heterogeneity, 50%–90% substantial heterogeneity, and 75%–100% considerable heterogeneity [15]. χ2 testing was used to examine differences in proportions, and a 2-way contingency table analysis was used to calculate relevant ORs.
RESULTS
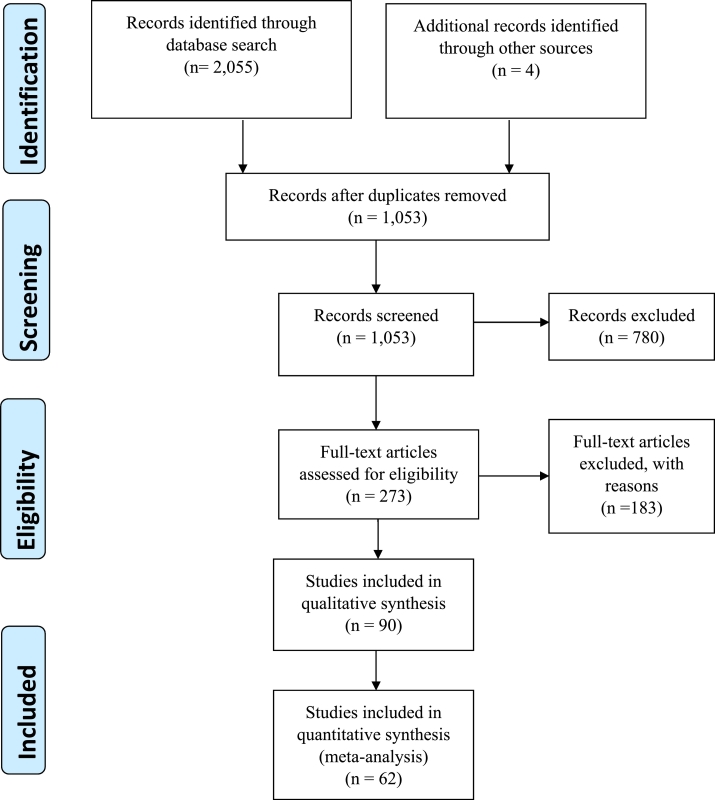
This study reviewed 2,059 articles of which 90 were potentially suitable. After applying the MINORS cutoff score, 62 were included for meta-analysis as shown in the PRISMA flowchart (Fig 1).
Fig 1.
Identification, review, and selection of articles included in the meta-analysis, shown by PRISMA flowchart.
The Rate of IOC Use During Cholecystectomy
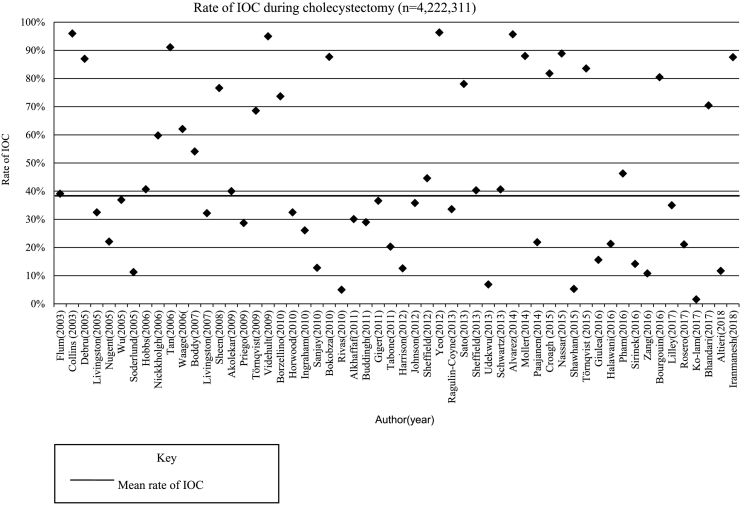
The rate of IOC use during cholecystectomy was analyzed across 56 studies (n = 4,221,311). Six studies were not included because the total number of cholecystectomies with and without planned IOC was not reported or the use of IOC was randomized to an intervention and control group. The mean rate of IOC use during cholecystectomy was 38.8% (range 1.6%–96.4%). There was marked variation in the use of IOC with studies reporting data from 19 countries (Fig 2). The mean operating time for IOC across 4 studies was 11 minutes (range 6–15 minutes) [[16], [17], [18], [19]].
Fig 2.
The rate of IOC during cholecystectomy, reported from 56 studies.
When analyzing 20 multicenter studies (96% of which were based on American and Swedish studies), the mean rate of IOC use was 38.5% (CI = 38.5–38.6), range 12% to 88% [6,8,11,[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]]. The use of IOC from 11 multicenter studies carried out in the United States [11,[20], [21], [22], [23], [24], [25], [26], [27], [28], [29]] revealed a mean rate of 33.2% (CI = 33.1–33.3) compared to a mean rate of 69.5% (CI = 69.4–69.6) from 4 multicenter Swedish studies [6,8,30,31].
Comparing Routine and Selective Policies of IOC
A selective policy of IOC use was adopted in 14 studies with a mean IOC use of 16.7% (2.8%–36.9%) in 12,064 patients [18,19,34,[37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47]]. Additionally, 14 studies adopted a policy of routine IOC with a mean average use of 88.3% (63.5%–99.2%) in 25,072 patients [17,19,34,37,42,[48], [49], [50], [51], [52], [53], [54], [55], [56]].
Eleven studies (n = 10,466) reported the incidence of CBD stones on routine IOC with a mean of 11.8%, ranging from 2.8% to 18.9% [19,34,37,38,[50], [51], [52], [53], [54], [55], [56]]. Eight studies (n = 4,556) reported the incidence of CBD stones on selective IOC with a mean of 3.9%, range 0.7% to 12.8% [18,19,34,[37], [38], [39],44,45]. A routine IOC policy significantly increased the rate of CBD stone detection (OR = 3.28, CI = 2.80–3.86, P value < .001).
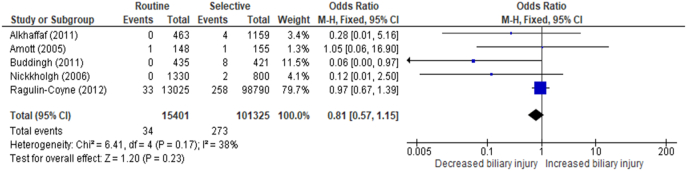
Five studies (n = 116,726) [19,34,37,38,57] reported findings of bile duct injury from routine and selective policies of IOC use (Fig 3). The average incidence of bile duct injury using a routine policy of IOC was 0.22% compared with 0.27% for a selective approach (OR = 0.81, CI = 0.57–1.15, P value = .23).
Fig 3.
The rate of biliary injury during cholecystectomy with routine IOC versus selective IOC.
In 25 studies (n = 71,191 patients) who reported successful IOC completion, the mean success rate was 95% (range 66%–99%) [5,6,[16], [17], [18], [19],34,[37], [38], [39], [40], [41], [42], [43], [44],[48], [49], [50], [51], [52], [53],55,56,59,60]. Successful completion of IOC was significantly greater with a routine IOC policy (95.2%) compared to a selective policy (90.6%) (OR = 2.09, CI = 1.73–2.51, P value < .001).
Comparing Bile Duct Injury and Readmission Rate With and Without the Use of IOC
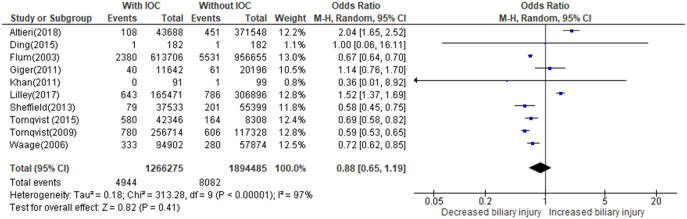
The incidence of bile duct injury during cholecystectomy with and without the use of IOC was assessed across 10 studies (n = 3,160,760 patients) as shown in Figure 4 [6,11,20,21,25,30,31,36,61,62]. The total number of cholecystectomy patients with IOC performed was 1,266,275, and the incidence of bile duct injury was 0.39%. The total number of patients undergoing cholecystectomy without cholangiography was 1,894,485, and the incidence of bile duct injury was 0.43%. Although IOC is potentially weakly associated with a lower incidence of bile duct injury, this effect is not significant (OR = 0.88, CI = 0.65–1.19, P value = .41). There was also considerable heterogeneity reported (I2 = 97%).
Fig 4.
The rate of biliary injury during cholecystectomy with IOC versus without IOC.
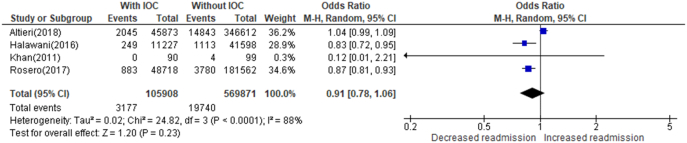
Four studies reported a readmission rate following cholecystectomy both with and without the use of IOC (Fig 5) [ 11,28,29,61]. The total number of patients undergoing cholecystectomy with IOC was 105,908, with an average readmission rate of 3.0%. The total number of patients undergoing cholecystectomy without IOC was 569,871, with an average readmission rate of 3.46%. IOC is not significantly associated with a decrease in readmissions (OR = 0.91, CI = 0.78–1.06, P value = .23, I2 = 88%).
Fig 5.
The rate of readmission following cholecystectomy with IOC versus without IOC.
DISCUSSION
This meta-analysis reviewed more than 2,000 publications identifying a wide variation in the performance of IOC, with variable detection of choledocholithiasis. Previously, there have been many studies of IOC, but the current meta-analysis is one of the first to assess the impact of the variable use of IOC during cholecystectomy.
Surgeons opting for the routine use of IOC feel that it aids detection of CBD stones and promotes surgical skills that facilitate cystic duct cannulation and transcystic single-stage bile duct exploration, which is a safe and efficacious treatment option in the management of choledocholithiasis [63,64]. In addition, it has been suggested that IOC is an effective tool for effectively reducing bile duct injury, but this has been the subject of major debate and the controversy remains [20,27]. With the advent of other imaging like ERCP and MRCP, the role of IOC has been challenged even further, with many surgeons opting for a selective policy of IOC use or not at all [46,65].
Different approaches have been advocated in the management of CBD stones from laparoscopic single-stage CBD clearance (LCBDC) to single- and dual-stage LCBDC with intraoperative ERCP [66,67]. In their meta-analysis, Pan and colleagues found that LCBDC during laparoscopic cholecystectomy (LC) has superior outcomes to a preoperative ERCP sphincterotomy followed by LC and should be considered as the optimal treatment choice for CBD stones [67]. Mohseni et al, in a recent retrospective study of more than 200 patients undergoing simultaneous intraoperative ERCP with LC, found that this approach was associated with few complications [68].
A key approach to single-stage or operative clearance requires IOC to be performed even in cases with preoperative MRCP. In a recent multicenter study of approaches to cholecystitis in fit patients undergoing a therapeutic sequence for the management of choledocholithiasis, 80% of the 25 centers reported that they favored a staged approach with upfront ERCP followed by cholecystectomy (either during the same admission or, more commonly, at an interval). A minority of survey respondents favored simultaneous cholecystectomy and either operative CBD exploration (4 of 25, 16%) or rendezvous intraoperative ERCP (5 of 25, 20%) as a 1-stage procedure [69]. Our study identified that IOC was performed in more than one third of patients (38.8%) undergoing cholecystectomy. This rate increased in Swedish and Australian compared to US cohorts. In Australia, the Royal Australasian College of Surgeons reported a 90% median use of IOC during cholecystectomy in their Surgical Variance Report 2017 [70]. A very recent multinational prospective evaluation of cholecystectomy outcomes in 504 patients in 16 countries found that the IOC rate was 13% and the preoperative ERCP rate was 16% [71]. These variations in IOC are truly remarkable, are hard to explain scientifically, and must in part be based on emotive learning by the surgeons involved.
Surgical opinion regarding the appropriate indications for the selective use of IOC varied considerably, contributing to the range of selective IOC rates recorded (2.8%–36.9%). Some studies reported that high-volume surgeons and high-volume hospitals were more likely to perform IOC [21,27,35]. Overall, these data were limited in the literature and not appropriate for statistical analysis.
Selective IOC based on preoperative indications is supportive as an alternative to routine IOC for the detection of choledocholithiasis [39,72]. A selective policy of IOC use results in an IOC rate of 16.7% compared to 88.3% in the routine policy institutions. The success of routine IOC is limited by occluded, friable, or very short cystic ducts and the required lead-lined operating rooms.
The principal goal of IOC is CBD stone detection, and this meta-analysis identified that routine IOC will detect more than 3-fold the number of CBD stones as selective IOC, with an average incidence of CBD stones during routine IOC reported as 12% compared to 4% on selective IOC (OR = 3.28, CI = 2.80–3.86, P value < .001). Up to 50% of CBD stones will pass spontaneously, and for this reason, some have argued for an expectant strategy based on spontaneous clearance rates of CBD stone [5,73]. The sequelae of persistent untreated stones are becoming clearer, with an increase in adverse outcomes if the stones are not removed [10,74]. However, these additional stones found on routine IOC may indeed be important, potentially causing further complications, recurrent cholangitis, pancreatitis, and readmission, as well as possibly contributing to a postcholecystectomy syndrome [75,76]. Recently, Hakuta et al revealed that the cumulative incidence of biliary complications related to asymptomatic stones picked up on incidental imaging was 6.1% at 1 year, 11% at 3 years, and 17% at 5 years [9]. Möller et al found that, among patients in whom no measures were taken intraoperatively or planned postoperatively (representing natural course), the risk for unfavorable outcomes ranged from 15.9% to 35.9% depending on stone size in a cohort of patients diagnosed with CBD stones using IOC [8]. Unfavorable outcome was defined as known incomplete clearance of bile ducts with any symptoms or complications related to bile duct stones within 30 days after cholecystectomy. This study also reported that 14.9% of patients diagnosed with CBD stones using IOC required postoperative ERCP for CBD stone clearance. Their data from the Swedish GallRiks Registry are some of the largest analyses reported and provide a cautionary note to those who disregard the importance of CBD stones diagnosed at the time of cholecystectomy.
Many now feel that MRCP will replace the use of IOC, and almost one third of UK patients have a preoperative magnetic resonance imaging. This was a stimulus for the Sunflower study, assessing the clinical effectiveness and cost-effectiveness of an expectant management versus preoperative imaging with MRCP in patients with symptomatic gallstones undergoing laparoscopic cholecystectomy at low or moderate risk of CBD stones [77]. Preoperative MRCP without IOC has been shown previously to be an effective and safe strategy in the treatment of gallstones, with an acceptable rate of retained CBD stones and bile duct injury (BDI) [46].
In patients with gallstone pancreatitis, intraoperative imaging modalities such as IOC or laparoscopic ultrasound (LUS) are important in ensuring that patients are not at risk of subsequent pancreatitis due to retained CBD stones [78]. The main benefit of IOC and LUS over MRCP is their ability to enable CBD imaging at the time of laparoscopic cholecystectomy. IOC has been reported to exhibit a higher diagnostic accuracy at detecting choledocholithiasis compared with MRCP (98% vs 85%) [79], whereas Richard et al concluded that there was no place for preoperative MRCP in patients with suspected choledocholithiasis because of the unacceptably elevated rate of false-negative results compared with IOC [80]. Thacoor et al similarly concluded that patients presenting with acute gallstone pancreatitis can be safely and successfully managed with laparoscopic cholecystectomy and IOC without requiring a preoperative MRCP [81].
In a randomized controlled trial, Lehrskov found that fluorescent cholangiography was not inferior to IOC in detecting the cystic junction with the CBD. This study was very selective, including 120 of a potential cohort of 1,889 patients with 60 in each arm in a single-surgeon study over 3 years [12].
The role of LUS in identifying biliary anatomy and preventing CBD injury is not well defined. LUS and IOC have similar success in visualizing the biliary anatomy, but they are not widely available and require significant experience [82,83].
There is evidence to support the routine use of IOC in the prevention, diagnosis, and management of bile duct injury [17,34,84]. During the transitioning period from open to laparoscopic cholecystectomy, a previous meta-analysis conveyed the effective role of routine IOC in the prevention of bile duct injury [85]. Since then, surgical approach to cholecystectomy has changed with the introduction of the critical view of safety (CVS) technique. It is has been suggested that implementation of a CVS could replace routine IOC, but this may reduce the detection rate of choledocholithiasis [45]. In many cases of severe cholecystitis, the CVS is not visible, and IOC may be difficult in those patients. In their retrospective study, 57 of 477 had IOC and 15 of 57 had choledocholithiasis. One must assume therefore that the incidence of missed CBD stones must have been significant. Other authors have argued that the 2 together provide optimal patient outcome [38]. In a recent consensus conference on prevention of bile duct injury during cholecystectomy, Brunt and colleagues recommended the use of IOC among surgeons to mitigate the risk of BDI [86]. In our study, although routine IOC was shown to reduce bile duct injury in most studies, it was an insignificant association. The definition of BDI in these included studies was lacking. For example, Törnqvist includes all forms of bile leakage and cystic duct leakage postcholecystectomy when reporting BDI rate of more than 1.3% [6].
Bile duct injury occurs in 0.3% of cholecystectomies, which results in 2,500 injuries per annum in the United States alone, with resultant 8.8-fold increase in mortality and a common cause for litigation [87,88]. The numbers to power a randomized controlled trial to finally answer the question of whether IOC reduces the rate of BDI at cholecystectomy would be near impossible [89]. For this reason, the best available evidence comes from large-scale retrospective analyses. However, these analyses are limited in their interpretation. Three retrospective studies reporting the smallest percentage use of IOC during cholecystectomy are also the 3 studies reporting an association of increased BDI with IOC [11,25,36]. The recent recommendation by the Prevention of Bile Duct Injury Consensus Work Group for the liberal use of IOC in acute hot gallbladder surgery could skew a potential association of IOC with a higher incidence of BDI because these cases are more prone to CBD injury [86]. Additionally, using IOC as a diagnostic tool after an injury has occurred makes the interpretation of the value of IOC uncertain on retrospective analysis.
This meta-analysis was hampered by considerable statistical heterogeneity reported in the analysis of bile duct injury (P value < .0001, I2 = 97%) and readmission rate (P value < .0001, I2 = 88%) (Fig 4, Fig 5). Clinical diversity relating to the differences associated with the participants, interventions, and outcomes, as well as methodological diversity, contributes to the statistical heterogeneity reported. Furthermore, IOC use extended widely, from routine, selective, to no use at all. A subgroup analysis of the 3 more routine policies allowed a reduction of I2 statistic to 64%, with all 3 reporting a significant protective effect [6,30,31]. The remaining 5 retrospective studies adopting a more selective IOC use reported an I2 statistic of 99% when grouped together, revealing an inconclusive effect of the relationship of IOC and BDI [11,20,21,61,62]. Further investigation of the participants analyzed in each of these studies revealed a difference in the average age, with 2 studies reporting outcomes only from patients aged more than 66 years and differences involving the indication for cholecystectomy [21,25]. Of the 10 studies analyzed, 2 were prospective randomized trials reporting outcomes from a small number of patients and therefore a much smaller number of events [61,62], whereas the remaining 8 were large retrospective studies using regional or national databases of registered cholecystectomies [6,11,20,21,25,30,31,36].
Recent new practice guidelines aimed at prevention of CBD injury make reference to an unpublished meta-analysis of 8 studies showing that the use of IOC was associated with increased intraoperative recognition of CBD injury compared to those without IOC (OR 2.92, 95% CI 1.55–5.68, P = .014) [86].
Readmission rate assessed across 4 studies revealed an insignificant association: with IOC (3%) lower than without IOC (3.5%) (P = .23) [11,28,29,61]. Recently, McIntyre et al, in a meta-analysis on readmission rate following LC, suggested that IOC might reduce readmission rate [90]. The differences in study design explain part of heterogeneity represented. However, differences in the clinical definition of readmission also existed. Readmission rate was defined according to 30 days [11,28,29] or 1 year [61]. The readmissions were defined in most cases as any referral or readmission to a hospital or clinic, whether they were related to the primary operation or not, usually not defined. One author appropriately defined readmission as being related to the primary operation, however, which is a more accurate definition but likely to record a smaller number of events [28].
There were some limitations to our study due to a lack of reported data on intraoperative complication and conversion rates related to both routine and selective policies of IOC and use of articles in English only. This meta-analysis was not tasked with assessment of the actual skill set required to undertake IOC and its potential benefit in facilitating transcystic CBD stone clearance.
Where routine IOC is planned, the success of the procedure is high (95%) and with a short time to complete (11 minutes). An important aspect of IOC is the ability of the general surgeon to interpret the results. Interpretation of anatomy was recently described in a study by Chehade which reported that 95% of IOCs adequately demonstrated biliary anatomy. Aberrant right sectoral ducts were identified in 15.2% of the complete IOCs, and 2.6% demonstrated left sectoral or confluence anomalies. Only 20.4% of these were reported intraoperatively [91]. Regarding the detection of CBD stones, the combined sensitivity and specificity of IOC in the detection of CBD stones are reported as 0.87 (95% CI: 0.83–0.89) and 0.98 (95% CI: 0.98–0.98), respectively [78].
We believe that IOC has benefits even in an era of increasing availability of MRCP. Other imaging techniques of the biliary tree will not provide a portal for stone removal. The effectiveness of LCBDC-LC varies between studies, with a recent series by Ballou et al reporting a success rate of completion and stone clearance of 66% [92], whereas others have reported success rates of 80%–98.5% [[93], [94], [95]]. With increasing use of 1-stage bile duct clearance, either with or without intraoperative ERCP, ability to cannulate the cystic duct is becoming increasingly important. IOC should be more widely and consistently used.
In conclusion, the use of IOC still has its place in cholecystectomy based on the detection of choledocholithiasis and the potential reduction of unfavorable outcomes associated with CBD stones.
Author Contribution
Eoin Donnellan: Conceptualization, Methodology, Formal analysis, Investigation, Project administration, Writing – review & editing. Jonathan Coulter: Validation, Formal analysis. Cherian Mathew: Investigation, Validation, Data curation. Michelle Choynowski: Methodology, Formal analysis. Louise Flanagan: Validation. Magda Bucholc: Methodology, Formal analysis. Alison Johnston: Conceptualization, Methodology, Funding acquisition, Writing – review & editing. Michael Sugrue: Conceptualization, Supervision, Funding acquisition, Writing – review & editing.
Conflict of Interest
None.
Funding Sources
This project is supported by the European Union’s INTERREG VA Programme managed by the Special EU Programmes Body, and Donegal Clinical and Research Academy.
Footnotes
PROSPERO trial registration number: CRD42018102154
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sopen.2020.07.004.
Appendix A. Supplementary data
Supplementary tables
References
- 1.Morgenstern L. Carl Langenbuch and the first cholecystectomy. Surg Endosc. 1992;6(3):113–114. doi: 10.1007/BF02309080. [DOI] [PubMed] [Google Scholar]
- 2.MacFadyen B.V. Intraoperative cholangiography: past, present, and future. Surg Endosc Other Interv Tech. 2006;20(2 SUPPL):436–440. doi: 10.1007/s00464-006-0053-0. [DOI] [PubMed] [Google Scholar]
- 3.Ricci C., Pagano N., Taffurelli G. Comparison of efficacy and safety of 4 combinations of laparoscopic and intraoperative techniques for management of gallstone disease with biliary duct calculi a systematic review and network meta-analysis. JAMA Surg. 2018;153(7):1–48. doi: 10.1001/jamasurg.2018.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown A.W.W., Wilson R.B. Legacy of Le Quesne: operative cholangiography in the modern era. ANZ J Surg. 2018;88(9):819–820. doi: 10.1111/ans.14501. [DOI] [PubMed] [Google Scholar]
- 5.Collins C., Maguire D., Ireland A., Fitzgerald E., O’Sullivan G.C. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg. 2004;239(1):28–33. doi: 10.1097/01.sla.0000103069.00170.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Törnqvist B., Strömberg C., Akre O., Enochsson L., Nilsson M. Selective intraoperative cholangiography and risk of bile duct injury during cholecystectomy. Br J Surg. 2015;102(8):952–958. doi: 10.1002/bjs.9832. [DOI] [PubMed] [Google Scholar]
- 7.Poh B., Cashin P., Bowers K. Management of choledocholithiasis in an emergency cohort undergoing laparoscopic cholecystectomy: a single-centre experience. HPB. 2014;16(7):629–634. doi: 10.1111/hpb.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Möller M., Gustafsson U., Rasmussen F., Persson G., Thorell A. Natural course vs interventions to clear common bile duct stones data from the Swedish registry for gallstone surgery and endoscopic retrograde cholangiopancreatography (GallRiks) JAMA Surg. 2014;149(10):1008–1014. doi: 10.1001/jamasurg.2014.249. [DOI] [PubMed] [Google Scholar]
- 9.Hakuta R., Hamada T., Nakai Y. Natural history of asymptomatic bile duct stones and association of endoscopic treatment with clinical outcomes. J Gastroenterol. 2019;31:1–8. doi: 10.1007/s00535-019-01612-7. [DOI] [PubMed] [Google Scholar]
- 10.Matsui Y., Hirooka S., Sakaguchi T. Bile duct stones predict a requirement for cholecystectomy in older patients. World J Surg. 2020;44(3):721–729. doi: 10.1007/s00268-019-05241-2. [DOI] [PubMed] [Google Scholar]
- 11.Altieri M.S., Yang J., Obeid N. Increasing bile duct injury and decreasing utilization of intraoperative cholangiogram and common bile duct exploration over 14 years: an analysis of outcomes in New York State. Surg Endosc. 2018;32(2):667–674. doi: 10.1007/s00464-017-5719-2. [DOI] [PubMed] [Google Scholar]
- 12.Lehrskov L.L., Westen M., Larsen S.S., Jensen A.B., Kristensen B.B., Bisgaard T. Fluorescence or X-ray cholangiography in elective laparoscopic cholecystectomy: a randomized clinical trial. Br J Surg. 2020 doi: 10.1002/bjs.11510. [DOI] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slim K., Nini E., Forestier D. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org March 2011].
- 16.Hope W.W., Bools L., Hooks W.B., Adams A., Kotwall C.A., Clancy T.V. Teaching cholangiography in a surgical residency program. J Surg Educ. 2013;70:243–247. doi: 10.1016/j.jsurg.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez F.A., De Santibañes M., Palavecino M., Sánchez Clariá R., Mazza O., Arbues G. Impact of routine intraoperative cholangiography during laparoscopic cholecystectomy on bile duct injury. Br J Surg. 2014;101:677–684. doi: 10.1002/bjs.9486. [DOI] [PubMed] [Google Scholar]
- 18.Giulea C., Enciu O., Bîrcǎ T., Miron A. Selective intraoperative cholangiography in laparoscopic cholecystectomy. Chir. 2016;111:26–32. doi: 10.1097/00129689-199206000-00037. [DOI] [PubMed] [Google Scholar]
- 19.Nickkholgh A., Soltaniyekta S., Kalbasi H. Routine versus selective intraoperative cholangiography during laparoscopic cholecystectomy: a survey of 2,130 patients undergoing laparoscopic cholecystectomy. Surg Endosc Other Interv Tech. 2006;20:868–874. doi: 10.1007/s00464-005-0425-x. [DOI] [PubMed] [Google Scholar]
- 20.Flum D.R., Dellinger E.P., Cheadle A. Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA. 2003;289:13. doi: 10.1001/jama.289.13.1639. [DOI] [PubMed] [Google Scholar]
- 21.Sheffield K.M., Riall T.S., Han Y., Kuo Y.F., Townsend C.M., Goodwin J.S. Association between cholecystectomy with vs without intraoperative cholangiography and risk of common duct injury. JAMA J Am Med Assoc. 2013;310:812–820. doi: 10.1001/jama.2013.276205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheffield K.M., Han Y., Kuo Y.F., Townsend C.M., Goodwin J.S., Riall T.S. Variation in the use of intraoperative cholangiography during cholecystectomy. J Am Coll Surg. 2012;214:668–679. doi: 10.1016/j.jamcollsurg.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livingston E.H., Miller J.A.G., Coan B., Rege R.V. Costs and utilization of intraoperative cholangiography. J Gastrointest Surg. 2007;11:1162–1167. doi: 10.1007/s11605-007-0209-9. [DOI] [PubMed] [Google Scholar]
- 24.Ragulin-Coyne E., Witkowski E.R., Chau Z., Ng S.C., Santry H.P., Callery M.P. Is routine intraoperative cholangiogram necessary in the twenty-first century? A national view. J Gastrointest Surg. 2013;17:434–442. doi: 10.1007/s11605-012-2119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lilley E.J., Scott J.W., Jiang W., Krasnova A., Raol N., Changoor N. Intraoperative cholangiography during cholecystectomy among hospitalized medicare beneficiaries with non-neoplastic biliary disease. Am J Surg. 2017;214:682–686. doi: 10.1016/j.amjsurg.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 26.Sirinek K.R., Willis R., Schwesinger W.H. Who will be able to perform open biliary surgery in 2025? J Am Coll Surg. 2016;223:110–115. doi: 10.1016/j.jamcollsurg.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Ingraham A.M., Cohen M.E., Ko C.Y., Hall B.L. A current profile and assessment of North American cholecystectomy: results from the American College of Surgeons national surgical quality improvement program. J Am Coll Surg. 2010;211:176–186. doi: 10.1016/j.jamcollsurg.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Halawani H.M., Tamim H., Khalifeh F., Mailhac A., Jamali F.R. Impact of intraoperative cholangiography on postoperative morbidity and readmission: analysis of the NSQIP database. Surg Endosc. 2016;30:5395–5403. doi: 10.1007/s00464-016-4896-8. [DOI] [PubMed] [Google Scholar]
- 29.Rosero E.B., Joshi G.P. Hospital readmission after ambulatory laparoscopic cholecystectomy: incidence and predictors. J Surg Res. 2017;219:108–115. doi: 10.1016/j.jss.2017.05.071. [DOI] [PubMed] [Google Scholar]
- 30.Waage A. Iatrogenic bile duct injury. Arch Surg. 2006;141:1207. doi: 10.1001/archsurg.141.12.1207. [DOI] [PubMed] [Google Scholar]
- 31.Törnqvist B., Zheng Z., Ye W., Waage A., Nilsson M. Long-term effects of iatrogenic bile duct injury during cholecystectomy. Clin Gastroenterol Hepatol. 2009;7:1013–1018. doi: 10.1016/j.cgh.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Croagh D.G., Devonshire D., Poh B., Berry R., Bowers K., Spilias D. Management of CBD stones in patients having laparoscopic cholecystectomy in a private setting in Australia. ANZ J Surg. 2015;85:53–57. doi: 10.1111/ans.12341. [DOI] [PubMed] [Google Scholar]
- 33.Hobbs M.S., Mai Q., Knuiman M.W., Fletcher D.R., Ridout S.C. Surgeon experience and trends in intraoperative complications in laparoscopic cholecystectomy. Br J Surg. 2006;93:844–853. doi: 10.1002/bjs.5333. [DOI] [PubMed] [Google Scholar]
- 34.Alkhaffaf B. Endoscopic retrograde cholangiopancreatography prior to laparoscopic cholecystectomy. Arch Surg. 2011;146:329. doi: 10.1001/archsurg.2011.30. [DOI] [PubMed] [Google Scholar]
- 35.Harrison E.M., O’Neill S., Meurs T.S., Wong P.L., Duxbury M., Paterson-Brown S. Hospital volume and patient outcomes after cholecystectomy in Scotland: retrospective, national population based study. BMJ. 2012;344:1–14. doi: 10.1136/bmj.e3330. [DOI] [PubMed] [Google Scholar]
- 36.Giger U., Ouaissi M., Schmitz S.F.H., Krähenbühl S., Krähenbühl L. Bile duct injury and use of cholangiography during laparoscopic cholecystectomy. Br J Surg. 2011;98:391–396. doi: 10.1002/bjs.7335. [DOI] [PubMed] [Google Scholar]
- 37.Amott D., Webb A., Tulloh B. Prospective comparison of routine and selective operative cholangiography. ANZ J Surg. 2005;75:378–382. doi: 10.1111/j.1445-2197.2005.03393.x. [DOI] [PubMed] [Google Scholar]
- 38.Buddingh K.T., Weersma R.K., Savenije R.A.J., Van Dam G.M., Nieuwenhuijs V.B. Lower rate of major bile duct injury and increased intraoperative management of common bile duct stones after implementation of routine intraoperative cholangiography. J Am Coll Surg. 2011;213:267–274. doi: 10.1016/j.jamcollsurg.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Horwood J., Akbar F., Katherine D., Morgan R. Prospective evaluation of a selective approach to cholangiography for suspected common bile duct stones. Ann R Coll Surg Engl. 2010;92:206–210. doi: 10.1308/003588410X12628812458293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nugent N., Doyle M., Mealy K. Low incidence of retained common bile duct stones using a selective policy of biliary imaging. Surgeon. 2005;3:352–356. doi: 10.1016/S1479-666X(05)80115-5. [DOI] [PubMed] [Google Scholar]
- 41.Priego P., Ramiro C., Molina J.M., Rodríguez Velasco G., Lobo E., Galindo J. Results of laparoscopic cholecystectomy in a third-level university hospital after 17 years of experience. Rev Esp Enferm Dig. 2009;101:20–25. doi: 10.4321/s1130-01082009000100003. [DOI] [PubMed] [Google Scholar]
- 42.Pham X.B.D., de Virgilio C., Al-Khouja L., Bermudez M.C., Schwed A.C., Kaji A.H. Routine intraoperative cholangiography is unnecessary in patients with mild gallstone pancreatitis and normalizing bilirubin levels. Am J Surg. 2016;212:1047–1053. doi: 10.1016/j.amjsurg.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Shawhan R.R., Porta C.R., Bingham J.R., McVay D.P., Nelson D.W., Causey M.W. Biliary leak rates after cholecystectomy and intraoperative cholangiogram in surgical residency. Mil Med. 2015;180:565–569. doi: 10.7205/milmed-d-14-00426. [DOI] [PubMed] [Google Scholar]
- 44.Wu S.C., Chen F.C., Lo C.J. Selective intraoperative cholangiography and single-stage management of common bile duct stone in laparoscopic cholecystectomy. World J Surg. 2005;29:1402–1408. doi: 10.1007/s00268-005-7694-3. [DOI] [PubMed] [Google Scholar]
- 45.Sanjay P., Fulke J.L., Exon D.J. “Critical view of safety” as an alternative to routine intraoperative cholangiography during laparoscopic cholecystectomy for acute biliary pathology. J Gastrointest Surg. 2010;14:1280–1284. doi: 10.1007/s11605-010-1251-6. [DOI] [PubMed] [Google Scholar]
- 46.Zang J., Yuan Y., Zhang C., Gao J. Elective laparoscopic cholecystectomy without intraoperative cholangiography: role of preoperative magnetic resonance cholangiopancreatography—a retrospective cohort study. BMC Surg. 2016;16:4–9. doi: 10.1186/s12893-016-0159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Söderlund C., Frozanpor F., Linder S. Bile duct injuries at laparoscopic cholecystectomy: a single-institution prospective study. Acute cholecystitis indicates an increased risk. World J Surg. 2005;29:987–993. doi: 10.1007/s00268-005-7871-4. [DOI] [PubMed] [Google Scholar]
- 48.Debru E., Dawson A., Leibman S., Richardson M., Glen L., Hollinshead J. Does routine intraoperative cholangiography prevent bile duct transection? Surg Endosc Other Interv Tech. 2005;19:589–593. doi: 10.1007/s00464-004-8711-6. [DOI] [PubMed] [Google Scholar]
- 49.Sato N., Shibao K., Akiyama Y., Inoue Y., Mori Y., Minagawa N. Routine intraoperative cholangiography during single-incision laparoscopic cholecystectomy: a review of 196 consecutive patients. J Gastrointest Surg. 2013;17:668–674. doi: 10.1007/s11605-012-2123-z. [DOI] [PubMed] [Google Scholar]
- 50.Tan J.T.H., Suyapto D.R., Neo E.L., Leong P.S.K. Prospective audit of laparoscopic cholecystectomy experience at a secondary referral centre in South Australia. ANZ J Surg. 2006;76:335–338. doi: 10.1111/j.1445-2197.2006.03721.x. [DOI] [PubMed] [Google Scholar]
- 51.Nassar A.H.M., Mirza A., Qandeel H., Ahmed Z., Zino S. Fluorocholangiography: reincarnation in the laparoscopic era—evaluation of intra-operative cholangiography in 3635 laparoscopic cholecystectomies. Surg Endosc. 2016;30:1804–1811. doi: 10.1007/s00464-015-4449-6. [DOI] [PubMed] [Google Scholar]
- 52.Videhult P., Sandblom G., Rasmussen I.C. How reliable is intraoperative cholangiography as a method for detecting common bile duct stones?: A prospective population-based study on 1171 patients. Surg Endosc. 2009;23:304–312. doi: 10.1007/s00464-008-9883-2. [DOI] [PubMed] [Google Scholar]
- 53.Photi E.S., El-Hadi A., Brown S., Swafe L., Ashford-Wilson S., Barwell J. The routine use of cholangiography for laparoscopic cholecystectomy in the modern era. JSLS J Soc Laparoendosc Surg. 2017;21:1–8. doi: 10.4293/JSLS.2017.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheen A.J., Asthana S., Al-Mukhtar A., Attia M., Toogood G.J. Preoperative determinants of common bile duct stones during laparoscopic cholecystectomy. Int J Clin Pract. 2008;62:1715–1719. doi: 10.1111/j.1742-1241.2007.01469.x. [DOI] [PubMed] [Google Scholar]
- 55.Iranmanesh P., Tobler O., De Sousa S., Andres A., Frossard J.L., Morel P. Feasibility, benefit and risk of systematic intraoperative cholangiogram in patients undergoing emergency cholecystectomy. PLoS One. 2018;13:1–12. doi: 10.1371/journal.pone.0199147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeo D., MacKay S., Martin D. Single-incision laparoscopic cholecystectomy with routine intraoperative cholangiography and common bile duct exploration via the umbilical port. Surg Endosc. 2012;26:1122–1127. doi: 10.1007/s00464-011-2009-2. [DOI] [PubMed] [Google Scholar]
- 57.Ragulin-Coyne E., Witkowski E.R., Chau Z., Ng S.C., Santry H.P., Callery M.P. Is routine intraoperative cholangiogram necessary in the twenty-first century? A national view. J Gastrointest Surg. 2013;17:434–442. doi: 10.1007/s11605-012-2119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bokobza B., Valverde A., Magne E., Delaby J., Rubay R., Bellouard A. Single umbilical incision laparoscopic cholecystectomy: initial experience of the Coelio Club. J Visc Surg. 2010;147:e253–e257. doi: 10.1016/j.jviscsurg.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 60.Kohn A., Creech S., Shayani V. Indicated cholangiography in patients operated on by routine versus selective cholangiographers. Am Surg. 2004;70:203–206. [PubMed] [Google Scholar]
- 61.Khan O.A., Balaji S., Branagan G., Bennett D.H., Davies N. Randomized clinical trial of routine on-table cholangiography during laparoscopic cholecystectomy. Br J Surg. 2011;98:362–367. doi: 10.1002/bjs.7356. [DOI] [PubMed] [Google Scholar]
- 62.Ding G.Q., Cai W., Qin M.F. Is intraoperative cholangiography necessary during laparoscopic cholecystectomy for cholelithiasis? World J Gastroenterol. 2015;21:2147–2151. doi: 10.3748/wjg.v21.i7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Memba R., González S., Coronado D. Single-stage approach for the management of choledocolithiasis with concomitant cholelithiasis. Implementation of a protocol in a secondary hospital. Surgeon. 2019;17(6):351–359. doi: 10.1016/j.surge.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 64.Zhu H.Y., Xu M., Shen H.J. A meta-analysis of single-stage versus two-stage management for concomitant gallstones and common bile duct stones. Clin Res Hepatol Gastroenterol. 2015;39(5):584–593. doi: 10.1016/j.clinre.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 65.Hamad M.A., Nada A.A., Abdel-Atty M.Y., Kawashti A.S. Major biliary complications in 2,714 cases of laparoscopic cholecystectomy without intraoperative cholangiography: a multicenter retrospective study. Surg Endosc. 2011;25(12):3747–3751. doi: 10.1007/s00464-011-1780-4. [DOI] [PubMed] [Google Scholar]
- 66.Platt T., Smith K., Nixon M. Success of intraoperative imaging and management of suspected choledocholithiasis without pre-operative bile duct imaging—a case series. Ann Med Surg. 2018;36(August):173–177. doi: 10.1016/j.amsu.2018.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pan L., Chen M., Ji L. The safety and efficacy of laparoscopic common bile duct exploration combined with cholecystectomy for the management of cholecysto-choledocholithiasis: an up-to-date meta-analysis. Ann Surg. 2018;268(2):247–253. doi: 10.1097/SLA.0000000000002731. [DOI] [PubMed] [Google Scholar]
- 68.Mohseni S., Ivarsson J., Ahl R. Simultaneous common bile duct clearance and laparoscopic cholecystectomy: experience of a one-stage approach. Eur J Trauma Emerg Surg. 2019;45(2):337–342. doi: 10.1007/s00068-018-0921-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bass G.A., Gillis A.E., Cao Y., Mohseni S., European Society for Trauma and Emergency Surgery (ESTES) Cohort Studies Group Self-reported and actual adherence to the Tokyo guidelines in the European snapshot audit of complicated calculous biliary disease. BJS Open. 2020 doi: 10.1002/bjs5.50294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.RACS Medibank surgical variance report (general surgery) https://surgeons.org/media/24091469/Surgical-Variance-Report-General-Surgery.pdf [Cited 10Dec 2017.] Available from URL:
- 71.Sugrue M., Coccolini F., Bucholc M. Intra-operative gallbladder scoring predicts conversion of laparoscopic to open cholecystectomy: a WSES prospective collaborative study. World J Emerg Surg. 2019;14(1):10–17. doi: 10.1186/s13017-019-0230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tabone L.E., Sarker S., Fisichella P.M., Conlon M. To ‘ gram or not ’? Indications for intraopertive cholangiogram. Surgery. 2009;150(4):810–819. doi: 10.1016/j.surg.2011.07.062. [DOI] [PubMed] [Google Scholar]
- 73.Frossard J.L., Hadengue A., Amouyal G. Choledocholithiasis: a prospective study of spontaneous common bile duct stone migration. Gastrointest Endosc. 2000;51(2):175–179. doi: 10.1016/S0016-5107(00)70414-7. [DOI] [PubMed] [Google Scholar]
- 74.Sugrue M., Watson A., Huan H., George G. Emergency cholecystectomy in the elderly chapter. In: in Latifi R., Catena F., Coccolini F., editors. Emergency general surgery in geriatrics—hot topics in acute care surgery and trauma series. Springer; 2020. [Google Scholar]
- 75.Cox M.R., Budge J.P.O., Eslick G.D. Timing and nature of presentation of unsuspected retained common bile duct stones after laparoscopic cholecystectomy: a retrospective study. Surg Endosc. 2015;29(7):2033–2038. doi: 10.1007/s00464-014-3907-x. [DOI] [PubMed] [Google Scholar]
- 76.Isherwood J., Oakland K., Khanna A. A systematic review of the aetiology and management of post cholecystectomy syndrome. Surgeon. 2019;17(1):33–42. doi: 10.1016/j.surge.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 77.Toogood G., Blazeby J.M. ISRCTN; 2019. A randomised controlled trial to establish the clinical and cost effectiveness of expectant management versus pre-operative imaging with MRCP in patients with symptomatic gallstones undergoing laparoscopic cholecystectomy at low ormoderate risk of common bile duct stones. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jamal K.N., Smith H., Ratnasingham K. Meta-analysis of the diagnostic accuracy of laparoscopic ultrasonography and intraoperative cholangiography in detection of common bile duct stones. Ann R Coll Surg Engl. 2016 1;98(4):244–249. doi: 10.1308/rcsann.2016.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tofigh A.M., Razmjoie F., Khabbaz A. Comparing the efficacy of preoperative magnetic resonance cholangiopancreatography with intra-operative cholangiography in patients suspicious to biliary stones. Gastroenterol Hepatol Bed Bench. 2013;6(2):80. [PMC free article] [PubMed] [Google Scholar]
- 80.Richard F., Boustany M., Britt L.D. Accuracy of magnetic resonance cholangiopancreatography for diagnosing stones in the common bile duct in patients with abnormal intraoperative cholangiograms. Am J Surg. 2013;205(4):371–373. doi: 10.1016/j.amjsurg.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 81.Thacoor A., Pike T.W., Pathak S. The role of intraoperative cholangiography in patients undergoing laparoscopic cholecystectomy for acute gallstone pancreatitis: is magnetic resonance cholangiopancreatography needed? Ann R Coll Surg Engl. 2019;101(6):428–431. doi: 10.1308/rcsann.2019.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Machi J., Johnson J.O., Deziel D.J. The routine use of laparoscopic ultrasound decreases bile duct injury: a multicenter study. Surg Endosc. 2009;23(2):384. doi: 10.1007/s00464-008-9985-x. [DOI] [PubMed] [Google Scholar]
- 83.Biffl W.L., Moore E.E., Offner P.J. Routine intraoperative laparoscopic ultrasonography with selective cholangiography reduces bile duct complications during laparoscopic cholecystectomy. J Am Coll Surg. 2001;193(3):272–280. doi: 10.1016/s1072-7515(01)00991-7. [DOI] [PubMed] [Google Scholar]
- 84.Törnqvist B., Strömberg C., Persson G., Nilsson M. Effect of intended intraoperative cholangiography and early detection of bile duct injury on survival after cholecystectomy: population based cohort study. BMJ. 2012;345(7880):1–10. doi: 10.1136/bmj.e6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ludwig K., Bernhardt J., Steffen H., Lorenz D. Contribution of intraoperative cholangiography to incidence and outcome of common bile duct injuries during laparoscopic cholecystectomy. Surg Endosc Other Interv Tech. 2002;16(7):1098–1104. doi: 10.1007/s00464-001-9183-6. [DOI] [PubMed] [Google Scholar]
- 86.Brunt L.M., Deziel D.J., Telem D.A. Safe cholecystectomy multi-society practice guideline and state of the art consensus conference on prevention of bile duct injury during cholecystectomy. Ann Surg. 2020;272(1):3–23. doi: 10.1097/SLA.0000000000003791. [DOI] [PubMed] [Google Scholar]
- 87.Halbert C., Pagkratis S., Yang J. Beyond the learning curve: incidence of bile duct injuries following laparoscopic cholecystectomy normalize to open in the modern era. Surg Endosc. 2016;30(6):2239–2243. doi: 10.1007/s00464-015-4485-2. [DOI] [PubMed] [Google Scholar]
- 88.MacLean T.R. Monetary lessons from litigation involving laparoscopic cholecystectomy. Am Surg. 2005;71:606–612. [PubMed] [Google Scholar]
- 89.Ford J.A., Soop M., Du J., Loveday B.P.T., Rodgers M. Systematic review of intraoperative cholangiography in cholecystectomy. Br J Surg. 2012;99(2):160–167. doi: 10.1002/bjs.7809. [DOI] [PubMed] [Google Scholar]
- 90.McIntyre C., Johnston A., Sugrue M. Readmission to hospital following laparoscopic cholecystectomy; a meta-analysis. Anaesthesiol Intensive Ther. 2019;52(1) doi: 10.5114/ait.2020.92967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chehade M., Kakala B., Sinclair J.L. Intraoperative detection of aberrant biliary anatomy via intraoperative cholangiography during laparoscopic cholecystectomy. ANZ J Surg. 2019;89(7–8):889–894. doi: 10.1111/ans.15267. [DOI] [PubMed] [Google Scholar]
- 92.Ballou J., Wang Y., Schreiber M., Kiraly L. 10 years of laparoscopic common bile duct exploration: a single tertiary institution experience. Am J Surg. 2019;217(5):970–973. doi: 10.1016/j.amjsurg.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 93.Bansal V.K., Misra M.C., Rajan K. Single-stage laparoscopic common bile duct exploration and cholecystectomy versus two-stage endoscopic stone extraction followed by laparoscopic cholecystectomy for patients with concomitant gallbladder stones and common bile duct stones: a randomized controlled trial. Surg Endosc. 2014;28(3):875e885. doi: 10.1007/s00464-013-3237-4. [DOI] [PubMed] [Google Scholar]
- 94.Karvounis E., Griniatsos J., Arnold J. Why does laparoscopic common bile duct exploration fail? Int Surg. 2006;91(2):90e93. [PubMed] [Google Scholar]
- 95.Bove A., Bongarzoni G., Palone G. Why is there recurrence after transcystic laparoscopic bile duct clearance? Risk factor analysis. Surg Endosc. 2009;23(7):1470e1475. doi: 10.1007/s00464-009-0377-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables