Abstract
BACKGROUND:
The majority of ovarian cancer cases are diagnosed at an advanced stage with poor prognosis. This study evaluates autoantibodies against tumor antigens to identify candidate biomarkers for early detection of ovarian cancer in women at increased risk.
OBJECTIVE:
To assess the immunoreactivity of paraneoplastic antigens and tumor associated antigens with high-grade serous ovarian cancer (HGSOC) samples.
METHODS:
Five paraneoplastic antigens along with three tumor-associated antigens were evaluated with HGSOC patient serum samples. Validation screening was performed with n = 164 serum samples consisting of: 50 late stage HGSOC, 14 early stage HGSOC, 50 benign ovarian cyst, and 50 healthy control samples on ELISA and western blot. The four markers TRIM21, NY-ESO-1, TP53, and PAX8 were evaluated on a second validation serum set, n = 150.
RESULTS:
TRIM21 achieved the highest sensitivity in the first validation screening of 33% with 100% specificity. Combining TRIM21 with NY-ESO-1, TP53, and PAX8 provided 67% sensitivity with 94% specificity, and 56% sensitivity at 98% specificity. These four markers resulted in 46% sensitivity with 98% specificity in the second validation cohort; TRIM21 achieved the highest individual sensitivity of 36%.
CONCLUSIONS:
Autoantibodies to TRIM21, NY-ESO-1, and TP53 may complement CA125 in screening of women at genetic risk for ovarian cancer.
Keywords: Autoantibody, biomarker, ovarian cancer, paraneoplastic antigen
1. Introduction
1.1. Early detection of ovarian cancer
Ovarian cancer is the fifth leading cause of cancer-related deaths in women. Stage I ovarian cancer is defined by localized cancer in the ovaries or fallopian tubes, with only 15% of cases diagnosed at this stage [1]. The majority of cases are diagnosed at an advanced stage, defined as stage II when the tumor has spread to organs within the pelvis, stage III which involves the peritoneal surface of the pelvis or abdomen and surrounding lymph nodes, and stage IV with metastasis beyond the abdominal cavity [1]. Worldwide, the 5-year age-standardized net survival for early stage ovarian cancer is 80%, which decreases to 30% for advanced disease [2].
Commonly, detection consists of a two-step test that involves measurement of circulating antigen CA125 either at a value > 35 U/mL or at an increased level relative to each patient’s baseline, followed by imaging such as trans-vaginal ultrasound (TVUS). Having a sequential test process of orthogonal measurements reduces the rate of false-positives. However, the initial step of CA125 detection in this two-step process misses approximately 20% of cases and can be improved by the addition of biomarkers such as autoantibodies [3]. Studies of autoantibody tests for ovarian cancer to complement CA125 measurements and TVUS are currently ongoing. A detailed literature review revealed that eighty-five autoantigens have been evaluated for the early diagnosis of ovarian cancer with ongoing studies seeking an optimal panel [4,5]. Due to inter-tumor heterogeneity and variable immune responses, it will be necessary to combine markers with individual sensitivities ranging from 10–30% to create a panel of antigens with sufficient sensitivity. In this study we evaluated a set of antigens associated with paraneoplastic syndromes for their use in the detection of autoantibodies for the early detection of ovarian cancer.
Screening in increased-risk populations has the potential to be beneficial given a higher incidence relative to the general population [3,6,7]. Those with a family history of ovarian cancer are at an increased-risk, including hereditary breast and ovarian cancer patients with BRCA1 and BRCA2 mutations which are frequently found in the germline of Type II high-grade serous ovarian cancers (HGSOC), and Lynch syndrome with mismatch repair gene mutations [8]. Seventy percent of all epithelial ovarian cancer cases are HGSOC; the present study evaluates a series of autoantibodies for use as diagnostic biomarkers in serum samples from patients with HGSOC [1].
1.2. Paraneoplastic autoantibodies associated with ovarian cancer
Paraneoplastic autoantibodies are associated with autoimmune syndromes that develop from the unregulated immune response initiated by tumor antigens. These antigens are also expressed by normal cells in the muscle or nervous system, resulting in a paraneoplastic syndrome indicated by neurological symptoms or muscle weakness. These syndromes present months to years prior to tumor diagnosis in an estimated 70% of cases; interestingly the autoimmune symptoms can resolve upon removal of the tumor, and return of symptoms can indicate tumor recurrence [9]. Cancers associated with paraneoplastic syndromes include thymoma, lung, breast, and ovarian adenocarcinomas, and groups of autoantibodies associated with various paraneoplastic syndromes are more specific for the tumor type than the syndrome [10]. Although paraneoplastic syndromes are rare, autoantibodies associated with paraneoplastic syndromes can be detected in patients with cancer who do not present with paraneoplastic syndromes [11]. For example, two paraneoplastic antigens associated with lung cancer, SOX2 and Hu-D, are included in an FDA-approved early detection autoantibody test for lung cancer [12]. It was previously reported that 36% of SCLC patients without paraneoplastic syndrome had reactivity to at least one SOX family member, and 16–25% of SCLC patients without a paraneoplastic syndrome harbored anti-Hu antibodies [11]. The aim of this study was to identify antigens to detect autoantibodies that may have individually low frequencies, but as a panel provide enhanced sensitivity to detect ovarian cancer. To that end, we evaluated a panel of five paraneoplastic antigens along with three tumor-associated antigens that were previously reported to have high immuno-reactivity or enhanced expression in ovarian cancer.
Two paraneoplastic syndromes that have been reported prior to diagnosis of ovarian cancer are polymyositis and paraneoplastic cerebellar degeneration [10]. Polymyositis is defined by muscle inflammation, and dermatomyositis by an accompanying skin rash. These myopathies are associated with the autoantibodies HARS, SRP-19, TRIM21, and Mi-2 [13]. Cerebellar degeneration results from damage to Purkinje cells and is associated with anti-neuronal antibodies (ANA), in particular CDR2 and CDR2L antigens [14]. CDR2 antibodies have previously been reported to have a low frequency, 2.3%, in ovarian cancer sera [15].
Of the eight antigens examined in a large-scale validation using western blot and ELISA, the antigens TRIM21, NY-ESO-1, TP53 and PAX8 combined to yield the highest sensitivity of 67% with 94% specificity, and 56% sensitivity with 98% specificity. A sec ond validation study of those four antigens on western blot with an independent sample set maintained 98% specificity with 46% sensitivity.
2. Methods
2.1. Collection of patient samples
Samples were obtained from patients at Karmanos Cancer Institute, St. John Hospital and Oakwood Hospital in Detroit, MI, and at the Mayo Clinic, Rochester, MN. Additional specimens were provided by the Cooperative Human Tissue Network (CHTN) and Gynecologic Oncology Group specimen banks. All samples were collected prior to surgery or therapy. Healthy control sera were collected as part of a large-scale community outreach project. Blood was collected via venipuncture, centrifuged at 2,500 rpm at 4δC, and the resulting serum stored at −80δC. Protocols were approved by the Institutional Review Boards of Wayne State University and the individual hospitals. Each patient provided written informed consent.
For the Validation I and Validation II studies, the early and late stage HGSOC group is comprised of females age 18 and over diagnosed with epithelial ovarian cancer. In these studies we used serum collected from 19 early stage and 95 late stage serous ovarian cancer patients prior to treatment or surgery. The benign ovarian cyst group included 100 samples. One hundred healthy controls were self-reported to be free of cancer and potentially confounding benign conditions such as ovarian cysts, uterine fibroids, or endometriosis (Table 1). The HARS antigen was not processed with the Validation II sample set, with the exception of n = 5 early stage HGSOC samples. Sample usage tracking ensured that the 314 samples selected for the two validation studies were not used initially to identify the biomarkers.
Table 1.
Serum sample patient population (n = 164), analyzed on western blot and ELISA
| Validation set I | ||
|---|---|---|
| Patient description | Number of samples | Age range (Avg) (median) |
| Late stage HGSOC at time of diagnosis, pre-treatment | 50 | 39–81* (62.6) (62) |
| Early stage HGSOC at time of diagnosis, pre-treatment | 14 | 44–76 (58.8) (57) |
| Benign gynecological condition (ovarian cyst) | 50 | 17–76 (48.8) (49.5) |
| Healthy volunteers from community outreach | 50 | 32–88 (56.2) (53) |
Patient population of serum samples analyzed on ELISA and western blot.
Indicates age not available for 5 cases.
2.2. Line blots
To determine which myositis-associated antigens were most useful as biomarkers for ovarian cancer, we evaluated line blots from Euroimmun (EUROIMMUN, Leubeck, Germany) and for onconeuronal antigens associated with paraneoplastic syndromes we evaluated line blots from Ravo Diagnostika (Ravo Diagnostika, Freiburg, Germany). Line blots were processed per manufacturer protocol, and incubated with a serum sample dilution of 1:100. The antigens included on the Euroimmun myositis profile line blots are: TRIM21, OJ, EJ, PL-12, PL-7, SRP, HARS, PM-SCL75, PM-SCL100, KU, and MI-2. The antigens included on Ravo Diagnostika paraneoplastic antigen line blots are: Hu-D, CDR2, RI, CRMP5, AMPHIPHYSIN, MA1, MA2, SOX1, and GAD65. Line blot reactivity was scored from 0–5 for each antigen by two independent observers blinded to the patient sample status.
2.3. Cloning strategy for recombinant expression vectors for protein expression
The tumor antigens were first PCR amplified using forward primers (containing a 6X Histidine tag and T7 tag) and reverse primers using cDNA template prepared from the ovarian cancer cell lines. The PCR products were column purified, restriction digested and ligated to pET-21b bacterial expression vector (EMD Millipore Corporation, San Diego, CA, USA). The ligated DNA was then transformed into BL21-DE3 strain and the positive colonies were sequenced. These expression vectors were employed for in vivo production of recombinant His-tagged proteins in BL21-DE3 bacterial strain. The SRP-19 expression plasmid was kindly gifted by Dr. Howard M. Fried, University of North Carolina at Chapel Hill [16]. The human TP53 (1–393) expression plasmid was a gift from Cheryl Arrowsmith (Addgene plasmid #24859; RRID:Addgene_24859; http://n2t.net/addgene: 24859) [17]. All cDNA expression plasmids used in this study were fully DNA sequenced.
2.4. Production and purification of recombinant His-tagged proteins
The BL21-DE3 bacterial cells bearing clones were grown overnight in 5 mL LB with 50 μg/mL ampicillin at 37δC. 0.5 mL of the overnight culture was added to 500 mL LB with 50 μg/mL ampicillin and grown at 37δC to OD between 0.4–0.5. IPTG was added to a final concentration of 0.6 mM to induce the production of T7 RNA polymerase within the BL21-DE3 expression host, which is required for RNA and subsequent protein synthesis and the culture was grown at 37δC for three hours. The cells were pelleted at 1,200 × g for 15 minutes and supernatant was discarded. The pellet was frozen at −80δC for at least 30 minutes and then lysed with Thermo Scientific Bacterial Protein Extraction Reagent lysis buffer, centrifuged at 15,000 × g and then transferred the supernatant. The pellet, containing the target protein that forms within inclusion bodies, was solubilized in 8 M urea. The crude His-tagged proteins were purified first using Ni-NTA beads (Thermo Fisher Scientific, Waltham, MA, USA) following manufacturer’s protocol. Ni-NTA beads bind to His residues that are contained at the N-terminus of the proteins and a single pass results in relatively pure protein. The Ni-NTA purified His-tagged proteins were further purified using T7Tag® Antibody Agarose (MilliporeSigma, Burlington, MA, USA) which bind the N-terminal 11 aa of the T7 gene 10 protein. The second round of purification with T7 Antibody bound agarose beads removes any contaminating bacterial poly-His containing proteins from first round of Ni-NTA bead purification. Following purification, proteins were processed through Zeba desalting columns (Thermo Fisher Scientific, Waltham, MA, USA). The recombinant proteins in this study are full-length, with the exception of the His-tagged recombinant PAX8 protein that consists of the amino acids 1–287 (Sino Biological, Wayne, PA, USA). The full-length AARS His-tagged recombinant protein (AARS-51H) was purchased, and was produced in Sf9 insect cells (Creative BioMart, Shirley, NY, USA).
2.5. Western blot
Western blots were performed with 0.3–0.5 μg of purified recombinant proteins separated on a 10% acrylamide SDS-PAGE. Protein loading concentration was within the linear range as calculated per antigen with anti-His tag antibody and positive control human serum samples with anti-human IgG. Proteins were transferred onto nitrocellulose membranes for one hour on ice at 250 mA. Membranes were blocked overnight at 4δC with 5% milk in TBS with 0.1% TWEEN 20 (Sigma-Aldrich, St. Louis, MO, USA). The next day, each serum sample was pre-incubated at a 1:300 dilution in 3 ml of 5% milk in TBS-T with 75 μg of BL21-DE3 E. coli lysate for one hour to reduce background reactions of human sera to E. coli proteins. The patient serum was then incubated with nitrocellulose membranes for one hour at room temperature. Following three washes with TBS-T, secondary IR-dye labeled mouse anti-His tag and goat anti-Human IgG antibodies were incubated for one hour at room temperature followed by three ten-minute washes with TBS-T and two washes with PBS. Autoantibody binding to the antigens was quantified on LiCor Image Studio software (LI-COR Biosciences, Lincoln, NE, USA) as background-corrected integrated intensity of anti-human IgG (IRDye800) normalized to anti-His tag antibody (IRDye700). Secondary anti-His tag antibody was quantified and used as a loading control to normalize the anti-human IgG value to each protein band. Day to day variation as calculated from the IRDye700 readings of the anti-His tag antibody was used to adjust data. Representative western blot images are shown in Figs 1 and 3.
Fig. 1.
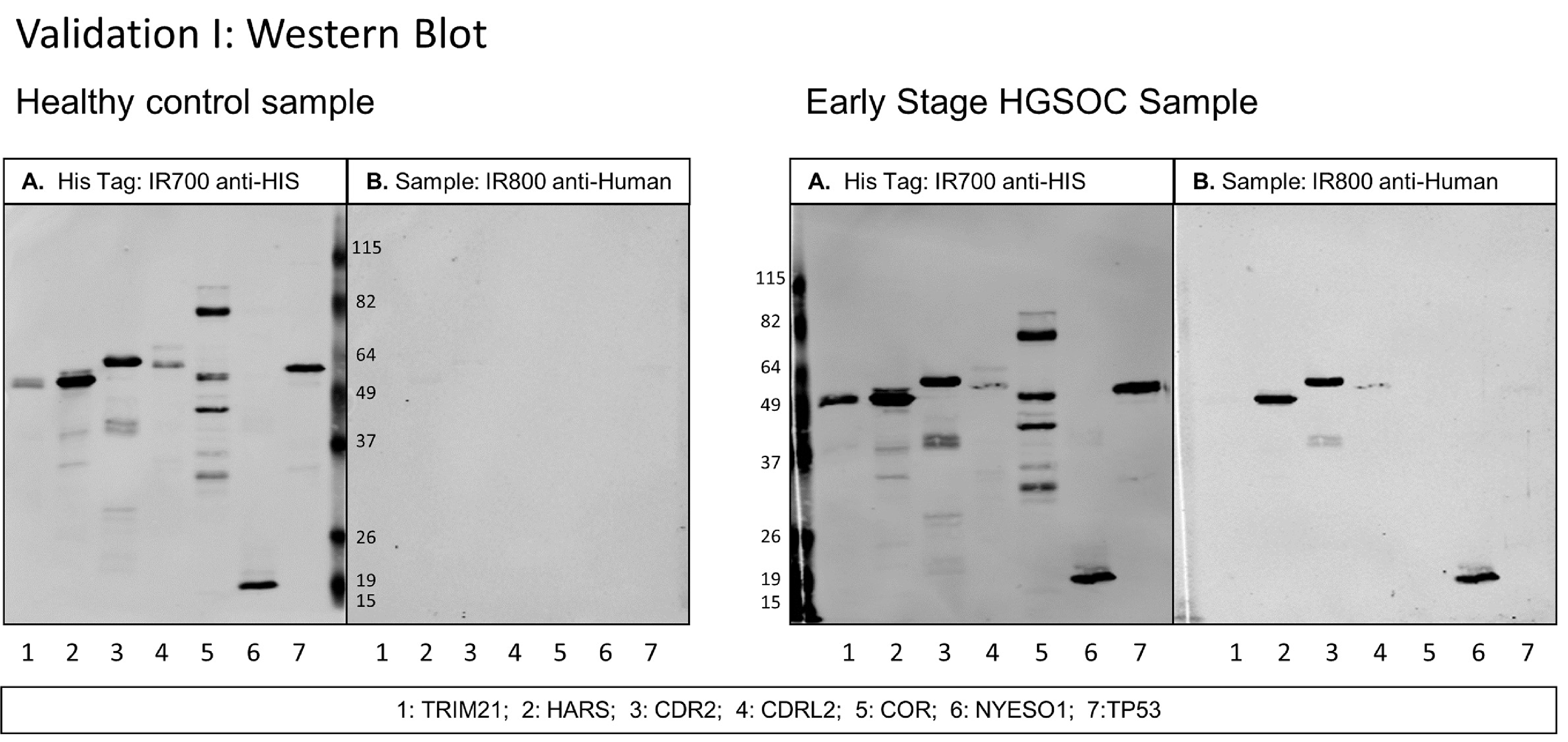
Western blot of healthy control serum and early stage HGSOC serum diluted at 1:300 with 7 antigens in Validation I study. A. Secondary antibody anti-HIS tag IgG loading control. B. Secondary antibody anti-human IgG. Scans quantified on Odyssey software; background-corrected integrated intensity of anti-human IgG antibody (IRDye800) normalized as ratio to anti-His tag antibody (IRDye700) per antigen.
Fig. 3.
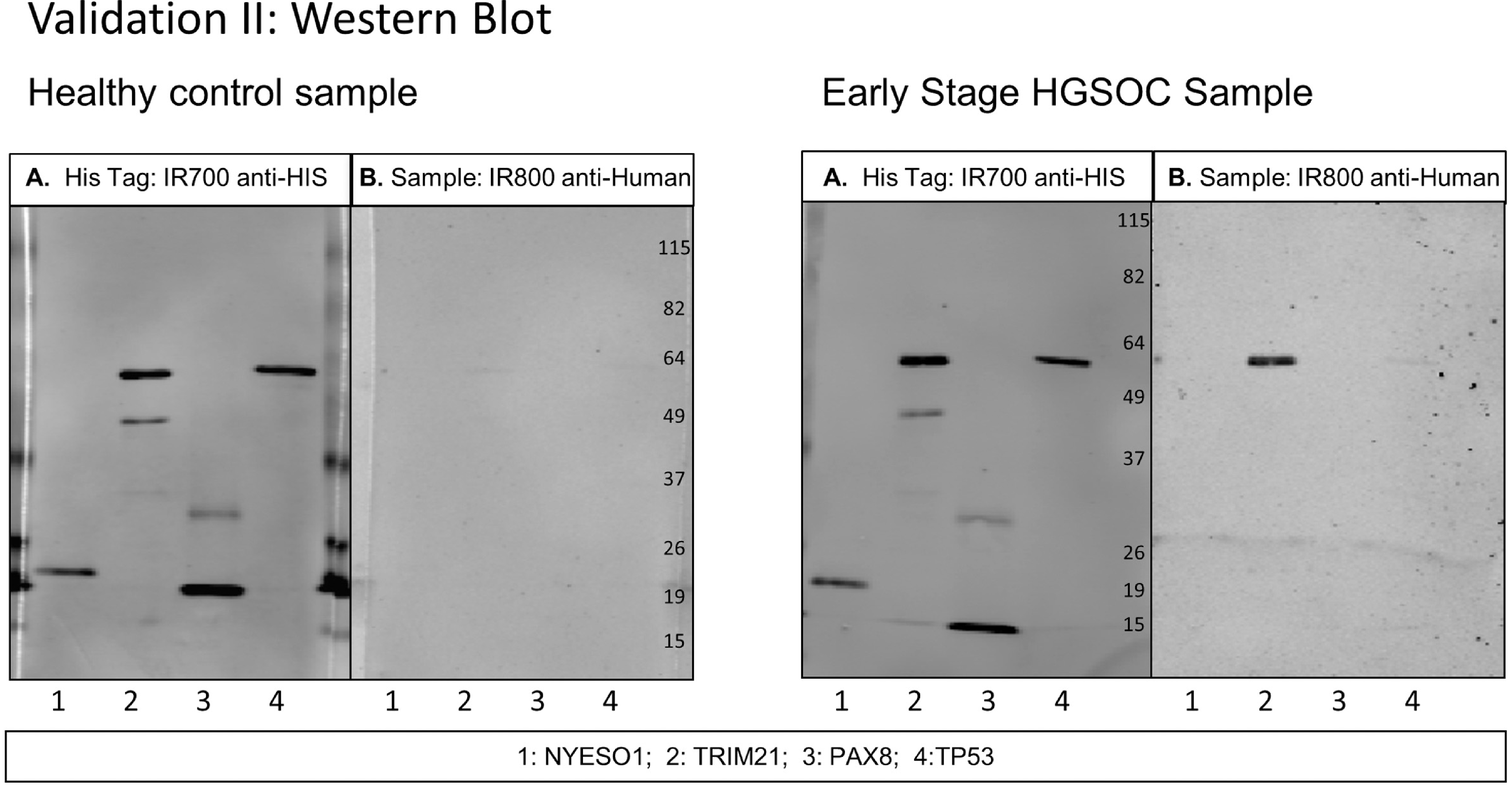
Western blot of healthy control serum and early stage HGSOC serum diluted at 1:300 with 4 antigens in Validation II study. A. Secondary antibody anti-HIS tag IgG loading control. B. Secondary antibody anti-human IgG. Scans quantified on Odyssey software; background-corrected integrated intensity of anti-human IgG antibody (IRDye800) normalized as ratio to anti-His tag antibody (IRDye700) per antigen.
2.6. ELISA
The serum set described in Table 1 was processed on ELISA as follows: purified antigens were coated in duplicate wells at concentrations from 0.3–1.5 μg/mL in PBS and incubated overnight at 4δC. All subsequent steps took place at room temperature. Wells were blocked for one hour with 5% donkey serum in PBS. To eliminate background of patient sera reactivity with donkey serum and nonspecific bacterial proteins, serum samples were diluted 1:100 in PBS with 5% donkey serum and 75 μg of BL21-DE3 E. coli lysate for one hour. Patient samples were incubated on the plate for one hour, followed by one-hour incubation of donkey anti-human HRP-conjugated secondary antibody. TMB (3,3’,5,5’-tetramethylbenzidine) ELISA substrate was added followed by sulfuric acid to stop the reaction after 20 minutes. The addition of blocking solution, washing steps, TMB and sulfuric acid addition were performed on the Biomek2000 automated liquid handling robot (Beckman Coulter, Brea, CA, USA).
A standard curve using serum with known reactivity to TRIM21 (The Binding Site, San Diego, CA, USA) at five dilutions ranging from 1:75 to 1:1200 was included on each plate to account for plate-to-plate and day-to-day variation. In addition, a pair of non-coated wells were blocked with donkey serum and incubated with each patient serum. These patient serum specific background values were subtracted from the antigen values for each patient sample. The serum set described in Table 1 was processed on ELISA. Each measurement was run in duplicate per plate, with four plates processed per day over 11 days. For 88 replicate measurements of the 1:75 dilution of the positive control standard curve, the coefficients of variation (CV) for the variance components in the ELISA assay are as follows: Intra-assay CV (within plate) 0.0281; Interassay CV (plate to plate) 0.0749; and CV day-to-day 0.0898.
2.7. Statistical methods
For the ELISA data analysis, to adjust for day-today variability among the 44 ELISA plates measured across 11 days, we utilized the positive control measurements to construct the standard curve, which was measured on all plates. The linear curve consisted of 6 dilutions of a positive control serum sample (The Binding Site, San Diego, CA, USA) measured against TRIM21 in duplicate. A linear mixed model with log optical density (OD) as the response; dilution (treated as a factor), protein (either TRIM21 or BKG) and their interaction as the fixed effects; plate nested within day as a random effect; an estimated correlation structure between duplicate observations; and unequal variance within each protein/dilution combination. From this model, we extracted the random effects terms to adjust the observed log (OD) values. After averaging the duplicates and exponenting resultant value, the appropriate control adjusted OD was subtracted to produce the normalized OD measurement. This normalized OD measurement was then used to compare to the values from the western blot analysis.
Quantification of autoantibody binding on western blot for each sample was measured over 15 days for Validation I for each of the 8 antigens, and measured over 16 days for Validation II for 4 antigens, utilizing multiple membranes per day. Samples were randomized per category of HGSOC, benign, and healthy with each category evenly distributed per day, and labeled so that the experimenters were blinded to the sample category. The quantification values for both the IRDye700 and IRDye800 channels were log transformed after the addition of a small constant (0.01) to ensure all values were positive. The difference between the log transformed IRDye700 and IRDye800 values for each antigen for each sample is the pre-adjustment analysis metric. We employed a mixed model to develop adjustment factors to account for the between-day variability. We utilized the estimated day-specific random effects (from a model including the log difference as the response, antigen as the fixed effect and day as the random effect) to account for day-to-day variability. The log difference minus the day-specific random effect was used as the final analysis metric. Subsequently, for each antigen, the mean and standard deviation of the analysis metric was computed using the healthy samples. For each antigen, the mean and standard deviation was used to create standardized values [(Obs-Mean)/StdDev)]. This standardized value was used in the figures and tables presented in this manuscript.
3. Results
3.1. Study design
3.1.1. Selection of 8 antigens from evaluation of full-length recombinant proteins and homologous epitopes on line blot, western blot, and ELISA
We previously identified ovarian cancer-related epitopes by screening unbiased random-peptide phage display cDNA libraries using ovarian cancer sera and found that many shared amino acid sequence homology with paraneoplastic antigens. Two phage-borne epitopes, 4B7 and 3A9, displayed 100% homology to the myositis associated antigens HARS and SRP-19, respectively [18]. Additionally, two ovarian cancer epitopes, 4F10 and 4E8, showed partial homology to the paraneoplastic antigens TRIM21 and Hu-D. An initial survey of autoantibodies to 20 paraneoplastic antigens in HGSOC sera [10,19] was performed, including the four antigens to which ovarian cancer epitopes showed homology; HARS, SRP-19, TRIM21, and Hu-D. This study utilized two commercially available line blots in which recombinant antigens were spotted onto a membrane, with one line blot test consisting of myositis-associated antigens, and a second line blot test consisting of onconeuronal antigens associated with paraneoplastic neurological syndrome (Supplemental Fig. 1).
In addition, selected antigens were purified for further analysis on western blot and ELISA. The following antigens were either expressed in E. coli and purified in house, or obtained commercially for immunoreaction in western blot and ELISA assays: SRP-19, HARS, AARS, CDR2, HuD, TRIM21, TRIM33, CDR2L, CORTACTIN, CKB, NY-ESO-1, PAX8 and TP53. In addition, the four phage display epitopes with homology to paraneoplastic antigens were sub-cloned, expressed in E. coli and purified [20]. Results from western blot and ELISA screening with a serum set of n = 36 samples are shown in Supplemental Ta ble 1. This sample set included 12 HGSOC samples, 12 healthy control samples, and 12 benign gynecologic condition samples. Both the homologous phage-borne epitopes and full-length protein pairs were evaluated; the full-length proteins provided increased sensitivity. From the commercial line blot, western blot, and ELISA screenings, three myositis-associated antigens, two paraneoplastic cerebellar degeneration (PCD) associated antigens, and three tumor-associated antigens were selected for validation on a large-scale western blot and ELISA study using an independent sample set. The 8 selected antigens are described in Table 2.
Table 2.
Description of paraneoplastic antigens evaluated against HGSOC sera
| Category | Antigens purified for western blot/ELISA screening | Epitope purified for western blot/ELISA screening | Autoantibodies targeting antigens | Autoimmune conditions associated with autoantibodies | Cancers types associated with autoantibodies | Reference |
|---|---|---|---|---|---|---|
| Myositis | HARS | 4B7: Epitope | Jo-1 | Myositis, Dermatomyositis | OVCA, lung | [13,35] |
| TRIM21 | 4F10: Epitope | Ro-52 | Myositis, Systemic lupus erythematosus, Sjogren’s Syndrome | ECC, basal-like breast cancer | [27,28] | |
| SRP-19 | 3A9: Epitope | Anti-SRP-19 | Myositis | Breast, endometrial, hepatocellular, bladder | [13,36] | |
| CORTACTIN | Anti-Cor | Myositis | [23] | |||
| AlaRS | PL-12 | Myositis | Lung, gastric | [13,37] | ||
| TRIM33 | Anti- TIF1-γ | Myositis | Lung, breast, OVCA, stomach | [13,35,38] | ||
| PCD | CDR2 | Yo | Paraneoplastic Cerebellar Degeneration | Breast, OVCA | [15,24] | |
| CDR2L | Yo | Paraneoplastic Cerebellar Degeneration | Breast, OVCA | [14,24] | ||
| CKB | Anti-CKB | Paraneoplastic Cerebellar Degeneration | OVCA | [39] | ||
| Other PNS | HuD | 4E8: Epitope | Anti-Hu | Encephalomyopathy, sensory neuronopathy | SCLC | [11,40] |
| P3F10 | Anti-P3F10 | OVCA epitope that binds PNS sera | OVCA | [20] | ||
| TAA | TP53 | Anti-TP53 | Systemic lupus erythematosus, Type I Diabetes, AI Thyroid Disease | Pancreatic, breast, OVCA | [30,41] | |
| NYESO-1 | Anti-NY-ESO-1 | N/A, Cancer/Testis Antigen | Lung, breast, OVCA | [42] | ||
| PAX8 | Anti-PAX8 | N/A | Antigen overexpression in OVCA | [43] | ||
The 8 antigens selected for the large-scale screening on western blot and ELISA are: HARS, TRIM21, CORTACTIN, CDR2, CDR2L, TP53, NY-ESO-1, and PAX8.
Epitopes identified by phage-display screening of ovarian cancer (OVCA) serum [20].
3.1.2. Validation I: Patient sample population, n = 164, processed on western blot and ELISA
To avoid experimental bias, an independent sample set of 164 samples that had not been used to identify the biomarkers initially was used for validation of the panel of antigens listed above. The sample population described in Table 1 consists of 50 healthy control samples, 50 benign ovarian cyst samples, 50 late stage HGSOC samples, and 14 early stage HGSOC samples.
3.2. ELISA and western blot correlation
A threshold based on the 50 healthy control values for each antigen defined positive results. The assay cutoff for both the ELISA assay and the western blot screening is defined as: Mean + 2 (StdDev) for the healthy controls. Using this criterion, NY-ESO-1, TP53, and TRIM21 had the highest sensivities and specificities on both platforms. Among all HGSOC samples (n = 64), 16/18 samples positive for NY-ESO-1 on ELISA were confirmed by western blot, 14/16 TP53 samples positive on ELISA were confirmed by western blot and 11/13 TRIM21 samples positive on ELISA were confirmed by western blot. However, for the marker TRIM21, 10 additional positive samples were identified on western blot that were not detected by ELISA. A representative image of a sample positive for TRIM21 on western blot that that was undetectable by ELISA is shown in Supplemental Fig. 2. The marker HARS did not react on ELISA whereas it showed reactivity on western blot. A number of patient samples retained an inherent high background on ELISA regardless of pre-incubation of the serum sample with blocking agent (5% donkey serum) and bacterial extract as described in Methods. It was concluded that western blot eliminates ambiguities introduced by samples with high background noise and is the more reliable platform for detection of patient au toantibodies. Therefore, the subsequent analyses employed the western blotting data.
3.3. TRIM21 provides highest sensitivity as an individual marker in HGSOC samples
Sensitivities and specificities as calculated by the standardized thresholds based on mean + 2 (StdDev) of the healthy controls and mean + 3 (StdDev) of the healthy controls per antigen are reported in Table 3. The resulting thresholds were applied to all patient groups: healthy, benign, early stage HGSOC, and late stage HGSOC. The ratio of anti-human IgG:anti-HIS IgG intensity values are plotted for each antigen for the late-stage HGSOC, early-stage HGSOC, benign ovarian cyst, and healthy control groups in Fig. 2. Applying the assay threshold = 2 for western blot to TRIM21 yields 21 positive samples out of 64 HGSOC cases, including both early and late stage. Notably, with 33% sensitivity TRIM21 achieves 100% specificity for healthy control samples. Four benign ovarian cyst samples had reactivity above the healthy control threshold. Individual sensitivities and specificities of all markers are shown in Table 3; the previously established biomarkers NY-ESO-1 and TP53 each detected 16/64 (25%) of HGSOC cases.
Table 3.
Sensitivity/specificity for TRIM21, NY-ESO-1, TP53, and PAX8, Validation I
| Late stage HGSOC (n = 50) | Early stage HGSOC (n = 14) | Late and early stage HGSOC (n = 64) | Benign (n = 50) | Healthy (n = 50) | Benign and healthy (n = 100) | |
|---|---|---|---|---|---|---|
| Positive threshold = 2 | ||||||
| Antigen | Sensitivity | Sensitivity | Sensitivity | Specificity | Specificity | Specificity |
| TRIM21 | 0.34 | 0.29 | 0.33 | 0.90 | 1.00 | 0.95 |
| TP53 | 0.28 | 0.14 | 0.25 | 0.98 | 0.96 | 0.97 |
| NYESO1 | 0.24 | 0.29 | 0.25 | 0.98 | 0.98 | 0.98 |
| PAX8 | 0.10 | 0.14 | 0.11 | 0.96 | 1.00 | 0.98 |
| HARS | 0.06 | 0.29 | 0.11 | 0.90 | 0.98 | 0.94 |
| CDR2L | 0.06 | 0.14 | 0.08 | 0.94 | 0.96 | 0.95 |
| CDR2 | 0.10 | 0.14 | 0.11 | 0.88 | 0.96 | 0.92 |
| COR | 0.02 | 0.00 | 0.02 | 1.00 | 0.96 | 0.98 |
| TRIM21, TP53, NYESO1 | 0.64 | 0.43 | 0.59 | 0.86 | 0.94 | 0.90 |
| TRIM21, TP53, NYESO1, PAX8 | 0.72 | 0.50 | 0.67 | 0.82 | 0.94 | 0.88 |
| Positive threshold = 3 | ||||||
| Antigen | Sensitivity | Sensitivity | Sensitivity | Specificity | Specificity | Specificity |
| TRIM21 | 0.28 | 0.21 | 0.27 | 0.92 | 1.00 | 0.96 |
| TP53 | 0.26 | 0.14 | 0.23 | 1.00 | 0.98 | 0.99 |
| NYESO1 | 0.22 | 0.29 | 0.23 | 1.00 | 1.00 | 1.00 |
| PAX8 | 0.04 | 0.00 | 0.03 | 0.96 | 1.00 | 0.98 |
| HARS | 0.02 | 0.21 | 0.06 | 0.98 | 1.00 | 0.99 |
| CDR2L | 0.02 | 0.14 | 0.05 | 0.96 | 1.00 | 0.98 |
| CDR2 | 0.06 | 0.14 | 0.08 | 0.94 | 0.98 | 0.96 |
| COR | 0.02 | 0.00 | 0.02 | 1.00 | 0.96 | 0.98 |
| TRIM21, TP53, NYESO1 | 0.58 | 0.36 | 0.53 | 0.94 | 0.98 | 0.96 |
| TRIM21, TP53, NYESO1, PAX8 | 0.62 | 0.36 | 0.56 | 0.88 | 0.98 | 0.93 |
Sensitivity and specificity for TRIM21, NY-ESO-1, TP53, PAX8, HARS, CDR2L, CDR2, and CORTACTIN. Threshold (2) = Mean + 2 (StdDev) for healthy controls, threshold (3) = Mean + 3 (StdDev) for healthy controls.
Fig. 2.
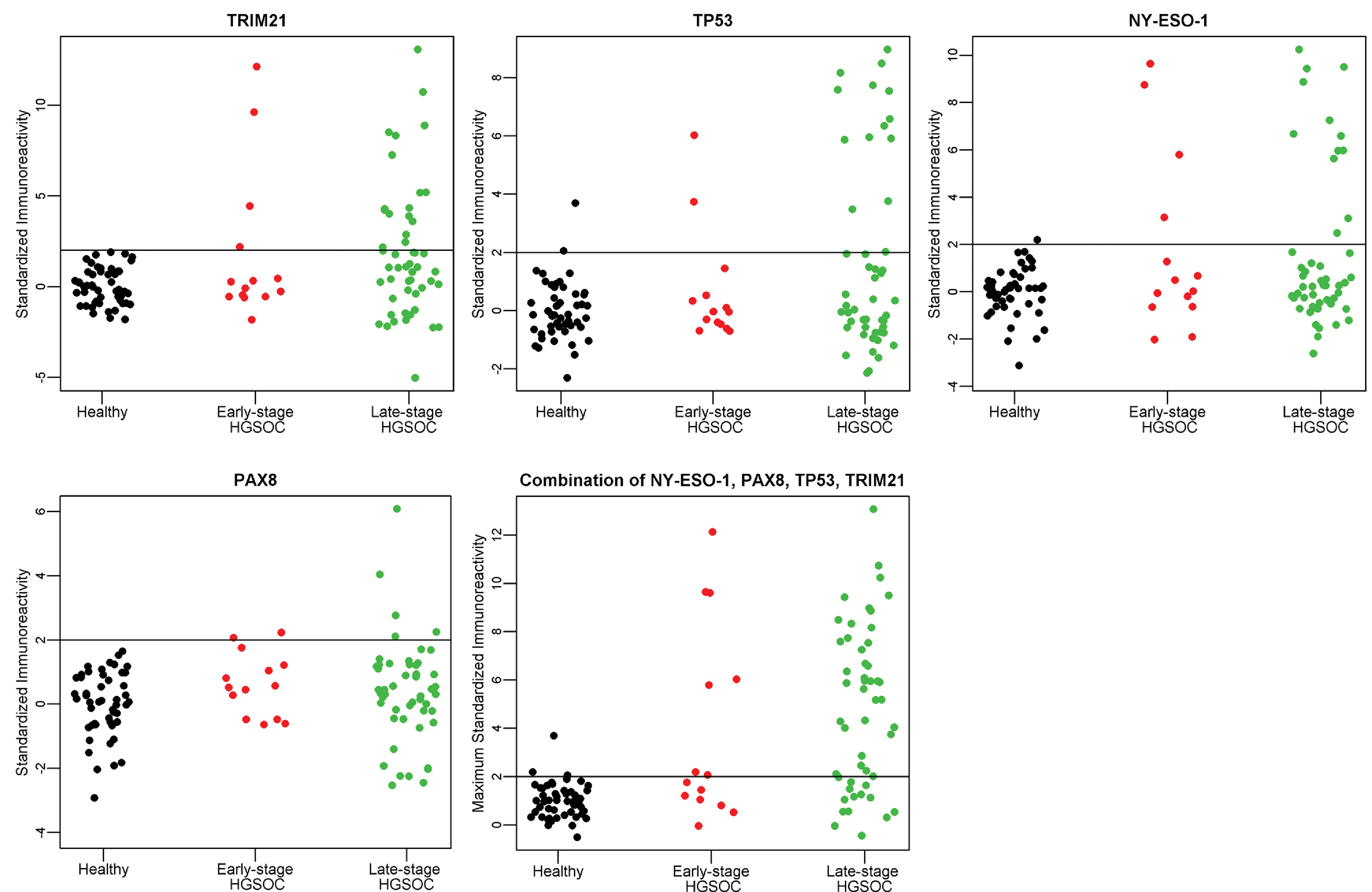
A–D. Individual antigen plots for 4 antigens, TRIM21, NY-ESO-1, TP53, and PAX8 in Validation I. E. Maximum value of TRIM21, NY-ESO-1, TP53, or PAX8 in Validation I. The immunoreactivity as defined by the ratio of 800/700 is standardized for each antigen to provide the threshold of Mean + 2 (StdDev) for healthy controls, as indicated by the horizontal line at Y = 2.
3.4. TRIM21, HARS, NYESO-1, PAX8, and TP53 detected in early stage HGSOC samples
The sample set included 14 early stage samples. Individually, NY-ESO-1 and HARS detected 4 early-stage HGSOC samples (29%), TRIM21 detected 3 early-stage HGSOC samples (21%), and TP53 and PAX8 each detected 2 early-stage HGSOC samples (14%). The combination of the 5 markers yielded 50% sensitivity for detecting early stage HGSOC with 94% specificity to discriminate healthy controls. Respective sensitivities and specificities within each sample group are shown in Table 3. The combination of markers PAX8, HARS, NY-ESO-1, TP53 and TRIM21 detected 7/14 early-stage HGSOC samples. Among these five antigens, all 7/7 of the positive early stage HGSOC samples were reactive with 2 or more antigens, com pared with 12/37 positive late stage HGSOC samples reacting with 2 or more antigens.
3.5. Panel of 4 markers achieves highest performance for HGSOC autoantibody detection: TRIM21, NY-ESO-1, TP53, and PAX8
The combination of the four markers TRIM21, NY-ESO-1, TP53, and PAX8 detected 42/64 HGSOC samples. With 94% specificity for the healthy control population, we can achieve 67% sensitivity (threshold = 2) for the 4-marker model. At 98% specificity relative to the healthy control population, this 4-marker combination achieves 56% sensitivity (threshold = 3). The maximum value among the 4 markers: TRIM21, NY-ESO-1, TP53, and PAX8 for each sample group is plotted in Fig. 2. Table 3 shows performance of the combinations of these 4 markers.
3.6. Validation II: Panel of 4 markers, TRIM21, NY-ESO-1, TP53, and PAX8, validated on independent serum set, n = 150
A separate serum set consisting of 50 healthy, 50 benign ovarian cyst, and 50 high-grade serous ovarian cancer samples was used for an independent validation of the 4 markers: TRIM21, NY-ESO-1, TP53, and PAX8. The patient population is described in Table 4. Patient samples were evaluated on western blot, shown in Fig. 3. In this sample set, PAX8 did not complement the 3 markers TRIM21, NY-ESO-1, and TP53. Standardized patient reactivity for each antigen is shown in Fig. 4. This validation screening of the 3 markers TRIM21, NY-ESO-1, and TP53 maintained a specificity of 98% with a sensitivity of 46% as described in Table 5. The sensitivity of the combination of the 4 markers: TRIM21, NY-ESO-1, TP53, and PAX8 for all HGSOC cases was lower in the second validation study, 50% vs. 67% with positive threshold = 2, and 46% vs. 56% with positive threshold = 3. This difference is not statistically significant at the 0.05 level (p-value = 0.096 and p-value = 0.369, respectively, using a 2X2 chi-square contingency table with Yates correction). Receiver Operating Characteristic (ROC) analysis resulted in area under the curve (AUC) of 0.832 for Validation I and 0.701 for Validation II, as shown in Fig. 5. For both sample sets, TRIM21 provided the highest AUC as an individual marker: 0.671 in Validation I and 0.618 in Validation II (Supplemental Fig. 3). Although the HARS antigen was not evaluated on this sample set, the 5 early stage HGSOC samples were processed with the HARS protein resulting in 1/5 positive samples. TRIM21 was the only other antigen positive in the 5 early stage HGSOC samples; combining TRIM21 and HARS resulted in 2/5 early stage HGSOC samples positive in this set.
Table 4.
Serum sample patient population (n = 150), analyzed on western blot
| Validation set II | ||
|---|---|---|
| Patient description | Number of samples | Age range (Avg) (median) |
| Late stage HGSOC at time of diagnosis, pre-treatment | 45 | 37–87 (63.6) (64) |
| Early stage HGSOC at time of diagnosis, pre-treatment | 5 | 43–66 (51.6) (47) |
| Benign gynecological condition (ovarian cyst) | 50 | 25–88* (56.5) (55) |
| Healthy volunteers from community outreach | 50 | 30–82 (55.4) (54) |
Patient population of serum samples analyzed on western blot.
Indicates age not available for 5 cases.
Fig. 4.
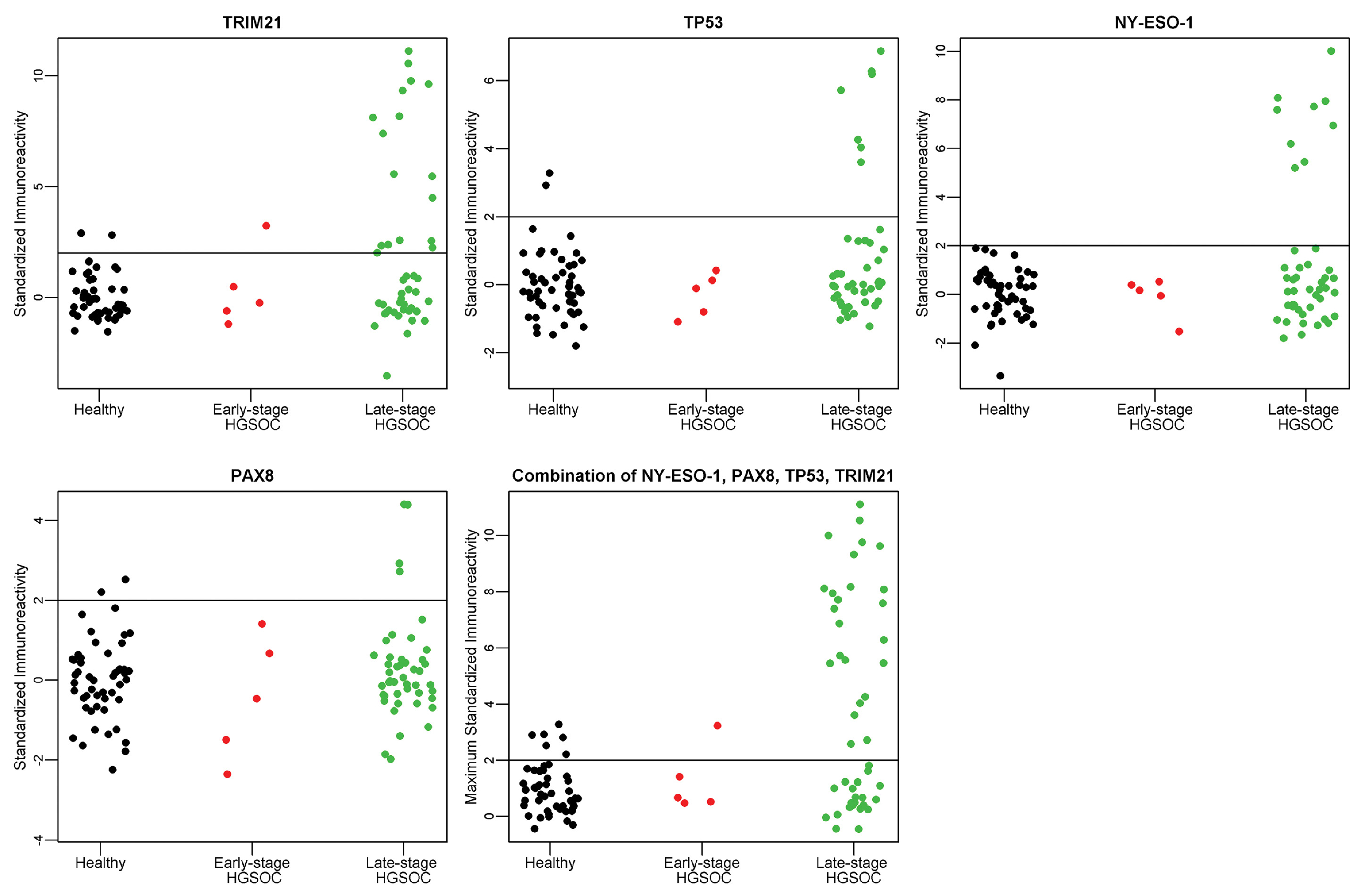
A–D. Individual antigen plots for 4 antigens, TRIM21, NY-ESO-1, TP53, and PAX8 in Validation II. E. Maximum value of TRIM21, NY-ESO-1, TP53, or PAX8 in Validation II. The immunoreactivity as defined by the ratio of 800/700 is standardized for each antigen to provide the threshold of Mean + 2 (StdDev) for healthy controls, as indicated by the horizontal line at Y = 2.
Table 5.
Sensitivity/specificity for TRIM21, NY-ESO-1, TP53, and PAX8, Validation II
| Late stage HGSOC (n = 45) | Early stage HGSOC (n = 5) | Late and early stage HGSOC (n = 50) | Benign (n = 50) | Healthy (n = 50) | Benign and healthy (n = 100) | |
|---|---|---|---|---|---|---|
| Positive threshold = 2 | ||||||
| Antigen | Sensitivity | Sensitivity | Sensitivity | Specificity | Specificity | Specificity |
| TRIM21 | 0.38 | 0.20 | 0.36 | 0.90 | 0.96 | 0.93 |
| TP53 | 0.16 | 0.00 | 0.14 | 0.96 | 0.96 | 0.96 |
| NYESO1 | 0.20 | 0.00 | 0.18 | 0.94 | 1.00 | 0.97 |
| PAX8 | 0.09 | 0.00 | 0.08 | 0.98 | 0.96 | 0.97 |
| TRIM21, TP53, NYESO1 | 0.51 | 0.20 | 0.48 | 0.80 | 0.92 | 0.86 |
| TRIM21, TP53, NYESO1, PAX8 | 0.53 | 0.20 | 0.50 | 0.80 | 0.88 | 0.84 |
| Positive threshold = 3 | ||||||
| Antigen | Sensitivity | Sensitivity | Sensitivity | Specificity | Specificity | Specificity |
| TRIM21 | 0.24 | 0.20 | 0.24 | 0.98 | 1.00 | 0.99 |
| TP53 | 0.16 | 0.00 | 0.14 | 1.00 | 0.98 | 0.99 |
| NYESO1 | 0.20 | 0.00 | 0.18 | 0.98 | 1.00 | 0.99 |
| PAX8 | 0.04 | 0.00 | 0.04 | 0.98 | 1.00 | 0.99 |
| TRIM21, TP53, NYESO1 | 0.49 | 0.20 | 0.46 | 0.96 | 0.98 | 0.97 |
| TRIM21, TP53, NYESO1, PAX8 | 0.49 | 0.20 | 0.46 | 0.94 | 0.98 | 0.96 |
Sensitivity and specificity for TRIM21, NY-ESO-1, TP53, and PAX8. Threshold (2) = Mean + 2 (StdDev) for healthy controls, threshold (3) = Mean + 3 (StdDev) for healthy controls.
Fig. 5.
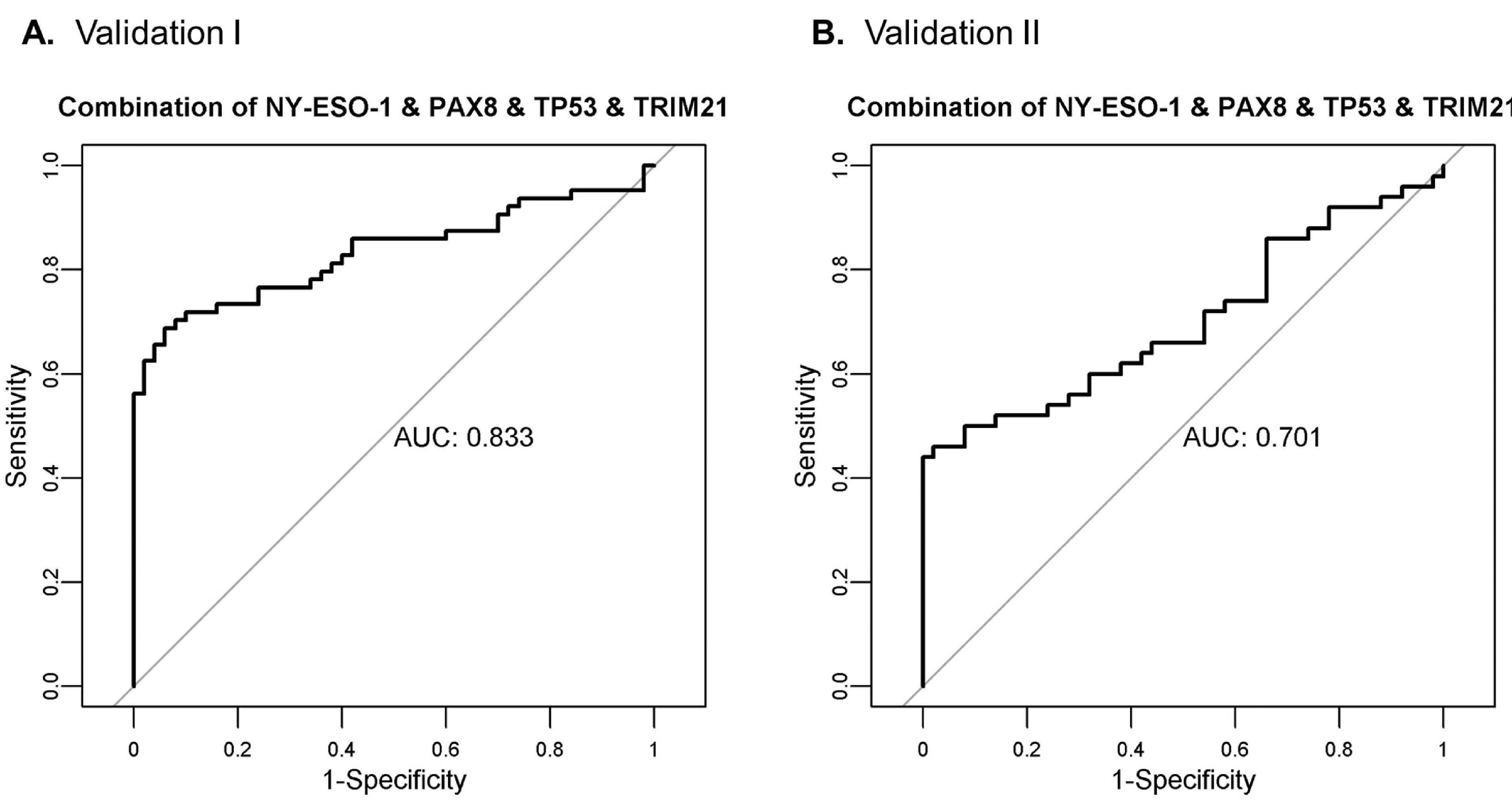
ROC curve analysis for the combination of the 4 markers: TRIM21, NY-ESO-1, TP53, and PAX8 in A. Validation I and B. Validation II.
4. Discussion
Early detection of ovarian cancer has the potential to improve patient outcome. In the UK Familial Ovarian Cancer Screening Study phase II trial, 4, 348 women at high-risk for ovarian cancer were screened for CA125 every 4 months with yearly transvaginal ultrasound, which resulted in a significant stage shift in diagnosis. Of the 19 total cases detected during the 5 years of screening, 10 were stage I–II [7]. Two prospective trials from the Cancer Genetics Network and the Gynecologic Oncology Group together screened 3, 962 women at high-risk for ovarian cancer with CA125 screening every 3 months, followed by transvaginal ultrasound upon increases in CA125 above the patient’s baseline. In these trials, 3 of the 6 incident cases were stage I–II [3]. Autoantibodies to tumor antigens, produced at small tumor volumes, can be combined with serum screening of CA125 to improve sensitivity in early detection.
We previously used phage-display screening to identify autoantibody biomarkers for both early detection and recurrence of ovarian cancer [18,20]. Two of our identified markers were epitopes for myositis-associated antigens, HARS and SRP-19. This study evaluates of a set of myositis associated and onconeuronal autoantigens for detection of autoantibodies in serum from ovarian cancer patients without a known paraneoplastic syndrome. We note that in our previous work, the antigens CDR2, TRIM21, and HARS were evaluated for reactivity to antibodies in sera from patients experiencing a recurrence of their HGSOC; however, in that study the levels of autoantibody were considered relative to a negative control antigen for each individual patient at three time points, with the goal of monitoring disease recurrence [19]. With the goal of early detection in the current study, autoantibodies were considered relative to healthy and benign control serum samples; therefore, this study reflects differences in the frequency of paraneoplastic autoantibodies in HGSOC sera relative to sera from healthy controls and women with benign ovarian cysts. As low-grade serous ovarian cancer (LGSOC) can develop step-wise from ovarian serous cystadenoma, we evaluated 22 samples from patients with early stage LGSOC with the antigens TRIM21, NY-ESO-1, TP53, PAX8, and HARS [21,22]. We found that 1/22 early stage LGSOC samples were positive for TP53 autoantibodies, and 1/22 samples were positive for TRIM21 autoantibodies (data not shown).
Cortactin (COR), a novel biomarker for myositis, did not show HGSOC specificity [23]. We found that CDR2 and CDR2L had reactivity with healthy and benign samples. Antibodies to CDR2 have previously been reported to have low frequency in ovarian cancer, we observed 5/50 late stage and 2/14 early stage samples positive for CDR2 autoantibodies at the threshold (2) = Mean + 2 (StdDev) [15]. Previous studies have shown that Yo-antibody positive patients with paraneoplastic cerebellar degeneration have anti-Yo antibodies that react with both CDR2 and CDR2L [24]. In a study evaluating ovarian cancer sera, anti-Yo positive sera reacted with CDR2L alone, or both CDR2 and CDR2L [24]. In our cohort, the three samples that were positive for both CDR2 and CDR2L were late-stage serous ovarian cancer cases. In cases of myositis, patients that had the combination of TRIM21 and HARS were more likely to have cancer [25,26]. In our cohort we observed 3/64 HGSOC patients with a combination of HARS and TRIM21 positive values.
TRIM21 was identified as a novel biomarker for ovarian cancer, with the highest individual sensitivity of 33% for all HGSOC samples at 100% specificity compared to healthy controls in Validation I, and 36% sensitivity at 96% specificity in Validation II. TRIM21 has previously been reported as a biomarker for esophageal squamous cell carcinoma and basal-like breast cancer [27,28]. In a study of HGSOC serum samples that utilized high-density programmable protein microarrays containing 10,247 antigens, TRIM21 was identified as one of the top 39 candidate tumor antigens, which passed three rounds of serum screening in independent sample sets [4]. In the present study, the combination of TRIM21 with TP53, NY-ESO-1, and PAX8 provided a sensitivity of 67% in Validation I sample set and 50% sensitivity in the Validation II sample set. Somatic mutations in TP53 are found in in 96% of HGSOC cases [29]; autoantibodies to TP53 in HGSOC can be detected against the wild type protein as a polyclonal response [30]. NY-ESO-1 is an immunotherapy target for ovarian cancer with numerous trials evaluating vaccines targeting NY-ESO-1 as well as adoptive transfer of NY-ESO-1 specific T-cells [31]. PAX8 is expressed in the majority of HGSOCs [32]. The present study is the first to detect anti-PAX8 autoantibodies. The full-length PAX8 protein is 450 aa; the recombinant PAX8 protein used in this study consists of the amino acids 1–287, which are present on isoforms C-E.
In three recent studies within the population of women with an increased risk of ovarian cancer that evaluated CA125 using the ROCA algorithm at intervals of 3 or 4 months, an increase in detection sensitivity for early stage tumors was observed [3,7]. The goal of identifying autoantibody biomarkers is both to complement CA125, and to provide lead-time to CA125 detection. The serum set used in this study had limited samples with data for CA125 values. Autoantibodies to TP53 have been shown by Yang et al. to be elevated in pre-diagnostic patient samples up to 11 months before detection of CA125, and in samples taken 23 months before diagnosis for CA125-negative cases [33]. In our cohort anti-TP53 antibodies were present in 2/19 early stage samples. Additional markers such as TRIM21, HARS, NY-ESO-1, and PAX8, which detected in combination 9/19 early stage samples, may show improved lead-time, thus addressing the ultimate goal of a diagnosis at an earlier stage. Determining whether TRIM21, HARS, NY-ESO-1, and PAX8 autoantibodies are also detectable in addition to TP53 in pre-diagnostic sera will be a critical step in evaluating these biomarkers for clinical use. Early detection of HGSOC has potential to provide a mortality reduction [34].
5. Conclusions
A panel of autoantibody biomarkers can be useful in complementing current screening methods for the early detection of ovarian cancer in women with an increased genetic risk of ovarian cancer. The presence of paraneoplastic antibodies in HGSOC sera was evaluated. In two independent sample sets, Validation I with n = 164 and Validation II with n = 150 samples, autoantibodies to TRIM21 were detected in HGSOC sera with the highest frequency relative to other antigens. In Validations I and II, autoantibodies to TRIM21 were detected at 33% and 36% sensitivity in all HGSOC cases with 100% and 96% specificity compared to healthy controls. Autoantibodies against the HARS antigen were more frequently detected in early stage sera than in late stage sera. TRIM21 and PAX8 autoantibodies enhance the sensitivity of previously established NY-ESO-1 and TP53 autoantibodies. Of the antigens evaluated, the combination of TRIM21, PAX8, NY-ESO-1, and TP53 provided a sensitivity of 67% with 94% specificity in the Validation I sample set, and 46% sensitivity with 98% specificity in the Validation II cohort.
Supplementary Material
Acknowledgments
This project was supported by The Barbara and Fred Erb Chair in Cancer Genetics. Laura Hurley was supported by the Ruth L. Kirschstein National Research Service Award T32-CA009531, the DeRoy Testamentary Foundation Predoctoral Fellowship in Cancer Research, and Wayne State University Rumble Graduate Research Assistantship. We would like to acknowledge Alyssa Moskala for her assistance with preparation of figures. A special thanks to the patients and healthy volunteers who donated serum and tissue for the study. This work could not be done without their willingness to participate in research.
Footnotes
Supplementary data
The supplementary files are available to download from http://dx.doi.org/10.3233/CBM-190988.
References
- [1].Prat J and FIGO Committee on Gynecologic Oncology, FIGO’s staging classification for cancer of the ovary, fallopian tube, and peritoneum: abridged republication, J Gynecol Oncol 26 (2015), 87–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Matz M, Coleman MP, Carreira H, Salmeron D, Chirlaque MD, Allemani C and Concord Working Group, Worldwide comparison of ovarian cancer survival: Histological group and stage at diagnosis (CONCORD-2), Gynecol Oncol 144 (2017), 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Skates SJ, Greene MH, Buys SS, Mai PL, Brown P, Piedmonte M, Rodriguez G, Schorge JO, Sherman M, Daly MB, Rutherford T, Brewster WR, O’Malley DM, Partridge E, Boggess J, Drescher CW, Isaacs C, Berchuck A, Domchek S, Davidson SA, Edwards R, Elg SA, Wake-ley K, Phillips KA, Armstrong D, Horowitz I, Fabian CJ, Walker J, Sluss PM, Welch W, Minasian L, Horick NK, Kasten CH, Nayfield S, Alberts D, Finkelstein DM and Lu KH, Early detection of ovarian cancer using the risk of ovarian cancer algorithm with frequent CA125 testing in women at increased familial risk – combined results from two screening trials, Clin Cancer Res 23 (2017), 3628–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Katchman BA, Chowell D, Wallstrom G, Vitonis AF, LaBaer J, Cramer DW and Anderson KS, Autoantibody biomarkers for the detection of serous ovarian cancer, Gynecol Oncol 146 (2017), 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fortner RT, Damms-Machado A and Kaaks R, Systematic review: Tumor-associated antigen autoantibodies and ovarian cancer early detection, Gynecol Oncol 147 (2017), 465–480. [DOI] [PubMed] [Google Scholar]
- [6].Henderson JT, Webber EM and Sawaya GF, Screening for ovarian cancer: Updated evidence report and systematic review for the US preventive services task force, JAMA 319 (2018), 595–606. [DOI] [PubMed] [Google Scholar]
- [7].Rosenthal AN, Fraser LSM, Philpott S, Manchanda R, Burnell M, Badman P, Hadwin R, Rizzuto I, Benjamin E, Singh N, Evans DG, Eccles DM, Ryan A, Liston R, Dawnay A, Ford J, Gunu R, Mackay J, Skates SJ, Menon U, Jacobs IJ and United Kingdom Familial Ovarian Cancer Screening Study collaborators, Evidence of stage shift in women diagnosed with ovarian cancer during phase II of the united kingdom familial ovarian cancer screening study, J Clin Oncol 35 (2017), 1411–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cunningham JM, Cicek MS, Larson NB, Davila J, Wang C, Larson MC, Song H, Dicks EM, Harrington P, Wick M, Winterhoff BJ, Hamidi H, Konecny GE, Chien J, Bibikova M, Fan JB, Kalli KR, Lindor NM, Fridley BL, Pharoah PP and Goode EL, Clinical characteristics of ovarian cancer classified by BRCA1, BRCA2, and RAD51C status, Sci Rep 4 (2014), 4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Graus F and Dalmau J, Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors, Nat Rev Clin Oncol 16 (2019), 535–548. [DOI] [PubMed] [Google Scholar]
- [10].Chatterjee M, Hurley LC and Tainsky MA, Paraneoplastic antigens as biomarkers for early diagnosis of ovarian cancer, Gynecol Oncol Rep 21 (2017), 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kazarian M and Laird-Offringa IA, Small-cell lung cancer-associated autoantibodies: potential applications to cancer diagnosis, early detection, and therapy, Mol Cancer 10 (2011), 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sullivan FM, Farmer E, Mair FS, Treweek S, Kendrick D, Jackson C, Robertson C, Briggs A, McCowan C, Bedford L, Young B, Vedhara K, Gallant S, Littleford R, Robertson J, Sewell H, Dorward A, Sarvesvaran J and Schembri S, Detection in blood of autoantibodies to tumour antigens as a case-finding method in lung cancer using the EarlyCDT(R)-Lung Test (ECLS): Study protocol for a randomized controlled trial, BMC Cancer 17 (2017), 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].van Dooren SH, van Venrooij WJ and Pruijn GJ, Myositis-specific autoantibodies: detection and clinical associations, Auto Immun Highlights 2 (2011), 5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Krakenes T, Herdlevaer I, Raspotnig M, Haugen M, Schubert M and Vedeler CA, CDR2L is the major yo antibody target in paraneoplastic cerebellar degeneration, Ann Neurol 86 (2019), 316–321. [DOI] [PubMed] [Google Scholar]
- [15].Monstad SE, Storstein A, Dorum A, Knudsen A, Lon-ning PE, Salvesen HB, Aarseth JH and Vedeler CA, Yo antibodies in ovarian and breast cancer patients detected by a sensitive immunoprecipitation technique, Clin Exp Immunol 144 (2006), 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Henry KA, Zwieb C and Fried HM, Purification and biochemical characterization of the 19-kDa signal recognition particle RNA-binding protein expressed as a hexahistidinetagged polypeptide in Escherichia coli, Protein Expr Purif 9 (1997), 15–26. [DOI] [PubMed] [Google Scholar]
- [17].Ayed A, Mulder FA, Yi GS, Lu Y, Kay LE and Arrowsmith CH, Latent and active p53 are identical in conformation, Nat Struct Biol 8 (2001), 756–760. [DOI] [PubMed] [Google Scholar]
- [18].Chatterjee M, Mohapatra S, Ionan A, Bawa G, Ali-Fehmi R, Wang X, Nowak J, Ye B, Nahhas FA, Lu K, Witkin SS, Fishman D, Munkarah A, Morris R, Levin NK, Shirley NN, Tromp G, Abrams J, Draghici S and Tainsky MA, Diagnostic markers of ovarian cancer by high-throughput antigen cloning and detection on arrays, Cancer Res 66 (2006), 1181–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chatterjee M, Hurley LC, Levin NK, Stack M and Tainsky MA, Utility of paraneoplastic antigens as biomarkers for surveillance and prediction of recurrence in ovarian cancer, Cancer Biomark 20 (2017), 369–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chatterjee M, Dyson G, Levin NK, Shah JP, Morris R, Munkarah A and Tainsky MA, Tumor autoantibodies as biomarkers for predicting ovarian cancer recurrence, Cancer Biomark 11 (2012), 59–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang Y, Hong S, Mu J, Wang Y, Lea J, Kong B and Zheng W, Tubal origin of “Ovaria” low-grade serous carcinoma: A gene expression profile study, J Oncol 2019 (2019), 8659754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vang R, Shih Ie M and Kurman RJ, Ovarian low-grade and high-grade serous carcinoma: Pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems, Adv Anat Pathol 16 (2009), 267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Labrador-Horrillo M, Martinez MA, Selva-O’Callaghan A, Trallero-Araguas E, Grau-Junyent JM, Vilardell-Tarres M and Juarez C, Identification of a novel myositis-associated antibody directed against cortactin, Autoimmun Rev 13 (2014), 1008–1012. [DOI] [PubMed] [Google Scholar]
- [24].Eichler TW, Totland C, Haugen M, Qvale TH, Mazen-gia K, Storstein A, Haukanes BI and Vedeler CA, CDR2L antibodies: A new player in paraneoplastic cerebellar degeneration, PLoS One 8 (2013), e66002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Marie I, Hatron PY, Dominique S, Cherin P, Mouthon L, Menard JF, Levesque H and Jouen F, Short-term and long-term outcome of anti-Jo1-positive patients with anti-Ro52 antibody, Semin Arthritis Rheum 41 (2012), 890–899. [DOI] [PubMed] [Google Scholar]
- [26].Rutjes SA, Vree Egberts WT, Jongen P, Van Den Hoogen F, Pruijn GJ and Van Venrooij WJ, Anti-Ro52 antibodies frequently co-occur with anti-Jo-1 antibodies in sera from patients with idiopathic inflammatory myopathy, Clin Exp Immunol 109 (1997), 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kuboshima M, Shimada H, Liu TL, Nomura F, Takiguchi M, Hiwasa T and Ochiai T, Presence of serum tripartite motif-containing 21 antibodies in patients with esophageal squamous cell carcinoma, Cancer Sci 97 (2006), 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang J, Figueroa JD, Wallstrom G, Barker K, Park JG, Demirkan G, Lissowska J, Anderson KS, Qiu J and LaBaer J, Plasma autoantibodies associated with basal-like breast cancers, Cancer Epidemiol Biomarkers Prev 24 (2015), 1332–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cole AJ, Dwight T, Gill AJ, Dickson KA, Zhu Y, Clarkson A, Gard GB, Maidens J, Valmadre S, Clifton-Bligh R and Marsh DJ, Assessing mutant p53 in primary high-grade serous ovarian cancer using immunohistochemistry and massively parallel sequencing, Sci Rep 6 (2016), 26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Katchman BA, Barderas R, Alam R, Chowell D, Field MS, Esserman LJ, Wallstrom G, LaBaer J, Cramer DW, Hollingsworth MA and Anderson KS, Proteomic mapping of p53 immunogenicity in pancreatic, ovarian, and breast cancers, Proteomics Clin Appl 10 (2016), 720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Odunsi K, Immunotherapy in ovarian cancer, Ann Oncol 28 (2017), viii1–viii7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bowtell DD, Bohm S, Ahmed AA, Aspuria PJ, Bast RC Jr., Beral V, Berek JS, Birrer MJ, Blagden S, Bookman MA, Brenton JD, Chiappinelli KB, Martins FC, Coukos G, Drapkin R, Edmondson R, Fotopoulou C, Gabra H, Galon J, Gourley C, Heong V, Huntsman DG, Iwanicki M, Karlan BY, Kaye A, Lengyel E, Levine DA, Lu KH, McNeish IA, Menon U, Narod SA, Nelson BH, Nephew KP, Pharoah P, Powell DJ Jr., Ramos P, Romero IL, Scott CL, Sood AK, Stronach EA and Balk-will FR, Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer, Nat Rev Cancer 15 (2015), 668–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yang WL, Gentry-Maharaj A, Simmons A, Ryan A, Fourkala EO, Lu Z, Baggerly KA, Zhao Y, Lu KH, Bowtell D, Jacobs I, Skates SJ, He WW, Menon U, Bast RC Jr. and AOCS Study Group, Elevation of TP53 autoantibody before CA125 in preclinical invasive epithelial ovarian cancer, Clin Cancer Res 23 (2017), 5912–5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, Amso NN, Apostolidou S, Benjamin E, Cruickshank D, Crump DN, Davies SK, Dawnay A, Dobbs S, Fletcher G, Ford J, Godfrey K, Gunu R, Habib M, Hallett R, Herod J, Jenkins H, Karpinskyj C, Lee-son S, Lewis SJ, Liston WR, Lopes A, Mould T, Mur-doch J, Oram D, Rabideau DJ, Reynolds K, Scott I, Seif MW, Sharma A, Singh N, Taylor J, Warburton F, Wid-schwendter M, Williamson K, Woolas R, Fallowfield L, McGuire AJ, Campbell S, Parmar M and Skates SJ, Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial, Lancet 387 (2016), 945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zahr ZA and Baer AN, Malignancy in myositis, Curr Rheumatol Rep 13 (2011), 208–215. [DOI] [PubMed] [Google Scholar]
- [36].Allenbach Y, Keraen J, Bouvier AM, Jooste V, Champ-tiaux N, Hervier B, Schoindre Y, Rigolet A, Gilardin L, Musset L, Charuel JL, Boyer O, Jouen F, Drouot L, Martinet J, Stojkovic T, Eymard B, Laforet P, Behin A, Salort-Campana E, Fain O, Meyer A, Schleinitz N, Mariampillai K, Grados A and Benveniste O, High risk of cancer in autoimmune necrotizing myopathies: usefulness of myositis specific antibody, Brain 139 (2016), 2131–2135. [DOI] [PubMed] [Google Scholar]
- [37].Yoichiro Akiyama TN, Iwamoto M and Minota S, Clinical features of seven Japanese patients with anti-PL-12 antibody: Frequent positivity for anti-cyclic citrullinated peptide antibody, Jichi Medical University Journal 38 (2015), 41–45. [Google Scholar]
- [38].Masiak A, Kulczycka J, Czuszynska Z and Zdrojewski Z, Clinical characteristics of patients with anti-TIF1-gamma antibodies, Reumatologia 54 (2016), 14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tetsuka S, Tominaga K, Ohta E, Kuroiwa K, Sakashita E, Kasashima K, Hamamoto T, Namekawa M, Morita M, Natsui S, Morita T, Tanaka K, Takiyama Y, Nakano I and Endo H, Paraneoplastic cerebellar degeneration associated with an onconeural antibody against creatine kinase, brain-type, J Neurol Sci 335 (2013), 48–57. [DOI] [PubMed] [Google Scholar]
- [40].Pignolet BS, Gebauer CM and Liblau RS, Immunopathogenesis of paraneoplastic neurological syndromes associated with anti-Hu antibodies: A beneficial antitumor immune response going awry, Oncoimmunology 2 (2013), e27384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chauhan R, Handa R, Das TP and Pati U, Over-expression of TATA binding protein (TBP) and p53 and autoantibodies to these antigens are features of systemic sclerosis, systemic lupus erythematosus and overlap syndromes, Clin Exp Immunol 136 (2004), 574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Thomas R, Al-Khadairi G, Roelands J, Hendrickx W, Dermime S, Bedognetti D and Decock J, NY-ESO-1 based immunotherapy of cancer: Current perspectives, Front Immunol 9 (2018), 947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Xiang L and Kong B, PAX8 is a novel marker for differentiating between various types of tumor, particularly ovarian epithelial carcinomas, Oncol Lett 5 (2013), 735–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


