Abstract
Ludwig’s angina is a deep neck space infection defined as a rapidly progressive bilateral cellulitis of the submandibular space. In spite of being an uncommon entity in developed countries and the reduction of mortality and morbidity due to modern era of antibiotics, improved imaging and airway management, it is still an important and potentially life-threatening condition. The authors present 3 cases of Ludwig’s angina that occurred in a developed country, and that required admission in intensive care unit and extensive surgical and medical treatment.
Keywords: drugs: infectious diseases, adult intensive care
Background
Ludwig’s angina is a deep neck space infection defined as a rapidly progressive bilateral cellulitis of the submandibular space. It was first described in 1836 by von Ludwig, and is still diagnosed by the criteria provided by Grodinsky in 1939 as a cellulitis originating in the floor of the mouth, invading bilaterally more than one compartment of the submandibular space that produces a serosanguinous infiltrate but little or no pus; it involves connective tissue fascia and muscle but not glandular structures, and is able to spread by contiguity, not through the lymphatic system.1
This type of infection is relatively uncommon in the postantibiotic era but is still an important and potentially life-threatening condition, primarily due to elevation and posterior displacement of the tongue causing airway obstruction unless early recognised and adequately treated.2 It is more frequent in male sex and in the fifth decade of life.3 4 The aetiology of Ludwig’s angina is primarily odontogenic5 and diabetes mellitus has been recognised as the systemic disease most commonly associated with deep neck infection.6 Early imaging is important to determine the extension of tissue infection or necrosis or the presence of an abscess, which will help to decide the most appropriate approach to the patient.7 CT scan may demonstrate either a diffusely infiltrative process or a more discrete low-attenuation area, reflecting abscess.8
The management of Ludwig’s angina involves maintenance of a secure airway with surgical tracheostomy or endotracheal intubation, antibiotics and surgical drainage, if necessary.9 Early antibiotic treatment should be broad spectrum to cover oral cavity flora Gram-positive, Gram-negative and anaerobic organisms.7 Before the availability of antibiotics, oedema frequently led to upper airway obstruction, and a mortality rate of 50% was reported. As a result of current antibiotic therapies and surgical techniques, current mortality estimates are in the range of 8%.10 Potentially fatal complications can occur and include descending necrotising mediastinitis and sepsis.11 Mortality associated to descending necrotising mediastinitis can reach 40%.12 Diagnostic delay and late or inappropriate drainage of the infected compartments are judged to be the main causes of death.13
Case presentation
Case 1
A 77-year-old male patient with a medical history of arterial hypertension, presented in the emergency department due to a neck and face swelling with a 3-day evolution. The physical examination showed an 8 cm soft tumefaction of the left anterior cervical triangle, with associated cervical and facial oedema. The vital signs showed low blood pressure (75/40 mm Hg), low saturation (85%) on room air and auricular temperature of 38.3°C. Relevant laboratory data included: leucocytosis (26.2×10⁹/L), elevated C reactive protein (CRP) (27.8 mg/dL) and acute kidney injury (blood urea nitrogen (BUN) of 56 mg/dL and creatinine of 1.55 mg/dL). CT scan was performed and revealed multiple liquid collections in the left masseter (with extension to the left hemiface), submentonian region and adjacent to the left jugular vein (with extension to the retropharyngeal space) compatible with Ludwig’s angina.
Case 2
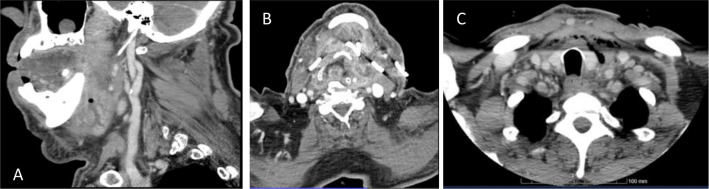
A 74-year-old male patient was admitted to the emergency department with a neck swelling and dysphagia with a 2-day evolution. He had multiple comorbidities, including arterial hypertension, type 2 diabetes mellitus, obesity, mild mitral regurgitation with right atrial dilatation and pulmonary hypertension and permanent atrial fibrillation. Physical examination showed a large bilateral mandibular tumefaction, limited mouth opening, macroglossia, floor of the mouth swelling and tooth decay in several teeth. He was haemodynamically stable with a body temperature of 38.7°C. Blood analysis showed CRP of 33.5 mg/dL, leucocytosis (20.4×10⁹/L) and acute kidney injury (BUN 37 mg/dL, creatinine 1.45 mg/dL). A CT scan of the neck showed cervical and submandibular soft-tissue and left parapharyngeal space oedema (figure 1A). It also showed an abscess adjacent to the internal side of the mandibula. These findings were compatible with Ludwig’s angina secondary to an odontogenic infection.
Figure 1.
(A) Sagittal CT image of patient of case 2 at emergency room admission showing submandibular soft-tissue and an abscess adjacent to the internal side of the mandibula. (B) Axial CT image of patient of case 1 after surgical drainage with bilateral tube drains in place. (C) Postoperative axial CT image of patient of case three showing the collection extending inferiorly to anterior mediastinal.
Case 3
A 61-year-old man was transferred from a district hospital with left hemiface swelling with cervical extension, with 3 days of evolution and progressive worsening. His medical history included a surgical exclusion of a left hypogastric aneurysm with stent placement and an uncontrolled arterial hypertension. The patient was haemodynamically stable and apyretic. His physical examination revealed an abscess on his left hemiface with submandibular and cervical bilateral extension; he also had a third quadrant molar with apparent crown fracture. Relevant laboratory data included: CRP 47.3 mg/dL, without leucocytosis and acute kidney injury (BUN of 28 mg/dL; creatinine of 1.85 mg/dL). A CT scan revealed a massive haematoma of the left malar region with probable dental origin.
Treatment
Case 1
Taken in account the respiratory distress and the inability to perform an orotracheal intubation due to a near complete obstruction of the airway, a tracheostomy was performed in the emergency room. The procedure was complicated by a false passage that led to extensive subcutaneous emphysema, bilateral pneumothorax and pneumomediastinum. The abscess was surgically drained using bilateral cervical approach and bilateral tube drains were left in place (figure 1B). Simple exodontia of teeth 37, bilateral chest drain placement and surgical revision of the tracheostomy were also performed. The patient was admitted in the intensive care unit (ICU) in septic shock and kept on vasopressor support and invasive mechanical ventilation. He was treated with empiric amoxicillin/clavulanate, linezolide and metronidazole for 15 days. After isolation of Pseudomonas aeruginosa on bronchial aspirate culture, antibiotic treatment was adjusted to ceftazidime and metronidazole with subsequent de-escalation of ceftazidime to ciprofloxacin according to the bacteria antibiotic sensibility test (table 1). The patient needed vasopressor support until the sixth day, was kept on mechanic ventilation for 28 days and was discharged to the maxillofacial surgery ward on the 31st day after admission in the ICU.
Table 1.
Antibiotic susceptibility testing of bacterial isolates from patients of cases 1, 2 and 3, using European Committee on Antimicrobial Susceptibility Testing clinical breakpoints
| Antibiotic | Case 1 | Case 2 | Case 3* |
| Pseudomonas aeruginosa | Streptococcus constellatus | Escherichia coli | |
| Ampicillin | R | ||
| Amoxicillin/clavulanic acid | R | ||
| Penicillin | S | ||
| Cefuroxime | R | ||
| Cefotaxime | R | ||
| Ceftazidime | I | ||
| Cefepime | R | ||
| Erythromycin | S | ||
| Levofloxacin | S | ||
| Ciprofloxacin | R | ||
| Clindamycin | S | ||
| Vancomycin | S | ||
| Ciprofloxacin | S | ||
| Gentamicin | S | S | |
| Meropenem | I | S | |
| Ceftazidime | S | ||
| Piperacillin/tazobactam | R | R | |
| Trimetropim/sulfamethoxazole | R | ||
| Amikacin | R | ||
| ESBL producing bacteria | Positive |
*In case 3, Streptococcus anginosus, Parvimonas micra, Prevotella melaninogenica and Actinomyces odontolyticus were not tested for antibiotic susceptibility.
ESBL, extended-spectrum beta-lactamase; I, intermediate; R, resistant; S, susceptible.
Case 2
The abscess was surgically drained using a submentonian approach and four tube drains were left in place and dental extraction was performed. The patient started therapy with dexamethasone and empirical antibiotic therapy with intravenous amoxicillin/clavulanate and metronidazole, which was escalated to meropenem and metronidazole 1 day after due to clinical deterioration. In the next day, patient’s condition worsens and due to the development of septic shock he was admitted in the ICU. Nasotracheal intubation was performed with fiberoptic bronchoscopy to initiate invasive mechanical ventilation. Sedation and analgesia were performed with propofol and fentanyl in perfusion, respectively. Vancomycin was added to the previously prescribed antibiotics and dexamethasone was stopped. There was only one positive culture of the abscess pus with isolation of Streptococcus constellatus (table 1). At day 8, the patient was submitted to a surgical tracheostomy and wound cleaning. At day 19, he developed a hypovolaemic shock due to oropharyngeal haemorrhage, demanding blood transfusion, which resolved with packing during 7 days. Norepinephrine perfusion was maintained for 28 days. The patient was discharged from ICU after 33 days to the oral and maxillofacial surgery ward.
Case 3
The patient was submitted to a submandibular and intraoral percutaneous drainage and subsequently tracheostomised. Postoperative CT scan showed a left malar abscess and tooth decay of the 28th tooth, confirming the odontogenic origin. This collection extended inferiorly to oropharynx and hypopharynx mucosal space, parapharyngeal bilaterally and anterior mediastinal space (figure 1C). The patient was diagnosed with descending necrotising mediastinitis due to odontogenic infection. A further surgical intervention was postponed as the superior mediastinal involvement was residual. He was first admitted at maxillofacial surgery ward and began empiric piperacillin/tazobactam plus metronidazole, switched to meropenem plus vancomycin and metronidazole due to persistent elevated inflammatory biomarkers. As he maintained an abundant upper airway purulent and bloody discharge, he was finally taken to surgical drainage of superior mediastinal collection, through an anterolateral thoracotomy and admitted to ICU postoperatively. Considering the septic shock associated with mediastinitis, he was kept on vasopressor support while maintained on invasive ventilatory support. In view of the isolation of Streptococcus anginosus, Parvimonas micra, Prevotella melaninogenica and Actinomyces odontolyticus, from the abscess content and Escherichia coli from wound exudate culture (table 1), antibiotic was changed to meropenem plus clindamycin and linezolide. During ICU stay, simple exodontia of teeth 28, 37 and 38 was performed with local anaesthesia. The submentonian, thoracic drains and ventilatory support were removed on 12th day. Although he began spontaneous ventilation, with minor supplementary oxygen needs, due to food leakage through traqueostomy ostium, patient was put on a percutaneous endoscopic gastrostomy, to assure adequate nutrition and hydration.
Outcome and follow-up
Case 1
In the ward, the cervical tumefaction resolved with satisfactory surgical wound healing by second intention. He was discharged to another hospital after 52 days of admission and died 18 days later from nosocomial respiratory infection.
Case 2
The tracheostomy cannula was removed, and physical rehabilitation was conducted in the ward. The patient was discharged home 44 days after hospital admission. At 1-month and 4-month follow-up visit, the patient had no complaints and no signs of recurrence.
Case 3
The patient was transferred to a surgical-intermediate care unit after 14 days at ICU. There he continued his recovery process without significant intercurrences. Thirty-one days after hospital admission, he was discharged to his local hospital, without airway compromise and tolerating per os feeding. He had follow-up visits 1 month and 3 months after discharge without relevant complications.
Discussion
Airway management is the first priority for a patient presenting with Ludwig’s angina. Conservative (close airway observation) or intervention (tracheostomy/cricothyroidotomy or endotracheal intubation) treatment may be chosen depending on the risk of obstruction. In the past, aggressive airway management by securing the airway with early emergency endotracheal intubation or surgically with a tracheostomy was the standard of care, but recent reports are more prone to a conservative management.7
When intervention is considered needed, surgical tracheostomy may be the first option. Routine orotracheal intubation may be complicated by trismus, distorted anatomy, immobility of the soft tissues, tissue friability and complete airway obstruction with anaesthetic induction.14 Airway compromise may be safely managed with advanced airway control techniques, such as fiberoptic intubation,15 but this requires higher level of expertise and available equipment. Although, tracheostomy is considered a safe route for airway protection it may also be associated with complications as described in case 1. In case of impeding loss of airway an emergence cricothyroidotomy may be the first option if complete obstruction is imminent.
When the airway is secured the next step is to treat the infection, using antibiotics alone or combined with appropriate source control, whether by surgical decompression or drainage, if necessary, and haemodynamic stabilisation. Failure in early and adequate infection control can lead to life-threatening complications such as a contiguous spread of the infection resulting in descending necrotising mediastinitis (case 3) or a systemic manifestation leading to septic shock (cases 1, 2 and 3), and need for admission in the ICU. The most common organisms isolated from head and neck abscesses are streptococci, Staphylococcus aureus, Klebsiella pneumonia and mixed anaerobes and often the aetiology is polymicrobial.16 Initial antibiotics should cover anaerobes, Gram-positive and negative bacteria and local bacterial resistance patterns should be considered. According to the 2018 Annual report on Surveillance of antimicrobial resistance in Europe,17 in Portugal, the incidence of methicillin-resistant S. aureus was 38.1%, K. pneumoniae resistant to carbapenems 11.7%, Enterococcus faecium resistant to vancomycin 4.4%, P. aeruginosa with combined resistance 15.3% and E. coli resistant to fluoroquinolones, third-generation cephalosporins and aminoglycosides 6.2%. In all the three cases described, initial coverage of different potential causative agents was assured. In cases 2 and 3, initial empiric antibiotics were appropriate for isolated microorganisms. In case 1, there were no microbiological isolates from wound exudate, but since there was clinical and analytical improvement appropriate antibiotic coverage combined with adequate source control can be assumed.
Adjunctive therapies such as steroids and nebulised epinephrine have also been described for initial management of Ludwig’s angina,18 although there is scarce information on the literature. A recent review of the literature on steroids use in the management of Ludwig’s angina found a limited number of cases with a total of only 31 patients, and concluded that its role remains uncertain.19
Learning points.
Ludwig’s angina can rapidly develop into a life-threatening complication and should not be undervalued.
Life-threatening complications may require prolonged intensive care unit and hospital stays.
Adequate source control and appropriate antibiotics are fundamental for successful treatment.
Nowadays Ludwig’s angina is still a potentially fatal entity despite differentiated care is provided.
Footnotes
Contributors: CMS, JP and PNT contributed to conceptualisation, analysis, interpretation of patient data and writing the original draft. JPB contributed to conceptualisation, methodology and article review. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Costain N, Marrie TJ. Ludwig’s Angina. Am J Med 2011;124:115–7. 10.1016/j.amjmed.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 2.Rowe DP, Ollapallil J. Does surgical decompression in Ludwig's angina decrease Hospital length of stay? ANZ J Surg 2011;81:168–71. 10.1111/j.1445-2197.2010.05496.x [DOI] [PubMed] [Google Scholar]
- 3.Allareddy V, Rampa S, Nalliah RP, et al. Longitudinal discharge trends and outcomes after hospitalization for mouth cellulitis and Ludwig angina. Oral Surg Oral Med Oral Pathol Oral Radiol 2014;118:524–31. 10.1016/j.oooo.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 4.McDonnough JA, Ladzekpo DA, Yi I, et al. Epidemiology and resource utilization of Ludwig's angina ED visits in the United States 2006-2014. Laryngoscope 2019;129:2041–4. 10.1002/lary.27734 [DOI] [PubMed] [Google Scholar]
- 5.Derber CJ, Troy SB. Head and neck emergencies: bacterial meningitis, encephalitis, brain abscess, upper airway obstruction, and jugular septic thrombophlebitis. Med Clin North Am 2012;96:1107–26. 10.1016/j.mcna.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 6.Hidaka H, Yamaguchi T, Hasegawa J, et al. Clinical and bacteriological influence of diabetes mellitus on deep neck infection: systematic review and meta-analysis. Head Neck 2015;37:1536–46. 10.1002/hed.23776 [DOI] [PubMed] [Google Scholar]
- 7.Hasan W, Leonard D, Russell J. Ludwig's Angina-A controversial surgical emergency: how we do it. Int J Otolaryngol 2011;2011:1–4. 10.1155/2011/231816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rana RS, Moonis G. Head and neck infection and inflammation. Radiol Clin North Am 2011;49:165–82. 10.1016/j.rcl.2010.07.013 [DOI] [PubMed] [Google Scholar]
- 9.Chueng K, Clinkard DJ, Enepekides D, et al. An unusual presentation of Ludwig's angina complicated by cervical necrotizing fasciitis: a case report and review of the literature. Case Rep Otolaryngol 2012;2012:931350. 10.1155/2012/931350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bansal A, Miskoff J, Lis RJ. Otolaryngologic critical care. Crit Care Clin 2003;19:55–72. 10.1016/S0749-0704(02)00062-3 [DOI] [PubMed] [Google Scholar]
- 11.Bross-Soriano D, Arrieta-Gómez JR, Prado-Calleros H, et al. Management of Ludwig's angina with small neck incisions: 18 years experience. Otolaryngol Head Neck Surg 2004;130:712–7. 10.1016/j.otohns.2003.09.036 [DOI] [PubMed] [Google Scholar]
- 12.Huang T-T, Liu T-C, Chen P-R, et al. Deep neck infection: analysis of 185 cases. Head Neck 2004;26:854–60. 10.1002/hed.20014 [DOI] [PubMed] [Google Scholar]
- 13.Kinzer S, Pfeiffer J, Becker S, et al. Severe deep neck space infections and mediastinitis of odontogenic origin: clinical relevance and implications for diagnosis and treatment. Acta Otolaryngol 2009;129:62–70. 10.1080/00016480802008181 [DOI] [PubMed] [Google Scholar]
- 14.Reynolds SC, Chow AW. Severe soft tissue infections of the head and neck: a primer for critical care physicians. Lung 2009;187:271–9. 10.1007/s00408-009-9153-7 [DOI] [PubMed] [Google Scholar]
- 15.Wolfe MM, Davis JW, Parks SN. Is surgical airway necessary for airway management in deep neck infections and Ludwig angina? J Crit Care 2011;26:11–14. 10.1016/j.jcrc.2010.02.016 [DOI] [PubMed] [Google Scholar]
- 16.Varghese L, Mathews SS, Antony Jude Prakash J, et al. Deep head and neck infections: outcome following empirical therapy with early generation antibiotics. Trop Doct 2018;48:179–82. 10.1177/0049475518774472 [DOI] [PubMed] [Google Scholar]
- 17.European Centre for Disease Prevention and Control . Surveillance of antimicrobial resistance in Europe 2018. Stockholm: ECDC, 2019. [Google Scholar]
- 18.Saifeldeen K, Evans R. Ludwig’s angina. Emerg Med J 2004;21:242–3. 10.1136/emj.2003.012336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tami A, Othman S, Sudhakar A, et al. Ludwig's angina and steroid use: a narrative review. Am J Otolaryngol 2020;41:102411. 10.1016/j.amjoto.2020.102411 [DOI] [PubMed] [Google Scholar]