Abstract
Cardiac resynchronisation therapy is an important intervention to reduce mortality and morbidity, but even in carefully selected patients approximately 30% fail to improve. This has led to alternative pacing approaches to improve patient outcomes. Left ventricular (LV) endocardial pacing allows pacing at site-specific locations that enable the operator to avoid myocardial scar and target areas of latest activation. Left bundle branch area pacing (LBBAP) provides a more physiological activation pattern and may allow effective cardiac resynchronisation. This article discusses LV endocardial pacing in detail, including the indications, techniques and outcomes. It discusses LBBAP, its potential benefits over His bundle pacing and procedural outcomes. Finally, it concludes with the future role of endocardial pacing and LBBAP in heart failure patients.
Keywords: Cardiac resynchronisation therapy, endocardial pacing, left bundle branch area pacing, WiSE-CRT
Cardiac resynchronisation therapy (CRT) is an established treatment for patients with symptomatic heart failure, severe left ventricular (LV) dysfunction and electrical dyssynchrony.[1,2] However, even in carefully selected patients, approximately 30% fail to respond, and this has led to the development of alternative pacing strategies to improve patient outcomes.[3,4] Conduction system pacing with His bundle pacing (HBP) or left bundle branch area pacing (LBBAP) provides physiological activation using the native conduction system.[5] LV endocardial pacing enables access to faster endocardial conduction and site-specific pacing, unlike conventional CRT.[6–9] This article will discuss LV endocardial pacing and LBBAP in detail, including the potential benefits and risks of each intervention and future directions.
Transvenous Epicardial CRT
Pacing within areas of myocardial scar is associated with poorer outcomes, whereas targeting areas of latest electrical and mechanical activation leads to improved patient outcomes.[10–15] Patient-specific pacing that avoids myocardial scar while targeting areas of latest activation is difficult with epicardial CRT because the pacing location is dependent on the coronary sinus anatomy, and the optimal pacing segment may not be subtended by a coronary vein or may result in phrenic nerve stimulation. Pacing in unfavourable locations will result in inadequate resynchronisation and a suboptimal response.[3,16] Furthermore, it is estimated that 8–10% of CRT procedures are unsuccessful due to anatomical constraints, such as failure to cannulate the coronary sinus.[17] Therefore, given the limitations of epicardial pacing and the need to improve response rates, the role of alternative pacing strategies has become increasingly important.
Endocardial Pacing
Endocardial pacing offers many advantages over epicardial pacing. It enables access to fast endocardial conduction, shorter path length for impulse conduction, a more physiological activation pattern by spreading from the endocardium to the epicardium, a lower pacing capture threshold and a lower risk of phrenic nerve stimulation.[6–9] Endocardial pacing is less arrhythmogenic than epicardial pacing and is less affected by myocardial scar location.[18] It is also less likely to result in phrenic nerve stimulation such as in epicardial pacing. The greatest potential benefit of endocardial pacing is the ability to pace anywhere inside the left ventricle, enabling the operator to select the optimal pacing site unrestricted by the coronary sinus anatomy. This is particularly attractive in patients with unfavourable characteristics, such as ischemic cardiomyopathy and transmural myocardial scar.
The haemodynamic changes with endocardial and epicardial pacing have been previously studied. In a study of eight anaesthetised dogs with experimental left bundle branch block (LBBB), endocardial pacing was associated with greater electrical resynchronisation, and increase in LV dP/dtmax and stroke work, compared with epicardial pacing.[19] Similarly in a study of 22 dogs, endocardial pacing resulted in better electrical resynchronisation and haemodynamic changes than epicardial pacing.[20] Large human studies comparing epicardial and endocardial pacing are lacking but smaller studies demonstrate the predominant benefit of endocardial pacing, which is its ability to access the optimal pacing site.[21–24]
Delivering Left Ventricular Endocardial Pacing
LV endocardial pacing was initially delivered using leads via an atrial transseptal, transventricular septal or transventricular apical approach. Several case series report their experience with lead-based endocardial pacing but are limited by the study design and a small patient cohort.[25] The ALSYNC study was the largest prospectively collected, multicentre registry investigating the feasibility and safety of LV endocardial pacing, enrolling 138 patients with a failed conventional LV lead, suboptimal coronary sinus anatomy or CRT non-response.[26] Patients were predominantly men, with non-ischaemic cardiomyopathy, LBBB, broad QRS duration and severely impaired LV systolic function. Successful procedures were achieved in 89% of patients, 82% of patients had freedom from complications related to the lead delivery system, implant procedure or lead, and 3.8% of patients had non-disabling strokes. At 6 months, the New York Heart Association (NYHA) functional class improved in 59% of patients, and 55% had a reduction in LV end-systolic volume (LVESV) ≥15%.[26] Although the response rate in this difficult patient group was promising, the main limitations were the significant rate of cerebrovascular accidents, the need for lifelong anticoagulation, and the low rate of optimal lead placement (leads could be placed in the desired location in only 81% of implants).
Leadless LV pacing offers many advantages over lead-based pacing, including a reduced risk of lead-related issues (including infection), no requirement for lifelong anticoagulation, and potentially a greater selection of pacing sites. Leadless LV pacemakers need to be compact to ensure that they do not interfere with anatomical structures within the left ventricle, the endocardial wall, or outflow tract. Longer devices with broad batteries are more likely to collide with intracardiac structures, and therefore, to reduce this risk of collision while maintaining the volume for the battery, devices must be shorter and thicker.[27] The current generation of leadless pacemakers used in the right ventricle are predicted to be able to be placed in only a limited number of LV endocardial sites due to their dimensions,[27] highlighting the importance of optimal length/device width ratio. Currently, the WiSE-CRT system (EBR Systems), in which the power is supplied from a remote battery, is the only commercially available leadless LV endocardial pacing system, and will be discussed further.
WiSE-CRT System
The WiSE-CRT system provides leadless LV endocardial pacing to achieve near simultaneous ventricular activation and resynchronisation. The system consists of three components: a submuscular transmitter, connected to a subcutaneous battery, and an endocardial receiver electrode (Figure 1). The system requires the patient to have a co-implant in situ that is capable of producing continuous right ventricular (RV) pacing. The transmitter and battery detect an RV pacing pulse emitted by the co-implant, and the transmitter emits a number of short ultrasound pulses to locate the electrode. Each pulse is converted into electrical energy to identify the electrode location but is of insufficient magnitude to pace the left ventricle. Once identified, the transmitter sends a focused beam of ultrasound energy to the electrode location and this is converted into electrical energy, causing LV capture and simultaneous biventricular pacing in 2–5 ms. The endocardial electrode can be placed anywhere inside the left ventricle but the energy reaching the electrode reduces with an increased angle and distance between the transmitter and electrode. Patients who have an obtuse angle between devices or increased distance, will have insufficient electrode capture, battery depletion and failure of biventricular pacing. The WiSE-CRT system is indicated in patients suitable for CRT.
Figure 1: Components of the WiSE-CRT System.
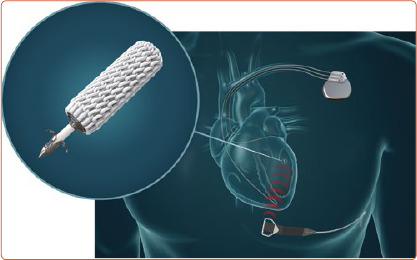
The WiSE-CRT system consists of three components: sub-muscular Transmitter connected to a subcutaneous Battery and an endocardial left ventricular Receiver Electrode. The system requires a co-implant in situ capable of right ventricular pacing.
Transmitter and Battery Implantation
Patients must undergo acoustic window screening to be eligible for the device. This involves placing an ultrasound probe in different intercostal spaces to determine if there is an adequate window. Acceptable windows have no lung encroachment during maximal inspiration (Figure 2) and an angle between the probe and basal posterolateral wall <45°, distance <12 cm and LV wall thickness ≥5 mm. These measurements are repeated with the patient lying supine, on their right side, and while sitting upright. Patients often have more than one intercostal space available for transmitter implantation, enabling the operator to select the optimal site.[28]
Figure 2: Acoustic Window Screening and WiSE-CRT Implantation.
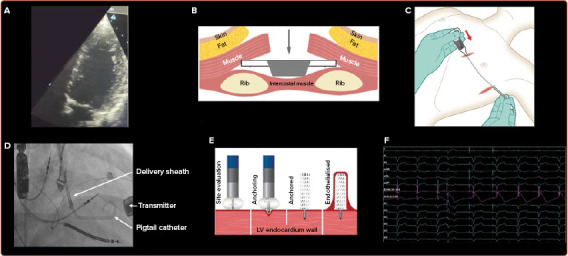
A: Evaluation of the intercostal spaces using an ultrasound probe during acoustic window screening. In this example the whole left ventricle can be viewed during maximal inspiration without any lung encroachment, demonstrating that this space is viable. B–E: WiSE-CRT implantation. The transmitter and battery are implanted first (B,C). B: The transmitter is implanted in the intercostal space identified pre-procedurally. It is placed on the internal intercostal muscle and the wings are sutured onto the costal cartilage of the ribs. C: The transmitter cable is tunnelled to the battery, which is implanted in the adjacent mid-axillary line. D: The electrode is implanted using an electrode delivery system, and the delivery sheath is positioned within the left ventricle using a pigtail catheter. E: Different endocardial sites are evaluated, and the electrode is implanted in three defined stages: anchoring, electrode detachment and electrode release. F: Change in left ventricular (LV) electrogram (EGM) and current of injury (COI), highlighted in pink, during anchoring. ST elevation indicates that the electrode is being anchored into viable myocardial tissue, and fluoroscopy with contrast is required to confirm that it is fully anchored.
Procedures are predominantly performed under general anaesthesia and can be undertaken in a single-stage or dual-stage procedure, with the latter involving implantation of the battery and transmitter, and the electrode on two separate occasions. The transmitter is always implanted first, is placed on the intercostal muscle and is secured to the costal cartilage, with the battery placed in the adjacent mid-axillary line (Figure 2). Intra-procedural confirmation of an adequate window to the left ventricle using echocardiography is advised to ensure there is no lung encroachment.
Electrode Implantation
The electrode can be implanted via a retrograde aortic approach using arterial access or a transseptal approach using venous access.[29] The electrode delivery system is a catheter-based system used for implanting the electrode, consisting of the electrode and delivery catheter (8 Fr) and a steerable delivery sheath (12 Fr). The delivery sheath has a diameter of 4 mm, therefore confirmation of adequate arterial access is recommended prior to the procedure, and this is possible with CT or ultrasound. Dual femoral arterial access can be used with the aid of an aortogram to ensure that the puncture site for the electrode delivery sheath is correctly sited, to reduce the risk of vascular complications. A trans-oesophageal or intracardiac echocardiogram is performed during electrode implantation to ensure that any complications are identified in a timely manner and to facilitate the implantation of the electrode.
The delivery sheath has a balloon at its distal tip, and once access has been achieved the balloon is inflated. The delivery sheath is positioned within the left ventricle and the electrode catheter is inserted. The delivery sheath is slowly advanced to the desired endocardial location. A tight seal between the balloon and the endocardium is confirmed by a flush of contrast, which should be seen coming around the sides of the balloon rather than forwards (Figure 3). The electrode is implanted in a number of defined stages, as follows:
Figure 3: Anchoring of the Endocardial Electrode.
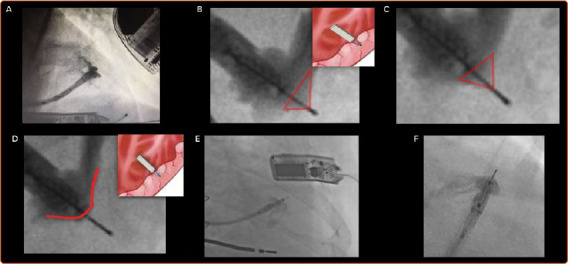
A: Good contact between the delivery sheath and endocardium, as demonstrated by the flow of contrast. B: The presence of contrast up to the tip of the electrode indicates inadequate anchoring. C: Partial tenting with contrast around the needle body caused by the five tines, which cannot be seen on fluoroscopy, and further advancement is required. D: No tenting, as indicated by the absence of contrast around the needle. This indicates that the electrode is now fully anchored. E,F: Troubleshooting during implantation of the electrode. E: The electrode has been advanced too far outside the delivery sheath and there is inadequate fluoroscopic magnification, therefore assessment of tenting cannot be reliably made. F: There is partial tenting present and the electrode is not fully anchored.
Anchoring: a tight seal is maintained between the balloon and the endocardium while the electrode catheter is advanced 1 mm at a time (Figure 3). Simultaneous live fluoroscopy and contrast flushes are used to look for LV tenting. Tenting demonstrates that the electrode tines are still within the cavity of the left ventricle. The electrode is then slowly advanced until there is no tenting, demonstrating that the tines are within the endocardium. The absence of tenting should be confirmed on two orthogonal views, with no contrast beyond the electrode body (Figure 3).
Electrode detachment: both the delivery sheath and electrode catheter are kept stable and the electrode is detached, resulting in an indicator change on the catheter and a disturbance on the intracardiac electrogram.
Electrode release: under continuous fluoroscopy, the delivery sheath is slowly retracted until it is aligned with the tip of the catheter; they are then withdrawn together. Satisfactory placement of the electrode can be seen on fluoroscopy, and pacing checks are undertaken to ensure that there is appropriate RV tracking and biventricular pacing.
Outcomes of the WiSE-CRT System
Experience and patient outcomes have been reported in three prospective multicentre trials: the WiSE-CRT study, the SELECT-LV study and the WiCS-LV Post Market Surveillance Registry.[30–32] These studies included patients who had a failed LV lead, were considered high-risk for a CRT upgrade or were non-responders to conventional CRT. The WiSE-CRT study was a first-in-man trial, published in 2014, which assessed the feasibility, safety and short-term outcomes of the system in 17 patients.[30] That study was stopped early due to a high incidence of pericardial tamponade, occurring in three patients (17.6%). Consequently, the delivery sheath was redesigned to incorporate a balloon at the distal tip to reduce traumatic engagement with the LV endocardium. The feasibility of the WiSE-CRT system using the re-designed delivery sheath was investigated in the SELECT-LV study, involving 35 patients across six centres and was published in 2017.[31] The recent publication of the WICS-LV Post Market Surveillance Registry in 2020 determined the safety and efficacy of the WiSE-CRT system in a real-world setting involving 90 patients from 14 European centres.[32] The outcomes of the WiSE-CRT system will be discussed further in the following sections using the latter two studies, which have utilised the latest iteration of the redesigned delivery sheath.
Procedural Success
Procedural success was reported in 34 of 35 patients (97.1%) in the SELECT-LV study, given that one patient had a ventricular arrhythmia.[31] Successful procedures occurred in 85 of 90 patients (94.4%) in the WICS-LV Post Market Surveillance Registry, with biventricular pacing confirmation after implantation.[32] Failure to achieve procedural success was due to failing to exclude unsuitable intercostal spaces during acoustic window screening, pericardial tamponade, transmitter displacement, and implantation of the electrode within suspected myocardial scar.
Response to CRT
Overall at 6 months, 84.8% of patients in the SELECT-LV study and 69.8% in the WICS-LV Post Market Surveillance Registry had an improvement in their clinical composite score.[31,32] There was also a significant reduction in NYHA functional class, QRS duration, improvement in LV ejection fraction (LVEF) and reduction in both LV end-diastolic volume and LVESV.[31,32] Overall, 52–55% of patients had a significant reduction in LVESV ≥15%. Additionally, guiding in WiSE-CRT procedures by targeting the electrode to areas of latest activation while avoiding myocardial scar using different imaging modalities has been shown to further improve clinical and echocardiographic outcomes.[33,34] In patients who fail to improve following conventional CRT and who undergo WiSE-CRT implantation, 55.6% show improvement in their clinical composite score and 66.7% have a reduction in LVESV ≥15% and/or absolute improvement in LVEF ≥5%.[35]
Complications
Procedure-related deaths occurred in three of 90 patients (3.3%), with acute complications ≤24 hours after the procedure in 4/90 patients (4.4%), intermediate complications 24 hours–1 month after the procedure in 17 of 90 patients (18.8%), and chronic complications 1–6 months after the procedure in six of 90 patients (6.7%).[32] The commonest complications included arterial access complications and cardiac tamponade.
Physiological Pacing and LBBAP
LBBAP and HBP restore physiological activation through the native conduction system, and LBBAP may be more feasible than HBP due to a wider target area.[5,36] Although HBP has been shown to lead to narrowing of the QRS duration and cardiac resynchronisation in clinical and simulation studies, implantation can be difficult and the success rates vary from 56% to 95%.[37–40] Follow-up can be problematic due to oversensing of atrial signals, undersensing of ventricular signals, lead displacement, and rising capture thresholds with premature battery depletion.[36] Indeed, robust long-term data on the outcomes of HBP are currently lacking.
Novel LBBAP was developed to bypass the left bundle branch conduction block by screwing a ventricular lead into the interventricular septum to provide LV resynchronisation.[41] Studies have shown it may overcome some of the limitations of HBP.[36,42,43] In a prospective study of 341 patients referred for pacing, 30 of whom (8.8%) required CRT, LBBAP was successful in 89% of procedures, and at 1-year follow up the pacing threshold and R waves remained stable.[43] Currently, LBBAP is usually delivered using a SelectSecure 3830 pacing lead (Medtronic), and confirmation is dependent on several criteria, which are currently being updated and validated.[5,36,44] The predominant complications of LBBAP relate to the risk of septal perforations and lead dislodgements. LBBAP may be affected by intrinsic conduction and programming optimal atrioventricular delays will be important.[40]
Several studies have shown this to be effective in improving acute haemodynamics and patient outcomes.[36,43,45] Several trials have demonstrated the feasibility of LBBAP for delivering CRT.[46–49] In a study of 63 patients with non-ischaemic cardiomyopathy, LVEF ≤50%, complete LBBB and who had an indication for CRT or ventricular pacing, left bundle branch pacing was successful in 97% of cases, and this resulted in a significant improvement in LVEF and NYHA functional class at 1 year.[48]
In a large international multicentre study of 325 patients with LVEF <50% and an indication for CRT or pacing, LBBAP was successful in 85% of patients, and this resulted in significant narrowing of the QRS duration, and improvement in LVEF and NYHA functional class at 6 months.[49] Unsuccessful procedures were due to failure to penetrate the septum or inadequate resynchronisation; and the presence of LBBB at baseline was found to be an independent predictor of echocardiographic response.[49] Additionally, biventricular pacing was compared with both LBBAP and HBP in a non-randomised observational study of 137 patients with LVEF ≤40%, typical LBBB and referral for CRT.[42] It was found that both HBP and left bundle branch pacing resulted in a significant improvement in LVEF and NYHA functional class compared with biventricular pacing at 1-year follow-up.[42]
LV septal pacing involves pacing the LV endocardial side of the interventricular septum, and this may provide an alternative approach for cardiac resynchronisation. In a study of 27 patients undergoing CRT, temporary LV septal pacing performed via a transaortic approach resulted in a significant reduction in QRS area and standard deviation of activation times, but similar LV dP/dtmax compared with biventricular pacing.[50] LV septal pacing may prove to be especially useful in patients who have failed LBBAP, particularly given that the pacing location is relatively large.
Future Directions
LV endocardial pacing with the WiSE-CRT system in prospective registries has demonstrated reliable resynchronisation, improvement of symptoms and reversal of LV remodelling, but the risk of procedural complications requires further evaluation. The ongoing SOLVE-CRT trial is a randomised controlled multicentre trial to assess the safety and efficacy of the WiSE-CRT system, and it will provide important outcome data on the safety and efficacy of leadless LV endocardial pacing.[51] In the future, completely leadless pacing and or CRT and defibrillation may be achievable with the incorporation of a Micra transcatheter pacing system (Medtronic), WiSE-CRT system and a subcutaneous ICD (Boston Scientific), but refinements in the technology will be needed before this becomes more widespread.[52]
LBBAP has the potential to improve outcomes in patients eligible for CRT, and future modifications to the equipment will likely further improve procedural success and patient outcomes. Data on the long-term safety profile and outcomes of heart failure patients who undergo LBBAP for CRT are needed to determine whether this will become a viable treatment intervention. Theoretically, the WiSE-CRT electrode could be targeted to achieve leadless left bundle branch stimulation from the LV endocardium, or HBP from the RV endocardium. However, it is likely that refinements of the technology, including modification of the electrode delivery system, will be required to enable targeted physiological pacing.
Conclusion
Endocardial pacing has many advantages over conventional CRT and has the potential to improve patient outcomes. The WiSE-CRT system allows pacing at a customised location and enables areas of latest activation to be targeted while avoiding myocardial scar. It can lead to clinical improvement, and the ongoing SOLVE-CRT trial will be important in determining its efficacy and safety profile. Physiological pacing with LBBAP has shown promising results in initial trials but its role in CRT requires further investigation. In the future, leadless LBBAP may be achievable but will require technological advances.
Clinical Perspective
Alternative pacing approaches including left ventricular (LV) endocardial pacing and left bundle branch area pacing (LBBAP) have the potential to provide superior resynchronisation to conventional cardiac resynchronisation therapy (CRT) and to improve response rates.
Patients undergoing WiSE-CRT implantation should have an assessment of their peripheral arterial vasculature to reduce potential complications. Guiding the electrode to the desired endocardial location can improve patient outcomes.
Prospective registries have shown that the WiSE-CRT system results in an improved clinical composite score in 70% of patients, and a reduction in LV end-systolic volume ≥15% in 55%.
LBBAP has the ability to provide physiological pacing and it overcomes many of the problems with His-bundle pacing but its role in CRT requires further investigation.
Acknowledgments
The authors thank Michael Lee and EBR Systems for providing images used in this manuscript.
References
- 1.Cleland JG,, Daubert JC,, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 2.Bristow MR,, Saxon LA,, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 3.European Heart Rhythm Association, European Society of Cardiology, Heart Rhythm Society, et al. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow-up recommendations and management. Europace. 2012;14:1236–86. doi: 10.1093/europace/eus222. [DOI] [PubMed] [Google Scholar]
- 4.Sidhu BS,, Gould J,, Sieniewicz BJ, et al. Complications associated with cardiac resynchronization therapy upgrades versus de novo implantations. Expert Rev Cardiovasc Ther. 2018;16:607–15. doi: 10.1080/14779072.2018.1498783. [DOI] [PubMed] [Google Scholar]
- 5.Arnold AD,, Whinnett ZI,, Vijayaraman P. His-Purkinje conduction system pacing: state of the art in 2020. Arrhythm Electrophysiol Rev. 2020;9:136–45. doi: 10.15420/aer.2020.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh JP,, Fan D,, Heist EK, et al. Left ventricular lead electrical delay predicts response to cardiac resynchronization therapy. Heart Rhythm. 2006;3:1285–92. doi: 10.1016/j.hrthm.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 7.Bordachar P,, Ploux S,, Lumens J. Endocardial pacing: the wave of the future? Curr Cardiol Rep. 2012;14:547–51. doi: 10.1007/s11886-012-0298-2. [DOI] [PubMed] [Google Scholar]
- 8.Huntjens PR,, Walmsley J,, Ploux S, et al. Influence of left ventricular lead position relative to scar location on response to cardiac resynchronization therapy: a model study. Europace. 2014;16((Suppl 4)):iv62–8. doi: 10.1093/europace/euu231. [DOI] [PubMed] [Google Scholar]
- 9.Mendonca Costa C,, Neic A,, Kerfoot E, et al. Pacing in proximity to scar during cardiac resynchronization therapy increases local dispersion of repolarization and susceptibility to ventricular arrhythmogenesis. Heart Rhythm. 2019;16:1475–83. doi: 10.1016/j.hrthm.2019.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleeker GB,, Schalij MJ,, Van Der Wall EE,, Bax JJ. Postero-lateral scar tissue resulting in non-response to cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2006;17:899–901. doi: 10.1111/j.1540-8167.2006.00499.x. [DOI] [PubMed] [Google Scholar]
- 11.Chalil S,, Foley PW,, Muyhaldeen SA, et al. Late gadolinium enhancement-cardiovascular magnetic resonance as a predictor of response to cardiac resynchronization therapy in patients with ischaemic cardiomyopathy. Europace. 2007;9:1031–7. doi: 10.1093/europace/eum133. [DOI] [PubMed] [Google Scholar]
- 12.Gold MR,, Birgersdotter-Green U,, Singh JP, et al. The relationship between ventricular electrical delay and left ventricular remodelling with cardiac resynchronization therapy. Eur Heart J. 2011;32:2516–24. doi: 10.1093/eurheartj/ehr329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sohal M,, Duckett SG,, Zhuang X, et al. A prospective evaluation of cardiovascular magnetic resonance measures of dyssynchrony in the prediction of response to cardiac resynchronization therapy. J Cardiovasc Magn Reson. 2014;16:58. doi: 10.1186/s12968-014-0058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behar JM,, Mountney P,, Toth D, et al. Real-Time X-MRI-guided left ventricular lead implantation for targeted delivery of cardiac resynchronization therapy. JACC Clin Electrophysiol. 2017;3:803–14. doi: 10.1016/j.jacep.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Behar JM,, Rajani R,, Pourmorteza A, et al. Comprehensive use of cardiac computed tomography to guide left ventricular lead placement in cardiac resynchronization therapy. Heart Rhythm. 2017;14:1364–72. doi: 10.1016/j.hrthm.2017.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullens W,, Grimm RA,, Verga T, et al. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol. 2009;53:765–73. doi: 10.1016/j.jacc.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 17.Morgan JM,, Delgado V. Lead positioning for cardiac resynchronization therapy: techniques and priorities. Europace. 2009;11((Suppl 5)):v22–8. doi: 10.1093/europace/eup306. [DOI] [PubMed] [Google Scholar]
- 18.Mendonca Costa C,, Neic A,, Gillette K, et al. Left ventricular endocardial pacing is less arrhythmogenic than conventional epicardial pacing when pacing in proximity to scar. Heart Rhythm. 2020;17:1262–70. doi: 10.1016/j.hrthm.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Deursen C,, van Geldorp IE,, Rademakers LM, et al. Left ventricular endocardial pacing improves resynchronization therapy in canine left bundle-branch hearts. Circ Arrhythm Electrophysiol. 2009;2:580–7. doi: 10.1161/CIRCEP.108.846022. [DOI] [PubMed] [Google Scholar]
- 20.Strik M,, Rademakers LM,, van Deursen CJ, et al. Endocardial left ventricular pacing improves cardiac resynchronization therapy in chronic asynchronous infarction and heart failure models. Circ Arrhythm Electrophysiol. 2012;5:191–200. doi: 10.1161/circep.111.965814. [DOI] [PubMed] [Google Scholar]
- 21.Spragg DD,, Dong J,, Fetics BJ, et al. Optimal left ventricular endocardial pacing sites for cardiac resynchronization therapy in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2010;56:774–81. doi: 10.1016/j.jacc.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Derval N,, Steendijk P,, Gula LJ, et al. Optimizing hemodynamics in heart failure patients by systematic screening of left ventricular pacing sites: the lateral left ventricular wall and the coronary sinus are rarely the best sites. J Am Coll Cardiol. 2010;55:566–75. doi: 10.1016/j.jacc.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 23.Behar JM,, Jackson T,, Hyde E, et al. Optimized left ventricular endocardial stimulation is superior to optimized epicardial stimulation in ischemic patients with poor response to cardiac resynchronization therapy: a combined magnetic resonance imaging, electroanatomic contact mapping, and hemodynamic study to target endocardial lead placement. JACC Clin Electrophysiol. 2016;2:799–809. doi: 10.1016/j.jacep.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sieniewicz BJ,, Behar JM,, Sohal M, et al. Electrical latency predicts the optimal left ventricular endocardial pacing site: results from a multicentre international registry. Europace. 2018;20:1989–96. doi: 10.1093/europace/euy052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gamble JHP,, Herring N,, Ginks M, et al. Endocardial left ventricular pacing for cardiac resynchronization: systematic review and meta-analysis. Europace. 2018;20:73–81. doi: 10.1093/europace/euw381. [DOI] [PubMed] [Google Scholar]
- 26.Morgan JM,, Biffi M,, Geller L, et al. ALternate Site Cardiac ResYNChronization (ALSYNC): a prospective and multicentre study of left ventricular endocardial pacing for cardiac resynchronization therapy. Eur Heart J. 2016;37:2118–27. doi: 10.1093/eurheartj/ehv723. [DOI] [PubMed] [Google Scholar]
- 27.Razeghi O,, Strocchi M,, Lee A, et al. Tracking the motion of intracardiac structures aids the development of future leadless pacing systems. J Cardiovasc Electrophysiol. 2020;31:2431–9. doi: 10.1111/jce.14657. [DOI] [PubMed] [Google Scholar]
- 28.DeFaria Yeh D,, Lonergan KL,, Fu D, et al. Clinical factors and echocardiographic techniques related to the presence, size, and location of acoustic windows for leadless cardiac pacing. Europace. 2011;13:1760–5. doi: 10.1093/europace/eur199. [DOI] [PubMed] [Google Scholar]
- 29.Sieniewicz BJ,, Gould JS,, Rimington HM, et al. Transseptal delivery of a leadless left ventricular endocardial pacing electrode. JACC Clin Electrophysiol. 2017;3:1333–5. doi: 10.1016/j.jacep.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Auricchio A,, Delnoy PP,, Butter C, et al. Feasibility, safety, and short-term outcome of leadless ultrasound-based endocardial left ventricular resynchronization in heart failure patients: results of the Wireless Stimulation Endocardially for CRT (WiSE-CRT) study. Europace. 2014;16:681–8. doi: 10.1093/europace/eut435. [DOI] [PubMed] [Google Scholar]
- 31.Reddy VY,, Miller MA,, Neuzil P, et al. Cardiac resynchronization therapy with wireless left ventricular endocardial pacing: the SELECT-LV Study. J Am Coll Cardiol. 2017;69:2119–29. doi: 10.1016/j.jacc.2017.02.059. [DOI] [PubMed] [Google Scholar]
- 32.Sieniewicz BJ,, Betts TR,, James S, et al. Real-world experience of leadless left ventricular endocardial cardiac resynchronization therapy: a multicenter international registry of the WiSE-CRT pacing system. Heart Rhythm. 2020;17:1291–7. doi: 10.1016/j.hrthm.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sieniewicz BJ,, Behar JM,, Gould J, et al. Guidance for optimal site selection of a leadless left ventricular endocardial electrode improves acute hemodynamic response and chronic remodeling. JACC Clin Electrophysiol. 2018;4:860–8. doi: 10.1016/j.jacep.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Sidhu BS,, Lee AWC,, Haberland U, et al. Combined computed tomographic perfusion and mechanics with predicted activation pattern can successfully guide implantation of a wireless endocardial pacing system. Europace. 2020;22:298. doi: 10.1093/europace/euz227. [DOI] [PubMed] [Google Scholar]
- 35.Sidhu BS,, Porter B,, Gould J, et al. Leadless left ventricular endocardial pacing in nonresponders to conventional cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2020;43:966–73. doi: 10.1111/pace.13926. [DOI] [PubMed] [Google Scholar]
- 36.Padala SK,, Ellenbogen KA. Left bundle branch pacing is the best approach to physiological pacing. Heart Rhythm O2. 2020;1:59–67. doi: 10.1016/j.hroo.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatt AG,, Musat DL,, Milstein N, et al. The efficacy of His bundle pacing: lessons learned from implementation for the first time at an experienced electrophysiology center. JACC Clin Electrophysiol. 2018;4:1397–1406. doi: 10.1016/j.jacep.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Vijayaraman P,, Chung MK,, Dandamudi G, et al. His bundle pacing. J Am Coll Cardiol. 2018;72:927–47. doi: 10.1016/j.jacc.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Sharma PS,, Vijayaraman P,, Ellenbogen KA. Permanent His bundle pacing: shaping the future of physiological ventricular pacing. Nat Rev Cardiol. 2020;17:22–36. doi: 10.1038/s41569-019-0224-z. [DOI] [PubMed] [Google Scholar]
- 40.Strocchi M,, Lee AWC,, Neic A, et al. His-bundle and left bundle pacing with optimized atrioventricular delay achieve superior electrical synchrony over endocardial and epicardial pacing in left bundle branch block patients. Heart Rhythm. 2020;17:1922–9. doi: 10.1016/j.hrthm.2020.06.028. [DOI] [PubMed] [Google Scholar]
- 41.Huang W,, Su L,, Wu S, et al. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol. 2017;33:1736.e1–3. doi: 10.1016/j.cjca.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Wu S,, Su L,, Vijayaraman P, et al. Left bundle branch pacing for cardiac resynchronization therapy: nonrandomized on-treatment comparison with His bundle pacing and biventricular pacing. Can J Cardiol. 2021;37:319–28. doi: 10.1016/j.cjca.2020.04.037. [DOI] [PubMed] [Google Scholar]
- 43.Padala SK,, Master VM,, Terricabras M, et al. Initial experience, safety, and feasibility of left bundle branch area pacing: a multicenter prospective study. JACC Clin Electrophysiol. 2020;6:1773–82. doi: 10.1016/j.jacep.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Huang W,, Chen X,, Su L, et al. A beginner’s guide to permanent left bundle branch pacing. Heart Rhythm. 2019;16:1791–6. doi: 10.1016/j.hrthm.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 45.Chen K,, Li Y,, Dai Y, et al. Comparison of electrocardiogram characteristics and pacing parameters between left bundle branch pacing and right ventricular pacing in patients receiving pacemaker therapy. Europace. 2019;21:673–80. doi: 10.1093/europace/euy252. [DOI] [PubMed] [Google Scholar]
- 46.Vijayaraman P,, Subzposh FA,, Naperkowski A, et al. Prospective evaluation of feasibility and electrophysiologic and echocardiographic characteristics of left bundle branch area pacing. Heart Rhythm. 2019;16:1774–82. doi: 10.1016/j.hrthm.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 47.Zhang W,, Huang J,, Qi Y, et al. Cardiac resynchronization therapy by left bundle branch area pacing in patients with heart failure and left bundle branch block. Heart Rhythm. 2019;16:1783–90. doi: 10.1016/j.hrthm.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Huang W,, Wu S,, Vijayaraman P, et al. Cardiac resynchronization therapy in patients with nonischemic cardiomyopathy using left bundle branch pacing. JACC Clin Electrophysiol. 2020;6:849–58. doi: 10.1016/j.jacep.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 49.Vijayaraman P,, Ponnusamy S,, Cano Ó, Left bundle branch area pacing for cardiac resynchronization therapy: results from the International LBBAP Collaborative Study Group. JACC Clin Electrophysiol. 2020. epub ahead of press. [DOI] [PubMed]
- 50.Salden F,, Luermans J,, Westra SW, et al. Short-term hemodynamic and electrophysiological effects of cardiac resynchronization by left ventricular septal pacing. J Am Coll Cardiol. 2020;75:347–59. doi: 10.1016/j.jacc.2019.11.040. [DOI] [PubMed] [Google Scholar]
- 51.Singh JP,, Abraham WT,, Auricchio A, et al. Design and rationale for the Stimulation Of the Left Ventricular Endocardium for Cardiac Resynchronization Therapy in non-responders and previously untreatable patients (SOLVE-CRT) trial. Am Heart J. 2019;217:13–22. doi: 10.1016/j.ahj.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Sidhu BS,, Gould J,, Porter B, et al. Completely leadless cardiac resynchronization defibrillator system. JACC Clin Electrophysiol. 2020;6:588–9. doi: 10.1016/j.jacep.2020.02.012. [DOI] [PubMed] [Google Scholar]


