Abstract
AIM
To assess the correlation between disorganization of the retinal inner layers (DRIL) and best-corrected visual acuity (BCVA) in patients with uveitis and macular edema (UME) who underwent systemic treatment using optical coherence tomography (OCT).
METHODS
A retrospective clinical study of 23 patients (30 eyes) with DRIL and 23 patients (31 eyes) without DRIL secondary to UME were included. All patients underwent comprehensive ophthalmic examinations at baseline, 3, 6, and 12mo after local and systemic treatment. The OCT-based parameters included foveal center point thickness (FCPT), mean thickness (MT), and diameters of DRIL in horizontal and vertical directions. BCVA and OCT-based parameters were compared between the two groups. The relationship between each OCT parameter and BCVA was evaluated using linear correlation and regression analysis.
RESULTS
At the initial visit, the mean baseline FCPT was 441.03±128.68 µm in the eyes with DRIL and 337.26±99.31 µm in the eyes without DRIL (P=0.001). No significant differences were observed in MT (P=0.357). The mean size of transverse and vertical diameters of DRIL was 684.07±267.51 and 267.07±104.61 µm at baseline, respectively. There was significant improvement in BCVA and OCT-based parameters at 3, 6, and 12mo in all cases (P<0.001 for each timepoint). In addition, significant differences were detected in BCVA and OCT parameters between eyes with and without DRIL at each time point (P<0.01 for each timepoint). A greater DRIL range at baseline was associated with a worse baseline BCVA (transverse diameter of DRIL: r=0.875, P<0.001; vertical diameter of DRIL: r=0.622, P<0.001). The transverse diameter of baseline DRIL was found to be significantly correlated with the final BCVA (P=0.003).
CONCLUSION
The improvement in BCVA is associated with DRIL in patients with UME. DRIL is an easy-to-determine and robust imaging biomarker that could help predict BCVA prognosis in eyes with UME.
Keywords: retinal inner layers, predictor, uveitis, macular edema
INTRODUCTION
The umbrella term of uveitis encompasses a group of intraocular inflammatory diseases that can lead to changes in the uveal tract and its adjacent structures. Uveitis is one of the most common etiologies of visual loss in the world and accounts for 10%-15%[1]–[2] and possibly as much as 25% of legal blindness in the developed and developing countries, respectively[3]–[4]. Macular edema (ME) is a typical, sight-threatening complication of uveitis that often occurs in patients showing retinal involvement[5]–[6].
ME is most often the clinical result of an accumulation of serous fluid in the retinal layers (the light-sensitive inner lining of the eye), which is caused by the disruption of the blood-retinal barrier. The pathogenesis of ME is still not completely understood; however, it is associated with the alteration of the functional cell relationship in the retina and promotion of inflammatory reparative responses. To improve the best-corrected visual acuity (BCVA), the management of ME via amelioration of both anatomical (by reducing central retinal thickness) and functional parameters is necessary[7]–[8]. Appropriate local and systemic use of corticosteroids may be the most widely used treatment for uveitic macular edema (UME), but resorting to other immunosuppressive drugs is also common. Delays in treatment can lead to worse visual outcomes.
Until recently, prediction of BCVA in patients with UME using structural markers has been unsuccessful because the correlation between retinal thickness on optical coherence tomography (OCT) and BCVA is only modest in retinal diseases. Recent studies reported that BCVA deteriorates as a result of the disorganization of retinal inner layers (DRIL), which has been explored as a new potential biological factor in numerous studies[9]–[17]. DRIL is an OCT feature represented by a disruption of any one of the two boundaries of inner nuclear layer (INL) with the ganglion cell-inner plexiform layer (GCIPL) and outer plexiform layer (OPL)[10]–[11]. Sun et al[10] first concluded that DRIL is significantly associated with the worsening of BCVA and has been identified as a prognostic biomarker for eyes with diabetic ME. Mimouni et al[11] recently reported a significant association between DRIL and BCVA in 136 eyes with retinal vein occlusion. Grewal et al[14] summarized the relationship between DRIL and BCVA in patients with uveitis cystoid ME. However, the dynamic relationship between DRIL and BCVA was not evaluated in those cases.
Therefore, it is not yet confirmed whether DRIL is a useful clinical biomarker for predicting BCVA in patients with UME. In this study, we evaluated the relationship between DRIL and BCVA. We found that a greater DRIL range at baseline was associated with a worse baseline BCVA, and the transverse diameter of baseline DRIL was significantly correlated with the final BCVA. Our results indicate that DRIL is a practical and reliable imaging biomarker that could help predict BCVA prognosis in eyes with UME.
SUBJECTS AND METHODS
Ethical Approval
In this retrospective noncomparative case series, patients were recruited from the Eye Hospital of Tianjin Medical University. The protocol was obtained through the institutional review board approval of the University of Tianjin Medical University Eye Hospital [No.2020KY (L)-26], and the procedures were performed in adherence to the tenets of the Declaration of Helsinki.
Study Participants
The patient files and OCT parameters of 46 patients (61 eyes) with UME who had been followed between March 2016 and December 2019 at Uveitis & Ocular Immunology Service of the Tianjin Medical University Eye Hospital were assessed retrospectively. Patients were included in the study if they had UME, had received systemic medical treatment and/or periocular injection corticosteroids or intravitreal injection of corticosteroids/anti-vascular endothelial growth factor (VEGF) agents, and if they were followed-up for at least 12mo. Exclusion criteria included the presence of significant cataracts or other substantial media opacities, a history of intraocular operation 6mo before undergoing treatment for uveitis and UME, other ocular comorbidities that may affect the results, extensive damage to the photoreceptors, retinal sub-foveal scar or atrophy confirmed by clinical examination and OCT. We extracted demographic data including age, sex, and preexisting systemic and ocular diseases from case records.
All patients had detailed ophthalmic examinations, including BCVA (logMAR), intraocular pressure, slit-lamp examination, fundus examination and imaging. BCVAs were evaluated using the Snellen chart, and logMAR equivalents were used for statistical analysis.
All OCT images were obtained with the radial macular 6.0-1024×12 scan mode using a Topcon DRI OCT machine (software version 9.20) by certified imaging technicians at baseline, 3, 6, and 12mo after treatment. Software version 9.20 of Topcon DRI OCT assumes central foveal fixation and generates a fast macular thickness map protocol. The protocol involves three tori of different diameters (1, 3, and 6 mm) and the division of the macula into nine sectors depending on the quadrant (superior, inferior, nasal, and temporal quadrants).We measured several parameters from the OCT images, including foveal center point thickness (FCPT), mean thickness (MT), and retinal thickness in each of the nine subfields. MT was automatically calculated by averaging retinal thickness in nine subfields.
DRIL was defined as the horizontal extent (per B-scan) in micrometers when any boundaries of the INL with the GCIPL complex and OPL could not be identified[10] (Figure 1). The presence and absence of DRIL was defined as DRIL extents of >20 µm and <20 µm, respectively[11] (Figure 1). All patients underwent DRIL extent evaluations both horizontally and vertically, and the measured values were recorded. Two observers who were blinded to the BCVA data independently judged and graded all OCT images. The diameter of DRIL in both directions was manually measured using calipers built into the OCT software. If the initial observers could not accurately distinguish the presence or absence of DRIL at a particular location, a second expert observer was consulted, and a consensus was reached by negotiation.
Figure 1. Optical coherence tomography for the definition of disorganization of retinal inner layers (DRIL).
The scan assumes central foveal fixation and generates the macular thickness map centered on this location. A: Patient with mild DRIL. The yellow arrow indicates the area that is difficult to distinguish the boundary between the ganglion cell-inner plexiform layer complex (GCIPL) and inner nuclear layer (INL). B: Patient with severe DRIL. No boundaries of the INL with the GCIPL and OPL can be identified and are irregular.
Statistical Analysis
SPSS version 22.0 (Prism 6.02, GraphPad, La Jolla, CA, USA) was used to perform the statistical analysis. The baseline characteristics of the study cohort were evaluated using descriptive statistics. Qualitative variables were expressed as percentages. Differences between two groups were assessed with a Chi-square test. A Kolmogorov-Smirnov test was used to explore the distribution of the numerical data. The data were distributed normally, and a two-tailed Student's t-test was used to compare means of continuous variables at baseline. Conversely, a Mann-Whitney test was used in the comparison of non-normal distribution variables. A Chi-square test was used for categorical analysis. Pearson's correlation analysis was performed to estimate the relationships between BCVA and OCT parameters associated with specific time points. A linear regression model was used to evaluate the independent effect of OCT parameters, which has been shown to have an effect on BCVA. A P value of <0.05 was considered statistically significant.
RESULTS
Patient Characteristics at Baseline
A total of 61 eyes from 46 patients (21 men) were included. Anatomic diagnosis classified 4 cases as anterior uveitis, 6 as intermediate, 10 as posterior, and 41 as panuveitis. There were several specific types of panuveitis, such as Behcet's disease (n=12), Vogt-Koyanagi-Harada syndrome (n=2), and retinal vasculitis (n=2). Table 1 shows the baseline characteristics of the patients. During the follow-up period, a total of 30 eyes of 23 patients with DRIL were compared with 31 eyes of 23 patients without DRIL. The mean age at diagnosis of the 23 patients (9 females and 14 males) with DRIL was 53.53±15.84y (range 14-79y). Eleven (47.8%) of 23 patients without DRIL were female, and 12 (52.2%) were male. The mean age at presentation was 49.48±15.07y (range 15-80y). Age and sex distribution were shown to be similar between the groups (P=0.310 and P=0.375, respectively).
Table 1. The baseline and demographic characteristics of the patients.
| Parameter | Data |
| Patients/eyes | 46/61 |
| Age, y, mean±SD (range) | 46.51±17.94 (11-80) |
| Male (%) | 21 (45.7) |
| Right eye (%) | 28 (45.9) |
| Uveitis type (%) | |
| Anterior | 4 (6.6) |
| Intermediate | 6 (9.8) |
| Posterior | 10 (16.4) |
| Panuveitis | 41 (67.2) |
| Behcet's disease | 12 (19.7) |
| Vogt-Koyanagi-Harada syndrome | 2 (3.3) |
| Retinal vasculitis | 2 (3.3) |
| Previous treatment (%) | 0 |
Comparison of FCPT and MT Between Eyes with and Without DRIL
The mean BCVA in the DRIL group was 0.55±0.23 logMAR at baseline and improved to 0.31±0.20, 0.25±0.17, and 0.20±0.16 logMAR at 3, 6, and 12mo after treatment, respectively (P<0.001 for each time point). The median BCVA in patients without DRIL was 0.19 logMAR at baseline and improved to 0.13, 0.09, and 0.05 logMAR at 3, 6, and 12mo after treatment, respectively (P=0.03, P<0.001 and P<0.001 respectively). There were significant differences between the groups with BCVA at all time points (all P<0.01).
At the initial visit, the mean baseline FCPT was 441.03±128.68 µm in the eyes with DRIL and 337.26±99.31 µm in the eyes without DRIL (P=0.001). No significant differences were observed in MT (P=0.357). We also analyzed the differences in FCPT and MT between the groups at 3, 6, and 12mo. Interestingly, there were significant differences in MT at 6 and 12mo (P=0.002 and P=0.004, respectively; Table 2).
Table 2. Comparison of FCPT and MT between eyes with DRIL and without DRIL.
| Follow up | DRIL+ | DRIL– | P |
| At baseline | |||
| FCPT | 441.03±128.68 | 337.26±99.31 | 0.001 |
| MT | 345.88±51.43 | 336.00±27.66 | 0.357 |
| At 3mo | |||
| FCPT | 312.17±100.58 | 293.71±31.17 | 0.343 |
| MT | 309.13±32.91 | 321.42±21.85 | 0.090 |
| At 6mo | |||
| FCPT | 276.80±54.96 | 286.10±35.05 | 0.432 |
| MT | 295.80±21.34 | 314.28±22.87 | 0.002 |
| At 12mo | |||
| FCPT | 264.10±39.88 | 271.06±33.35 | 0.462 |
| MT | 289.78±21.44 | 307.63±25.15 | 0.004 |
DRIL: Disorganization of the retinal inner layers; FCPT: Foveal center point thickness; MT: Mean thickness.
mean±SD, µm
Associations of DRIL with BCVA at Baseline
The mean size of transverse diameter of DRIL was 684.07±267.51 µm, and the mean size of vertical diameter of DRIL was 267.07±104.61 µm at baseline. The scatter plots in Figure 2 demonstrate the linear relationships of DRIL, FCPT, and MT with BCVA at baseline in the DRIL group. A strong correlation was found between BCVA and the parameters of DRIL, including transverse diameter of DRIL (r=0.875, P<0.001) and vertical diameter of DRIL (r=0.622, P<0.001). Briefly, a greater DRIL range at baseline was associated with a worse baseline BCVA. MT was also significantly associated with BCVA (r=0.591, P=0.001). Estimates for further linear regression analysis of BCVA and OCT parameters are shown in Table 3. Stepwise linear regression analysis indicated that the transverse diameter of DRIL had significant effects on BCVA (B=0.875, P<0.001).
Figure 2. Bivariate analyses of OCT parameters and BCVA at baseline.
A: Association of FCPT with BCVA (r=0.357, P=0.052); B: Association of MT with BCVA (r=0.591, P=0.001); C: Association of transverse diameter of DRIL with BCVA (r=0.875, P<0.001); D: Association of vertical diameter of DRIL with BCVA (r=0.622, P<0.001). FCPT: Foveal center point thickness; MT: Mean thickness; DRIL: Disorganization of retinal inner layers.
Table 3. Associations of OCT Parameters with BCVA at baseline.
| BCVA | Coefficient (95%CI) | P |
| MT | 0.292 (0.000-0.003) | 0.040 |
| Transverse diameter of DRIL | 0.824 (0.001-0.001) | 0.000 |
| Vertical diameter of DRIL | -0.144 (-0.001-0.000) | 0.364 |
BCVA: Best corrected visual acuity; CI: Confidence intervals; DRIL: Disorganization of the retinal inner layers; MT: Mean thickness.
Associations of Baseline DRIL with BCVA During Follow-up
BCVA at the 3-month time point was strongly associated with transverse diameter of DRIL (r=0.789, P<0.001), vertical diameter of DRIL (r=0.689, P<0.001), FCPT (r=0.464, P=0.010), and MT (r=0.569, P=0.001) at baseline. In addition, BCVA at 6mo was strongly association with transverse diameter of DRIL (r=0.754, P<0.001), vertical diameter of DRIL (r=0.592, P=0.001), and MT (r=0.471, P=0.009) at baseline. The linear regression model highlighted the correlations of BCVA at 6mo with the transverse diameter of DRIL at baseline (P=0.040). Neither FCPT nor MT indicated a clear significant correlation with the improvement in BCVA at 6mo.
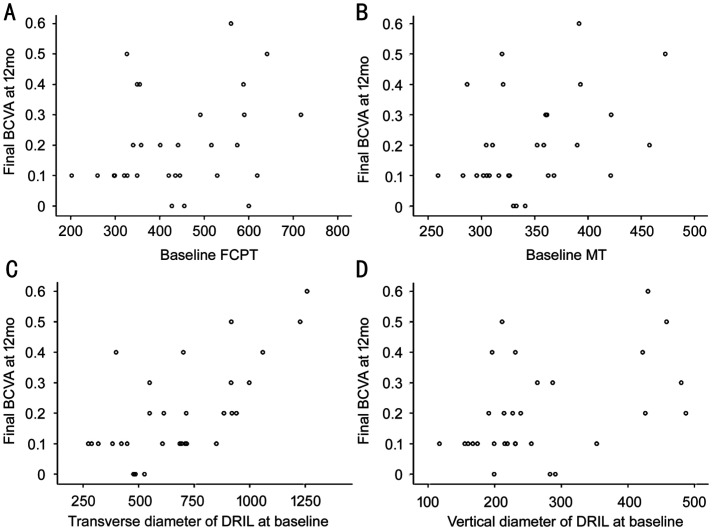
The relationships of DRIL and other OCT parameters at baseline and final BCVA at 12mo is shown in Figure 3. Bivariate analyses demonstrated that BCVA was significantly correlated with transverse and vertical diameters of DRIL (r=0.666, P<0.001; r=0.450, P=0.013) and MT (r=0.363, P=0.048) at baseline. No strong correlation was found between FCPT and BCVA (r=0.273, P=0.144). When these variables were evaluated using linear regression analysis, the transverse diameter of baseline DRIL was found to be significantly correlated with the final BCVA (P=0.003).
Figure 3. Bivariate analyses of change in OCT parameters and change in BCVA at 3mo.
A: Association of baseline FCPT with final BCVA; B: Association of baseline MT with final BCVA; C: Association of baseline transverse diameter of DRIL with final BCVA; D: Association of baseline vertical diameter of DRIL with final BCVA. FCPT: Foveal center point thickness; MT: Mean thickness; DRIL: Disorganization of retinal inner layers.
DISCUSSION
The presence of DRIL is a common reaction to retinal stress in a range of retinal disorders, including vein occlusion[11], central retinal artery occlusion[12], diabetic retinopathy[13], uveitis[14], retinitis pigmentosa[15], epiretinal membrane[16], and closed globe trauma[17]. This study aimed to dynamically evaluate the relevance of BCVA and the transverse/vertical diameter of DRIL in patients with UME. We found that the extent of DRIL at baseline was negatively associated with worse initial and final BCVAs.
In our study, the eyes with DRIL were associated with worse BCVA at baseline, 3, 6, and 12mo compared with eyes without DRIL. Although both MT and the parameters of DRIL were significantly associated with BCVA, the line regression model indicated that only the transverse diameter of baseline DRIL was significantly associated with the BCVA at the different time points. These results indicate that the transverse diameter of baseline DRIL can be a reliable biomarker for predicting the BCVA prognosis. Being distinct from other OCT-based biomarkers, DRIL can not only qualitatively evaluate the structural disruption in inner retinal layers but also quantify the extent.
The exact pathogenesis of DRIL and the mechanism by which DRIL affects BCVA is unknown. The main potential causes of DRIL in UME are inflammation and ischemia. Nicholson et al[18] revealed that DRIL was strongly correlated with retinal capillary nonperfusion on fundus fluorescein angiography. The intact organization of retinal cellular pathways determines the quality of visual function. Because bipolar cells constitute the only transmission pathway between ganglion cells and photoreceptors, any destruction of bipolar cells will compromise visual acuity. Sun et al[10] suggested that the possible mechanism of DRIL may be a disruption of pathways that transmit visual information from the photoreceptors to the ganglion cells. It has been shown that the higher the increase in DRIL, the greater the number of axons of bipolar cells that stretch or even break[19]. Given the elasticity of biological material, the continuity of bipolar cells will be maintained within elastic limits. However, if sufficient swelling exceeds the elastic limits, neuronal axons may be disrupted, leading to the loss of the transmission pathway[20]. This concept may explain why DRIL was strongly associated with visual acuity. The range of DRIL was the most important parameter correlated with a worse BCVA prognosis, indicating that worse BCVA may result from long-term overstretching of bipolar axons.
This study has several limitations. Uveitis is a heterogeneous and relatively uncommon ophthalmic disease, and it is extremely difficult to conduct prospective randomized clinical trials, considering the challenges associated with recruiting and achieving a sufficient sample size. This study had a relatively small sample size with a 12-month follow-up period. Nevertheless, diagnosis, examination, and treatment were based on the common protocol used by all clinicians, and good interobserver reliability strengthens the study. Second, this study did not include subgroups. Therefore, the difference between eyes showing different degrees of DRIL is unknown. Third, the degree of DRIL described in our case is difficult to compare with that of DRIL identified in other studies because we evaluated a larger foveal area (diameter of 6 mm) than previous studies (1.5 mm ring)[14]. Using 6-mm OCT scans may help detect DRIL in eyes with early or minimal UME. With an improvement in technology, it has become possible to assess DRIL outside the macular area. Finally, there is no automated algorithm to measure DRIL integrity rapidly. However, our study found firm evidence that DRIL may be an important predictor of visual acuity outcomes, which could accelerate the development of such algorithms.
In conclusion, the findings of our study further emphasize that DRIL is strongly correlated with BCVA. Unlike other variables, DRIL includes not only qualitative grading of the regularity but also quantitative measurements. Therefore, we believe that DRIL could be used as a biological marker to predict the outcome of UME post-treatment.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81671642; No.81870651).
Conflicts of Interest: Liu Z, None; Tao QQ, None; Li XR, None; Zhang XM, None.
REFERENCES
- 1.Durrani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica. 2004;218(4):223–236. doi: 10.1159/000078612. [DOI] [PubMed] [Google Scholar]
- 2.Williams GJ, Brannan S, Forrester JV, Gavin MP, Paterson-Brown SP, Purdie AT, Virdi M, Olson JA. The prevalence of sight-threatening uveitis in Scotland. Br J Ophthalmol. 2007;91(1):33–36. doi: 10.1136/bjo.2006.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdulaal MR, Abiad BH, Hamam RN. Uveitis in the aging eye: incidence, patterns, and differential diagnosis. J Ophthalmol. 2015;2015:509456. doi: 10.1155/2015/509456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao NA. Uveitis in developing countries. Indian J Ophthalmol. 2013;61(6):253–254. doi: 10.4103/0301-4738.114090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin MH, Pistilli M, Daniel E, Gangaputra SS, Nussenblatt RB, Rosenbaum JT, Suhler EB, Thorne JE, Foster CS, Jabs DA, Levy-Clarke GA, Kempen JH, Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study Incidence of visual improvement in uveitis cases with visual impairment caused by macular edema. Ophthalmology. 2014;121(2):588–595.e1. doi: 10.1016/j.ophtha.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iannetti L, Spinucci G, Abbouda A, De Geronimo D, Tortorella P, Accorinti M. Spectral-domain optical coherence tomography in uveitic macular edema: morphological features and prognostic factors. Ophthalmologica. 2012;228(1):13–18. doi: 10.1159/000337234. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, Berg K, Chakravarthy U, Gerendas BS, Jonas J, Larsen M, Tadayoni R, Loewenstein A. Guidelines for the management of diabetic macular edema by the European society of retina specialists (EURETINA) Ophthalmologica. 2017;237(4):185–222. doi: 10.1159/000458539. [DOI] [PubMed] [Google Scholar]
- 8.Wong TY, Sun J, Kawasaki R, Ruamviboonsuk P, Gupta N, Lansingh VC, Maia M, Mathenge W, Moreker S, Muqit MMK, Resnikoff S, Verdaguer J, Zhao PQ, Ferris F, Aiello LP, Taylor HR. Guidelines on diabetic eye care: the international council of ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018;125(10):1608–1622. doi: 10.1016/j.ophtha.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Radwan SH, Soliman AZ, Tokarev J, Zhang L, van Kuijk FJ, Koozekanani DD. Association of disorganization of retinal inner layers with vision after resolution of center-involved diabetic macular edema. JAMA Ophthalmol. 2015;133(7):820–825. doi: 10.1001/jamaophthalmol.2015.0972. [DOI] [PubMed] [Google Scholar]
- 10.Sun JK, Lin MM, Lammer J, Prager S, Sarangi R, Silva PS, Aiello LP. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132(11):1309–1316. doi: 10.1001/jamaophthalmol.2014.2350. [DOI] [PubMed] [Google Scholar]
- 11.Mimouni M, Segev O, Dori D, Geffen N, Flores V, Segal O. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with macular edema secondary to vein occlusion. Am J Ophthalmol. 2017;182:160–167. doi: 10.1016/j.ajo.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Yilmaz H, Durukan AH. Disorganization of the retinal inner layers as a prognostic factor in eyes with central retinal artery occlusion. Int J Ophthalmol. 2019;12(6):990–995. doi: 10.18240/ijo.2019.06.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishibashi T, Sakimoto S, Shiraki N, Nishida K, Sakaguchi H, Nishida K. Association between disorganization of retinal inner layers and visual acuity after proliferative diabetic retinopathy surgery. Sci Rep. 2019;9(1):12230. doi: 10.1038/s41598-019-48679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grewal DS, O'Sullivan ML, Kron M, Jaffe GJ. Association of disorganization of retinal inner layers with visual acuity in eyes with uveitic cystoid macular edema. Am J Ophthalmol. 2017;177:116–125. doi: 10.1016/j.ajo.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Jones BW, Pfeiffer RL, Ferrell WD, Watt CB, Marmor M, Marc RE. Retinal remodeling in human retinitis pigmentosa. Exp Eye Res. 2016;150:149–165. doi: 10.1016/j.exer.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garnavou-Xirou C, Xirou, Gkizis I, Kabanarou SA, Dimitriou E, Theodossiadis P, Chatziralli I. The role of disorganization of retinal inner layers as predictive factor of postoperative outcome in patients with epiretinal membrane. Ophthalmic Res. 2020;63(1):13–17. doi: 10.1159/000499370. [DOI] [PubMed] [Google Scholar]
- 17.Chen HY, Lu YF, Huang HC, Zheng JL, Hou P, Chen WQ. Prediction of visual prognosis with spectral-domain optical coherence tomography in outer retinal atrophy secondary to closed globe trauma. Retina. 2013;33(6):1258–1262. doi: 10.1097/IAE.0b013e31827b63ba. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson L, Ramu J, Triantafyllopoulou I, Patrao NV, Comyn O, Hykin P, Sivaprasad S. Diagnostic accuracy of disorganization of the retinal inner layers in detecting macular capillary non-perfusion in diabetic retinopathy. Clin Exp Ophthalmol. 2015;43(8):735–741. doi: 10.1111/ceo.12557. [DOI] [PubMed] [Google Scholar]
- 19.Zur D, Iglicki M, Feldinger L, Schwartz S, Goldstein M, Loewenstein A, Barak A. Disorganization of retinal inner layers as a biomarker for idiopathic epiretinal membrane after macular surgery-the DREAM study. Am J Ophthalmol. 2018;196:129–135. doi: 10.1016/j.ajo.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 20.Pelosini L, Hull CC, Boyce JF, McHugh D, Stanford MR, Marshall J. Optical coherence tomography may be used to predict visual acuity in patients with macular edema. Invest Ophthalmol Vis Sci. 2011;52(5):2741–2748. doi: 10.1167/iovs.09-4493. [DOI] [PubMed] [Google Scholar]