Abstract
AIM
To compare the corneal outcome in Fuchs' endothelial dystrophy (FED) patients between femtosecond laser-assisted cataract surgery (FLACS) and conventional phaco surgery (CPS).
METHODS
This was a randomized controlled study comparing one eye surgery by FLACS and the contralateral eye operated by CPS (stop and chop technique) in FED patients. Central corneal thickness, corneal light backscatter, corneal densitometry, and central corneal endothelial cell count and hexagonality (noncontact endothelial cell microscope), and corrected distance visual acuity (CDVA) were assessed preoperatively and at day 1, 40, and 180 postoperatively.
RESULTS
Totally 31 patients (16 women) were included. At day 40 postoperatively, the mean endothelial cell loss (ECL) was 23.67% by FLACS and 17.30% by CPS (P=0.53). At day 180 postoperatively, ECL was 25.58% in FLACS and 21.32% in CPS (P=0.69). Densitometry data in all layers and all annuli from anterior layer to posterior layer in annuli 0-2, 2-6, 6-10 and 10-12, total densitometry with all layers and all annuli was performed. A significant difference was found in 6-10 (posterior layer) at day 1 with -1.42 grayscale units (GSU; 95%CI: -2.66 to -0.19, P=0.02). In 10-12 (anterior layer, central layer and all layers) at day 40 were significant different with 7.7 (95%CI: 1.89 to 13.50, P=0.009), 3.97 (95%CI: 0.23 to 7.71, P=0.03), 4.73 GSU (95%CI: 0.71 to 8.75, P=0.02), respectively. In the remaining parameters we found no difference between the two groups (P>0.05). Three CPS eyes suffered from corneal decompensation.
CONCLUSION
There is no significant difference in corneal outcome between FLACS and CPS. Endothelial cell density and pentacam corneal outcome may be inadequate as outcome parameters in FED patients.
Keywords: cataract surgery, femtosecond-assisted cataract surgery, corneal endothelial cell loss, central corneal thickness, pentacam, Fuchs' endothelial dystrophy
INTRODUCTION
Femtosecond laser-assisted cataract surgery (FLACS) was introduced to cataract surgery in 2009[1]. FLACS technology can perform corneal incisions, consistent capsulorhexis and laser fragmentation of the lens. FLACS reduces effective phacoemulsification time (EPT) and energy use[2]. The endothelial cells in the cornea are vulnerable towards EPT and phaco energy and it is believed that a decrease in EPT and energy use could lead to less endothelial cell loss (ECL)[3]. The reported difference in cell loss between FLACS and CPS is relatively small and should not be of any clinical consequence for a patient with a healthy endothelium[3]. Patients with Fuchs' endothelial dystrophy (FED) suffers from progressive loss of endothelial cells and are in risk of developing chronic edema with poor visual outcome following cataract surgery[4]. For these patients it is important to maintain as many endothelial cells as possible after cataract surgery. As FLACS causes less energy use and less phacoemulsification time it might be beneficial for FED patients resulting in less corneal damage compared to CPS. Currently endothelial cell density (ECD) and central corneal thickness (CCT) are the most common used outcomes when monitoring FED, but they may not be the best suitable measurement to monitor FED. ECD is based on a tiny fraction of the corneal inner surface and is subject to severe sampling bias; i.e., variation depending on which area is selected for cell counting[5]. CCT is normally used to define the presence of corneal edema but the relationship between endothelial cell count and CCT is highly non-linear, and there are biological variations in CCT making establishment of a cut off value difficult[6]. Newer suggestions to monitor FED patients are corneal periphery ratio thickness (CPRT) where a ratio between the central and peripheral corneal thickness are calculated to adjust for the variation in CCT[7]. Another suggestion is corneal light back scatter where corneal transparency is measured[8]–[9]. These outcomes are potential future endpoints to monitor clinical outcomes in FED, however, no studies have examined if these parameters are affected by cataract surgery and therefore their clinical relevance is unknown.
This pilot study aimed to examine if FLACS is more beneficial in regard to corneal outcome for FED patients compared to conventional phaco surgery (CPS) and evaluate how present surgical corneal outcomes presents in FED patients after surgery.
SUBJECTS AND METHODS
Ethical Approval
All patients participated on a voluntary basis and signed an informed consent. The study was performed according to the Declaration of Helsinki and was approved by the Local Committee of Ethics (No.H-16020650) and the Danish Data Protection Agency. Patients of the public were not involved in the study design or conduct of the research.
We performed a randomized prospective clinical trial with a consecutive cohort of 34 patients that were offered FLACS on one eye and CPS on the contralateral eye at the Department of Ophthalmology, Glostrup, University Hospital of Copenhagen, Denmark.
Inclusion criteria were: FED based on findings of guttae >5[10], visually significant cataract of any type and degree, and age older than 18y. Exclusion criteria were the following: severe dry eye, corneal scars, keratoconus, history of herpetic keratitis, history of uveitis, pseudoexfoliation syndrome, uncontrolled glaucoma, vitreomacular traction, lack of cooperation or tremor and previous ocular surgery. If patients failed to have both eyes operated (one eye with FLACS and the other eye with standard CPS), they were also excluded.
Preoperative assessment where the following examinations were performed: autorefraction, corrected distance visual acuity (CDVA), intraocular lens (IOL) power calculation, slit lamp examination with grading of FED, specular microscopy, Pentacam measurement and corneal density measurement.
Postoperative Assessment
Postoperatively follow up was performed after 1, 40, and 180d. The following measurements were performed by the same optometrist whom were blinded to operation method: autorefraction, CDVA with subjective refraction, Pentacam measurements with evaluation of corneal density, CCT, CPRT at 4 and 6 mm and endothelial imaging with ECD, hexagonality and rate of polymegethism described by coefficiency of variance.
Fuchs' Endothelial Dystrophy Grading
This was done clinically by the surgeon (Mensah AMA). Diagnosis was based on Krachmers grading system with findings of nonconfluent or confluent central guttate with or without edema in the slit lamp[10]. Specular microscopy was performed by an experienced optometrist: ECD, percentage of hexagonal cells and coefficient of variance were analysed using a noncontact specular microscope (SP 3000P, Topcon, Tokyo, Japan) with the Image-Net imaging system (version 4.0 Topcon, Tokyo, Japan). Corneal decompensation was defined as corneal edema causing a visual acuity lower than 20/50 in more than three months or the need of corneal transplantation[11].
Pentacam
Cataract grading, corneal densitometry, CCT and CPRT at 4 and 6 mm were analysed using Pentacam software (Oculus, Wetzlar, Germany). Cataract grading were performed objectively using lens density measurement from the Pentacam. This measurement has been shown to have high correlation to the LOCS III nuclear opacity classification[12]. The lens density measurement was taken as the peak value on an image at 120°-300° for the right eye and at 240°-260° for the left eye as recommended[12]. Corneal densitometry can be measured in 4 annular zones centred on the apex of the cornea (0-2, 2-6, 6-10, and 10-12 mm in diameter). We included all 4 zones in our data analysis. The densitometry measurement can also be provided for the anterior layer (first 120 µm; AL), central layer (from the first 120 µm to the posterior 60 µm; CL), and the posterior layer of the cornea (60 µm; PL). Densitometry is expressed in grayscale units (GSU), ranging from a minimum light scatter of 0 (maximum transparency) to a maximum light scatter of 100 (minimum transparency). We included all three layers in our dataanalysis.
Densitometry change was calculated by:
Densitometrypreop – Densitometrypostop
CPRT is a ratio determined by CCT divided by the mean of corneal thickness measurement at 4 or 6 mm superiorly, inferiorly, nasally and temporally. CPRT is expressed in percentage. Change in CCT or CPRT was calculated by subtracting the postoperative result (day 1, day 40, or day 180) from the preoperative result.
Endothelial Cell Imaging
Endothelial cell imaging was done as previously described[13]. Preoperatively and at day 40 and 180, three central photographs of each cornea were taken and analysed automatically by the Image-Net imaging system. Afterwards, a blinded observer chose the clearest image of the three and discarded the remaining two images. Hereafter the cell count performed by Image-Net was manually corrected according to the golden standard[14]–[15].
We calculated ECL by subtracting ECD on day 40 or 180 from the preoperative ECD. When calculating percentage ECL, the following formula was used:
 |
Randomization Technique
All patients were randomized using block randomization by a computer. Operation method was noted on a file and on operation day the surgeon would open the file and see what operation method was to be performed.
Surgery
Ultrasound energy
Infiniti® Vision System (Alcon Laboratories Inc, Fort Worth, TX, USA) uses cumulative dissipated energy (CDE) as a value for phaco energy. This is calculated as (phaco time×average phaco power)+(torsional time×0.4×average torsional amplitude). The factor 0.4 represents approximate reduction of heat dissipated at the incision as compared to non-torsional phaco.
Surgical technique
All patients were operated by the same experienced surgeon (Mensah AMA) and was considered past learning curve. All patients had their operation method randomized by a computer. The eye with the worst vision was operated first.
Femtosecond laser-assisted cataract surgery
The laser LensAR® (Topcon, Gamagori, Japan) procedure started with the docking of the laser with a 3D imaging of the anterior chamber. The software divides cataract types in 4 grades of density to which the surgeon can couple a definite nucleus fragmentation pattern and adapt the laser treatment to the cataract density. The nucleus patterns are various and can be combined as circles, radiuses and cubes. Treatment plan and images were confirmed before the laser procedure. The laser was performed capsulorhexis (5.0 mm) and nucleus fragmentation with various cutting pattern depending on the cataract density was chosen as suggested by the laser machine. Hereafter, the laser was disconnected, and the remaining surgery was done as CPS.
Conventional phaco surgery
Procedure was done as previously described[13]: A 1-mm side port was created with a keratome followed by instillation of 0.5 mL Lidocaine (10 mg/mL) and an ophthalmic viscosurgical device (Healon, Abbott Medical Optics, Santa Ana, USA). Then, a clear cornea main incision was fashioned with a 2.4-mm angled keratome. A continuous curvilinear capsulorhexis with an intended diameter of 5 mm was created (Corydon Forceps, Moria, France).
Phacoemulsification (Infiniti® Vision System, Alcon Laboratories Inc, Fort Worth, TX, USA) using the technique “stop and chop” and irrigation/aspiration (I/A) was performed. An aspheric, hydrophobic IOL (ZCB00, Abbott Medical Optics, Santa Ana, USA) was implanted using Healon, which was then aspirated. The procedure was concluded by instillation of 1 mL cefuroxime (2.5 mg/mL) and hydration of the incisions.
Statistical Analysis
All statistical analyses were performed using R software (version 1.0.136, Boston, USA). All baseline values are presented with median and range when not normally distributed and with a mean and standard deviation when normally distributed. Normally distributed results are presented with mean and 95% confidence interval (CI) and two tailed paired Student's t-test was used for between group comparative baseline statistics. Any P-value below 0.05 was found significant. To evaluate the repeated measurement Pentacam data and Image-Net data we used a linear mixed model with patients as random effect with covariance structure compound symmetry. There were no missing values. We used the same linear mixed model for the subgroup analysis on cataract grade. Finally, the pooled effect of CPS and FLACS was evaluated by comparing changes from baseline to follow up at day 1, day 40, and day 180, respectively, using a linear mixed model with patients as random effect to account for the correlation between measurements on two eyes from the same patient. To evaluate visual outcome all decimal values was converted to logMAR values for statistical analysis. Afterward the results were back-transformed into decimal values for an easier interpretation of the results for the reader.
RESULTS
This study included 68 eyes of 34 patients. Median age 75 (range 68-86)y. Seventeen females.
Complications
Totally 2 patients suffered from complications. One patient had an anterior capsule tear during nucleus fragmentation and had a sulcus IOL installed. This patient withstood from operation of the second eye. One patient suffered from branch vein occlusion after surgery and withstood from operation of the second eye. Both patients had FLACS procedure. One patient could not cooperate to dock the laser and had to be excluded for FLACS surgery. The remaining 31 patients all completed follow up at day 1, day 40, and day 180. One patient's Pentacam data for both eyes was accidently erased from the software thus only 30 patients were included in the Pentacam data analysis.
Three CPS patients suffered from corneal decompensation following surgery, all three were offered operation on the second eye as per protocol. Two withstood from further operations and one had Descemet membrane endothelial keratoplasty (DMEK).
Preoperative Data
Pentacam data
The two groups were comparable in preoperative measurements in all thickness and density measurements: CCT, CPRT4, CPRT6, density measurement in AL, CL, PL and annuli 0-2, 2-6, 6-10 and 10-12 (P>0.05; Table 1).
Table 1. Preoperative data.
| Parameters | FLACS (95%CI) | CPS (95%CI) | Pa |
| Mean cataract grade density (%) | 20.8 (18-22) | 20.5 (18-22) | 0.74 |
| Mean CCT preop (µm) | 576 (561-591) | 577 (563-591) | 0.66 |
| Mean CPRT4 (%) | 0.95 (0.95-0.95) | 0.95 (0.95-0:96) | 0.42 |
| Mean CPRT6 (%) | 0.90 (0.89-0.90) | 0.90 (0.89-0.91) | 0.87 |
| Mean ECD (cells/mm2) | 2486 (2234-2629) | 2613 (2395-2830) | 0.02 |
| Mean hexagonality (%) | 59 (55-63) | 59 (53-65) | 0.76 |
| Mean covariance | 28 (26-30) | 28 (27-30) | 0.59 |
aPaired t-test. Cataract grade, CCT, CPRT4, CPRT6, n=31; ECD, hexagonality and covariance, n=20. FLACS: Femto-assisted laser cataract surgery; CPS: Conventional phacoemulsification surgery; CCT: Central corneal thickness; CPRT: Corneal periphery ratio thickness; ECD: Endothelial cell density.
Surgical data
The cataract grade was comparable in the two groups (Table 1). Fluid use was 25% higher in FLACS compared to CPS. EPT and CDE was significantly lower in FLACS compared to CPS. Knife time and total procedure time was significantly higher in FLACS compared to CPS (Table 2).
Table 2. Baseline characteristics of cataract grade and surgical data.
| Parameters | FLACS (95%CI) | CPS (95%CI) | Pa |
| CDE (U/S) | 1.17 (0.64-1.71) | 7.9 (6.58-9.14) | 2.40×10−11 |
| Fluid use (mL) | 60.98 (54.54-67.39) | 45.0 (40.69-49.31) | 0.00014 |
| Phacoemulsification time (s) | 11.50 (6.35-16.65) | 47.25 (42.0-52.5) | 3.01×10−11 |
| Laser time (s) | 57.87 (45-70) | ||
| Knife time (min) | 12.38 (11.30-13.47) | 10.51 (9.55-11.48) | 0.01 |
| Docking time (min) | 2.36 (2.17-2.57) | ||
| Total procedure time (min) | 14.9 (13.7-16.1) | 10.51 (9.55-11.48) | 2.53×10−6 |
aPaired t-test. CDE in the FLACS group was decreased by 85% compared to CPS. Total procedure time includes laser time and docking time. FLACS: Femto-assisted laser cataract surgery; CPS: Conventional phacoemulsification surgery; CDE: Cumulative dissipated energy.
n=31
Postoperative Outcomes
Visual outcome
There was no difference in visual results between the two groups at any follow up. At day 1 CDVA was 0.5 (95%CI: 0.36-0.69) in FLACS and 0.60 (95%CI: 0.45-0.81) in CPS, at day 40, 1.03 (95%CI: 0.93-1.13) and 0.94 (95%CI: 0.83-1.04), at day 180, 1.01 (95%CI: 1.07-1.1) and 1.00 (95%CI: 0.89-1.13) (P>0.1), respectively.
Corneal outcome
Pentacam data
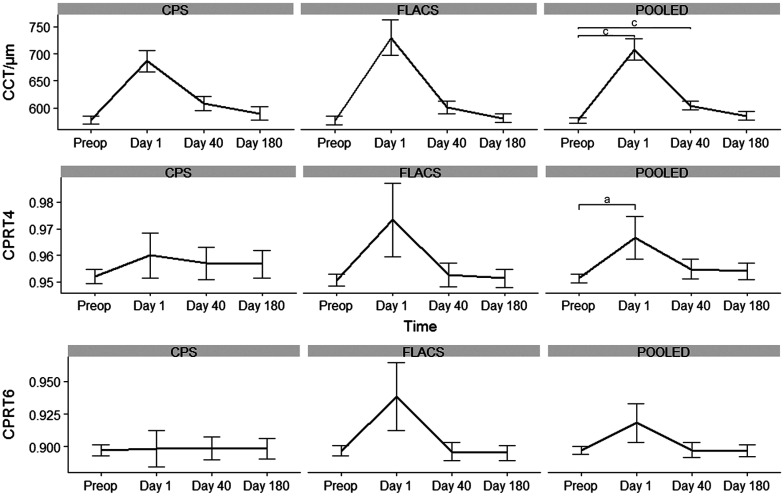
CPS compared to FLACS: no significant differences in CCT, CPRT4, CPRT6 outcome between the two methods were found. Densitometry data in all layers and all annuli from anterior layer to posterior layer in annuli 0-2, 2-6, 6-10 and 10-12 as well as total densitometry with all layers and all annuli were examined. There was a significant difference in annuli 6-10 and 10-12. In 6-10 the difference was in the posterior layer at day 1 with -1.42 GSU (95%CI: -2.66 to -0.19, P=0.02). In 10-12 the difference was in both the anterior layer, central layer and all layers combined at day 40, 7.7 (95%CI: 1.89-13.50, P=0.009), 3.97 (95%CI: 0.23-7.71, P=0.03), 4.73 GSU (95%CI: 0.71-8.75, P=0.02). In the remaining parameters no difference between the two groups was detected (P>0.05). There was a trend towards higher CCT, CPRT and corneal light backscatter at day 1 in the FLACS group compared to CPS (Figures 1 and 2).
Figure 1. Corneal thickness and central to periphery ratio 4 and 6 mm outcome at days 1, 40, and 180 in FLACS, CPS and data pooled.
Pooled: FLACS and CPS combined. Error bars: Standard error of the mean. aP<0.05; bP<0.01; cP<0.001. FLACS: Femto-assisted laser cataract surgery; CPS: Conventional phacoemulsification surgery; CCT: Central corneal thickness; CPRT: Corneal periphery ratio thickness.
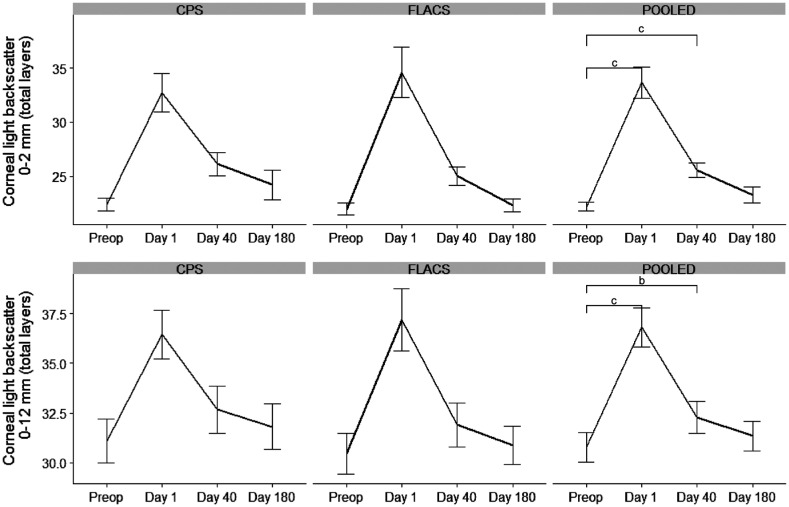
Figure 2. Corneal light backscatter at 0-2 mm annulus all layers and 0-12 mm annulus all layers.
There was no difference between CPS and FLACS. When pooling data there was a significant difference between preoperative and day 1 and day 40. The difference became insignificant at day 180. Error bars: standard error of the mean. Significance level: aP<0.05; bP<0.01; cP<0.001. FLACS: Femto-assisted laser cataract surgery; CPS: Conventional phacoemulsification surgery.
CPS and FLACS combined: To examine if surgery itself affects the corneal outcome, data from FLACS and CPS was combined to examine if follow up results differed from preoperative status. There was a significant difference from baseline to day 1 and from baseline to day 40 in all layers in 0-2 and 2-6 annuli as well as in total densitometry with all layers and all annuli (Figure 2). In annuli 6-10 there was significance in all layers between baseline and day 1 and in annuli 10-12 there was a significant difference at day 1 in the anterior layer and at days 1, 40, and 180 in the posterior layer (Table 3). CCT was significant different from baseline at day 1 and day 40 (131 µm, 95%CI: 96-165, P=3.12×10−8; 27 µm, 95%CI: 15-39, P=7.33×10−5). CPRT4 was significant difference at day 1 (0.015%, 95%CI: 0.004-0.03, P=0.049) but not at day 40 or 180. CPRT6 was not significant different from baseline at any follow up (Figure 1).
Table 3. Image-Net endothelial cell data for 20 patients pre- and post-operatively.
| Endothelial cell results | FLACS (95%CI) | CPS (95%CI) | Pa |
| Mean 40d ECL (cells/mm2) | 550 (330-770) | 450 (315-585) | 0.53 |
| ECL (%) | 23.67 | 17.30 | |
| Mean 180d ECL (cells/mm2) | 606 (440-772) | 559 (428-690) | 0.69 |
| ECL (%) | 25.58 | 21.32 | |
| Mean 40d hexagonality (%) | 56 (47-65) | 53 (45-60) | 0.78 |
| Mean 180d hexagonality (%) | 54 (44-62) | 56 (47-65) | 0.49 |
| Mean 40d covariance (%) | 29 (27-31) | 28 (26-30) | 0.52 |
| Mean 180d covariance (%) | 28 (26-30) | 27 (25-30) | 0.70 |
aPaired t-test. Preoperatively (n=20); Day 40 (n=20); Day 180 (n=20). FLACS: Femto-assisted laser cataract surgery; CPS: Conventional phacoemulsification surgery; ECL: Endothelial cell loss.
Image-Net data
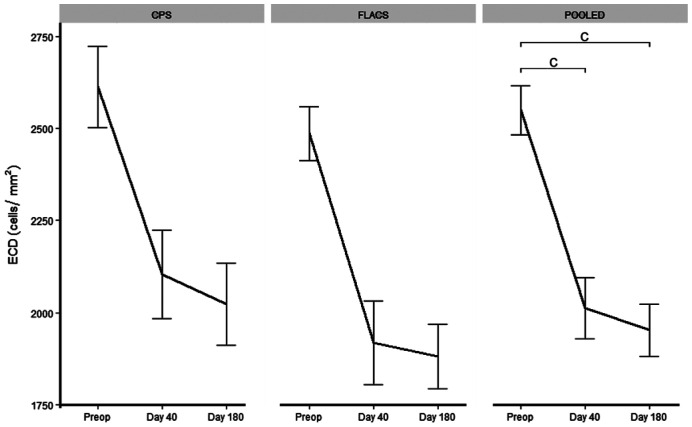
Totally 11 patients had uncountable preoperative ECD on both eyes. Of the remaining 20 patients the FLACS group had a lower ECD preoperatively (Table 3). The two groups had no significant difference in ECD, hexagonality or coefficient of variance at follow up day 40 and day 180. FLACS had ECL loss of 23.67% at day 40 and 25.58% at day 180 vs CPS with 17.30% at day 40 and 21.32% at day 180. The difference in ECL was non-significant (P>0.05). There was no difference in hexagonality change over time (P>0.05). An analysis on relatively change in ECD count was also performed with no difference at day 40 or day 180 between the two groups (P>0.05).
FLACS and CPS combined: ECD was significantly different from baseline at both day 40 and at day 180 with -530 cells/mm2, 95%CI: -705 to 357, P=1.62×10−5 and -610 cells/mm2, 95%CI: -749 to 472, P=1.86×10−7, respectively (Figure 3).
Figure 3. ECD in 20 patients.
There was no difference between CPS and FLACS. When pooling data there was a significant difference between preoperative status and day 40 and day 180. Error bars: Standard error of the mean. aP<0.05; bP<0.01; cP<0.001. FLACS: Femto-assisted laser cataract surgery; CPS: Conventional phacoemulsification surgery; ECD: Endothelial cell density.
Effect of Cataract Grade on Corneal Outcome
A sub analysis on cataract grade on all ImageNet and pentacam densitometry data was performed and there was no difference in any outcome between the two operation methods at any follow up (P>0.05).
DISCUSSION
This pilot study examined the effect of cataract surgery on the cornea in FED patients and compare the impact of FLACS compared to CPS.
There is no general agreement on which parameters to monitor FED patients with. Corneal thickness is known to vary widely in FED patients so a patient might have severe FED even though the CCT is below 600 µm[16]–[17]. Patients with FED above grade 3 has guttae with central confluence making cells borders difficult to be accurately seen and therefore ECD might not be accurately estimated when counting the cells by endothelial cell imaging[18]. Another problem with ECD is the large variation regionally from the centre to the periphery so there is a significant risk of sampling error when choosing this method. This was confirmed in our study where we had 11 patients out of 31 unable to evaluate ECD due to lack of visible cells. McLaren et al[5] developed a technique adjusting for the heterogenicity in ECD images. They calculated local ECD by plotting a small area without guttae and then counted the number of cells in this area. Here after effective ECD was found by dividing local ECD by the fraction of the image which was guttae free. This technique showed good correlation with subjective grading and seems more adequate; though it stills requires images where cells are visible thereby excluding images mainly containing guttae. Corneal light backscatter quantifies corneal opacities and has been used to assess visual outcomes in DMEK and Descemet's stripping automated endothelial keratoplasty (DSAEK) patients[9],[19]–[20]. In our study we found a significant difference in corneal light backscatter (0-6 annuli, all layers) when comparing all patients preoperative status to day 1 and day 40 indicating that these two endpoints might be suitable to evaluate short terms outcomes in FED patients. The effect was diminished at day 180 in all parameters albeit they might not be suitable for long term monitoring.
The peripheral cornea swells less compared to the CCT[21] and therefore it has been suggested that the ratio between CPRT could be a better objective outcome compared to CCT to diagnose and monitor FED patients. Repp et al[7] found CPRT to be a reliable objective and repeatable metric to assess FED. Repp et al[7] developed a formula which predicts FED using CPRT with a sensitivity of 78% and a specificity of 90% when a cut off value of 64 is used. All our patients had results above the cutoff number of 64 indicating that all patients included had relevant FED[7]. CPRT6 has been suggested as being superior to CPRT4[22]. Despite having patients with relevant FED and including CPRT6 in our analysis, we did not find any difference in CPRT6 outcome. We found a significant difference between CPRT4 preoperatively and at day 1 (Figure 1, pooled data), however, there was no difference at day 40 or day 180. CCT was significantly different at day 1 and day 40, but there was no difference between preoperative CCT preoperative and at day 180 (Figure 1). The lack of effect on corneal outcome at day 180 might be due to a redistribution of cells from the periphery to the center when corneal damage occurs[23] which could explain the near normalization of results at day 180.
We included all current corneal outcomes and in general the results of this study indicate that they might be affected shortly after cataract surgery but stabilizes and therefore they might not be suitable to monitor clinical outcome in FED patients.
Earlier studies examining ECL in FLACS compared to CPS in eyes without pre exiting endothelial dystrophy found ECL percentages of 4.3%-17.06%[24]–[28]. There have only been a few case studies examining ECL in FED patients and they found an ECL of 0.7% to 13.5%[29]–[30]. We found no difference in ECL between FLACS and CPS. However, we only had results of 20 patients which could influence the result. Recently, Yong et al[31] examined ECL in 68 FLACS compared to 72 CPS in a retrospective study. They found no difference in CCT outcome between the two groups but found a significantly less ECL in FLACS (14.2% ECL in CPS and 6.5% in FLACS). Difference in the two groups was more pronounced in patients with hard cataracts. Their study has several weaknesses: use of different surgeons with different expertise, large differences in follow up with some patients seen after 3mo and others after 17mo and they excluded 125 patients out of 265 without further explanation. Concerns of the methodology and the conclusion presented in the study has been questioned by others[32]. A retrospective study by Zhu et al[11] examined CCT, corneal edema and decompensation between 143 CPS patients and 64 FLACS patients. They found a significantly higher proportion of edema at one month in the FLACS group, but no difference between the two groups after 3 and 6mo. They reported no significant difference in corneal decompensation, CDVA outcome or CCT outcome. They found that higher grade of FED and cataract correlated with greater corneal edema duration and severe edema. Weakness was they only had preoperative CCT measurement for 18 FLACS patients, they excluded advanced FED and the clinical FED grading was done by several clinicians. In the two retrospective studies they found more corneal decompensations in the FLACS group compared to the CPS group.
We had three patients with corneal decompensation in the CPS groups while no decompensation in the FLACS group. As both ECD and pentacam data seem inferior to monitor FED patients, it might be that the only reliable clinical outcome for FED patients is corneal decompensation. By combining the number of decompensations found in this study and the two retrospective studies we have a total of 6 decompensation out of 166 FLACS patients (risk of 0.03) and 16 decompensations out of 249 CPS patients (risk of 0.06). A sample size calculation based on a Chi-squared statistics states that to detect a difference between FLACS and CPS in FED patients with a power of 0.8, we would need 815 in each group, a total of 1630 patients.
EPT and phaco energy is believed to be the main factors for ECL. We found that EPT was reduced by 75% and CDE with 85% in FLACS compared to CPS. However, the significantly lower EPT and CDE in FLACS compared to CPS was not reflected in our results. Fluid use is also believed to cause endothelial damage due to turbulence[33]–[34]. A few studies have reported fluid use[26],[35] in FLACS and CPS and found no difference in fluid use between the two methods. We found a 25% higher fluid use in FLACS compared to CPS, however despite the difference in fluid use, both groups used substantially less fluid than reported in the earlier studies, 45 mL in CPS and 61 mL in FLACS compared to reports of 85-91 mL. We also found significantly higher fluid use in a previous study comparing FLACS with CPS in healthy eyes[13]. We report a significantly higher knife time in FLACS compared to CPS. This is in contrast with previous findings where knife time is shorter in FLACS compared to CPS primarily explained by the preoperative laser treatment in FLACS patients[24],[26],[28],[31]. Our knife time was 12min in FLACS and 10min in CPS, so both are still relatively short. However, it might be that the longer knife time and higher fluid use in our FLACS eyes caused more manipulation in the eyes and thereby diminishes the positive effect of the lower CDE and EPT on the endothelium. The longer knife time and fluid use could be due to extra manipulation and aspiration irrigation performed after FLACS laser procedure due to difficulty in removal of cortex[33],[36].
At day 1 we saw a trend towards higher corneal impact in the FLACS groups (Figures 1 and 2). FLACS capsulotomy causes an increase in prostaglandin release and this can lead to miosis and an increase in CCT. We treated the patients with ketorolac one day before the operation as this should decrease the prostaglandin release[37]–[41]. However, the surgeon still experienced miosis in all FLACS patients and therefore there might still have been an increase in prostaglandin release which, alongside a longer knife time and extra fluid use, might explain the trend in corneal outcome at day 1.
Our study is strengthened by its prospective design, no missing values at follow up, objective evaluating of FED by the method suggested by Repp et al[7], analysis of most common used and latest recommended FED outcome, use of a single experienced surgeon, intra-individual control and randomization. This study is limited by its small sample size as well as having mainly LOCS grade 3 cataract and only having Image-Net data for 20 patients.
In conclusion, our results did not demonstrate a significant benefit for FLACS in FED patients. We found that both ECD and pentacam corneal data are inadequate as outcome parameters in FED patients. Our results suggest that corneal decompensation should be the main outcome measure for clinical studies of FED patients.
Acknowledgments
Conflicts of Interest: Krarup T, None; Rose K, None; Mensah AMA, None; la Cour M, None; Holm LM, None.
REFERENCES
- 1.Nagy Z, Takacs A, Filkorn T, Sarayba M. Initial clinical evaluation of an intraocular femtosecond laser in cataract surgery. J Refract Surg. 2009;25(12):1053–1060. doi: 10.3928/1081597X-20091117-04. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Xiao W, Ye S, Chen W, Liu Y. Efficacy and safety of femtosecond laser-assisted cataract surgery versus conventional phacoemulsification for cataract: a meta-analysis of randomized controlled trials. Sci Rep. 2015;5:13123. doi: 10.1038/srep13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abell RG, Kerr NM, Vote BJ. Toward zero effective phacoemulsification time using femtosecond laser pretreatment. Ophthalmology. 2013;120(5):942–948. doi: 10.1016/j.ophtha.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 4.Seitzman GD, Gottsch JD, Stark WJ. Cataract surgery in patients with Fuchs' corneal dystrophy: expanding recommendations for cataract surgery without simultaneous keratoplasty. Ophthalmology. 2005;112(3):441–446. doi: 10.1016/j.ophtha.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 5.McLaren JW, Bachman LA, Kane KM, Patel SV. Objective assessment of the corneal endothelium in Fuchs' endothelial dystrophy. Invest Ophthalmol Vis Sci. 2014;55(2):1184–1190. doi: 10.1167/iovs.13-13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopplin LJ, Przepyszny K, Schmotzer B, Rudo K, Babineau DC, Patel SV, Verdier DD, Jurkunas U, Iyengar SK, Lass JH, Fuchs' Endothelial Corneal Dystrophy Genetics Multi-Center Study Group Relationship of Fuchs endothelial corneal dystrophy severity to central corneal thickness. Arch Ophthalmol. 2012;130(4):433–439. doi: 10.1001/archophthalmol.2011.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Repp DJ, Hodge DO, Baratz KH, McLaren JW, Patel SV. Fuchs' endothelial corneal dystrophy: subjective grading versus objective grading based on the central-to-peripheral thickness ratio. Ophthalmology. 2013;120(4):687–694. doi: 10.1016/j.ophtha.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Dijk K, Rodriguez-Calvo-de-Mora M, van Esch H, Frank L, Dapena I, Baydoun L, Oellerich S, Melles GR. Two-year refractive outcomes after descemet membrane endothelial keratoplasty. Cornea. 2016;35(12):1548–1555. doi: 10.1097/ICO.0000000000001022. [DOI] [PubMed] [Google Scholar]
- 9.Alnawaiseh M, Rosentreter A, Prokosch V, Eveslage M, Eter N, Zumhagen L. Changes in corneal densitometry in patients with fuchs endothelial dystrophy after endothelial keratoplasty. Curr Eye Res. 2017;42(2):163–167. doi: 10.3109/02713683.2016.1146774. [DOI] [PubMed] [Google Scholar]
- 10.Krachmer JH. Corneal endothelial dystrophy. Arch Ophthalmol. 1978;96(11):2036. doi: 10.1001/archopht.1978.03910060424004. [DOI] [PubMed] [Google Scholar]
- 11.Zhu DC, Shah P, Feuer WJ, Shi W, Koo EH. Outcomes of conventional phacoemulsification versus femtosecond laser-assisted cataract surgery in eyes with Fuchs endothelial corneal dystrophy. J Cataract Refract Surg. 2018;44(5):534–540. doi: 10.1016/j.jcrs.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pei X, Bao Y, Chen Y, Li X. Correlation of lens density measured using the Pentacam Scheimpflug system with the Lens Opacities Classification System III grading score and visual acuity in age-related nuclear cataract. Br J Ophthalmol. 2008;92(11):1471–1475. doi: 10.1136/bjo.2007.136978. [DOI] [PubMed] [Google Scholar]
- 13.Krarup T, Ejstrup R, Mortensen A, la Cour M, Holm LM. Comparison of refractive predictability and endothelial cell loss in femtosecond laser-assisted cataract surgery and conventional phaco surgery: prospective randomised trial with 6 months of follow-up. BMJ Open Ophthalmol. 2019;4(1):e000233. doi: 10.1136/bmjophth-2018-000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Schaick W, van Dooren BT, Mulder PG, Völker-Dieben HJ. Validity of endothelial cell analysis methods and recommendations for calibration in Topcon SP-2000P specular microscopy. Cornea. 2005;24(5):538–544. doi: 10.1097/01.ico.0000151505.03824.6c. [DOI] [PubMed] [Google Scholar]
- 15.Cheung SW, Cho P. Endothelial cells analysis with the TOPCON specular microscope SP-2000P and IMAGEnet system. Curr Eye Res. 2000;21(4):788–798. doi: 10.1076/ceyr.21.4.788.5548. [DOI] [PubMed] [Google Scholar]
- 16.Prasad A, Fry K, Hersh PS. Relationship of age and refraction to central corneal thickness. Cornea. 2011;30(5):553–555. doi: 10.1097/ICO.0b013e3181fb880c. [DOI] [PubMed] [Google Scholar]
- 17.Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol. 2000;44(5):367–408. doi: 10.1016/s0039-6257(00)00110-7. [DOI] [PubMed] [Google Scholar]
- 18.Syed ZA, Tran JA, Jurkunas UV. Peripheral endothelial cell count is a predictor of disease severity in advanced fuchs endothelial corneal dystrophy. Cornea. 2017;36(10):1166–1171. doi: 10.1097/ICO.0000000000001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivarsen A, Hjortdal J. Recipient corneal thickness and visual outcome after Descemet's stripping automated endothelial keratoplasty. Br J Ophthalmol. 2014;98(1):30–34. doi: 10.1136/bjophthalmol-2013-304042. [DOI] [PubMed] [Google Scholar]
- 20.Chu HY, Hsiao CH, Chen PY, Ma DH, Chang CJ, Tan HY. Corneal backscatters as an objective index for assessing Fuchs' endothelial corneal dystrophy: a pilot study. J Ophthalmol. 2017;2017:8747013. doi: 10.1155/2017/8747013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunette I, Sherknies D, Terry MA, Chagnon M, Bourges JL, Meunier J. 3-D characterization of the corneal shape in Fuchs dystrophy and pseudophakic keratopathy. Invest Ophthalmol Vis Sci. 2011;52(1):206–214. doi: 10.1167/iovs.09-4101. [DOI] [PubMed] [Google Scholar]
- 22.Alnawaiseh M, Zumhagen L, Wirths G, Eveslage M, Eter N, Rosentreter A. Corneal densitometry, central corneal thickness, and corneal central-to-peripheral thickness ratio in patients with Fuchs endothelial dystrophy. Cornea. 2016;35(3):358–362. doi: 10.1097/ICO.0000000000000711. [DOI] [PubMed] [Google Scholar]
- 23.Cho YK, Chang HS, Kim MS. Risk factors for endothelial cell loss after phacoemulsification: comparison in different anterior chamber depth groups. Korean J Ophthalmol. 2010;24(1):10–15. doi: 10.3341/kjo.2010.24.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu YH, Chen XY, Hua HX, Wu MH, Lai KR, Yao K. Comparative outcomes of femtosecond laser-assisted cataract surgery and manual phacoemusification: a six-month follow-up. Clin Exp Ophthalmol. 2016;44(6):472–480. doi: 10.1111/ceo.12695. [DOI] [PubMed] [Google Scholar]
- 25.Krarup T, Holm LM, la Cour M, Kjaerbo H. Endothelial cell loss and refractive predictability in femtosecond laser-assisted cataract surgery compared with conventional cataract surgery. Acta Ophthalmol. 2014;92(7):617–622. doi: 10.1111/aos.12406. [DOI] [PubMed] [Google Scholar]
- 26.Conrad-Hengerer I, Al Juburi M, Schultz T, Hengerer FH, Dick HB. Corneal endothelial cell loss and corneal thickness in conventional compared with femtosecond laser-assisted cataract surgery: three-month follow-up. J Cataract Refract Surg. 2013;39(9):1307–1313. doi: 10.1016/j.jcrs.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 27.Takács AI, Kovács I, Miháltz K, Filkorn T, Knorz MC, Nagy ZZ. Central corneal volume and endothelial cell count following femtosecond laser-assisted refractive cataract surgery compared to conventional phacoemulsification. J Refract Surg. 2012;28(6):387–391. doi: 10.3928/1081597X-20120508-02. [DOI] [PubMed] [Google Scholar]
- 28.Abell RG, Kerr NM, Howie AR, Mustaffa Kamal MA, Allen PL, Vote BJ. Effect of femtosecond laser-assisted cataract surgery on the corneal endothelium. J Cataract Refract Surg. 2014;40(11):1777–1783. doi: 10.1016/j.jcrs.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 29.Gavriş M, Horge I, Avram E, Belicioiu R, Olteanu IA, Kedves H. Fuchs endothelial corneal dystrophy: is femtosecond laser-assisted cataract surgery the right approach? Rom J Ophthalmol. 2015;59(3):159–163. [PMC free article] [PubMed] [Google Scholar]
- 30.Yamazoe K, Yamaguchi T, Hotta K, Satake Y, Konomi K, Den S, Shimazaki J. Outcomes of cataract surgery in eyes with a low corneal endothelial cell density. J Cataract Refract Surg. 2011;37(12):2130–2136. doi: 10.1016/j.jcrs.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 31.Yong WWD, Chai HC, Shen L, Manotosh R, Anna Tan WT. Comparing outcomes of phacoemulsification with femtosecond laser-assisted cataract surgery in patients with fuchs endothelial dystrophy. Am J Ophthalmol. 2018;196:173–180. doi: 10.1016/j.ajo.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Patel SV, Baratz KH. Comparing outcomes of phacoemulsification with femtosecond laser-assisted cataract surgery in patients with Fuchs endothelial dystrophy. Am J Ophthalmol. 2019;199:258–259. doi: 10.1016/j.ajo.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi K, Hayashi H, Nakao F, Hayashi F. Risk factors for corneal endothelial injury during phacoemulsification. J Cataract Refract Surg. 1996;22(8):1079–1084. doi: 10.1016/s0886-3350(96)80121-0. [DOI] [PubMed] [Google Scholar]
- 34.Mahdy MA, Eid MZ, Mohammed MA, Hafez A, Bhatia J. Relationship between endothelial cell loss and microcoaxial phacoemulsification parameters in noncomplicated cataract surgery. Clin Ophthalmol. 2012;6:503–510. doi: 10.2147/OPTH.S29865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy KP, Kandulla J, Auffarth GU. Effectiveness and safety of femtosecond laser-assisted lens fragmentation and anterior capsulotomy versus the manual technique in cataract surgery. J Cataract Refract Surg. 2013;39(9):1297–1306. doi: 10.1016/j.jcrs.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 36.Bali SJ, Hodge C, Lawless M, Roberts TV, Sutton G. Early experience with the femtosecond laser for cataract surgery. Ophthalmology. 2012;119(5):891–899. doi: 10.1016/j.ophtha.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 37.Schultz T, Joachim SC, Stellbogen M, Dick HB. Prostaglandin release during femtosecond laser-assisted cataract surgery: main inducer. J Refract Surg. 2015;31(2):78–81. doi: 10.3928/1081597X-20150122-01. [DOI] [PubMed] [Google Scholar]
- 38.Schultz T, Joachim SC, Kuehn M, Dick HB. Changes in prostaglandin levels in patients undergoing femtosecond laser-assisted cataract surgery. J Refract Surg. 2013;29(11):742–747. doi: 10.3928/1081597X-20131021-03. [DOI] [PubMed] [Google Scholar]
- 39.Tsikripis P, Papaconstantinou D, Koutsandrea C, Apostolopoulos M, Georgalas I. The effect of prostaglandin analogs on the biomechanical properties and central thickness of the cornea of patients with open-angle glaucoma: a 3-year study on 108 eyes. Drug Des Devel Ther. 2013;7:1149–1156. doi: 10.2147/DDDT.S50622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiss HJ, Takacs AI, Kranitz K, Sandor GL, Toth G, Gilanyi B, Nagy ZZ. One-day use of preoperative topical nonsteroidal anti-inflammatory drug prevents intraoperative prostaglandin level elevation during femtosecond laser-assisted cataract surgery. Curr Eye Res. 2016;41(8):1064–1067. doi: 10.3109/02713683.2015.1092556. [DOI] [PubMed] [Google Scholar]
- 41.Diakonis VF, Anagnostopoulos AG, Moutsiopoulou A, Yesilirmak N, Cabot F, Waren DP, O'Brien TP, Yoo SH, Weinstock RJ, Donaldson KE. The effect of NSAID pretreatment on aqueous humor prostaglandin E2 concentration in eyes undergoing femtosecond laser-assisted capsulotomy. J Ophthalmol. 2018;2018:1891249. doi: 10.1155/2018/1891249. [DOI] [PMC free article] [PubMed] [Google Scholar]