Abstract
Introduction
Alarming outcomes have been reported following infected endovascular aortic aneurysm repair (EVAR) device explantation. Infected fenestrated EVAR (FEVAR) exposes patients to even worse procedural risks.
Report
A 67 year old man with a prior history of FEVAR presented with impaired general condition, abdominal and back pain, and increased C reactive protein. Computed tomography angiography revealed a collection around the aortic graft bifurcation and 18F-fluorodeoxyglucose–positron emission tomography (FDG-PET) revealed increased FDG uptake at this level, confirmed by labelled white blood cells, all favouring graft infection. A thoracophrenolumbotomy was performed and revealed an aorto-enteric fistula which was treated by small bowel resection. The left renal artery was transected at the distal end of the bridging stent and a thoracorenal bypass was performed. The thoracic aorta was cross clamped above the coeliac trunk for complete graft excision. Meanwhile, the right kidney was perfused with 4°C Ringer lactate solution. In situ reconstruction was accomplished with a bifurcated antimicrobial graft sutured below the superior mesenteric artery with re-implantation of the right renal artery. The patient was left with a laparostomy for definitive abdominal closure, restoration of the digestive tract, and omental wrap 72 hours later. Broad spectrum antibiotic therapy was initiated peri-operatively and reduced to sulfamethoxazole/trimethoprim for a total duration of six weeks after one sample was positive for Moraxella osloensis. Eleven months later, the patient was free from re-infection, with no fever or inflammatory syndrome.
Discussion
Total explantation of stent grafts with tissue debridement and post-operative antibiotic therapy is the gold standard when dealing with infected EVAR. As with type IV thoraco-abdominal aneurysm open repair, FEVAR device explantation requires additional protective measures to prevent visceral ischaemia and renal impairment. In agreement with the European Society for Vascular Surgery guidelines, such patients should be referred to dedicated vascular centres with expertise in surgical repair, anaesthetics, and post-operative intensive care.
Keywords: Aorto-enteric fistula, Fenestrated endovascular aneurysm repair, Graft infection, Renal bypass, Visceral protection
Highlights
-
•
Alarming outcomes have been reported following infected endovascular aortic aneurysm repair (EVAR) explantation.
-
•
Total explantation of stent grafts with tissue debridement and post-operative antibiotic therapy is the gold standard.
-
•
FEVAR explantation requires additional protective measures to prevent visceral ischaemia and kidney impairment.
Introduction
Widespread use of endovascular aortic aneurysm repair (EVAR) is accompanied by an increase in aortic graft infection with an incidence of 1% to 8%,1 occurring as a primary event as a result of peri-operative contamination, haematogenous seeding, or mechanical erosion. Non-specific symptoms may delay the diagnosis. Progression affects the aneurysm sac, stent, and peri-aortic tissues. If not interrupted, it evolves into rupture or disseminated sepsis.2 A third of cases present as chronic/acute sepsis and secondary aorto-enteric fistula (SAEF), which can lead to gastro-intestinal bleeding and haemorrhagic shock.2 It represents one of the most threatening complications and a therapeutic challenge since over 60% of patients die if left untreated.3,4 As reported in the latest European Society for Vascular Surgery (ESVS) guidelines, treatment goals are to eradicate the infectious source by “complete excision of all graft material and infected tissue”.5 Infected fenestrated EVAR (FEVAR) exposes patients to even worse procedural risks, as reported in this case of complete FEVAR excision for SAEF.
Report
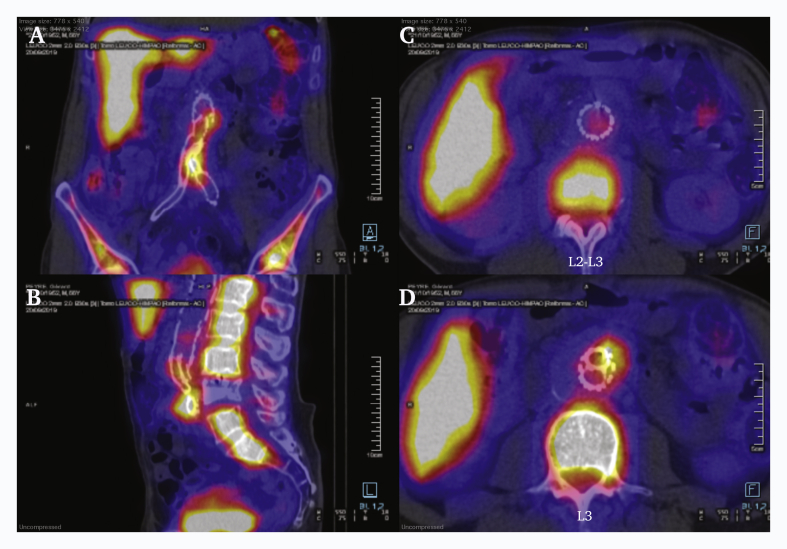
A 67 year old man presented with impaired general condition, and abdominal and lower back pain on day one following his latest endovascular procedure. One year before, he had undergone FEVAR with a Zenith fenestrated graft (Cook Medical, Bloomington, IN, USA), including a superior mesenteric artery (SMA) scallop, fenestration and bridging stent for each renal artery, and a bifurcated endograft to treat a proximal pseudo-aneurysm following an aorto-iliac Dacron bypass implanted 12 years previously for an infrarenal abdominal aortic aneurysm. Multiple distal revision procedures were subsequently required and are summarised in Table 1. C reactive protein (CRP) was 330 mg/L, and white blood cell count (WBC) was 7.2 × 109/L, without fever. He had suffered significant weight loss (18 kg) over the previous three months. In hospital, urine, blood cultures and pulmonary radiography were negative, with no ultrasound evidence of endocarditis. Computed tomography angiography (CTA) revealed a collection around the aortic graft bifurcation, and positron emission tomography (PET) revealed increased 18F-fluorodeoxyglucose (FDG) uptake at this level (Fig. 1), confirmed by labelled WBC scintigraphy.
Table 1.
Sequence of vascular surgery procedures and indications.
| Date of procedure | Type of procedure | Indication |
|---|---|---|
| 12 years ago | Aorto-iliac bypass with Dacron graft implanted distally to the right external iliac artery (ligature of hypogastric artery) and left common iliac artery (laparotomy) | Infrarenal abdominal aortic aneurysm |
| 11 years ago | Right supragenicular femoropopliteal PTFE bypass | Severe claudication after SFA occlusion |
| 9 years ago | Angioplasty and stenting of right femoropopliteal bypass distal anastomosis | Severe claudication after myointimal hyperplasia stenosis |
| 7 years ago | Right ilioprofunda bypass | Acute limb ischaemia after occlusion of right femoropopliteal bypass due to graft degeneration and severe calcific stenosis of the common femoral artery and the profunda |
| 1 year ago | FEVAR (Zenith fenestrated, Cook Medical, Bloomington, IN, USA):
|
Proximal pseudo-aneurysm |
| 6 months ago | Angioplasty and stenting of proximal anastomosis of right ilioprofunda bypass | Critical limb ischaemia after myointimal hyperplasia stenosis |
| Present | Complete excision of endografts, bridging stents, and ancient Dacron graft Left thoracorenal bypass in situ reconstruction with bifurcated antimicrobial graft implanted below SMA, re-implantation of right renal artery to main body graft (thoracophrenolumbotomy, ligature of left hypogastric artery) |
Graft infection (of note, there were no symptoms or signs of bowel ischaemia at any time point) |
PTFE = polytetrafluoroethylene; SFA = superficial femoral artery; FEVAR = fenestrated endovascular aortic repair; SMA = superior mesenteric artery.
Figure 1.
Pre-operative computed tomography and fluorodeoxyglucose (FDG) positron emission tomography. (A, B, D) All three locations had clear elevations in maximum standard uptake value (SUVmax) and tissue to background ratio with focal FDG accumulation in the graft. (C) The proximal part of the graft, located in the para-renal segment, appears to be free from focal FDG accumulation (A, B, D) While the mid and distal part of the graft show clear signs of infection.
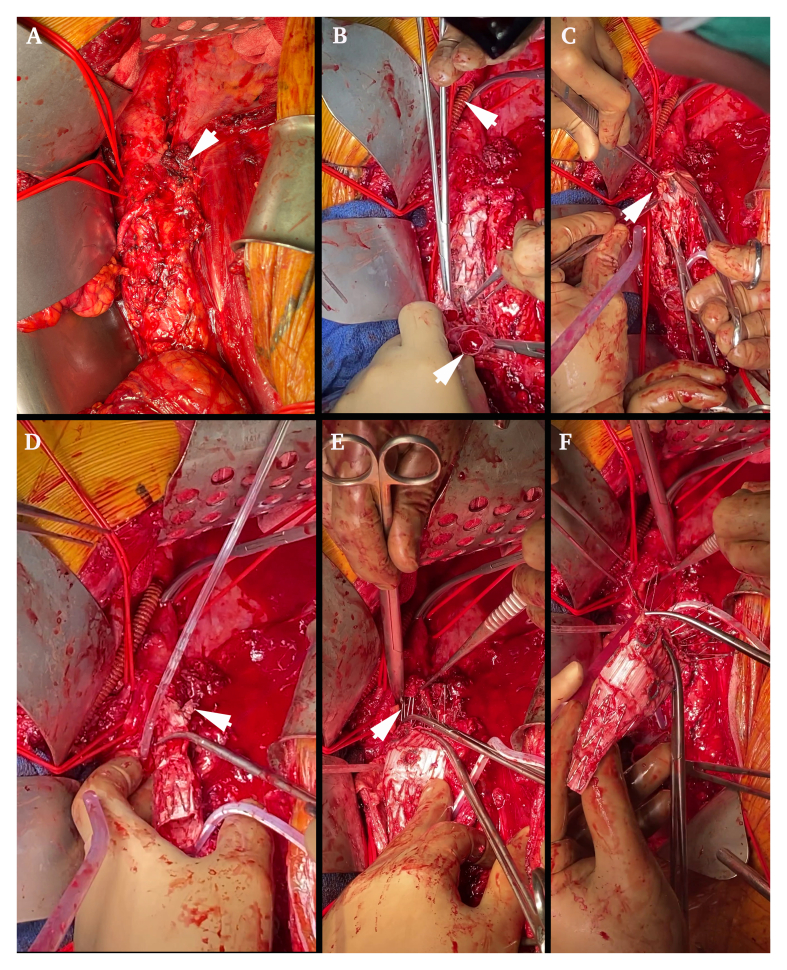
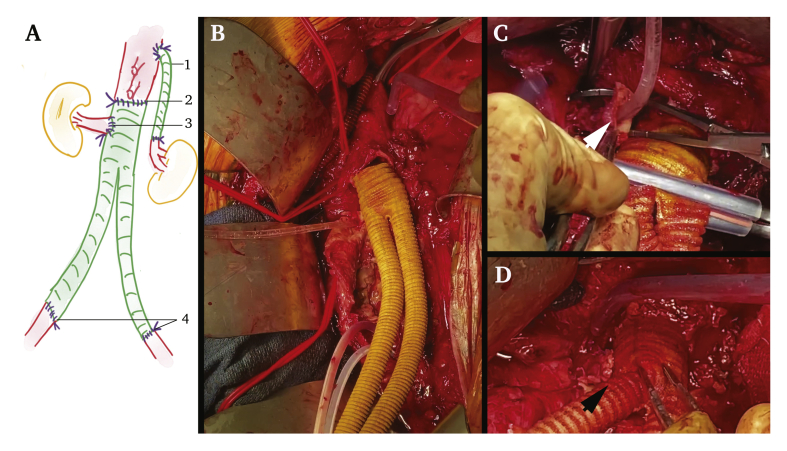
After a multidisciplinary meeting, surgical management without pre-operative antibiotic therapy was selected. A thoracophrenolumbotomy in the ninth intercostal space and circumferential phrenotomy were performed. Dissection was difficult near the graft attachment sites, with a reactive process similar to that encountered in inflammatory aneurysms, especially near the right iliac artery, revealing a small bowel fistula (Fig. 2) with enteral leak and peri-graft collections without clear purulence. The fistula was resected through a transperitoneal window and the stumps were left for secondary anastomosis. The left renal artery was transected at the distal end of the bridging stent and a thoracorenal bypass was performed using a non-ringed 7 mm diameter Synergy antimicrobial graft (AMG) (Getinge, Bensheim, Germany) to limit the left renal warm ischaemia time to 25 minutes (Fig. 3A 1). The thoracic aorta was cross clamped below the thoracorenal bypass to perform complete excision of the grafts (endograft, bridging stents, and Dacron graft). Haemodynamic changes were managed with the surgical “clamp and go technique” and anticipated with preventive amine administration and fluid resuscitation. Complete debridement of all of the macroscopically infected tissues was performed with complete aortectomy and resection of the peri-aortic tissues. The right renal bridging stent was gently avulsed, and the right kidney was perfused using 4°C Ringer lactate solution (Fig. 3B). The proximal portion of the stent graft was retrieved by squeezing the upper stent and pushing the hooks in a proximal direction. In situ reconstruction was accomplished with a bifurcated AMG of 20/10 mm diameter sutured just below the SMA with a warm ischaemic time of 30 minutes for both visceral arteries (Fig. 3A 2 and 3C). The right renal artery was re-implanted in the main body after one hour of cold ischaemia (Fig. 3A 3 and 3D). Distal anastomoses were performed to both external iliac arteries (the right hypogastric artery had been ligated 12 years previously and the left was ligated during this procedure) (Fig. 3A 4). A left chest drain was left in place for 48 hours and the patient was left with a laparostomy with negative pressure wound therapy (ABthera®, KCI, USA) and retroperitoneal packing on account of hypothermia (34.8°C), length of surgery (6 hours and 8 minutes), and the need for a second look. The procedure was supported by intra- and post-operative broad spectrum antibiotic therapy with meropenem, linezolid, and caspofungin.
Figure 2.
Explantation of the fenestrated endograft. (A) Picture of the aorto-enteric fistula (arrowhead) and the important local inflammation responsible for increased difficulty in surgical debridement. (B) Thoracic aorta to left renal bypass with an antimicrobial graft performed before explantation (upper arrowhead), and explantation of the limbs first by unplugging the distal modules (lower arrowhead). (C) Explantation of the left renal stent graft (arrowhead). (D) Explantation of the right renal stent graft (arrowhead) and perfusion of the right kidney using a 4°C Ringer lactate solution. (E) Explantation of the barbs and the hooks of the fenestrated graft by squeezing the upper stent and gently pushing the stent hooks in a proximal direction (arrowhead). (F) Complete explantation of the fenestrated module.
Figure 3.
Reconstruction with an aorto-bi-iliac antimicrobial graft. (A) Sketch of the aortic reconstruction, always recorded in the patient's files: (1) left thoracorenal bypass using a 7 mm diameter antimicrobial graft to limit left renal warm ischaemia time to 25 minutes; (2) cross clamping of the thoracic aorta above the coeliac trunk and below the thoraco-renal bypass to perform complete excision of grafts and debridement of devitalised tissue while perfusing the right kidney using a 4°C Ringer lactate solution. In situ reconstruction with a bifurcated antimicrobial graft of 20/10 mm in diameter sutured below the superior mesenteric artery, with a warm ischaemic time of 30 minutes for the coeliac trunk and superior mesenteric artery; (3) direct re-implantation of the right renal artery into the main body after one hour of cold ischaemia; (4) distal anastomoses on both external iliac arteries. (B) Proximal aortic anastomosis performed below the superior mesenteric artery and the coeliac trunk (identified with the two upper red silastic slings) but above the renal arteries, using an antimicrobial bifurcated graft. (C) Cross clamping of the right renal artery just before performing its re-implantation. (D) Re-implantation of the right renal artery to the main body of the aortic graft.
In the intensive care unit, he presented with lactate acidosis secondary to acute pneumonia acquired under mechanical ventilation, which was treated with sulfamethoxazole/trimethoprim after bronchial samples were positive for Stenotrophomonas pneumoniae. A second look was performed 72 hours later, with restoration of the digestive tract through manual lateral iso-peristaltic ileo-ileal anastomosis (absorbable 4/0 single stranded sutures) after continuous running sutures to bury the previous rows of ileal staples, omental wrap, and definitive fascial closure. Renal function declined from 78 mL/min/1.73 m2 pre-operatively to 38.4 mL/min/1.73 m2 on day three but recovered before discharge (Fig. S1) without the need for temporary extrarenal support. The hospital stay was 30 days, including 20 days in a surgical resuscitation ward.
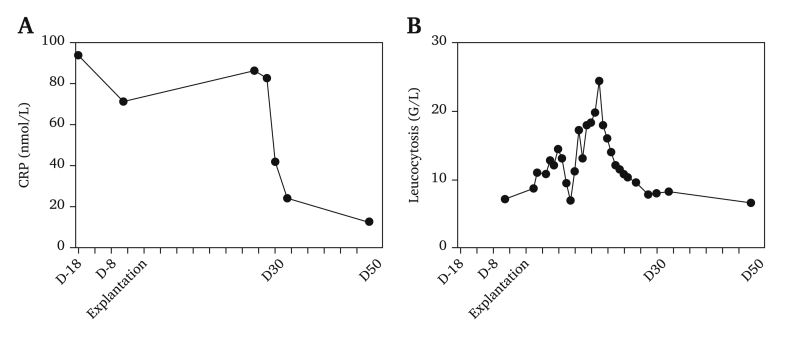
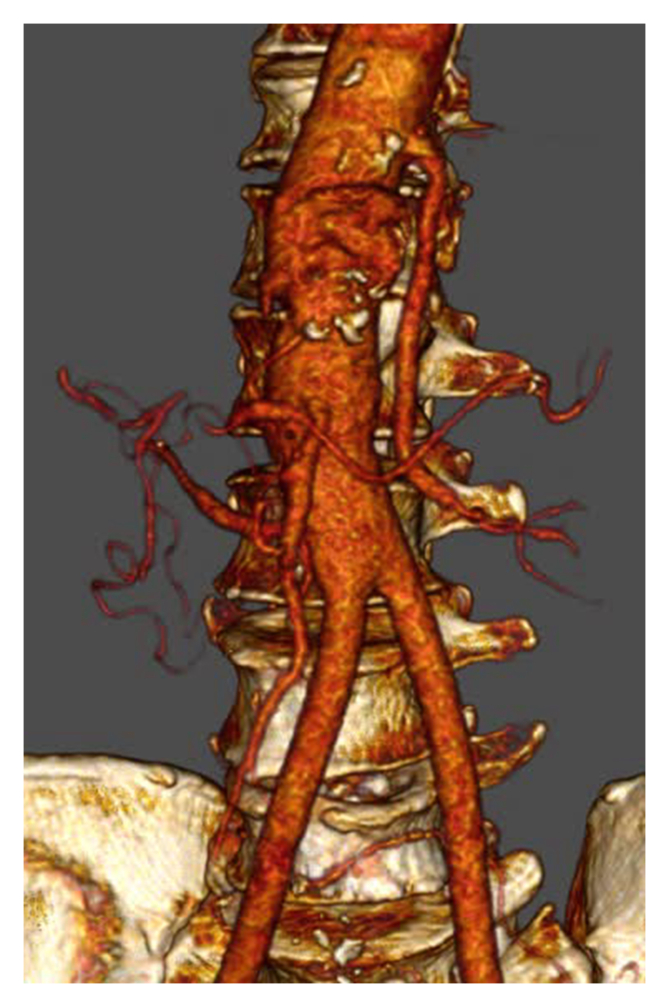
Peri-operative samples (peritoneal fluids, endograft, bridging stents, Dacron graft, peri-aortic collections) were sent for bacteriological and mycological analysis. Only one peri-aortic collection was positive for Moraxella osloensis. The antibiotic regimen was changed to piperacillin/tazobactam while maintaining linezolid and caspofungin for 13 days and was then switched to sulfamethoxazole/trimethoprim for a total of six weeks, which is the usual duration as soon as a sample returns positive. Eleven months later, the patient was free from re-infection (CRP: 13 mg/L, WBC: 6.7 × 109/L, Fig. 4), with normalised renal function (70 mL/min/1.73 m2), good general condition, a patent reconstruction (Fig. S2, Fig. 5) and no increased 18F-FDG uptake on control PET. However, at 12 months, he presented with acute occlusion of the left renal artery and a decline in renal function to 25 mL/min/1.73 m2.
Figure 4.
Graph of the evolution of the inflammatory biological syndrome. (A) Evolution of the C reactive protein before and after explantation. (B) Evolution of leucocytosis before and after explantation.
Figure 5.
Patency of the aortic reconstruction on computed tomography angiography.
Written informed consent for publication of his clinical details and clinical images was obtained from the patient.
Discussion
This is the first report of successful FEVAR explantation managed as a type IV thoraco-abdominal aneurysm (TAA). Two other FEVAR explantations have been reported: (1) a juxtarenal aneurysm with SAEF 26 months after the primary procedure,6 cross clamped over the renal arteries through median laparotomy, and sutured below the renal arteries with two year follow up patency; (2) a pararenal aneurysm with aorto-enteric fistula one month after the primary procedure who died three days post-operatively.7 The aortoduodenal fistula was closed through a thoracophrenolaparotomy with a total operation time of 8.5 hours, renal ischaemia time after intra-arterial cooling of 45 and 75 minutes, and bowel ischaemia time of 90 minutes. Three revisions were needed, two for bleeding and one for subtotal colectomy and segmental small bowel resection.
Interval vascular procedures can potentially seed the endograft1 but an aorto-enteric fistula is present in one third of EVAR device explantations.8 The MAEFISTO registry showed that the risk of EVAR infection is significantly increased when EVAR is performed for a post-surgical pseudo-aneurysm.2 As such, the SAEF was probably present when FEVAR was proposed, secondary to erosion of the digestive tract by the proximal anastomotic pseudo-aneurysm. Nuclear imaging might be a good option to differentiate between proximal progression of aneurysmal disease and evolution secondary to aortitis. Depending on this result and the patient's general condition, a multidisciplinary meeting should then help decide which solution seems the most suitable.
Since 2011, the unit has introduced a multidisciplinary consultation meeting (Recommendation 15)5 and this fit patient was offered complete excision of all graft material (Recommendation 38),5 which leads to improved mortality compared with conservative therapy. This allowed the SAEF to be detected and treated with microbiological identification (Recommendation 2) and adaptation of the antibiotic regimen.5 While awaiting those results, it is of the utmost importance to initiate and maintain intra-operative broad spectrum antibiotic therapy, and the addition of antifungal agents should be considered in the presence of an aorto-enteric fistula.5 At the unit, pre-operative antimicrobial treatment is only administered in the event of uncontrolled sepsis, it is usually initiated immediately after device explantation to optimise the chances of microbiological documentation. To this end, a protocol has also been implemented including sonication of vascular grafts and inoculation in blood culture bottles.
The graft enteric erosion was located in the aneurysm wall and not near the endograft hooks, and it was a small bowel rather than duodenal fistula, which may explain the three month delay in diagnosis. Cultures were positive to only one aerobic Gram negative coccobacillus commensal of human skin and mucosa, which can be isolated from healthy human respiratory tracts, environmental sources, and anaesthetic agents. Fewer than 40 cases of M. osloensis infection have been reported to date, including endocarditis but no aortitis. It is usually only pathogenic in immunocompromised individuals, which was not the case with the patient.
Due to extensive repair of the visceral arteries, biological substitutes such as deep veins, bovine pericardium or allograft arteries were turned down in favour of AMGs combining silver with triclosan. Since more than half of the patients are treated in an emergency setting, these AMGs have the advantage of “off the shelf” availability and offer versatility in diameter and length with prompt adaptation to any situation. Furthermore, the recent in vitro tests with clinical micro-organisms obtained from infected explants demonstrated satisfactory antimicrobial properties of AMGs regarding Methicillin resistant Staphylococcus aureus, Escherichia coli, and Candida albicans.9
The use of a thoracic aorta to left renal bypass offered two advantages: (1) the possibility to secure one warm kidney perfusion while the grafts were explanted; (2) sectioning of the renal artery after the bridging stent precluded the risk of dissection following blind extraction and repair from the ostium. Importantly, two fenestrated graft explantations have been performed so far; the intima of the renal arteries was intact after removing the bridging stent. The use of a Dacron ringed graft to cross the diaphragm might have precluded the occlusion of this bypass. The choice not to perform a thoracorenal bypass would have required the infusion of both kidneys at 4°C with an increased risk of renal failure after prolonged cold ischaemia for the second re-implanted kidney. Partial extracorporeal circulation would probably have been preferable but remains difficult to organise in a delayed emergency.
At the unit, complete explantation with in situ reconstruction has become the standard of care, with a staged approach including sequential cross clamping and protective measures for visceral arteries to reduce the ischaemic time whenever possible. It avoids the risk of aortic stump blow out and renal vessel occlusion, and offers increased patency.5 Indeed, extra-anatomical reconstruction using an axillofemoral bypass can reduce the operative time and maintain limb perfusion during explantation but is associated with a 30% complication rate.10
Total explantation of stent grafts with tissue debridement and post-operative antibiotic therapy is the gold standard when dealing with infected EVAR. As with a type IV TAA open repair, FEVAR device explantation requires additional protective measures to prevent visceral ischaemia and kidney impairment. In agreement with the ESVS guidelines, such patients should be referred to dedicated vascular centres with expertise in surgical repair, anaesthetics, and post-operative intensive care.
Funding
The authors received no financial support for the research or authorship. The publication fees were funded by an educational grant from Boston Scientific, United States.
Conflict of Interest
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ejvsvf.2020.12.020.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Graph of the evolution of the estimated glomerular filtration rate (eGFR) according to the CKD-EPI formula.
Complete healing of the surgical approach, with no evidence of eventration.
References
- 1.Smeds M.R., Duncan A.A., Harlander-Locke M.P., Lawrence P.F., Lyden S., Fatima J. Treatment and outcomes of aortic endograft infection. J Vasc Surg. 2016;63:332–340. doi: 10.1016/j.jvs.2015.08.113. [DOI] [PubMed] [Google Scholar]
- 2.Kahlberg A., Rinaldi E., Piffaretti G., Speziale F., Trimarchi S., Bonardelli S. Results from the Multicenter Study on Aortoenteric Fistulization After Stent Grafting of the Abdominal Aorta (MAEFISTO) J Vasc Surg. 2016;64:313–320.e1. doi: 10.1016/j.jvs.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Schaefers J.F., Donas K.P., Panuccio G., Kasprzak B., Heine B., Torsello G.B. Outcomes of surgical explantation of infected aortic grafts after endovascular and open abdominal aneurysm repair. Eur J Vasc Endovasc Surg. 2019;57:130–136. doi: 10.1016/j.ejvs.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Argyriou C., Georgiadis G.S., Lazarides M.K., Georgakarakos E., Antoniou G.A. Endograft infection after endovascular abdominal aortic aneurysm repair: a systematic review and meta-analysis. J Endovasc Ther. 2017;24:688–697. doi: 10.1177/1526602817722018. [DOI] [PubMed] [Google Scholar]
- 5.Chakfé N., Diener H., Lejay A., Assadian O., Berard X., Caillon J. Editor's Choice – European Society for Vascular Surgery (ESVS) 2020 clinical practice guidelines on the management of vascular graft and endograft infections. Eur J Vasc Endovasc Surg. 2020;59:339–384. doi: 10.1016/j.ejvs.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Nordanstig J., Törngren K., Smidfelt K., Roos H., Langenskiöld M. Deep femoral vein reconstruction of the abdominal aorta and adaptation of the neo-aortoiliac system bypass technique in an endovascular era. Vasc Endovascular Surg. 2019;53:28–34. doi: 10.1177/1538574418801100. [DOI] [PubMed] [Google Scholar]
- 7.Terry C., Houthoofd S., Maleux G., Fourneau I. Explantation of an infected fenestrated abdominal endograft with autologous venous reconstruction. EJVES Short Rep. 2017;34:21–23. doi: 10.1016/j.ejvssr.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaufour X., Gaudric J., Goueffic Y., Khodja R.H., Feugier P., Malikov S. A multicenter experience with infected abdominal aortic endograft explantation. J Vasc Surg. 2017;65:372–380. doi: 10.1016/j.jvs.2016.07.126. [DOI] [PubMed] [Google Scholar]
- 9.Berard X., Stecken L., Pinaquy J.-B., Cazanave C., Puges M., Pereyre S. Comparison of the antimicrobial properties of silver impregnated vascular grafts with and without triclosan. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2016;51:285–292. doi: 10.1016/j.ejvs.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Lyons O.T.A., Patel A.S., Saha P., Clough R.E., Price N., Taylor P.R. A 14 year experience with aortic endograft infection: management and results. Eur J Vasc Endovasc Surg. 2013;46:306–313. doi: 10.1016/j.ejvs.2013.04.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Graph of the evolution of the estimated glomerular filtration rate (eGFR) according to the CKD-EPI formula.
Complete healing of the surgical approach, with no evidence of eventration.