Abstract
Patients undergoing cardiac surgery are placed under intense physiologic stress. Blood and urine biomarkers measured peri-operatively may help identify patients at higher risk for adverse long-term kidney outcomes. We sought to determine the independent associations of various biomarkers with development or progression of CKD following cardiac surgery.
In this sub-study of the prospective cohort TRIBE AKI Study, we evaluated 613 adult patients undergoing cardiac surgery from 2007–2010 in Canada in our primary analysis. We tested the association of 40 blood and urinary biomarkers with the primary composite outcome of CKD incidence or progression. In those with baseline eGFR>60 mL/min/1.73m2, we defined CKD incidence as a 25% reduction and eGFR <60. In those with baseline baseline eGFR <60 mL/min/1.73m2, we defined CKD progression as a 50% reduction in eGFR or eGFR<15. We evaluated our results in a replication cohort of 310 patients from one study site in the US. Over a median (IQR) follow-up of 5.6 (4.3-6.8) years, 172 (28.1%) patients developed the primary outcome. Each log increase in basic fibroblast growth factor (aHR [95% CI] 1.52 [1.19, 1.93]), Kidney Injury Molecule-1 (1.51 [0.98, 2.32]), N-terminal prohormone of brain natriuretic peptide (1.19 [1.01, 1.41]), and tumor necrosis factor receptor 1 (1.75 [1.18, 2.59]) were associated with the outcome after adjustment for demographic factors, serum creatinine, and albuminuria. Similar results were noted in the replication cohort. Although there was no interaction by AKI, mortality was higher in the no AKI group by biomarker tertiles.
Elevated post-operative levels of blood biomarkers following cardiac surgery were independently associated with the development of CKD. These biomarkers can provide additional value in evaluating CKD incidence and progression after cardiac surgery.
Keywords: biomarkers, CKD, cardiac surgery, subclinical AKI
Introduction
Over one million cardiac surgeries are performed annually worldwide.1, 2 Patients receiving cardiac surgery undergo intense physiologic stress and are at increased risk for adverse outcomes. Acute kidney injury (AKI) is a frequent complication following cardiac surgery, affecting up to 30% of patients.1 It is well known that AKI is associated with an increased risk of all-cause mortality as well as adverse cardiovascular outcomes following cardiac surgery.3-5 AKI has also been increasingly recognized as a major risk factor for chronic kidney disease (CKD).6 Notably, however, only a fraction of patients who develop AKI progress to CKD, whereas some patients who do not develop AKI subsequently develop CKD.6 It remains unclear how to identify those patients at greatest risk for CKD after surgery.
The use of serum creatinine-based definitions of AKI has a number of important limitations when evaluating a long-term outcome like CKD, particularly in the inpatient setting. Acute changes in serum creatinine may not accurately reflect the severity or nature of kidney injury due to the influence of factors such as age, sex, muscle mass, nutritional status, medication effects on creatinine kinetics, IV fluid administration, and hemodynamic changes with subsequent oxygen supply-demand mismatch.7 Therefore, serum creatinine measured in the hospital setting can vary significantly and unpredictably. Further, elevations in serum creatinine are known to occur 48-72 hours following the episode of kidney injury.8
Previous research has demonstrated that patients with subclinical AKI, namely those without AKI by serum creatinine but with elevated levels of kidney injury biomarkers, show clear structural kidney injury on histology.9 Patients with subclinical AKI have greater long-term morbidity and mortality risk compared to individuals with biomarker levels in the normal range.4, 10 A number of blood and urine biomarkers reflecting hemodynamic and cardiac function, as well as markers for structural injury, inflammation, and repair, have been investigated previously with long-term cardiovascular outcomes and mortality.5
To our knowledge, no studies have investigated the relationship between blood and urine biomarkers of injury, inflammation, or repair with CKD independent of serum creatinine in the setting of cardiac surgery. Therefore, in this study, we aimed to examine the independent associations of biomarkers specific for structural injury, inflammation, and repair, with long-term CKD in patients following either coronary artery bypass grafting (CABG) or valvular cardiac surgery. We hypothesized that kidney injury and repair biomarkers would be associated with either incident CKD in patients with eGFR ≥60, or progression of CKD in patients with GFR <60 mL/min/1.73m2, whereas cardiac biomarkers would not have any significant independent associations with the primary outcome.
Results
Study Population
After excluding 127 (17.2%) participants with missing data during follow-up or unable to link to follow-up data, the analytic population consisted of 613 patients in the primary cohort (Figure 1). There were no significant differences in baseline characteristics in those with versus without follow-up serum creatinine values available (Table S1). Table 1 outlines the baseline characteristics for participants by composite primary outcome in the primary cohort. The mean age of patients at the time of surgery was 71.0 (8.8) years, and 168 (27%) patients were female. The mean baseline eGFR was 71.0 (18.2) mL/min/1.73m2, and there were no significant differences in baseline eGFR between participants who developed the primary outcome and those who did not. Similarly, there were no significant differences in baseline hypertension, congestive heart failure (CHF), prior myocardial infarction, or differences in surgery type and indication.
Figure 1:
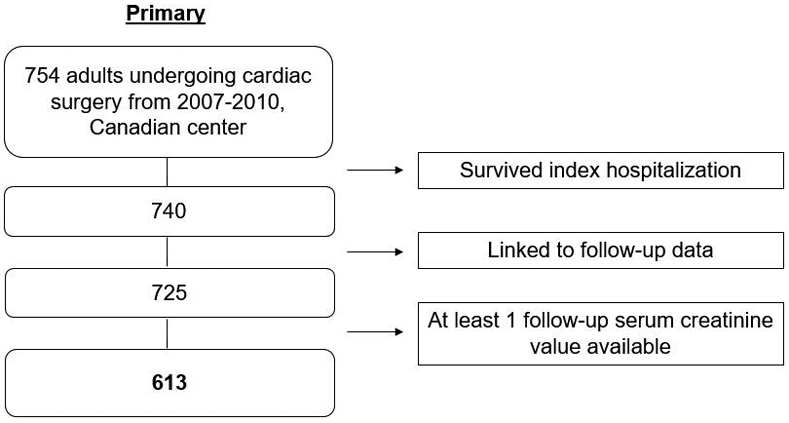
Flowchart of study population from primary cohort
Table 1:
Baseline Demographics, Primary Cohort
| Variable | ALL | No primary outcome |
Developed primary outcome |
P- value |
|
|---|---|---|---|---|---|
| (N=613) | (N=441) | (N=172) | |||
| Age at the time of surgery | 70.97 ± 8.84 | 70.68 ± 9.14 | 71.70 ± 8.01 | 0.20 | |
| Sex (female, %) | 168 (27.4%) | 113 (25.6%) | 55 (32.0%) | 0.11 | |
| Race (white, %) | 589 (96.1%) | 427 (96.8%) | 162 (94.2%) | 0.13 | |
| Diabetes | 256 (41.8%) | 173 (39.2%) | 83 (48.3%) | 0.03 | |
| Hypertension | 484 (79.0%) | 345 (78.2%) | 139 (80.8%) | 0.20 | |
| Congestive Heart Failure | 62 (10.1%) | 40 (9.1%) | 22 (12.8%) | 0.11 | |
| Left ventricular ejection fraction <40% | 48 (7.8%) | 29 (6.6%) | 19 (11.0%) | 0.048 | |
| Previous myocardial infarction | 173 (28.2%) | 123 (27.9%) | 50 (29.1%) | 0.26 | |
| eGFR, mL/min per 1.73m2 | 71.00 ± 18.23 | 70.79 ± 19.32 | 71.55 ± 15.12 | 0.64 | |
| eGFR, mL/min per 1.73m2 | >60 | 447 (72.9%) | 301 (68.3%) | 146 (84.9%) | <0.001 |
| <60 | 165 (26.9%) | 140 (31.7%) | 25 (14.4%) | ||
| Serum creatinine, mg/dL | 1.03 ± 0.31 | 1.04 ± 0.31 | 1.01 ± 0.32 | 0.25 | |
| Urine Microalbumin, pre-operative (>30 mg/g) | 365 (59.5%) | 248 (56.2%) | 117 (68.0%) | 0.027 | |
| STS Score32 | 9.08 ± 3.49 | 8.99 ± 3.52 | 9.33 ± 3.40 | 0.27 | |
| Elective surgery | 561 (91.5%) | 404 (91.6%) | 157 (91.3%) | 0.28 | |
| Isolated CABG or valve surgery | 480 (78.3%) | 347 (78.7%) | 133 (77.3%) | 0.75 | |
| Off-pump | 69 (11.3%) | 53 (12.0%) | 16 (9.3%) | 0.20 | |
| Reoperation | 6 (1.0%) | .. | .. | .. | |
| Perfusion time, min | 107.12 ± 57.93 | 106.93 ± 57.57 | 107.63 ± 59.02 | 0.89 | |
| Crossclamp time, min | 71.59 ± 43.86 | 71.89 ± 43.48 | 70.80 ± 44.97 | 0.78 | |
| AKIN Stage | 0 | 403 (65.7%) | 307 (69.6%) | 96 (55.8%) | 0.001 |
| 1 | 186 (30.3%) | 122 (27.7%) | 64 (37.2%) | ||
| 2 or 3 | 24 (3.9%) | 12 (2.7%) | 12 (7.0%) | ||
| AKI Duration (days) | 1 to 2 | 143 (23.3%) | 94 (21.3%) | 49 (28.5%) | 0.015 |
| 3 to 6 | 50 (8.2%) | 31 (7.0%) | 19 (11.0%) | ||
| >6 | 16 (2.6%) | 9 (2.0%) | 7 (4.1%) | ||
| Last serum Creatinine before discharge | 1.03 ± 0.45 | 1.01 ± 0.31 | 1.07 ± 0.67 | 0.15 | |
Mean (SD) and frequency (%) are presented for the continuous and categorical variables, respectively Ellipses (..) indicate small cell counts that cannot be presented due to privacy concerns
The primary outcome was a composite of CKD incidence or progression:
- CKD incidence (pre-operative eGFR ≥60): 25% reduction in eGFR and a fall below 60 mL/min/1.73m2
- CKD progression (pre-operative eGFR <60):50% reduction in eGFR or a fall below 15 mL/min/1.73m2
Abbreviations: eGFR: estimated glomerular filtration rate. STS: Society of Thoracic Surgeons. CABG: coronary artery bypass graft. AKI: acute kidney injury. AKIN: acute kidney injury network
Results from the primary cohort
Over a median follow-up of 5.6 (IQR 4.3-8.6) years, 172 (28%) patients developed the primary outcome of CKD incidence or progression at a rate of 53.2 per 1,000 person-years based on at least one follow-up serum creatinine. We noted a higher rate of the primary outcome in patients with increasing AKI stage, from 96 (23.8%) of patients without in-hospital AKI developing the primary outcome to 24 (50%) of those with stage 2 or 3 AKI developing the primary outcome.
Of the 172 patients who developed the primary outcome, 144 (84%) patients had at least two serum creatinine measurements during follow-up spaced 90 days or more apart. In total, a median of 21 (IQR 12-34) serum creatinine values were measured per patient over the course of follow-up, with those who developed AKI stages 2 or 3 having notably more follow-up creatinine values (31 [IQR 13-34]).
Forty blood and urine biomarkers were analyzed for association with the primary outcome. In unadjusted analyses following natural log transformation, higher post-operative values of blood basic fibroblast growth factor (bFGF), interleukin-2 (IL-2), interleukin-10 (IL-10), kidney injury molecule 1 (KIM-1), N-terminal pro-hormone of brain natriuretic peptide (NT pro-BNP), tumor necrosis factor receptor 1 (TNF-r1), vascular endothelial growth factor receptor 1 (VEGF-r1), and YKL-40 were significantly associated with the primary outcome (Table 2). After adjustment for age, sex, AKI stage, preoperative albuminuria, preoperative serum creatinine, and discharge serum creatinine, the biomarkers bFGF, NT pro-BNP, and TNF-r1 remained significantly associated with an increased risk of the CKD incidence or progression (Table 2). Additionally, KIM-1 was associated with an increased risk of CKD incidence or progression that was approaching statistical significance (aHR 1.51 [0.98, 2.32]; p=0.07). The association of these four post-operative biomarkers with the primary outcome was then evaluated by tertiles, using the first tertile as the reference group. In categorical analysis, only participants in the highest tertile of bFGF had a significantly higher risk of the primary outcome compared to those in the lowest tertile (HR = 1.89 [1.26, 2.82], Table S2). Adjustment for preoperative biomarker levels yielded similar point estimates for bFGF, KIM-1, NT pro-BNP, and TNF-r1, but with wider confidence intervals such that pro-BNP no longer reached statistical significance (Table S3).
Table 2:
Risk of CKD Incidence or Progression by Post-operative Biomarker Level
| Blood biomarkers (natural log- transformed) |
N | Hazard Ratio (95% CI) | |
|---|---|---|---|
| Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
||
| bFGF | 612 | 1.50 (1.20, 1.87) | 1.52 (1.19, 1.93) * |
| KIM-1 | 612 | 1.55 (1.14, 2.10) | 1.51 (0.98, 2.32)^ |
| NT pro-BNP | 387 | 1.21 (1.05, 1.39) | 1.19 (1.01, 1.41) * |
| TNF-r1 | 612 | 1.77 (1.26, 1.49) | 1.75 (1.18, 2.59) * |
| IL-10 | 612 | 1.14 (1.01, 1.29) | 1.11 (0.98, 1.27) |
| IL-2 | 612 | 1.19 (1.01, 1.40) | 1.08 (0.91, 1.28) |
| VEGFr1 | 612 | 1.31 (1.08, 1.59) | 1.21 (0.98, 1.49) |
| YKL-40 | 612 | 1.27 (1.03, 1.56) | 1.10 (0.88, 1.38) |
n=613
p<0.05
p<=0.07
Adjusted for age, sex, AKI stage, pre-operative albuminuria, pre-operative serum creatinine, discharge serum creatinine
Results from the replication cohort
Table S4 lists the baseline characteristics of the replication cohort, based on data from the largest US center in the TRIBE-AKI cohort. Over a median follow-up of 6.5 (4.2-8.6) years, 60 (19%) patients developed the primary outcome of CKD incidence or progression at a rate of 61.3 per 1,000 person-years based on at least one follow-up serum creatinine. In addition to bFGF, NT pro-BNP, and TNF-r1, we also evaluated the associations between KIM-1 and our primary outcome in the replication cohort, given its established association with kidney injury from prior literature and the strength of association relative to other biomarkers. Similar to the primary cohort, higher post-operative blood levels of bFGF, KIM-1, and NT pro-BNP remained significantly associated with the primary kidney outcome after adjustment in the replication cohort (Table S5). Increases in post-operative TNF-r1 concentration were not significantly associated with an increased risk of CKD incidence or progression (HR = 1.73 [0.94, 3.18]). Patients in the third tertile of all three biomarkers had a significantly higher risk compared to those in first tertile (Table S5).
In a meta-analysis of both cohorts, after natural log transformation, the pooled hazard ratios for all four biomarkers were significantly associated with our primary outcome (Figure 2).
Figure 2:
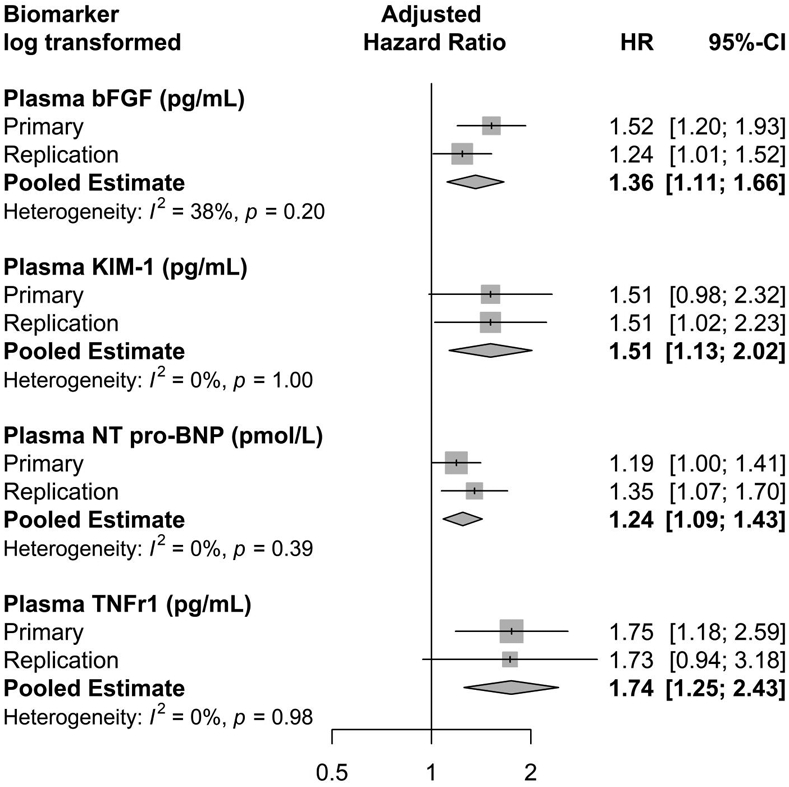
Forest plot with pooled hazard ratio of CKD incidence or progression by postoperative biomarker level, primary and replication cohorts
Additional analyses
There were a total of 78 (12.7%) deaths in the primary cohort and 31 (10%) deaths in the replication cohort. We performed a competing-risk analysis for death in the primary cohort. There was no significant difference in the primary outcome after accounting for competing risk of death using Fine and Gray’s subdistribution model (Table S6). We performed interaction testing to evaluate the effect of clinical AKI. There was no significant interaction between biomarker level and our primary outcome by AKI status in continuous analysis. However, in categorical analysis, patients without clinical AKI in the highest tertiles of bFGF, KIM-1, and TNFr1 level had similar to higher rates of the primary outcome compared to patients with clinical AKI in the lowest tertiles (Figure 3). We additionally evaluated for interaction by pre-operative CKD status and did not find any significant difference in outcome for any of the four biomarkers (Table S7). Notably, the analysis of TNF-r1 produced wide confidence intervals thought the interaction p-value was not significant, likely as a result of limited power for this analysis. Within each cohort, we examined model performance by calculating the net reclassification index for models with biomarker measurements added to a base model with clinical parameters alone (Table S8). In sensitivity analysis, we additionally examined the association between peak postoperative biomarker level (Table S9) and mean post-operative biomarker level (Table S10) with CKD incidence or progression. We found generally similar results compared to our primary analysis using first post-operative biomarker levels.
Figure 3: CKD incidence or progression event rates per 1000 patient-years by biomarker tertile and clinical AKI status.
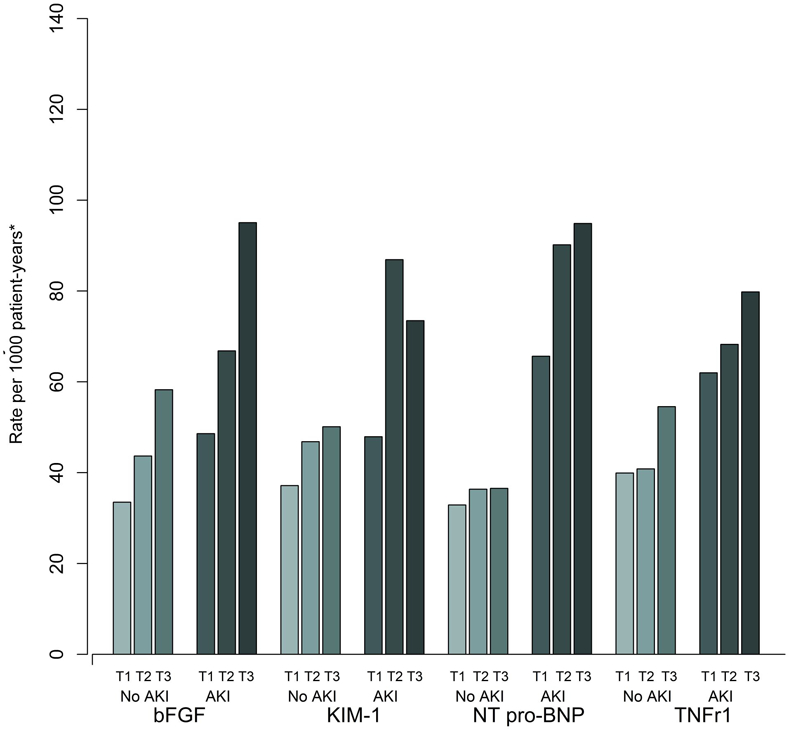
*The event rate in the highest tertile of the injury biomarkers (bFGF, KIM-1, TNFr1) in those without clinical AKI are similar to the event rate in the lowest tertile of the biomarker in those with clinical AKI. The event rates for all tertiles of NT pro-BNP are lower in those without clinical AKI compared to those with clinical AKI.
Discussion
In this prospective cohort study of adults undergoing cardiac surgery, we evaluated 40 blood and urine biomarkers and found that elevated post-operative levels of blood bFGF, KIM-1, NT pro-BNP, and TNF-r1 were independently associated with an increased risk of CKD incidence or progression regardless of AKI status after surgery.
It is well established that patients who develop AKI are at greater risk for long-term adverse outcomes including mortality and cardiovascular disease compared to those who do not.6, 11, 12 In recent years several studies have evaluated the risk of cardiovascular outcomes following subclinical AKI, with elevated levels of kidney injury or repair biomarkers in the setting of normal serum creatinine.9, 10 Elevations in biomarkers of injury, inflammation, and repair have been associated with long-term risk of overall mortality and cardiovascular disease.3, 5, 13, 14 This is the first study to evaluate a large number of blood and urine biomarkers establish their association with increased risk of CKD incidence or progression in a cardiac surgery cohort.
There are a number of plausible mechanisms by which levels of bFGF, KIM-1, NT pro-BNP, and TNF-r1 can associate with CKD incidence or progression. Basic FGF, also referred to as FGF2, is a member of the fibroblast growth factor family distinct from FGF-21 and FGF-23. Similar to other members of the FGF family, bFGF serves multiple roles in cellular differentiation and function through various signaling pathways, several of which include inhibition of bone mineralization, angiogenesis, and cell proliferation.15 Basic FGF has been implicated in the response to inflammation by upregulation of endothelial cell adhesion molecules as well, with chronic inflammation being a known factor in CKD progression.16
KIM-1 is a trans-membrane protein located in the proximal tubule of the kidney, and its expression is significantly up-regulated in the setting of proximal tubular injury.17, 18 Urinary KIM-1 has been shown to be a robust marker for acute tubular injury, with localization to the proximal tubule, in both animal and human studies.17-19 However, elevated blood levels of KIM-1 have been associated with progression to CKD in patients with Type 1 diabetes mellitus and even among healthy adults.20, 21
NT pro-BNP has also been independently associated with progression of CKD.22 Elevations in NT pro-BNP may help distinguish patients at increased risk for a cardio-renal phenotype of CKD.23 One study in patients with type 2 diabetes demonstrated the association between both TNF-r1 and NT pro-BNP with progression of CKD.24 These findings are consistent with these prior studies supporting the association between elevated levels of these markers with kidney function decline.
TNF-r1 serves as a cell membrane receptor which binds TNF-α and accentuates endothelial inflammation. TNF-r1 has been associated with progression to end-stage kidney disease and mortality in patients with diabetes mellitus type 1 and type 2 beyond established risk factors including proteinuria.25, 26 Elevated levels of TNF-r1 have been implicated in other kidney diseases including various glomerulonephritides, obstructive kidney injury, and kidney transplant rejection.25, 26 The presence of higher circulating TNF-r1 is a very acute and sensitive marker for inflammation, up-regulate in the setting of elevated TNF-α. We did see a loss of significance evaluating the risk of the primary outcome by TNF-r1 level in the replication cohort after full adjustment. However, the direction of association was similar to what was observed in the primary cohort, and the loss of significance may be due to sample size limitations.
These findings suggest that these four biomarkers are associated with CKD incidence or progression even after adjustment for serum creatinine and AKI stage. Moreover, our sub-group analysis evaluating patients by clinical AKI status indicated that even without clinical AKI, patients in the highest tertile of bFGF, KIM-1, and TNFr1 level had similar to higher risk of the primary outcome compared to those with clinical AKI who had lower post-operative biomarker levels. These results further highlight the importance of using biomarkers to detect sub-clinical AKI, given its association with CKD incidence or progression. The results of this research may have practical applications in follow-up care after hospital discharge. Patients at greater risk for CKD incidence or progression could be seen sooner and more frequently, with greater emphasis on reducing other known risk factors for CKD. Further, these patients represent a unique subset of individuals with known high risk of CKD and could be enrolled in clinical trials to evaluate interventions to reduce or prevent CKD.
This study has a number of strengths. With the use of two cohorts with unique follow-up after discharge from surgery, we were able to replicate findings from the primary cohort. We were also able to evaluate a large number of biomarkers. One strength of this study is the measurement of 40 blood and urine biomarkers at multiple time points post-operatively. We were therefore able to perform additional analyses evaluating the association between peak and mean biomarker levels with CKD incidence or progression, finding generally similar results compared to our primary analysis and demonstrating that biomarkers measured immediately after cardiac surgery are equally informative. This study benefits from the use of high-quality data, sample collection, processing, and retention beyond hospital discharge and short-term follow-up.
There are a number of limitations in this study. The replication cohort had a relatively small final analytic population due to challenges in obtaining post-discharge laboratory data across multiple centers. This loss to follow-up may not be at random and could have biased our cohort towards inclusion of those at higher risk for CKD. Similarly, we were only able to evaluate our primary outcome as a composite of CKD incidence or progression, and not as individual outcomes, due to concerns with adequate power. Our clinical AKI definition incorporated serum creatinine at baseline but did not account for urine output over a 48-hour window. Most participants in the TRIBE-AKI Study were male and Caucasian, limiting the generalizability of our findings. Also, the average age of participants in both cohorts were relatively older, with a mean age above 70 years. Further, we did not have information to ascertain the phenotype of CKD over the course of follow-up in those who had decline in kidney function. The definition of incident CKD was based on the first serum creatinine value that satisfied the end-point. We additionally do not have information on the etiology of CKD in patients on follow-up. While we were able to obtain serum creatinine levels over the course of follow-up, we do not have information on biomarker levels throughout follow-up to evaluate how these may have changed over time. Finally, since serum creatinine values were primarily followed clinically and not in a protocolized fashion across centers, there is some concern for ascertainment bias.
In summary, we provide evidence for an association between post-operative biomarker levels of blood bFGF, KIM-1, NT pro-BNP, and TNF-r1 with CKD incidence or progression, independent of serum creatinine or AKI stage during hospitalization. In the future, measurement of these biomarkers in the immediate post-operative setting may help guide management of patients following cardiac surgery and other procedures who are at greater risk for CKD outcomes, who could benefit from closer outpatient follow-up.
Methods
Study design and data sources
We conducted a sub-study of the Translational Research Investigating Biomarker Endpoints in AKI (TRIBE-AKI) Study, a longitudinal prospective cohort study of adults who underwent cardiac surgery in academic centers in North America. The detailed study enrollment methods have been described previously.13 The study was approved by the institutional review board of each participating site, and written informed consent was obtained from all participants.
Population
Adults undergoing cardiac surgery, either CABG or valvular surgery, at high risk for AKI were prospectively enrolled between July 2007 and December 2010.13, 27 Data from the largest TRIBE-affiliated academic medical center in Canada were used for our primary analysis, given the relatively larger sample size and near complete ascertainment of post-discharge laboratory data compared to other centers. Patients enrolled in the Canadian academic medical center who had consented to linkage with administrative data for long-term follow-up and had at least one follow-up serum creatinine measurement after discharge were included in the primary cohort. This patient cohort had rigorous follow-up on most patients due to its universal publicly funded health care system. Data from one US center was additionally included with available post-discharge laboratory data for replication.
Sample collection and biomarker measurement
Details of sample collection and processing have been described previously.13 Urine and blood samples were collected preoperatively and then daily, for up to five days following cardiac surgery. Immediate post-operative urine and blood samples were collected within six hours following the conclusion of the patient’s surgery. Blood samples were collected in EDTA tubes, centrifuged to separate plasma, and subsequently stored at −80°C. Seven urine biomarkers and 33 blood biomarkers were measured as previously described (Table S11).13, 27-29 All analyses for this current study use the immediate post-operative biomarker measurements to minimize missingness across biomarkers that were present in subsequent sample collections. In the primary cohort, AKI stage was not available for 1 participant, pre-op albuminuria measurement for 2 participants, and discharge serum creatinine for 8 participants.
Covariate measurement
Clinical AKI was defined as an increase of at least ≥50% or 0.3 mg/dL in serum creatinine within the index hospitalization, using preoperative serum creatinine as the baseline. All preoperative serum creatinine values were measured within two months prior to surgery. Serum creatinine values were obtained during routine clinical care for every patient throughout the hospitalization. Estimated glomerular filtration rate was estimated using the Chronic Kidney Disease Epidemiology Collaboration Equation (CKD-EPI).30 The severity of AKI was classified by the Acute Kidney Injury Network staging criteria on the basis of the peak serum creatinine within the index hospitalization.31 Society of Thoracic Surgeons (STS) risk scores were calculated based on pre- and post-operative details including demographics, surgical details, and post-operative complications based on prior literature.32
Outcomes
The primary outcome of the study was a composite of CKD incidence or progression. In those individuals with an eGFR ≥ 60 mL/min/1.73m2 pre-operatively, CKD incidence was defined as a 25% reduction in eGFR and a fall below 60 mL/min/1.73m2. In those individuals with an eGFR < 60 mL/min/1.73m2 pre-operatively, CKD progression was defined as a 50% reduction in eGFR or a fall below 15 mL/min/1.73m2. These definitions were based on established cutoffs as outlined by the multi-center Assessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) Study.33, 34
In the primary cohort, vital status was obtained on all patients from the Registered Persons Database. Follow-up serum creatinine values were obtained using the Ontario Laboratories Information System (OLIS), a province-wide integrated laboratory database incorporating outpatient and inpatient test results with serum creatinine values available from 2007-2015. These datasets were linked using unique encoded identifiers and analyzed at ICES. Participants still alive on September 30, 2015 without the primary outcome were censored due to end of data availability.
For patients in the replication cohort, follow-up creatinine measurements were ascertained through the Yale Joint Data Analytics Team’s HELIX data repository, which includes data from all Yale-New Haven Health-affiliated hospitals and outpatient practices with serum creatinine values available from 2012-2018. Participants were censored at the time of their last serum creatinine measurement. In a subset of all participants (Canadian and American), home visits were conducted in the first year after discharge and collected samples for serum creatinine.
Statistical analysis
Descriptive characteristics were reported using mean (standard deviation) or median (interquartile range) for continuous variables and frequency (percentage) for categorical variables. First post-operative biomarker levels were modeled continuously (after natural log transformation) in unadjusted analysis, and those that were statistically significant were also modelled categorically as tertiles, with the first tertile serving as a reference group. Tertiles were defined based on biomarker levels within each cohort. Cubic spline plots were used to explore the functional relationship of the biomarker with the outcome (Figure S1). Models were adjusted for age, sex, AKI stage, pre-operative albuminuria, pre-operative serum creatinine, and discharge serum creatinine. The selection of these variables was based on related work investigating progression to CKD following an episode of AKI, which showed strong predictive ability using those 6 variables alone.35 Cox proportional hazards regression was used to examine the cause-specific association between post-operative biomarker levels immediately following surgery and the primary outcome. Kolmogorov-type supremum tests were used to evaluate proportional hazards assumptions for all models. The subset of biomarkers with statistical significance after adjustment, and those with the strongest point estimates, were examined in the US replication cohort. This strategy facilitated selection of the most promising biomarkers among the 40 candidate blood and urine biomarkers measured post-operatively, thereby reducing re-substitution and model selection biases, largely addressing the concern of multiple testing.36 We additionally estimated subdistribution hazard ratios using Fine and Gray’s subdistribution hazard model, accounting for the competing risk of death. We performed additional analysis evaluating the interaction between AKI and our primary outcome, as well as between pre-operative CKD and our primary outcome.
We combined the results of the two cohorts and used the I2 test statistic to quantify the magnitude of heterogeneity. A pooled estimate was used for all comparisons where the Q test was not statistically significant. All pooled hazard ratio estimates were calculated using the random effects meta-analysis method. All analyses were performed in SAS (version 9.4; SAS Institute, Cary, NC), Stata (version 14; StataCorp LLC, College Station, TX), and R (version 3.1.2; R foundation for statistical Computing, Vienna, Austria). All tests of statistical significance were two-sided, with p<0.05 considered significant.
Supplementary Material
Table S1: Comparison of Baseline Demographics in Patients with Vs. without Follow-up Serum Creatinine Measurements.
Table S2: Risk of CKD Incidence or Progression by Post-operative Biomarker Tertiles
Table S3: Risk of CKD Incidence or Progression by Post-operative Biomarker Level, Adjusted for Pre-operative Level
Table S4: Baseline Demographics, Replication Cohort
Table S5: Risk of CKD Incidence or Progression by Post-operative Biomarker Level, Replication Cohort
Table S6: Risk of CKD Incidence or Progression by Post-operative Biomarker Level Using Sub-distribution Hazard Model, Primary Cohort
Table S7. Evaluation for Interactions Between Biomarker Level and Pre-Operative CKD on the Risk of CKD Incidence or Progression
Table S8. Model Performance using Net Reclassification Index
Table S9: Risk of CKD Incidence or Progression by Peak Biomarker Level, Primary Cohort
Table S10: Risk of CKD Incidence or Progression by Mean Biomarker Level, Primary Cohort
Table S11: Biomarker Measurement Details
Figure S1: Post-operative biomarker spline plots using 3 cubic spline knots
Acknowledgements
SPM is supported by NIH T32 grant (HL007024). DGM is supported by NIH K23 grant (K23DK117065) and by the Yale O’Brien Kidney Center (P30DK079310). SGM is supported by AHA (18CDA34110151) and Patterson Trust Fund. NIH (R01-HL-085757 to Dr Parikh) funded the TRIBE-AKI Consortium. CRP is supported by NIH grant K24-DK-090203 and the P30-DK-079310 O’Brien Kidney Center Grant. SGC has salary support from NIH grants R01 DK115562, UO1 DK106962, R01 HL085757, R01 DK112258, and U01 OH011326. Dr. Wilson is supported by NIH grant R01-DK113191. JLK is supported by NIH grant R21-DK113420. SGC, AXG, and CRP are members of the NIH-sponsored Assessment, Serial Evaluation, and Subsequent Sequelae of Acute Kidney Injury (ASSESS-AKI) Consortium (U01-DK-082185). This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). Parts of this material are based on data and information compiled and provided by Canadian Institute for Health Information. The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. Furthermore, these funding organizations had no role in the study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Footnotes
Disclosures
MGS has received grant support from Cricket Health, Inc, has received consultancy fees from University of Washington, and has equity in TAI Diagnostics and Cricket Health, Inc. SGC and CRP are members of the advisory board of RenalytixAI and own equity in the same. In the past 3 years, SGC has received consulting fees from Goldfinch Bio, CHF Solutions, Quark Biopharma, Janssen Pharmaceuticals, Takeda Pharmaceuticals, and Relypsa. JLK has received research fees from Bioporto and Astute Medical, and consulting fees from Baxter, Astute Medical and Sphingotec. The other authors declare that they have no relevant financial interests.
References
- 1.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006; 1: 19–32. [DOI] [PubMed] [Google Scholar]
- 2.Vervoort D, Meuris B, Meyns B, et al. Global cardiac surgery: Access to cardiac surgical care around the world. J Thorac Cardiovasc Surg 2019. [DOI] [PubMed] [Google Scholar]
- 3.Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation 2009; 53: 961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coca SG, Garg AX, Thiessen-Philbrook H, et al. Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. Journal of the American Society of Nephrology : JASN 2014; 25: 1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parikh CR, Puthumana J, Shlipak MG, et al. Relationship of Kidney Injury Biomarkers with Long-Term Cardiovascular Outcomes after Cardiac Surgery. Journal of the American Society of Nephrology : JASN 2017; 28: 3699–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 2012; 82: 516–524. [DOI] [PubMed] [Google Scholar]
- 7.Bullen A, Liu ZZ, Hepokoski M, et al. Renal Oxygenation and Hemodynamics in Kidney Injury. Nephron 2017; 137: 260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parikh CR, Mishra J, Thiessen-Philbrook H, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int 2006; 70: 199–203. [DOI] [PubMed] [Google Scholar]
- 9.Moledina DG, Hall IE, Thiessen-Philbrook H, et al. Performance of Serum Creatinine and Kidney Injury Biomarkers for Diagnosing Histologic Acute Tubular Injury. Am J Kidney Dis 2017; 70: 807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haase M, Kellum JA, Ronco C. Subclinical AKI--an emerging syndrome with important consequences. Nat Rev Nephrol, vol. 8: England, 2012, pp 735–739. [DOI] [PubMed] [Google Scholar]
- 11.Coca SG. Outcomes and renal function trajectory after acute kidney injury: the narrow road to perdition. Kidney Int 2017; 92: 288–291. [DOI] [PubMed] [Google Scholar]
- 12.See EJ, Jayasinghe K, Glassford N, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int 2019; 95: 160–172. [DOI] [PubMed] [Google Scholar]
- 13.Parikh CR, Coca SG, Thiessen-Philbrook H, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. Journal of the American Society of Nephrology : JASN 2011; 22: 1748–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang WR, Garg AX, Coca SG, et al. Plasma IL-6 and IL-10 Concentrations Predict AKI and Long-Term Mortality in Adults after Cardiac Surgery. J Am Soc Nephrol 2015; 26: 3123–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang W, Liang Q, Du L, et al. Sequential application of bFGF and BMP-2 facilitates osteogenic differentiation of human periodontal ligament stem cells. J Periodontal Res 2019. [DOI] [PubMed] [Google Scholar]
- 16.Zittermann SI, Issekutz AC. Basic fibroblast growth factor (bFGF, FGF-2) potentiates leukocyte recruitment to inflammation by enhancing endothelial adhesion molecule expression. Am J Pathol 2006; 168: 835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han WK, Bailly V, Abichandani R, et al. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 2002; 62: 237–244. [DOI] [PubMed] [Google Scholar]
- 18.Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 1998; 273: 4135–4142. [DOI] [PubMed] [Google Scholar]
- 19.Parikh CR, Thiessen-Philbrook H, Garg AX, et al. Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clinical journal of the American Society of Nephrology : CJASN 2013; 8: 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz CA, Engstrom G, Nilsson J, et al. Plasma kidney injury molecule-1 (p-KIM-1) levels and deterioration of kidney function over 16 years. Nephrol Dial Transplant 2020; 35: 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowak N, Skupien J, Niewczas MA, et al. Increased plasma kidney injury molecule-1 suggests early progressive renal decline in non-proteinuric patients with type 1 diabetes. Kidney Int 2016; 89: 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim Y, Matsushita K, Sang Y, et al. Association of high-sensitivity cardiac troponin T and natriuretic peptide with incident ESRD: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis 2015; 65: 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choudhary R, Gopal D, Kipper BA, et al. Cardiorenal biomarkers in acute heart failure. J Geriatr Cardiol 2012; 9: 292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bansal N, Hyre Anderson A, Yang W, et al. High-sensitivity troponin T and N-terminal pro-B-type natriuretic peptide (NT-proBNP) and risk of incident heart failure in patients with CKD: the Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol 2015; 26: 946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niewczas MA, Gohda T, Skupien J, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol 2012; 23: 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saulnier PJ, Gand E, Ragot S, et al. Association of serum concentration of TNFR1 with all-cause mortality in patients with type 2 diabetes and chronic kidney disease: follow-up of the SURDIAGENE Cohort. Diabetes Care 2014; 37: 1425–1431. [DOI] [PubMed] [Google Scholar]
- 27.Mansour SG, Zhang WR, Moledina D, et al. The Association of Angiogenesis Markers With Acute Kidney Injury and Mortality After Cardiac Surgery. American Journal of Kidney Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koyner JL, Garg AX, Shlipak MG, et al. Urinary cystatin C and acute kidney injury after cardiac surgery. Am J Kidney Dis 2013; 61: 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moledina DG, Isguven S, McArthur E, et al. Plasma Monocyte Chemotactic Protein-1 Is Associated With Acute Kidney Injury and Death After Cardiac Operations. Ann Thorac Surg 2017; 104: 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of Internal Medicine 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care, vol. 11: England, 2007, p R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez FG, Falcoz PE, Kozower BD, et al. The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases: joint standardization of variable definitions and terminology. Ann Thorac Surg 2015; 99: 368–376. [DOI] [PubMed] [Google Scholar]
- 33.Go AS, Parikh CR, Ikizler TA, et al. The assessment, serial evaluation, and subsequent sequelae of acute kidney injury (ASSESS-AKI) study: design and methods. BMC Nephrol 2010; 11: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu CY, Chinchilli VM, Coca S, et al. Post-Acute Kidney Injury Proteinuria and Subsequent Kidney Disease Progression: The Assessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) Study. JAMA Intern Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.James MT, Pannu N, Hemmelgarn BR, et al. Derivation and External Validation of Prediction Models for Advanced Chronic Kidney Disease Following Acute Kidney Injury. Jama 2017; 318: 1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meisner A, Kerr KF, Thiessen-Philbrook H, et al. Methodological issues in current practice may lead to bias in the development of biomarker combinations for predicting acute kidney injury. Kidney Int 2016; 89: 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Comparison of Baseline Demographics in Patients with Vs. without Follow-up Serum Creatinine Measurements.
Table S2: Risk of CKD Incidence or Progression by Post-operative Biomarker Tertiles
Table S3: Risk of CKD Incidence or Progression by Post-operative Biomarker Level, Adjusted for Pre-operative Level
Table S4: Baseline Demographics, Replication Cohort
Table S5: Risk of CKD Incidence or Progression by Post-operative Biomarker Level, Replication Cohort
Table S6: Risk of CKD Incidence or Progression by Post-operative Biomarker Level Using Sub-distribution Hazard Model, Primary Cohort
Table S7. Evaluation for Interactions Between Biomarker Level and Pre-Operative CKD on the Risk of CKD Incidence or Progression
Table S8. Model Performance using Net Reclassification Index
Table S9: Risk of CKD Incidence or Progression by Peak Biomarker Level, Primary Cohort
Table S10: Risk of CKD Incidence or Progression by Mean Biomarker Level, Primary Cohort
Table S11: Biomarker Measurement Details
Figure S1: Post-operative biomarker spline plots using 3 cubic spline knots


