Abstract
Objective
We performed a meta-analysis of randomized double-blinded placebo controlled trials (DB-RCTs) to investigate efficacy and safety of intranasal esketamine in treating major depressive disorder (MDD) including treatment resistant depression (TRD) and major depression with suicide ideation (MDSI).
Methods
Mean change in total scores on Montgomery-Åsberg Depression Rating Scale (MADRS) from baseline to different time-points were our primary outcome measure. Secondary efficacy measures included rate of remission of depression and resolution of suicidality.
Results
Eight DB-RCTs (seven published and one un-published) covering 1,488 patients with MDD were included. Esketamine more significantly improved MADRS total scores than placebo starting from 2−4 hours after the first administration (standardized mean difference, −0.41 [95% CI, −0.58 to −0.25], p < 0.00001), and this superiority maintained until end of double-blinded period (28 days). Sub-group analysis showed that superior antidepressant effects of esketamine over placebo in TRD and MDSI was observed from 2−4 hours, which was maintained until 28 days. Resolution of suicide in MDSI was also greater for esketamine than for placebo at 2−4 hours (OR of 2.04, 95% CIs, 1.37 to 3.05, p = 0.0005), but two groups did not statistically differ at 24 hours and day 28. Total adverse events (AEs), and other common AEs including dissociation, blood pressure increment, nausea, vertigo, dysgeusia, dizziness, and somnolence were more frequent in esketamine than in placebo group.
Conclusion
Esketamine showed rapid antidepressant effects in patients with MDD, including TRD and MDSI. The study also suggested that esketamine might be associated with rapid anti-suicidal effects for patients with MDSI.
Keywords: Esketamine, Depression, Treatment resistant depression, Suicide, Meta-analysis.
INTRODUCTION
Major depressive disorder (MDD) is a common debilitating disease with a lifetime prevalence of 15−20%, and it is known to cause severe functional impairment [1,2]. Multiple antidepressants are available, but approximately one-third of patients with MDD fail to achieve adequate response or remission and become treatment-resistant depression (TRD) [3]. Besides low remission and response rate, delayed onset of efficacy is another important limitation of conventional antidepressants [4,5]. Moreover, around 10−20% of patients with MDD attempt suicide over their lifetimee, and 3.4% of patients with MDD actually commit or complete suicide [6,7]. However, due to therapeutic lag between administration of antidepressant and onset of clinical improvement, patients having major depression with suicide ideation (MDSI) remain symptomatic and at risk of suicidal behavior and self-harm for more than two weeks [8]. The monoamine hypothesis of depression received criticisms for more than a decade, and studies suggested that patients with TRD may need novel antidepressants with different mechanisms of action [9,10]. Thus, additional antidepressant having novel mechanism of action, higher potency, faster onset of action, and anti-suicidal effect are urgently needed [11].
Esketamine is a nonselective, noncompetitive antagonist at the N-methyl-D-aspartate (NMDA) receptor which modu-lates glutamatergic transmission [12]. It is an S-enantiomer of ketamine, which is known to have a higher affinity for the NMDA receptor than the R-enantiomer [13]. The US Food and Drug Administration (FDA) approved intranasal esketamine in conjunction (augmentation) with an oral antidepressant first for the treatment of TRD in 2019 [14]. Three double-blinded randomized placebo controlled trials (DB-RCTs) have shown its anti-suicidal effect [15-17], so the US FDA further approved intranasal esketamine augmentation to treatment depressive symptoms in adults with MDD having suicidal ideation or behavior [18].
An earlier study of intranasal esketamine showed its rapid onset of action in TRD [19]. However, findings from subsequent DB-RCTs of intranasal esketamine have been mixed in terms of its rapid antidepressant effect [20,21]. Although studies confirmed its anti-suicidal effects in patients with MDSI, but whether or not the anti-suicidal effects are rapid is still obscure. In terms of understanding efficacy and safety of a new drug, meta-analysis is important because it can overcome limitation of small sample sizes, increase statistical power of group comparisons, enhance generalizability of DB-RCTs, and quantify inconsistencies of DB-RCTs [22,23]. An initial meta-analysis for intranasal esketamine showed that significant superiority of intranasal esketamine over placebo with regard to response and remission in patients with MDD were noted as early as two hours [24]. However, the study included only four DB-RCTs which precluded more detailed elucidation of publication bias for outcome measures. In addition, due to small sample size, the study was unable to confirm its effects in MDSI. A more recent study by Papakostas et al. [25] also showed that adjunctive intranasal esketamine was significantly more effective than placebo for Montgomery-Åsberg Depression Rating Scale (MADRS) score change, response, and remission. However, besides having small study numbers (5 DB-RCTs), the timing of primary outcome or end-point measurements differed depending on the studies, but the meta-analysis did not specify their efficacies according to different time after esketamine administration. The study also failed to address whether or not esketamine have rapid antidepressant effect.
We performed a meta-analysis and studied efficacy and safety of intranasal esketamine in treatment of patients with MDD. We also aimed to investigate its rapid antidepressive actions in patients with TRD and MDSI.
METHODS
Sources of Data
Three investigators (SMW, NKK, and YSW) independently searched from December 1st, 2020 to January 10th, 2021 using following terms: “esketamine,” and “depression” (Mesh) at PubMed, Embase, PubMed, PsycINFO, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Web of Science for published articles. No restrictions were utilized for publication date. In terms of clinical trials, Cochrane Central Register of Controlled Trials Library and ClinicalTrials.gov (www.clinicaltrials.gov) was explored. We also manually searched reference lists from identified articles and reviews to find additional studies. Two other authors (HRN and HKL) re-evaluated potentially eligible papers to determine whether they truly met the selection criteria. The last two authors (CUP and WMB) discussed and reached a consensus for disagreements.
Study Criteria and Data Extraction
Primary inclusion criteria were all DB-RCTs comparing adjunctive treatment of intranasal esketamine with standard antidepressants for MDD. To be included in our meta-analysis, studies were required to: 1) have placebo as a comparator, regardless of having an active comparator, 2) exclusively focused on patients with MDD 3) have clearly described all inclusion and exclusion criteria. No restrictions were utilized for severity of MDD, gender, treatment basis (i.e., inpatient or outpatient), dose range, or study location. Three investigators (SMW, NKK, and YSW) who conducted initial data search also extracted the data. In addition, if a DB-RCT contained multiple double-blinded phases (i.e., Daly et al. [19]), only data from the first period were extracted and analyzed. We also assessed quality of DB-RCTs based on recommendations of Cochrane Review [26].
Study Outcomes
The primary outcome measures were change of MADRS total score from baseline to different time points until the end of double blinded phase. The secondary efficacy measures were rate of study-defined remission and resolution of suicidality at different time points during the double blinded phase. In terms of safety and tolerability, total number of adverse events (AEs) and common AEs including dissociation, blood pressure increment, nausea, vertigo, dysgeusia, dizziness, somnolence, and headache were included in the meta-analysis.
Statistical Analysis
Review Manager version 5.4 software (Cochrane Collaboration, Oxford, UK) was used to undertake statistical analysis. Standardized mean difference (SMD) using method developed by Hedges (Hedges g) with 95% confidence intervals (95% CIs) and odds ratio (OR) with 95% CIs using Mantel−Haenszel method were used for continuous and dichotomous outcome measures respectively. Cohen’s classification can be used to assess effect size: small = SMD < 0.2, medium = SMD of 0.5, and large = SMD > 0.8 [27]. In terms of heterogeneity, we used I2 statistic and evaluated what degree of variance between studies can be attributed to actual differences between the studies rather than to chance [28]. Studies suggested that I2 of 75−100% indicate considerable heterogeneity, and the heterogeneity threshold was defined as 50% or more in I2 value and p < 0.10.
RESULTS
Study Characteristics
Initially 804 abstracts were identified with use of Embase, PubMed, Psychinfo, and Web of Science. After a preliminary review, 754 papers were excluded because they were either duplicates, irrelevant, or non-full articles. The remaining 50 full-text articles were retrieved for a more detailed evaluation. Among them seven published DB-RCTs were included in the meta-analysis. Of the 35 records obtained from ClinicalTrials.gov and 132 studies from Cochrane Central Register of Controlled Trials, we found one DB-RCT having full reports which were not published. Thus, a total of eight DB-RCTs (seven published and one un-published) were finally selected for our meta-analysis (Fig. 1).
Fig. 1.
Schematic presentation of studies selected in the present meta- analysis. DB-RCT, double-blinded, random-ized, placebo-controlled clinical trial; ICTRP, international clinical trials registry platform.
Table 1 presents main characteristics of these eight DB-RCTs. All studies were multi-centered, and six studies [15,16,19-21,29] were multi-national while two were conducted either in Japan [30] or US [17] only. Five trials [19-21,29,30] involved TRD while other three [15-17] involved patients with MDSI. A total of 1,488 participants were included, and number of patients included in placebo and intranasal esketamine groups were 661 and 827 respectively. Four [15,16,20,21] studies used flexible doses while other four [17,19,29,30] used fixed doses of intranasal esketamine. Risk of bias assessment showed that all studies included were good in quality in terms of their methodologies (Supplementary Fig. 1; available online). Publication bias could not be tested because only one trial was un-published.
Table 1.
General characteristics of double-blinded randomized clinical trials included in the meta-analysis
| Study name (trial number) |
Length of DB | Mean age (SD) | Subjects | Clinical phase | Number of participants | Primary outcome measure | Study location | Intervention Frequency | Remission | Augmentation/ Monotherapy |
|---|---|---|---|---|---|---|---|---|---|---|
| NCT02918318a | 4 wk | 43.4 (10.35) | MDD with treatment resistant to more than 2 antidepressants | II | PBO: 80 ESK 28 mg: 41 ESK 56: 40 ESK 84: 41 |
MADRS change at week 4 | Japan only | Twice weekly Fixed dose |
MADRS < 12 | Augmentation |
| Daly et al. [19] (NCT01998958) | 2 wk | 44.7 (10.0) | MDD with treatment resistant to more than 2 antidepressants | II | PBO: 33 ESK 28 mg: 11 ESK 56: 10 ESK 84: 12 |
MADRS change at day 8 | 13 in US 1 in Belgium |
Twice weekly Fixed dose |
MADRS < 10 | Augmentation |
| Canuso et al. [17] (NCT02133001) | 4 wk | 35.8 (13.03) | MDD with imminent suicide risk | II | PBO: 31 ESK 84: 35 |
MADRS change at 4 hours | 11 in US | Twice weekly Fixed dose |
MADRS < 12 | Augmentation |
| Fedgchin et al. [29] TRANSFORM-1 (NCT02417064) |
4 wk | 46.3 (11.16) | MDD with treatment resistant to more than 2 antidepressants | III | PBO: 80 ESK 28 mg: 41 ESK 56: 40 ESK 84: 41 |
MADRS change at week 4 | 91 centers in 9 countries |
Twice weekly Fixed dose |
MADRS < 12 | Augmentation |
| Popova et al. [20] TRANSFORM-2 (NCT02418585) |
4 wk | 45.7 (11.89) | MDD with treatment resistant to more than 2 antidepressants | III | PBO: 114 ESK 56−84 mg: 109 |
MADRS change at week 4 | 39 centers in 5 countries | Twice weekly Flexible dose |
MADRS < 12 | Augmentation |
| Ochs-Ross et al. [21] TRANSFORM-3 (NCT02422186) |
4 wk | 70 (4.52) | MDD (age > 65) with treatment resistant to more than 2 antidepressants | III | PBO: 65 ESK 28−84 mg: 72 |
MADRS change at week 4 | 69 centers in 12 countries | Twice weekly Flexible dose |
MADRS < 12 | Augmentation |
| Fu et al. [15] ASPIRE-1 (NCT03039192) |
25-day | 39.3 (12.88) | MDD with suicide intent/idea | III | PBO: 112 ESK 56−84 mg: 112 |
MADRS change at 24 hrs | 51 sites in US, Europe, Asia, and South Africa | Twice weekly Flexible dose |
MADRS < 12 | Augmentation |
| Ionescu et al. [16] ASPIRE-2 (NCT03097133) |
25-day | 40.8 (13.07) | MDD with suicide intent/idea | III | PBO: 113 ESK 56−84 mg: 114 |
MADRS change at 24 hrs | 47 centers in 13 countries | Twice weekly Flexible dose |
MADRS < 12 | Augmentation |
DB, double-blinded phse; SD, standard deviation; MDD, major depressive disorder; PBO, placebo; ESK, esketamine; MADRS, Montgomery-Åsberg depression rating scale; US, United States.
aUnpublished study.
Efficacy
Primary endpoint: mean change of MADRS
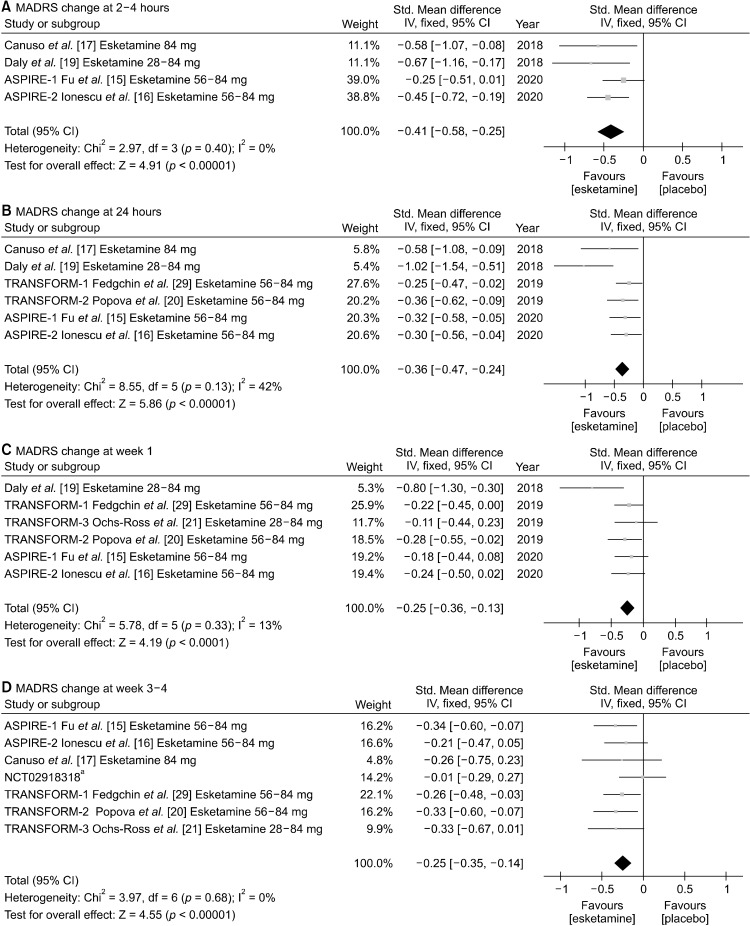
Mean change of MADRS total score from baseline to 2−4 hours, 24 hours, week 1, and week 3−4 are presented as forest plots (Fig. 2). Intranasal esketamine more significantly improved MADRS total scores than placebo for treating MDD starting from 2−4 hours after the first injection (SMD, −0.41 [95% CI, −0.58 to −0.25], p < 0.00001), and the significant superiority maintained at 24 hours (SMD, −0.36 [95% CI, −0.47 to −0.24], p < 0.00001), week 1 (SMD, −0.25 [95% CI, −0.36 to −0.13], p < 0.0001), and end of double-blinded period (week 3−4) (SMD, −0.25 [95% CI, −0.35 to −0.14], p < 0.00001). Significant heterogeneities were not reported for 2−4 hours (I2 = 0%, p = 0.40), 24 hours (I2 = 42%, p = 0.13), week 1 (I2 = 13%, p = 0.33), and week 3−4 (I2 = 0%, p = 0.68), so we used fixed effect model for all analyses.
Fig. 2.
Mean change of Montgomery-Åsberg depression rating scale (MADRS) at (A) 2−4 hours, (B) 24 hours, (C) week 1, and (D) week 3−4 between intranasal esketamine and placebo. Std., standard deviation; CI, confidence interval; IV, inverse variance. aUnpublished study.
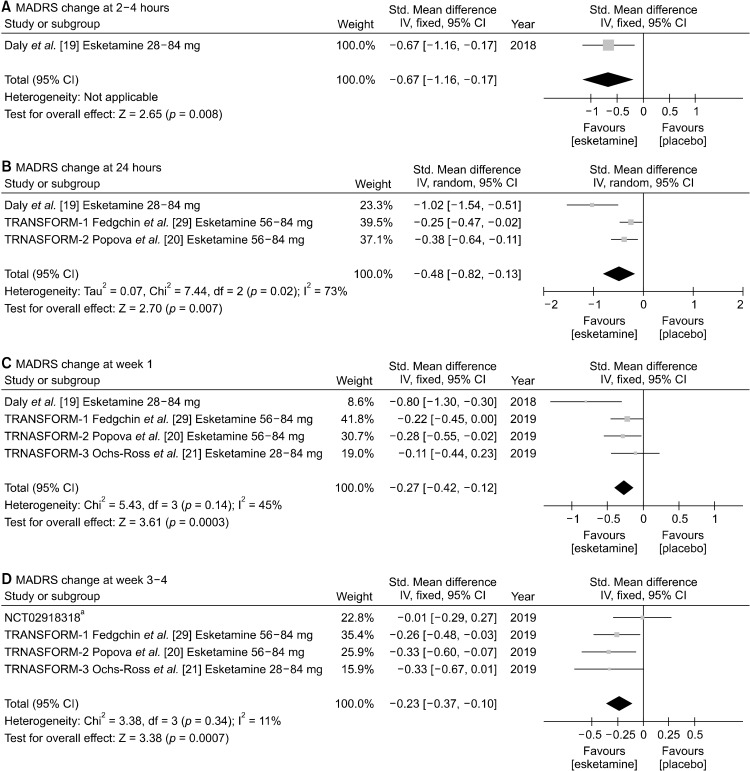
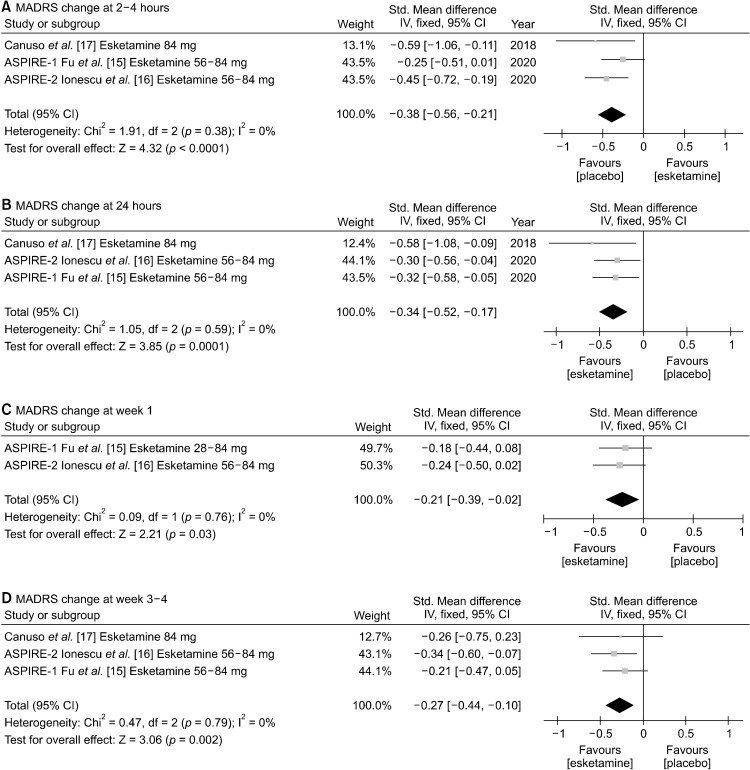
We conducted sub-group analysis for patients with TRD and MDSI. In terms of patients with TRD, MADRS improvement was significantly more superior in intranasal esketamine group than in placebo group from 2−4 hours (SMD, −0.67 [95% CI, −1.16 to −0.17], p = 0.008) to 24 hours (SMD, −0.48 [95% CI, −0.82 to −0.13], p = 0.007), week 1 (SMD, −0.27 [95% CI, −0.42 to −0.12], p = 0.0003), and week 3−4 (SMD, −0.23 [95% CI, −0.37 to −0.10], p = 0.0007). However, only one study assessed MADRS at 2−4 hours after the first injection (Fig. 3). Significant heterogeneity was noted for 24 hours (I2 = 73%, p = 0.02), so random effect model was used. For 2−4 hours, week 1, and week 3−4 fixed effect model was utilized because no significant heterogeneity was observed. Similar trends of rapid antidepressive effects were noted for subgroup analysis involving patients with MDSI at 2−4 hours (SMD, −0.38 [95% CI, −0.56 to −0.21], p < 0.0001), 24 hours (SMD, −0.34 [95%CI, −0.52 to −0.17], p = 0.0001), week 1 (SMD, −0.21 [95% CI, −0.39 to −0.02], p = 0.03), and week 3−4 (SMD, −0.27 [95% CI, −0.44 to −0.10], p = 0.002) (for all heterogeneity = 0), which is illustrated in Figure 4.
Fig. 3.
Mean change of Montgomery-Åsberg depression rating scale (MADRS) at (A) 2−4 hours, (B) 24 hours, (C) week 1, and (D) week 3−4 between intranasal esketamine and placebo in patients with treatment resistant depression (TRD). Std., standard deviation; CI, confidence interval; IV, inverse variance. aUnpublished study.
Fig. 4.
Mean change of Montgomery-Åsberg depression rating scale (MADRS) at (A) 2−4 hours, (B) 24 hours, (C) week 1, and (D) week 3−4 between intranasal esketamine and placebo in major depression with suicide ideation (MDSI). Std., standard deviation; CI, confidence interval; IV, inverse variance.
Resolution of suicide
Esketamine showed superior efficacy over placebo in resolution of suicide at 2−4 hours after initial nasal infusion with OR of 2.04 (95% CIs, 1.37 to 3.05, p = 0.0005; heterogeneity = 0%), but the two groups did not statistically differ at 24 hours (OR = 1.15, 95% CIs, 0.80 to 1.65, p = 0.46; heterogeneity = 0%) and week 3−4 (OR = 1.32, 95% CIs, 0.91 to 1.90, p = 0.44; heterogeneity = 0%) (Supplementary Fig. 2; available online).
Rate of remission
A total of seven studies were included for comparing rate of remission between intranasal esketamine and placebo groups at week 3−4. Intranasal esketamine group showed superior remission rate than placebo with OR of 1.64 (95% CIs, 1.30 to 2.07, p < 0.0001; heterogeneity = 0%) at week 3−4. In addition, superior efficacy was noted at 2−4 hours (OR = 2.43, 95% CIs, 1.27 to 4.67, p = 0.007; heterogeneity = 41%, p = 0.18) and 24 hours (OR = 2.47, 95% CIs, 1.58 to 3.85, p < 0.0001; heterogeneity = 0%) after initial nasal esketamine infusion, but the two groups did not differ at day 8 (OR = 1.46, 95% CIs, 0.96 to 2.23, p = 0.08; heterogeneity = 0%) (Supplementary Fig. 3; available online).
Safety and Tolerability
In terms of commonly observed side effects, esketamine showed higher incidence of total AEs (OR = 4.23, 95% CIs, 2.85 to 6.27, p < 0.00001; heterogeneity = 55%, p = 0.04), dissociation (OR = 7.93, 95% CIs, 5.36 to 11.72, p < 0.00001; heterogeneity = 0%), blood pressure increment (OR = 7.18, 95% CIs, 4.82 to 10.69, p < 0.00001; heterogeneity = 0%), nausea (OR = 3.28, 95% CIs, 2.40 to 4.48, p < 0.00001; heterogeneity = 30%, p = 0.20), vertigo (OR = 6.22, 95% CIs, 3.97 to 9.73, p < 0.00001; heterogeneity = 43%, p = 0.10), dysgeusia (OR = 1.67, 95% CIs, 1.21 to 2.31, p = 0.002; heterogeneity = 0%), dizziness (OR = 4.47, 95% CIs, 3.27 to 6.11, p < 0.00001; heterogeneity = 0%), and somnolence (OR = 2.08, 95% CIs, 1.49 to 2.89, p < 0.0001; heterogeneity = 0%) compared with placebo (Fig. 5). Although headache was numerically more common in esketamine group than in placebo group, the two groups did not differ statistically (OR = 1.33, 95% CIs, 1.00 to 1.77, p = 0.05; heterogeneity = 5%, p = 0.39).
Fig. 5.
Safety and tolerability: Rate of (A) total, (B) dissociation, (C) blood pressure increment, (D) nausea, (E) vertigo, (F) dysgeusia, (G) dizziness, (H) somnolence, and (I) headache during the double-blind phase. CI, confidence interval. aUnpublished study.
DISCUSSION
To the best of our knowledge, this is the largest meta- analysis (eight DB-RCTs with 1,488 subjects) comparing efficacy and safety of intranasal esketamine and placebo in patients with MDD. Our study confirmed previous research by showing that augmentation of antidepressants with intranasal esketamine was significantly more effective than with placebo for MADRS score change and depression remission [24,25]. In addition, the superior treatment response and remission of intranasal esketamine were noticeable as early as 2−4 hours after the first intranasal esketamine, and this superior efficacy lasted until end of double blinded phase, which is week 3−4.
By conducting subgroup analysis, we are the first one to show that the rapid improvement of depressive symptoms was evident in patients with TRD and MDSI. Our results also extended previous studies and showed rapid anti-suicidal effect of intranasal esketamine (resolution of suicidality 2−4 hours after the 1st injection) in MDSI. However, although intranasal esketamine showed trend of superior efficacy over placebo, the statistical significance was not maintained at 24 hours and week 3−4. Only three studies were conducted in MDSI, so small number of clinical trials might have been the main cause. More DB-RCTS are needed to define rapid anti-suicidal effects of intra-nasal esketamine.
The SMD in MADRS across different time ranged from 0.25−0.41, which equal to small~medium effect size according to Cohen’s classification [31]. More importantly, all efficacies including MADRS score change, remission of depression, and resolution of suicidality were greatest either at 2−4 hours or 24 hours after the 1st administration of intranasal esketamine. All patients in the eight DB-RCTS were taking oral antidepressants in addition to intranasal esketamine or placebo, so the efficacy difference between the two groups might have decreased or attenuated as the onset of actions for oral antidepressants started to show effects. In line with our hypothesis, the mean change of MADRS from baseline to week 3−4 was less than four points difference in all eight DB-RCTs. Thus, as Canuso et al. has suggested, intranasal esketamine could be used to overcome the efficacy gap observed between drug administration and onset of action for the conventional antidepressants [17]. This therapeutic role could be particularly important in patients having MDSI.
In terms of safety and tolerability, intranasal esketamine showed higher total AEs than the placebo group. Since esketamine was initially introduced medically as an anesthetic in Germany in 1997 [32], its higher risk of causing somnolence, dizziness, and vertigo are not surprising. The intranasal esketamine also had significantly higher rate of nausea and dysgeusia.
The risk of intranasal esketamine causing dissociation [33] and blood pressure increment [34] have been well documented. Likewise, the rate of dissociation and blood pressure increment was higher in intranasal esketamine than in placebo with particularly higher odd ratios (7.18−7.93) compared with AEs. Previous studies showed that dissociation and perceptual change symptoms peaked shortly after esketamine administration, which generally resolved by 2 hours after dosing [21,29]. Evidence also showed that rate and intensity of dissociation lowered with repeated administrations of intranasal esketamine. Similarly, studies consistently illustrated that blood pressure increment following intranasal esketamine were transient, asymptomatic, and not associated with serious cardiovascular complications [34]. However, number of studies investing long-term safety and tolerability of intranasal esketamine are scarce [35]. Therefore, whether or not intranasal esketamine result in long-terms adverse events needs further varication.
Our study contained several limitations. First, we combined all doses of intranasal esketamine (28−84 mg/day) so were not able to conduct meta-regression and investigate its dose related efficacy, tolerability, and safety. Second, we found one unpublished DB-RCT whish showed negative results. There could have been more unpublished negative trials and possibility of publication bias. Third, we did not investigate rate and severity of diverse side effects across different time. As a result, we were not able to confirm that important side effects such as dissociation and blood pressure increment resolved shortly after and attenuated as the administration of intranasal esketamine repeated.
In conclusion, the present meta-analysis confirmed that intranasal esketamine was effective in patients with MDD including TRD and MDSI. Our meta-analysis further showed that intranasal esketamine was associated with rapid antidepressant effect for patients with TRD and MDSI. The study also suggested that esketamine might have rapid anti-suicidal effects for patients with MDSI.
Supplemental Materials
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2019R1C1C1011664).
Footnotes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: Sheng-Min Wang, Won-Myong Bahk. Data acquisition: Nak-Young Kim, Hae-Ran Na. data analysis: Chi-Un Pae, Hyun Kook Lim, Young Sup Woo. Writing article: Sheng-Min Wang. All authors reviewed and approved for publication.
References
- 1.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen H -U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabbri C, Serretti A. Genetics of treatment outcomes in major depressive disorder: present and future. Clin Psychopharmacol Neurosci. 2020;18:1–9. doi: 10.9758/cpn.2020.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–659. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- 4.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 5.Fabbri C, Serretti A. How to utilize clinical and genetic information for personalized treatment of major depressive disorder: step by step strategic approach. Clin Psychopharmacol Neurosci. 2020;18:484–492. doi: 10.9758/cpn.2020.18.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blair-West GW, Cantor CH, Mellsop GW, Eyeson-Annan ML. Lifetime suicide risk in major depression: sex and age deter-minants. J Affect Disord. 1999;55:171–178. doi: 10.1016/s0165-0327(99)00004-x. [DOI] [PubMed] [Google Scholar]
- 7.Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75:336–346. doi: 10.1001/jamapsychiatry.2017.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leon AC, Solomon DA, Li C, Fiedorowicz JG, Coryell WH, Endicott J, et al. Antidepressants and risks of suicide and suicide attempts: a 27-year observational study. J Clin Psychiatry. 2011;72:580–586. doi: 10.4088/JCP.10m06552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Leary TG, Dinan TG, Cryan JF. Faster, better, stronger: towards new antidepressant therapeutic strategies. Eur J Pharmacol. 2015;753:32–50. doi: 10.1016/j.ejphar.2014.07.046,. [DOI] [PubMed] [Google Scholar]
- 10.Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, Pae CU. Five potential therapeutic agents as antidepressants: a brief review and future directions. Expert Rev Neurother. 2015;15:1015–1029. doi: 10.1586/14737175.2015.1071192. [DOI] [PubMed] [Google Scholar]
- 11.Wang SM, Han C, Pae CU. Criticisms of drugs in early development for the treatment of depression: what can be improved? Expert Opin Investig Drugs. 2015;24:445–453. doi: 10.1517/13543784.2014.985784. [DOI] [PubMed] [Google Scholar]
- 12.Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–811. doi: 10.1038/mp.2017.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molero P, Ramos-Quiroga JA, Martin-Santos R, Martin-Santos R, Calvo-Sánchez E, Gutiérrez-Rojas L, Meana JJ. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32:411–420. doi: 10.1007/s40263-018-0519-3. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Farchione T, Potter A, Chen Q, Temple R. Esketamine for treatment-resistant depression - first FDA-approved antidepressant in a new class. N Engl J Med. 2019;381:1–4. doi: 10.1056/NEJMp1903305. [DOI] [PubMed] [Google Scholar]
- 15.Fu DJ, Ionescu DF, Li X, Lane R, Lim P, Sanacora G, et al. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I) J Clin Psychiatry. 2020;81:19m13191. doi: 10.4088/JCP.19m13191. [DOI] [PubMed] [Google Scholar]
- 16.Ionescu DF, Fu DJ, Qiu X, Lane R, Lim P, Kasper S, et al. Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a phase 3, double-blind, randomized study (ASPIRE II) Int J Neuropsy-chopharmacol. 2021;24:22–31. doi: 10.1093/ijnp/pyaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175:620–630. doi: 10.1176/appi.ajp.2018.17060720. [DOI] [PubMed] [Google Scholar]
- 18.FDA approves a nasal spray to treat patients who are suicidal [Internet] [cited at 2021 Jan 10];NPR-National Public Radio. 2020 Aug 4; Available from: https://www.npr.org/2020/08/04/899060885/fda-approves-a-nasal-spray-to-treat-patients-who-are-suicidal .
- 19.Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75:139–148. doi: 10.1001/jamapsychiatry.2017.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 2019;176:428–438. doi: 10.1176/appi.ajp.2019.19020172. [DOI] [PubMed] [Google Scholar]
- 21.Ochs-Ross R, Daly EJ, Zhang Y, Lane R, Lim P, Morrison RL, et al. Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression-TRANSFORM-3. Am J Geriatr Psychiatry. 2020;28:121–141. doi: 10.1016/j.jagp.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Wang SM, Woo YS, Kim NY, Na HR, Lim HK, Bahk WM. Agomelatine for the treatment of generalized anxiety disorder: a meta-analysis. Clin Psychopharmacol Neurosci. 2020;18:423–433. doi: 10.9758/cpn.2020.18.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyman GH, Kuderer NM. The strengths and limitations of meta-analyses based on aggregate data. BMC Med Res Methodol. 2005;5:14. doi: 10.1186/1471-2288-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng W, Sim K, Ning YP, Xiang YT. Adjunctive intranasal esketamine for major depressive disorder: a systematic review of randomized double-blind controlled-placebo studies- authors’ reply. J Affect Disord. 2020;274:955. doi: 10.1016/j.jad.2020.05.154. [DOI] [PubMed] [Google Scholar]
- 25.Papakostas GI, Salloum NC, Hock RS, Jha MK, Murrough JW, Mathew SJ, et al. Efficacy of esketamine augmentation in major depressive disorder: a meta-analysis. J Clin Psychiatry. 2020;81:19r12889. doi: 10.4088/JCP.19r12889. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakagawa S, Noble DW, Senior AM, Lagisz M. Meta-evaluation of meta-analysis: ten appraisal questions for biologists. BMC Biol. 2017;15:18. doi: 10.1186/s12915-017-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20:123–129. doi: 10.1111/1469-0691.12494. [DOI] [PubMed] [Google Scholar]
- 29.Fedgchin M, Trivedi M, Daly EJ, Melkote R, Lane R, Lim P, et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active- controlled study (TRANSFORM-1) Int J Neuropsychopharmacol. 2019;22:616–630. doi: 10.1093/ijnp/pyz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.A study to evaluate the efficacy, safety and tolerability of fixed doses of intranasal esketamine in Japanese participants with treatment resistant depression [Internet] [cited at 2021 Jan 2];U.S. National Library of Medicine. 2019 Dec 13; Available from: https://clinicaltrials.gov/ct2/show/NCT02918318 .
- 31.Faraone SV. Interpreting estimates of treatment effects: implications for managed care. P T. 2008;33:700–711. [PMC free article] [PubMed] [Google Scholar]
- 32.Himmelseher S, Pfenninger E. [The clinical use of S-(+)-ketamine--a determination of its place] Anasthesiol Intensivmed Notfallmed Schmerzther. 1998;33:764–770. doi: 10.1055/s-2007-994851. German. [DOI] [PubMed] [Google Scholar]
- 33.Pereira S, Brennan E, Patel A, Moran M, Wallier J, Liebowitz MR. Managing dissociative symptoms following the use of esketamine nasal spray: a case report. Int Clin Psychopharmacol. 2021;36:54–57. doi: 10.1097/YIC.0000000000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doherty T, Wajs E, Melkote R, Miller J, Singh JB, Weber MA. Cardiac safety of esketamine nasal spray in treatment-resistant depression: results from the clinical development program. CNS Drugs. 2020;34:299–310. doi: 10.1007/s40263-020-00699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wajs E, Aluisio L, Holder R, Daly EJ, Lane R, Lim P, et al. Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: assessment of long-term safety in a phase 3, open-label study (SUSTAIN-2) J Clin Psychiatry. 2020;81:19m12891. doi: 10.4088/JCP.19m12891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.