Abstract
Background:
We aimed to investigate the impact of reducing drinking in patients with unhealthy alcohol use on improvement of chronic pain interference, substance use, and psychiatric symptoms.
Methods:
We analyzed longitudinal data from 2003–2015 in the Veterans Aging Cohort Study, a prospective, multisite observational study of US veterans, by emulating a hypothetical randomized trial (a target trial). Alcohol use was assessed using the AUDIT questionnaire and outcome conditions were assessed via validated survey items. Individuals were followed from the first time their AUDIT score was ≥8 (baseline), a threshold consistent with unhealthy alcohol use. We compared individuals who reduced drinking (AUDIT<8) at the next follow-up visit with individuals who did not (AUDIT≥8). We fit separate logistic regression models to estimate odds ratios for improvement of each condition 2-years post-baseline among individuals who had that condition at baseline: moderate or severe pain interference symptoms, tobacco smoking, cannabis use, cocaine use, depressive symptoms, and anxiety symptoms. Inverse probability weighting was used to account for potential selection bias and confounding.
Results:
Adjusted 2-year odds ratios (95% confidence intervals) for associations between reducing drinking and improvement or resolution of each condition were: 1.49 (0.91, 2.42) for pain interference symptoms, 1.57 (0.93, 2.63) for tobacco smoking, 1.65 (0.92, 2.95) for cannabis use, 1.83 (1.03, 3.27) for cocaine use, 1.11 (0.64, 1.92) for depressive symptoms, and 1.33 (0.80, 2.22) for anxiety symptoms.
Conclusions:
We found some evidence for improvement of pain interference symptoms and substance use after reducing drinking among US veterans with unhealthy alcohol use, but confidence intervals were wide.
Keywords: unhealthy alcohol use, chronic pain, substance use, psychiatric condition
INTRODUCTION
Unhealthy alcohol use, chronic pain, substance use (i.e., smoking, opioid misuse, stimulant misuse), and psychiatric conditions (i.e., depression and anxiety) are substantial sources of preventable morbidity and mortality in the United States (US) and frequently co-occur (Grant et al., 2017, Kessler et al., 1994, Kessler et al., 2005, Nahin, 2015, Centers for Disease Control and Prevention, 2017). Due to the high prevalence and adverse health effects of alcohol use, with 13% of adults meeting the criteria for unhealthy alcohol use (Grant et al., 2017), screening for and treatment of alcohol use disorders have received significant attention from practitioners, policy makers, and health researchers (U. S. Preventive Services Task Force, 2018). Unhealthy alcohol use, however, also often co-occurs with other substance use and psychiatric conditions that are not included in current United States Preventive Services Task Force screening guidelines (U.S. Preventive Services Task Force, 2019), and are therefore identified and treated less frequently (Kushner et al., 2000, Boden and Fergusson, 2011, Brennan et al., 2005, Substance Abuse and Mental Health Services Administration, 2013, Reed et al., 2007, McCabe et al., 2006, Anglin et al., 1989, Welte and Barnes, 1982, Substance Abuse and Mental Health Services Administration, 2014, Gossop et al., 2006, Marks et al., 2015).
Integrating the screening and/or treatment of pain, substance use, and psychiatric conditions co-occurring with unhealthy alcohol use could improve health and economic outcomes. Potential advantages of integrated screening and treatment include economies of scope (i.e., leveraging an existing screening infrastructure for a new screening target may reduce cost), and patient-centeredness (i.e., fewer distinct clinic visits at separate sites and times). Screening for one condition might provide incidental information regarding a second condition, because the conditions co-occur and/or because the screening test for the first condition has unrecognized diagnostic value for the second condition (Khan et al., 2020). Similarly, treating one condition might treat a second condition because resolution of the first condition may have an incidental “spillover” effect on the co-occurrence of the second condition and/or because the treatment is effective against both conditions.
Treating unhealthy alcohol use in particular could help address a second condition if resolution of unhealthy alcohol use has an incidental, beneficial effect on the co-occurrence of the second condition (via neurologic or behavioral pathways) or if the treatment for unhealthy alcohol use is effective against both conditions (e.g., naltrexone is approved for the treatment of both alcohol and opioid use disorder (Oslin et al., 2015, Krupitsky et al., 2011); varenicline for alcohol and smoking cessation (de Bejczy et al., 2015, Mitchell et al., 2012, Litten et al., 2013); and gabapentin for alcohol and chronic pain (Mason et al., 2014)). A previous study involving the Veterans Aging Cohort Study (VACS) demonstrated that reducing drinking in patients with unhealthy alcohol use was associated with discontinuation of tobacco smoking and improvement of depressive symptoms (Braithwaite et al., 2015), but did not include some elements of a causal inference approach (i.e. robust adjustment for confounding and direct specification of a hypothetical randomized trial or a target trial) (Hernán et al., 2016, Hernan and Robins, 2016). In addition, the previous study did not evaluate the impact of reducing drinking in patients with unhealthy alcohol use on discontinuation of other substances and improvement of other psychiatric disorders.
The use of alcohol and other substances to self-medicate for or mask symptoms of pain, anxiety, and depression has been well documented in the literature as a potential explanation for the co-occurring nature of these conditions (Bolton et al., 2009, Quitkin et al., 1972, Bolton et al., 2006, Riley and King, 2009, Alford et al., 2016). In a large, nationally representative survey of mental illness in community-dwelling adults in the U.S. (i.e., the National Epidemiologic Survey on Alcohol and Related Conditions), reported self-medication with alcohol or drugs was common, with 18% of individuals with generalized anxiety disorder (Robinson et al., 2009) and 15% of individuals with major depressive disorder self-medicating with alcohol (Bolton et al., 2009). In this population, self-medication with alcohol for depression and other mood disorders was associated with higher odds of comorbid anxiety, self-medication for anxiety was associated with higher odds of comorbid mood disorders, and self-medication was associated with future alcohol use disorder, signaling any perceived benefits of self-medication may be unwarranted (Bolton et al., 2009, Robinson et al., 2009, Crum et al., 2013). An additional study in a primary care setting found that the majority of patients misusing drugs and alcohol had chronic pain and were using substances to self-medicate (Alford et al., 2016). While the link between alcohol misuse and chronic pain is well documented, it is often difficult to determine the direction of causality between these conditions, and it is also possible that alcohol misuse could exacerbate pain or increase injury risk (Egli et al., 2012, Justice et al., 2016).
Here, we attempt to emulate a hypothetical randomized trial (a target trial) (Hernan and Robins, 2016, Hernán et al., 2016) to evaluate whether reducing drinking could lead to improvement or resolution of pain interference symptoms, tobacco use, illicit opioid and stimulant use, depressive symptoms, and anxiety symptoms among individuals with unhealthy alcohol use. The target trial framework explicitly outlines key components of a hypothetical randomized trial -- e.g., eligibility criteria, treatment strategies, start and end of follow-up, and outcomes of interest -- and how each component may be emulated using observational data. The framework is helpful to avoid selection and confounding biases that are consequences of common flaws in observational studies’ design and analyses, to identify potential limitations of the data and analytic plan, and to guide interpretation of study findings. We use data from HIV-positive and HIV-negative veterans in the VACS, a population with relatively high prevalence of these target conditions who may benefit substantially from interventions to reduce unhealthy alcohol use.
MATERIALS AND METHODS
Study population
The VACS includes US veterans receiving healthcare in nine Veteran Administration (VA) centers: Atlanta, Baltimore, the Bronx, Dallas, Houston, Los Angeles, Manhattan/Brooklyn, Pittsburgh, and Washington, D.C. The VACS includes clinical, administrative, and survey data on approximately 3,500 HIV-positive veterans and 3,500 HIV-negative controls, frequency matched by age, race, gender, and site (Justice et al., 2001b). Surveys were administered approximately annually from 2003 to 2015 in Atlanta, the Bronx, Houston, Los Angeles, Manhattan/Brooklyn, and Pittsburg and from 2004 to 2015 in Baltimore and Washington. The surveys collected demographic and clinical information including HIV risk factors, alcohol use, moderate or severe pain interference symptoms, use of other substances, and symptoms of anxiety and depression. HIV status was ascertained via the medical record. Institutional review boards at each site as well as New York University and Yale University approved all study activities.
Alcohol assessment
The alcohol use disorder identification test (AUDIT) is a 10-item questionnaire designed to detect hazardous or harmful drinking (Gache et al., 2005). The AUDIT assesses past year alcohol consumption, dependence symptoms, and consequences of use, in addition to past year or lifetime alcohol-related injury and others’ concern about use. Each item is scored from 0–4 for a total score ranging from 0–40. Those reporting no alcohol use in the past year are given a score of 0 on all items with the exception of items 9 and 10, which are not restricted to the past year, and those with missing AUDIT items who report never drinking are given a score of 0.(Khan et al., 2020) The World Health Organization guidelines consider AUDIT scores ≥8 consistent with at-risk, hazardous, or harmful drinking (which we considered consistent with “unhealthy alcohol use” (Saitz, 2005)) and scores <8 consistent with low-risk drinking and/or abstinence (World Health Organization, 2001).
Current pain, smoking, depression, and anxiety assessment
Current pain interference symptoms were ascertained using a single question from the Health Survey Short-Form 12, which asked the participant: “During the last month, how much has pain interfered with your normal work (including work outside and inside the home)?” We classified individuals who answered “moderately”, “quite a bit”, or “extremely” as having moderate or severe pain interference (Becker et al., 2009, Novak et al., 2009, Ware et al., 1996, Stevens, 2020). Current smoking status was ascertained at each survey with a single question: “Do you currently smoke cigarettes?” Current (past 2 weeks) depressive symptoms were measured using the Patient Health Questionnaire (PHQ-9), where a score of 10 or more was classified as current depression (Kroenke et al., 2001). Current anxiety symptoms were assessed by a single survey item which asked if the participant had or had not “felt nervous or anxious” in the four weeks before the survey. We classified individuals who stated the symptom at least “bothers me a little” as having anxiety symptoms (Justice et al., 2001a). Single-item screening tools for anxiety have shown robust test performance in detection of validated measures of anxiety symptoms (Young et al., 2015).
Past-year cannabis, cocaine, other stimulants, and illicit opioid use
At each survey, individuals were asked about their substance use over the past-year, including use of cannabis, crack/cocaine, other stimulants (e.g., amphetamine), heroin, and prescription opioids (e.g., Oxycontin, Vicodin, Percocet). We defined self-reported illicit opioid use as non-medical use of prescription opioids or heroin use (Edelman et al., 2020, Khan et al., 2020).
Eligibility criteria, treatment strategies, and start of follow-up
We included individuals with an AUDIT score ≥8 at one or more follow-up visits in our analysis and classified these individuals as meeting criteria for “unhealthy alcohol use”. We defined the start of follow-up (baseline) as the first survey during the study period at which the AUDIT score was ≥8. Our treatment strategies of interest were: (1) reduce AUDIT score to <8 at the next scheduled follow-up visit (“reduce drinking”) and (2) retain an AUDIT score ≥8 at the next scheduled follow-up visit (“do not reduce drinking”). At the next follow-up visit (1-year post baseline), individuals with an AUDIT score <8 were classified as reducing drinking and individuals with an AUDIT score ≥8 were classified as not reducing drinking. Individuals who were missing AUDIT items 9 and/or 10 but reported no drinking in the past year and individuals who were missing one AUDIT item but the sum of the remaining 9 AUDIT items was less than or equal to 3 were also classified as having an AUDIT score less than 8 (Khan et al., 2020). Individuals who had a missing AUDIT score at the next follow-up visit or who did not attend the next follow-up visit were classified as having an unknown drinking reduction status. The definition of our treatment strategies was agnostic to AUDIT score after 1-year post-baseline.
Outcome definitions and end of follow-up
Our primary outcomes were improvement of moderate or severe pain interference symptoms (answering “not at all” or “a little bit” to the same pain question), stopping smoking, improvement of depressive symptoms (defined as PHQ-9≤9), and resolution of anxiety symptoms (answering “I do not have this symptom” to the same anxiety question) at 1-year and 2-years post baseline, and discontinuation of cannabis and cocaine (no use in the past year) at 2-years post baseline. We did not consider cannabis and past-year cocaine use at 1-year post baseline because the survey asked about past-year rather than current substance use. Individuals were followed from baseline until 2-years post-baseline, the administrative end of follow-up, death, or censoring (defined as having an unknown drinking reduction status at 1-year post baseline).
Statistical analyses
We fit separate logistic regression models to estimate odds ratios (ORs) for resolution of each outcome at 1 and 2 years post-baseline comparing participants who reduced their drinking (AUDIT score <8) to those who did not reduce their drinking (AUDIT score ≥8). For each outcome, the analyses were restricted to individuals who had that condition at baseline. For example, the tobacco smoking analysis assessed smoking status at 1-year and at 2-years among individuals who were tobacco smokers at baseline.
Individuals who were classified as having an unknown drinking reduction status at 1-year post baseline were censored in our analyses as it was unknown what treatment strategy they followed. Inverse probability weights were estimated to account for potential selection bias induced by censoring individuals who did not have an AUDIT measured at 1-year post baseline and for confounding by baseline co-occurring condition status and demographic factors (Hernán and Robins, 2019). To estimate the weights, two separate models were fit. First, we fit a logistic regression model for having an AUDIT measured at 1-year versus not having an AUDIT measured at 1-year, conditional on the following baseline variables: HIV status, race, education, income, AUDIT score, current moderate or severe pain interference symptoms, smoking, depressive symptoms, and anxiety symptoms, and past-year cannabis, cocaine, other stimulant, and illicit opioid use. Second, we fit a logistic regression model for reducing AUDIT score to <8 at 1-year versus not reducing AUDIT score to <8 at 1-year, conditional on having an AUDIT measured at 1-year, the baseline variables mentioned above, and the following time-varying covariates measured at 1-year: past-year cannabis, cocaine, other stimulant, and illicit opioid use. We assumed changes in these time-varying covariates occurred prior to or at the same time as (and caused or shared common causes with) changes in 1-year AUDIT score, rather than occurring after (and being caused by) changes in 1-year AUDIT score (Figure 1a). We did not include time-varying measures of current moderate or severe pain interference symptoms, smoking, depressive symptoms, and anxiety symptoms measured at 1-year because these items could be caused by 1-year AUDIT score. All uncensored individuals (those with an AUDIT measured at 1-year) received a weight inversely proportional to the product of their conditional probability of having an AUDIT measured and having the 1-year AUDIT score (≥8 or <8) that they did indeed have. The weights were stabilized and truncated at the 99th percentile.
Figure 1a. Causal DAG for estimating the effect of reducing AUDIT score to <8 (A) on co-occurring conditions (Y) under various assumptions about the causal structure of the data (shown at one time point only).
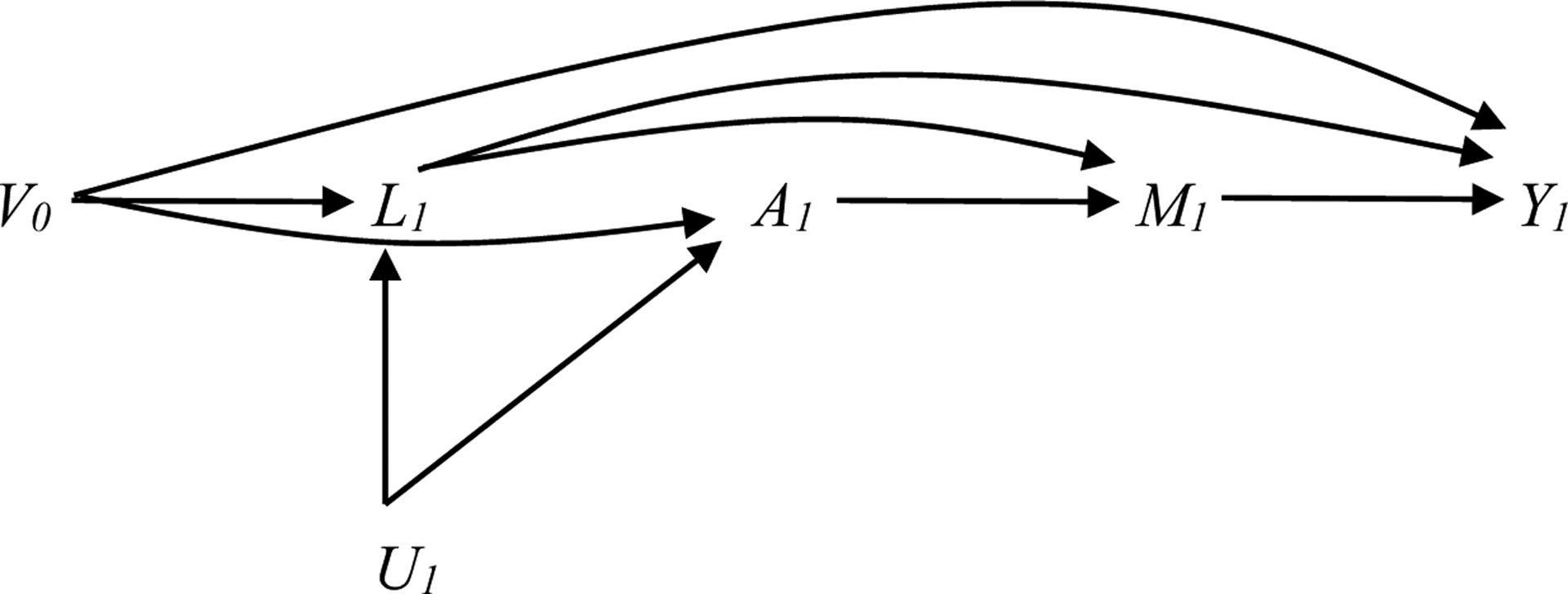
assumes the following temporal ordering of variables: L1, A1, M1. L1 and V0 are included in the weight model.
V0 includes: baseline covariates (HIV status, race, education, income); past year cannabis, cocaine, other stimulants, and illicit opioids measured at time 0; past year alcohol measured at time 0; and current depression, anxiety, smoking, and pain measured at time 0
L1 past year cannabis, cocaine, other stimulants, and opioids measured at time 1
U1 Potential unmeasured common causes at time 1
A1 past year alcohol measured at time 1
M1 current depression, anxiety, smoking, and pain measured at time 1 (exclude outcome)
Y1 outcome measured at time 1 (for depression, anxiety, smoking, and pain as outcome only)
The logistic regression models to estimate the odds ratios for each outcome at each time were then fit in the pseudo-population created by the inverse probability weights. Under the assumption of no model misspecification for all of the fitted models, the inverse probability weights create a pseudo-population where confounding and selection bias by the measured covariates no longer exist (Robins, 1997).
Among individuals who reduced their AUDIT score between baseline and the next follow-up visit, the exact time of AUDIT score reduction is unknown. This makes confounding adjustment challenging as it is unknown whether changes in co-occurring conditions between baseline and the next follow-up are causes of reduction in AUDIT score (i.e. co-occurring conditions measured at the next follow-up should be included in the model) or consequences of reduction in AUDIT score (i.e. co-occurring conditions measured at the next follow-up should be omitted from the model). We evaluated the sensitivity of our estimates to assumptions about the causal structure of our data. First, we assumed changes in AUDIT score between baseline and the next follow-up occurred prior to changes in cannabis, cocaine, other stimulant, and illicit opioid use reported at next follow-up and excluded these variables from the model for the weights (Figure 1b). Second, we assumed changes in AUDIT score occurred after changes in moderate or severe pain interference symptoms, smoking, depressive symptoms, and anxiety symptoms, and included these variables in the model for the weights (Figure 1c). Finally, we evaluated effect modification by HIV status in secondary analyses to understand if findings may differ by HIV status.
Figure 1b. Causal DAG for estimating the effect of reducing AUDIT score to <8 (A) on co-occurring conditions (Y) under various assumptions about the causal structure of the data (shown at one time point only).
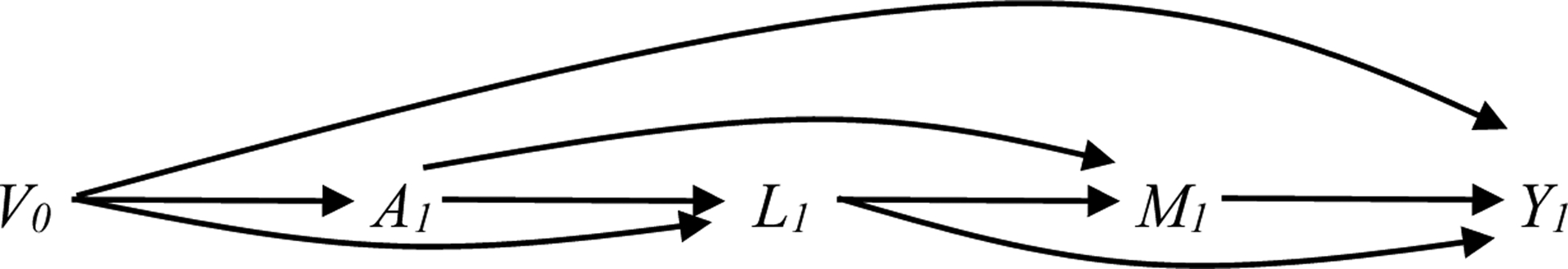
assumes the following temporal ordering of variables: A1, L1, M1. V0 is included in the weight model.
V0 includes: baseline covariates (HIV status, race, education, income); past year cannabis, cocaine, other stimulants, and illicit opioids measured at time 0; past year alcohol measured at time 0; and current depression, anxiety, smoking, and pain measured at time 0
L1 past year cannabis, cocaine, other stimulants, and opioids measured at time 1
U1 Potential unmeasured common causes at time 1
A1 past year alcohol measured at time 1
M1 current depression, anxiety, smoking, and pain measured at time 1 (exclude outcome)
Y1 outcome measured at time 1 (for depression, anxiety, smoking, and pain as outcome only)
Figure 1c. Causal DAG for estimating the effect of reducing AUDIT score to <8 (A) on co-occurring conditions (Y) under various assumptions about the causal structure of the data (shown at one time point only).
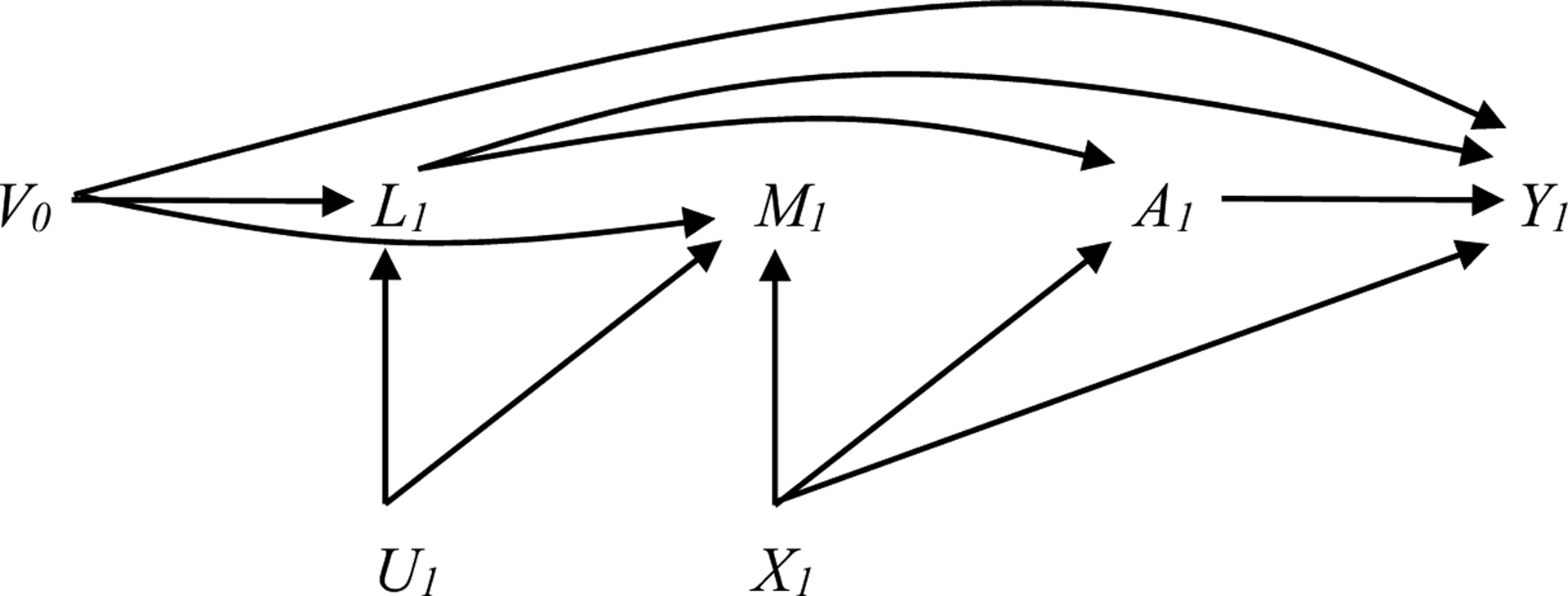
assumes the following temporal ordering of variables: L1, M1, A1. L1, M1, and V0 are included in the weight model.
V0 includes: baseline covariates (HIV status, race, education, income); past year cannabis, cocaine, other stimulants, and illicit opioids measured at time 0; past year alcohol measured at time 0; and current depression, anxiety, smoking, and pain measured at time 0
L1 past year cannabis, cocaine, other stimulants, and opioids measured at time 1
U1 Potential unmeasured common causes at time 1
A1 past year alcohol measured at time 1
M1 current depression, anxiety, smoking, and pain measured at time 1 (exclude outcome)
X1 Potential common cause(s) of M1 and A1
Y1 outcome measured at time 1 (for depression, anxiety, smoking, and pain as outcome only)
RESULTS
A total of 1,491 individuals met criteria for unhealthy alcohol use (AUDIT≥8) and were included in our analysis. 97.7% of individuals were male, 70.6% of individuals were African-American, 88.9% received a high school education or more, and 54.9% had an annual household income less than $12,000. 69% of individuals had an AUDIT score of 8–15, 11% had an AUDIT score of 16–19, and 20% had an AUDIT score of 20 or greater. Co-occurring conditions were common, ranging from 5.9% to 67.5% depending on the condition. At baseline, 44.7% of individuals had current moderate or severe pain interference symptoms. More than two thirds of individuals were current smokers, one third of individuals reported past-year cannabis use, and one third of individuals reported past-year cocaine use. Reported past-year use of other stimulants and illicit opioids was less common: 5.9% and 18.2%, respectively. 33.5% of individuals had current depressive symptoms and 53.7% had current anxiety symptoms (Table 1). Baseline year was between 2003 and 2007 (VACS survey waves 1–3) for the majority (69%) of individuals, whereas 15% had a baseline year in 2009 or later (VACS survey waves 5–6).
Table 1.
Baseline characteristics of included individuals, overall and by AUDIT score at the next survey, VACS
| Baseline characteristic Number (%) |
All individuals n=1,491 |
AUDIT<8 at next survey n=455 |
AUDIT≥8 at next survey n=556 |
No AUDIT at next survey n=480 |
|---|---|---|---|---|
| HIV status | ||||
| Positive | 737 (49.4) | 244 (53.6) | 266 (47.8) | 227 (47.3) |
| Negative | 754 (50.6) | 211 (46.4) | 290 (52.2) | 253 (52.7) |
| Race | ||||
| African-American | 1,052 (70.6) | 329 (72.3) | 401 (72.1) | 322 (67.1) |
| Other | 439 (29.4) | 126 (27.7) | 155 (27.9) | 158 (32.9) |
| Highest educational attainment | ||||
| Less than high school | 142 (9.5) | 35 (7.7) | 55 (9.9) | 52 (10.8) |
| High school or more | 1,325 (88.9) | 414 (91.0) | 493 (88.7) | 418 (87.1) |
| Missing | 24 (1.6) | 6 (1.3) | 8 (1.4) | 10 (2.1) |
| Annual household income | ||||
| <$12,000 | 818 (54.9) | 267 (58.7) | 311 (55.9) | 240 (50.0) |
| ≥12,000 | 624 (41.9) | 171 (37.6) | 230 (41.4) | 223 (46.5) |
| Missing | 49 (3.3) | 17 (3.7) | 15 (2.7) | 17 (3.5) |
| Current (past month) moderate or severe pain interference symptoms | ||||
| Yes | 667 (44.7) | 203 (44.6) | 241 (43.4) | 223 (46.5) |
| No | 798 (53.5) | 244 (53.6) | 307 (55.2) | 247 (51.5) |
| Missing | 26 (1.7) | 8 (1.8) | 8 (1.4) | 10 (2.1) |
| Current smoker | ||||
| Yes | 1,006 (67.5) | 276 (60.7) | 404 (72.7) | 326 (67.9) |
| No | 485 (32.5) | 179 (39.3) | 152 (27.3) | 154 (32.1) |
| Current (past year) cannabis use | ||||
| Yes | 502 (33.7) | 142 (31.2) | 194 (34.9) | 166 (34.6) |
| No | 945 (63.4) | 303 (66.6) | 346 (62.2) | 296 (61.7) |
| Missing | 44 (3.0) | 10 (2.2) | 16 (2.9) | 18 (3.8) |
| Current (past year) cocaine use | ||||
| Yes | 499 (33.5) | 149 (32.8) | 192 (34.5) | 158 (32.9) |
| No | 953 (63.9) | 289 (63.5) | 351 (63.1) | 313 (65.2) |
| Missing | 39 (2.6) | 17 (3.7) | 13 (2.3) | 9 (1.9) |
| Current (past year) other stimulant use | ||||
| Yes | 88 (5.9) | 31 (6.8) | 26 (4.7) | 31 (6.5) |
| No | 1,354 (90.8) | 407 (89.5) | 512 (92.1) | 435 (90.6) |
| Missing | 49 (3.3) | 17 (3.7) | 18 (3.2) | 14 (2.9) |
| Current (past year) illicit opioid use | ||||
| Yes | 272 (18.2) | 89 (19.6) | 92 (16.6) | 91 (19.0) |
| No | 1,143 (76.9) | 344 (75.6) | 430 (77.3) | 373 (77.7) |
| Missing | 72 (4.8) | 16 (3.3) | 34 (6.1) | 16 (3.3) |
| Current (past 2 weeks) depressive symptoms | ||||
| Yes | 499 (33.5) | 150 (33.0) | 185 (33.3) | 164 (34.2) |
| No | 971 (65.1) | 301 (66.2) | 364 (65.5) | 306 (63.8) |
| Missing | 21 (1.4) | 4 (0.9) | 7 (1.3) | 10 (2.1) |
| Current (past 4 weeks) anxiety symptoms | ||||
| Yes | 801 (53.7) | 234 (51.4) | 308 (55.4) | 259 (54.0) |
| No | 515 (34.5) | 163 (35.8) | 191 (34.4) | 161 (33.5) |
| Missing | 175 (11.7) | 58 (12.8) | 57 (10.3) | 60 (12.5) |
At the next follow-up visit after baseline, 455 (30.5%) individuals reduced their drinking (AUDIT<8), 556 (37.3%) did not reduce their drinking (AUDIT ≥8), 102 (6.8%) had a missing AUDIT score, and 378 (25.4%) did not attend the visit. Individuals who reduced their drinking at the follow-up visit were more likely to be HIV-positive at baseline, and were less likely to have had anxiety symptoms and have been tobacco smokers at baseline than individuals who did not reduce their drinking at the next follow-up visit (Table 1). Individuals without an AUDIT score at the next follow-up visit were less likely to be African-American and less likely to have an annual household income less than $12,000 than individuals with an AUDIT score. Of the 1,011 with a non-missing AUDIT score at 1-year post baseline, 784 (77.5%) remained under follow up at 2-years post baseline (75.2% of those who reduced their drinking and 79.5% of those who did not reduce their drinking at 1-year). Sixty-four (14.1%) of the individuals who had an AUDIT<8 at 1-year had an AUDIT≥8 at 2-years and 136 (24.5%) of the individuals who had an AUDIT≥8 at 1-year had an AUDIT<8 at 2-years.
The results of the multivariable regression analyses are shown in Table 2. Among individuals with moderate or severe pain interference symptoms at baseline, the adjusted odds ratio (95% confidence interval) for improvement of moderate or severe pain interference symptoms was 1.31 (0.84, 2.07) at 1-year and 1.49 (0.91, 2.42) at 2-years post baseline comparing reducing drinking (AUDIT<8) versus not reducing drinking (AUDIT≥8). Among individuals who were tobacco smokers at baseline, the adjusted odds ratio for stopping smoking was 1.67 (0.98, 2.84) at 1-year and 1.57 (0.93, 2.63) at 2-years post baseline, comparing reducing drinking to not reducing drinking. Among individuals with depressive symptoms at baseline, the adjusted odds ratio for improvement of depressive symptoms was 1.10 (0.68, 1.79) at 1-year and 1.11 (0.64, 1.92) at 2-years post baseline, comparing reducing drinking versus not reducing drinking. Among individuals with anxiety symptoms at baseline, the adjusted odds ratio for no longer having anxiety symptoms was 1.33 (0.82, 2.15) at 1-year and 1.33 (0.80, 2.22) at 2-years post baseline, comparing reducing drinking versus not reducing drinking (Table 2).
Table 2.
Odds ratios and 95% confidence intervals for each condition improving or resolving comparing those who reduce drinking (AUDIT<8) at 1-year with those who do not reduce drinking (AUDIT≥8) at 1-year
| Condition improves | Analysis | Odds ratios* (95% CIs) | |
|---|---|---|---|
| At 1-year | At 2-years | ||
| Current moderate or severe pain | Unadjusted | 1.45 (0.97, 2.17) | 1.89 (1.20, 2.99) |
| Interference symptoms | Adjusted | 1.31 (0.84, 2.07) | 1.49 (0.91, 2.42) |
| Current smoking | Unadjusted | 2.10 (1.28, 3.44) | 1.64 (1.01, 2.68) |
| Adjusted | 1.67 (0.98, 2.84) | 1.57 (0.93, 2.63) | |
| Current depressive symptoms | Unadjusted | 1.18 (0.76, 1.82) | 1.33 (0.80, 2.22) |
| Adjusted | 1.10 (0.68, 1.79) | 1.11 (0.64, 1.92) | |
| Current anxiety symptoms | Unadjusted | 1.03 (0.73, 1.45) | 1.38 (0.86, 2.22) |
| Adjusted | 1.33 (0.82, 2.15) | 1.33 (0.80, 2.22) | |
| Past year cannabis use | Unadjusted | -- | 1.66 (1.00, 2.76) |
| Adjusted | -- | 1.65 (0.92, 2.95) | |
| Past year cocaine use | Unadjusted | -- | 2.05 (1.24, 3.37) |
| Adjusted | -- | 1.83 (1.03, 3.27) | |
Adjusted for HIV status, race, education, income, baseline conditions (AUDIT score, depressive symptoms, anxiety symptoms, moderate or severe pain interference symptoms, current smoking, and past-year cannabis, cocaine, other stimulant, and illicit opioid use), and time-varying covariates measured at 1-year (past-year cannabis, cocaine, other stimulant, and illicit opioid use).
Cannot evaluate “past year” outcomes at 1-year since exposure is “past year” alcohol.
Among individuals reporting cannabis use in the past year at baseline, the adjusted odds ratio for reporting no longer using cannabis in the past year at 2-years post baseline was 1.65 (0.92, 2.95) comparing reducing drinking versus not reducing drinking. Among individuals reporting past year cocaine use at baseline, the adjusted odds ratio for reporting no longer using cocaine in the past year at 2-years post baseline was 1.83 (1.03, 3.27) comparing reducing drinking versus not reducing drinking (Table 2).
Different assumptions about the causal structure of the data yielded variable estimates. Assuming changes in AUDIT score between baseline and the next follow-up occurred prior to changes in cannabis, cocaine, other stimulant use, and illicit opioid use reported at the next follow-up (and thus excluding these variables from the model for the weights) resulted in 2-year estimates that were generally larger than our primary estimates, ranging from 1.21 (0.70, 2.06) for improvement of depressive symptoms to 2.27 (1.31, 3.94) for no longer using cocaine. In contrast, assuming changes in AUDIT score occurred after changes in moderate or severe pain interference symptoms, smoking, depressive symptoms, and anxiety symptoms (and thus including these variables in the model for the weights) resulted in 2-year estimates that were generally smaller, ranging from 0.98 (0.56, 1.72) for improvement of depressive symptoms to 1.69 (0.94, 3.05) for no longer using cocaine (Table 3).
Table 3.
Odds ratios and 95% confidence intervals for each condition improving or resolving at 2-years post-baseline comparing those who reduce drinking (AUDIT<8) at 1-year with those who do not reduce drinking (AUDIT≥8) at 1-year, under different assumptions about the causal structure of the data
| Condition improves | Analysis | Odds ratios (95% CIs) |
|---|---|---|
| Current moderate or severe pain | Figure 1a* | 1.49 (0.91, 2.42) |
| Interference symptoms | Figure 1b** | 1.77 (1.11, 2.84) |
| Figure 1c*** | 1.48 (0.91, 2.42) | |
| Current smoking | Figure 1a* | 1.57 (0.93, 2.63) |
| Figure 1b** | 1.66 (1.00, 2.75) | |
| Figure 1c*** | 1.21 (0.72, 2.03) | |
| Current depressive symptoms | Figure 1a* | 1.11 (0.64, 1.92) |
| Figure 1b** | 1.21 (0.70, 2.06) | |
| Figure 1c*** | 0.98 (0.56, 1.72) | |
| Current anxiety symptoms | Figure 1a* | 1.33 (0.80, 2.22) |
| Figure 1b** | 1.28 (0.78, 2.11) | |
| Figure 1c*** | 1.23 (0.73, 2.07) | |
| Past year cannabis use | Figure 1a* | 1.65 (0.92, 2.95) |
| Figure 1b** | 1.85 (1.05, 3.25) | |
| Figure 1c*** | 1.66 (0.92, 3.01) | |
| Past year cocaine | Figure 1a* | 1.83 (1.03, 3.27) |
| Figure 1b** | 2.27 (1.31, 3.94) | |
| Figure 1c*** | 1.69 (0.94, 3.05) |
Primary weighted analysis reported in Table 2. Adjusted for HIV status, race, education, income, baseline conditions (AUDIT score, depressive symptoms, anxiety symptoms, moderate or severe pain interference symptoms, current smoking, and past-year cannabis, cocaine, other stimulant, and illicit opioid use), and some time-varying covariates measured at 1-year (past-year cannabis, cocaine, other stimulant, and illicit opioid use).
All time-varying covariates measured at 1-year (past-year cannabis, cocaine, other stimulant, and illicit opioid use, depressive symptoms, anxiety, symptoms, smoking, and moderate or severe pain interference symptoms) excluded from the model for the weights.
All time-varying covariates measured at 1-year included in the model for the weights.
When we stratified our analyses by HIV status, our 2-year estimates were similar for no longer using cannabis, improvement of depressive symptoms, and resolution of anxiety symptoms. However, 2-year estimates for stopping smoking and no longer using cocaine were larger among HIV-positive compared with HIV-negative individuals, while 2-year estimates for no longer having moderate or severe pain interference symptoms were larger among HIV-negative individuals than HIV-positive individuals (Table 4).
Table 4.
Odds ratios and 95% confidence intervals for each condition improving or resolving at 2-years post-baseline comparing those who reduce drinking (AUDIT<8) at 1-year with those who do not reduce drinking (AUDIT≥8) at 1-year, by HIV-status
| Condition improves | Odds ratios* (95% CIs) | |
|---|---|---|
| HIV-negative | HIV-positive | |
| Current moderate or severe pain interference symptoms | 2.03 (1.07, 3.85) | 0.93 (0.43, 2.03) |
| Current smoking | 0.86 (0.40, 1.85) | 2.44 (1.18, 5.03) |
| Current depressive symptoms | 1.00 (0.45, 2.21) | 1.15 (0.53, 2.53) |
| Current anxiety symptoms | 1.21 (0.57, 2.56) | 1.38 (0.68, 2.80) |
| Past year cannabis use | 2.25 (0.86, 5.87) | 1.36 (0.65, 2.84) |
| Past year cocaine | 1.42 (0.56, 3.62) | 2.12 (1.02, 4.43) |
Adjusted for, race, education, income, baseline conditions (AUDIT score, depressive symptoms, anxiety symptoms, moderate or severe pain interference symptoms, current smoking, and past-year cannabis, cocaine, other stimulant, and illicit opioid use), and time-varying covariates measured at 1-year. For simplicity, we show one time point only. The DAG could be extended to include time zero and time two.
DISCUSSION
Our study is the first to specify and attempt to emulate a target trial of reducing drinking in patients with unhealthy alcohol use to improve or resolve co-occurring pain, use of other substances, and psychiatric symptoms. We found some evidence for greater improvement of pain interference symptoms and greater discontinuation of co-occurring substance use (i.e. smoking, cocaine, and cannabis) after reducing drinking (reducing AUDIT score to <8) compared with not reducing drinking (retaining an AUDIT score ≥8). In contrast, we found little evidence for greater improvement of depressive symptoms or greater resolution of anxiety symptoms after reducing drinking. Comparing results in HIV-positive individuals with results in HIV-negative individuals, odds ratios were larger for stopping smoking and discontinuing cocaine but smaller for improving pain interference symptoms, but confidence intervals were wide. However, a causal interpretation of our findings is not warranted due to unmeasured and residual confounding resulting from missing data on the timing and reasons for reducing drinking.
Our results are consistent with other studies that have demonstrated an association between reducing drinking in patients with unhealthy alcohol use and a decrease in the use of other substances (Dermody et al., 2018, Braithwaite et al., 2015, Edelman et al., Ruggles et al., 2016). A previous analysis in the VACS found that reducing drinking (defined as reducing AUDIT-C from ≥4 to <4) was associated with discontinuation of tobacco smoking and improvement of depressive symptoms, with larger odds ratios for smoking than for depression (Braithwaite et al., 2015). While our analysis included updated data, analyzed additional outcomes, and used a target trial framework, the results for smoking and depression were markedly similar.
To estimate a causal effect of reducing drinking in patients with unhealthy alcohol use on the improvement or resolution of other co-occurring conditions, all common causes of reducing drinking and the incidence of co-occurring conditions must be measured and adjusted for. Since information on alcohol use and other co-occurring conditions was assessed at each survey, the precise timing of reductions in drinking vis-a-vis other co-occurring conditions was unknown. For example, if an individual with unhealthy alcohol use reduced their drinking and stopped smoking between the first and second survey, it is unknown which event occurred first. Similarly, if an individual reduced their drinking and experienced improvement of pain interference symptoms between the first and second survey, it is unknown which event occurred first. As the precise date at which drinking was reduced and other conditions were resolved or improved is not available, we were not able to adequately adjust for potential causes of reducing drinking. We addressed this uncertainty by varying our assumptions about the causal structure of the data in sensitivity analyses. Different assumptions about the temporal ordering of changes in alcohol, smoking, symptoms of pain interference, depression, and anxiety, and other substance use resulted in variable estimates of the relationship between reducing drinking and resolution or improvement of co-occurring conditions. Assuming changes in all of the other conditions reported at the next follow-up visit occurred after changes in drinking reported at the next follow-up visit resulted in larger estimates whereas assuming changes in all of the other conditions occurred prior to changes in drinking resulted in smaller estimates. However, the point estimates from these analyses were in the same direction and did not vary by more than 25% of the estimates from our primary analysis. Since the assumptions about the causal structure of the data cannot be tested, our analyses provide a range of estimates of the associations of interest.
Even if the timing of reducing drinking were known, our results could still be biased by unmeasured confounding. While our analyses controlled for HIV status and several demographic factors, diagnoses of chronic diseases or other changes in health status, receipt of pharmacotherapy or other treatment, and other changes in social and economic circumstances could be related to reducing drinking and the outcomes of interest. Finally, a causal interpretation of our findings would rely on the additional assumption that the treatment strategies were sufficiently well-defined, that is, that no meaningful vagueness remains in the intervention’s definition. Since behavioral and pharmaceutical interventions to reduce AUDIT score may have different impacts on the outcomes of interest, this assumption may not hold (Hernán, 2016).
A number of other study limitations should be noted. First, the presence of unhealthy alcohol use and co-occurring conditions were assessed using brief screening tools (i.e., AUDIT, PHQ) or self-reported endorsement (e.g., anxiety, drug use) based on questionnaires that changed over time rather than clinical diagnoses. Pain interference and anxiety symptoms were both assessed using single-item screening tools. This exposure and outcome measurement error and misclassification could be differential or non-differential, though these measures are widely used (Khan et al., 2020, Edelman et al., 2020, Becker et al., 2009, Novak et al., 2009). Second, our analyses dichotomized unhealthy alcohol use and co-occurring conditions based on clinically relevant but somewhat arbitrary cut-points. It is possible that the relationship between reducing drinking and co-occurring conditions could vary according to baseline AUDIT score and according to classifications of co-occurring conditions based on alternative cut-points. Future analyses could consider reduction in AUDIT score as a continuous variable. Third, differential loss to follow-up or death could induce selection bias. While we were able to adjust for potential selection bias induced by not having an AUDIT score at the follow-up visit after baseline, information on the co-occurring conditions over follow-up was incomplete (82–100% at one-year, 61–78% at two-years). We were not able to evaluate outcomes after two years as the VACS cohort is ongoing and many VACS participants have not yet been followed for more than two years after our study’s baseline. Fourth, our sample size was limited and confidence intervals were wide. Finally, since our analysis was conducted in a sample of HIV-positive and HIV-negative matched veterans receiving care in the VA, our findings may not be generalizable to younger individuals, women (for whom a lower AUDIT score may indicate unhealthy alcohol use), or individuals with less access to medical care. However, this population has a high prevalence of the target conditions and may benefit substantially from interventions to reduce unhealthy alcohol use.
A causal interpretation of our findings is not warranted and we cannot conclude that reducing drinking improved certain co-occurring conditions. However, the observed associations suggest that reducing drinking in patients with unhealthy alcohol use does not decrease the likelihood of improving pain interference symptoms or discontinuing other substance use. Randomized trials, simulation studies, and observational studies with more granular data on timing and reasons for changes in alcohol use and co-occurring conditions are needed to evaluate the impact of treatment and screening strategies for unhealthy alcohol use and other co-occurring conditions. Future studies should also investigate whether changes in co-occurring conditions are sustained over time, and whether the impact of reducing unhealthy alcohol use differs according to the severity and duration of unhealthy alcohol use. Our findings provide support for continued efforts to reduce drinking in those with unhealthy alcohol use in this population and may provide reassurance that reducing drinking in those with unhealthy alcohol use will not worsen symptoms of pain interference or decrease the likelihood of discontinuing other substance use.
Funding:
This work was supported by the National Institutes of Health [NIAAA R01AA024706].
Footnotes
Declarations of interest: None
REFERENCES
- ALFORD DP, GERMAN JS, SAMET JH, CHENG DM, LLOYD-TRAVAGLINI CA & SAITZ R 2016. Primary Care Patients with Drug Use Report Chronic Pain and Self-Medicate with Alcohol and Other Drugs. J Gen Intern Med, 31, 486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANGLIN MD, ALMOG IJ, FISHER DG & PETERS KR 1989. Alcohol-Use by Heroin-Addicts - Evidence for an Inverse Relationship - a Study of Methadone-Maintenance and Drug-Free Treatment Samples. American Journal of Drug and Alcohol Abuse, 15, 191–207. [DOI] [PubMed] [Google Scholar]
- BECKER WC, FIELLIN DA, GALLAGHER RM, BARTH KS, ROSS JT & OSLIN DW 2009. The association between chronic pain and prescription drug abuse in Veterans. Pain Med, 10, 531–6. [DOI] [PubMed] [Google Scholar]
- BODEN JM & FERGUSSON DM 2011. Alcohol and depression. Addiction, 106, 906–914. [DOI] [PubMed] [Google Scholar]
- BOLTON J, COX B, CLARA I & SAREEN J 2006. Use of alcohol and drugs to self-medicate anxiety disorders in a nationally representative sample. J Nerv Ment Dis, 194, 818–25. [DOI] [PubMed] [Google Scholar]
- BOLTON JM, ROBINSON J & SAREEN J 2009. Self-medication of mood disorders with alcohol and drugs in the National Epidemiologic Survey on Alcohol and Related Conditions. J Affect Disord, 115, 367–75. [DOI] [PubMed] [Google Scholar]
- BRAITHWAITE RS, FANG Y, TATE J, MENTOR SM, BRYANT KJ, FIELLIN DA & JUSTICE AC 2015. Do Alcohol Misuse, Smoking, and Depression Vary Concordantly or Sequentially? A Longitudinal Study of HIV-Infected and Matched Uninfected Veterans in Care. AIDS Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRENNAN PL, SCHUTTE KK & MOOS RH 2005. Pain and use of alcohol to manage pain: prevalence and 3-year outcomes among older problem and non-problem drinkers. Addiction, 100, 777–786. [DOI] [PubMed] [Google Scholar]
- CENTERS FOR DISEASE CONTROL AND PREVENTION. 2017. Illegal Drug Use [Online]. Available: https://www.cdc.gov/nchs/fastats/drug-use-illegal.htm [Accessed March 11, 2019].
- CRUM RM, MOJTABAI R, LAZARECK S, BOLTON JM, ROBINSON J, SAREEN J, GREEN KM, STUART EA, LA FLAIR L, ALVANZO AA & STORR CL 2013. A prospective assessment of reports of drinking to self-medicate mood symptoms with the incidence and persistence of alcohol dependence. JAMA Psychiatry, 70, 718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE BEJCZY A, LOF E, WALTHER L, GUTERSTAM J, HAMMARBERG A, ASANOVSKA G, FRANCK J, ISAKSSON A & SODERPALM B 2015. Varenicline for treatment of alcohol dependence: a randomized, placebo-controlled trial. Alcohol Clin Exp Res, 39, 2189–99. [DOI] [PubMed] [Google Scholar]
- DERMODY SS, HENDERSHOT CS, ANDRADE AK, NOVALEN M & TYNDALE RF 2018. Changes in Nicotine Metabolite Ratio among Daily Smokers receiving Treatment for Alcohol Use Disorder. Nicotine Tob Res. [DOI] [PubMed] [Google Scholar]
- EDELMAN EJ, LI Y, DECLAN B, BRADEN JB, CRYSTAL S, KERNS RD, GAITHER JR, GORDON KS, MANHAPRA A, MERLIN JS, MOORE BA, OLDFIELD BJ, PARK LS, RENTSCH CT, SKANDERSON M, WILLIAMS EC, JUSTICE AC, TATE J, BECKER WC & MARSHALL BDL 2020. Trajectories of self-reported opioid use among patients with HIV engaged in care: Results from a national cohort study. Journal of Acquired Immune Deficiency Syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN EJ, MAISTO SA, HANSEN NB, CUTTER CJ, DZIURA J, DENG Y, FIELLIN LE, O’CONNOR PG, BEDIMO R, GIBERT CL, MARCONI VC, RIMLAND D, RODRIGUEZ-BARRADAS MC, SIMBERKOFF MS, TATE JP, JUSTICE AC, BRYANT KJ & FIELLIN DA Integrated stepped alcohol treatment for patients with HIV and alcohol use disorder: a randomised controlled trial. The Lancet HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGLI M, KOOB GF & EDWARDS S 2012. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev, 36, 2179–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GACHE P, MICHAUD P, LANDRY U, ACCIETTO C, ARFAOUI S, WENGER O & DAEPPEN JB 2005. The Alcohol Use Disorders Identification Test (AUDIT) as a screening tool for excessive drinking in primary care: reliability and validity of a French version. Alcohol Clin Exp Res, 29, 2001–7. [DOI] [PubMed] [Google Scholar]
- GOSSOP M, MANNING V & RIDGE G 2006. Concurrent use and order of use of cocaine and alcohol: behavioural differences between users of crack cocaine and cocaine powder. Addiction, 101, 1292–1298. [DOI] [PubMed] [Google Scholar]
- GRANT BF, CHOU S, SAHA TD & et al. 2017. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: Results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatry, 74, 911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNÁN MA 2016. Does water kill? A call for less casual causal inferences. Ann Epidemiol, 26, 674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNAN MA & ROBINS JM 2016. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am J Epidemiol, 183, 758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNÁN MA & ROBINS JM 2019. Causal Inference, Boca Raton Florida: Chapman & Hall/CRC, forthcoming. [Google Scholar]
- HERNÁN MA, SAUER BC, HERNANDEZ-DIAZ S, PLATT R & SHRIER I 2016. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol, 79, 70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUSTICE AC, HOLMES W, GIFFORD AL, RABENECK L, ZACKIN R, SINCLAIR G, WEISSMAN S, NEIDIG J, MARCUS C, CHESNEY M, COHN SE & WU AW 2001a. Development and validation of a self-completed HIV symptom index. J Clin Epidemiol, 54 Suppl 1, S77–90. [DOI] [PubMed] [Google Scholar]
- JUSTICE AC, LANDEFELD CS, ASCH SM, GIFFORD AL, WHALEN CC & COVINSKY KE 2001b. Justification for a new cohort study of people aging with and without HIV infection. J Clin Epidemiol, 54 Suppl 1, S3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUSTICE AC, MCGINNIS KA, TATE JP, BRAITHWAITE RS, BRYANT KJ, COOK RL, EDELMAN EJ, FIELLIN LE, FREIBERG MS, GORDON AJ, KRAEMER KL, MARSHALL BD, WILLIAMS EC & FIELLIN DA 2016. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend, 161, 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSLER RC, BERGLUND P, DEMLER O, JIN R, MERIKANGAS KR & WALTERS EE 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry, 62, 593–602. [DOI] [PubMed] [Google Scholar]
- KESSLER RC, MCGONAGLE KA, ZHAO S, NELSON CB, HUGHES M, ESHLEMAN S, WITTCHEN H-U & KENDLER KS 1994. Lifetime and 12-Month Prevalence of DSM-III-R Psychiatric Disorders in the United States: Results From the National Comorbidity Survey. Archives of General Psychiatry, 51, 8–19. [DOI] [PubMed] [Google Scholar]
- KHAN MR, YOUNG KE, CANIGLIA EC, FIELLIN DA, MAISTO SA, MARSHALL BDL, EDELMAN EJ, GAITHER JR, CHICHETTO NE, TATE J, BRYANT K, SEVERE M, STEVENS ER, JUSTICE AC & BRAITHWAITE RS 2020. Associations between alcohol screening scores and adverse mental health conditions and substance use among US adults JAMA Network Open. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROENKE K, SPITZER RL & WILLIAMS JB 2001. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med, 16, 606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRUPITSKY E, NUNES EV, LING W, ILLEPERUMA A, GASTFRIEND DR & SILVERMAN BL 2011. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet, 377, 1506–13. [DOI] [PubMed] [Google Scholar]
- KUSHNER MG, ABRAMS K & BORCHARDT C 2000. The relationship between anxiety disorders and alcohol use disorders: A review of major perspectives and findings. Clinical Psychology Review, 20, 149–171. [DOI] [PubMed] [Google Scholar]
- LITTEN RZ, RYAN ML, FERTIG JB, FALK DE, JOHNSON B, DUNN KE, GREEN AI, PETTINATI HM, CIRAULO DA, SARID-SEGAL O, KAMPMAN K, BRUNETTE MF, STRAIN EC, TIOURIRINE NA, RANSOM J, SCOTT C & STOUT R 2013. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med, 7, 277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKS KR, PIKE E, STOOPS WW & RUSH CR 2015. Alcohol Administration Increases Cocaine Craving But Not Cocaine Cue Attentional Bias. Alcohol Clin Exp Res, 39, 1823–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASON BJ, QUELLO S, GOODELL V, SHADAN F, KYLE M & BEGOVIC A 2014. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med, 174, 70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCABE SE, CRANFORD JA & BOYD CJ 2006. The relationship between past-year drinking behaviors and nonmedical use of prescription drugs: Prevalence of co-occurrence in a national sample. Drug and Alcohol Dependence, 84, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL JM, TEAGUE CH, KAYSER AS, BARTLETT SE & FIELDS HL 2012. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology (Berl), 223, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAHIN RL 2015. Estimates of pain prevalence and severity in adults: United States, 2012. The Journal of Pain, 16, 769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVAK SP, HERMAN-STAHL M, FLANNERY B & ZIMMERMAN M 2009. Physical pain, common psychiatric and substance use disorders, and the non-medical use of prescription analgesics in the United States. Drug Alcohol Depend, 100, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSLIN DW, LEONG SH, LYNCH KG, BERRETTINI W, O’BRIEN CP, GORDON AJ & RUKSTALIS M 2015. Naltrexone vs Placebo for the Treatment of Alcohol Dependence: A Randomized Clinical Trial. JAMA Psychiatry, 72, 430–7. [DOI] [PubMed] [Google Scholar]
- QUITKIN FM, RIFKIN A, KAPLAN J & KLEIN DF 1972. Phobic anxiety syndrome complicated by drug dependence and addiction. A treatable form of drug abuse. Arch Gen Psychiatry, 27, 159–62. [DOI] [PubMed] [Google Scholar]
- REED MB, WANG R, SHILLINGTON AM, CLAPP JD & LANGE JE 2007. The relationship between alcohol use and cigarette smoking in a sample of undergraduate college students. Addictive Behaviors, 32, 449–464. [DOI] [PubMed] [Google Scholar]
- RILEY JL 3RD & KING C 2009. Self-report of alcohol use for pain in a multi-ethnic community sample. J Pain, 10, 944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINS J 1997. Marginal structural models. American Statistical Association Alexandria, VA. [Google Scholar]
- ROBINSON J, SAREEN J, COX BJ & BOLTON J 2009. Self-medication of anxiety disorders with alcohol and drugs: Results from a nationally representative sample. J Anxiety Disord, 23, 38–45. [DOI] [PubMed] [Google Scholar]
- RUGGLES KV, FANG Y, TATE J, MENTOR SM, BRYANT KJ, FIELLIN DA, JUSTICE AC & BRAITHWAITE RS 2016. What are the Patterns Between Depression, Smoking, Unhealthy Alcohol Use, and Other Substance Use Among Individuals Receiving Medical Care? A Longitudinal Study of 5479 Participants. AIDS Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITZ R 2005. Clinical practice. Unhealthy alcohol use. N Engl J Med, 352, 596–607. [DOI] [PubMed] [Google Scholar]
- STEVENS ER, MAZUMDAR M, CANIGLIA EC, KHAN MR, YOUNG KE, EDELMAN EJ, GORDON AJ, FIELLIN DA, MAISTO SA, CHICHETTO NE, CRYSTAL S, GAITHER JR, JUSTICE AC, BRAITHWAITE RS 2020. Insights provided by depression screening regarding pain, anxiety, and substance use in a veteran population. Journal of Primary Care and Community Health, Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUBSTANCE ABUSE AND MENTAL HEALTH SERVICES ADMINISTRATION 2013. Results from the 2012 National Survey on Drug Use and Health: Summary of national findings (NSDUH Series H-46, HHS Publication No. (SMA; ) 13–4795). Rockville, MD. [Google Scholar]
- SUBSTANCE ABUSE AND MENTAL HEALTH SERVICES ADMINISTRATION 2014. National survey on drug use and health. [Google Scholar]
- U. S. PREVENTIVE SERVICES TASK FORCE 2018. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: Us preventive services task force recommendation statement. JAMA, 320, 1899–1909. [DOI] [PubMed] [Google Scholar]
- U.S. PREVENTIVE SERVICES TASK FORCE. 2019. USPSTF A and B Recommendations [Online]. Available: https://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-recommendations/ [Accessed March 11, 2019].
- WARE J JR., KOSINSKI M & KELLER SD 1996. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care, 34, 220–33. [DOI] [PubMed] [Google Scholar]
- WELTE JW & BARNES GM 1982. The Relationship between Alcohol-Use and Other Drug-Use among New-York State College-Students. Drug and Alcohol Dependence, 9, 191–199. [DOI] [PubMed] [Google Scholar]
- WORLD HEALTH ORGANIZATION; 2001. The alcohol use disorders identification test. Guidelines for Use in Primary Care 2nd edn WHO: Geneva, Switzerland. [Google Scholar]
- YOUNG QR, NGUYEN M, ROTH S, BROADBERRY A & MACKAY MH 2015. Single-item measures for depression and anxiety: Validation of the Screening Tool for Psychological Distress in an inpatient cardiology setting. Eur J Cardiovasc Nurs, 14, 544–51. [DOI] [PubMed] [Google Scholar]


