Abstract
Rheumatoid arthritis (RA) is an autoimmune disease characterized by symmetrical and chronic polyarthritis. Fibroblast-like synoviocytes are mainly involved in joint inflammation and cartilage and bone destruction by inflammatory cytokines and matrix-degrading enzymes in RA. Approaches that induce various cellular growth alterations of synoviocytes are considered as potential strategies for treating RA. However, since synoviocytes play a critical role in RA, the mechanism and hyperplastic modulation of synoviocytes and their motility need to be addressed. In this review, we focus on the alteration of synoviocyte signalling and cell fate provided by signalling proteins, various antioxidant molecules, enzymes, compounds, clinical candidates, to understand the pathology of the synoviocytes, and finally to achieve developed therapeutic strategies of RA.
Cite this article: Bone Joint Res 2021;10(4):285–297.
Keywords: Rheumatoid arthritis, Synoviocytes, Inflammation, Hyperplasia, Joint destruction
Article focus
Apoptosis or proliferation of fibroblastic synoviocytes (FLS) controls excessive multiplication of FLS, which causes pannus formation of joint.
The inflammation and migration of FLS involved in rheumatoid arthritis (RA) pathogenesis.
Current strategies for targeting inflammation and pathognomonic parameters for RA FLS.
Key messages
FLS-related signalling is involved in FLS hyperplasia, joint destruction, and pain. To regulate the excessive multiplication of FLS, mechanisms of various signalling molecules related to proliferation and apoptosis of FLS are summarized in this review.
Activated FLS tends to migrate to and invade synovial tissues following aggravation of RA. We elucidate the signalling molecules, enzymes, and various compounds focused on the migration and invasion of FLS.
We also describe our current knowledge of FLS signalling and several agents related to FLS signalling as candidates for therapeutic opportunities.
Strengths and limitations
We describe the numerous molecules and mechanisms, which are directly involved in RA, to regulate FLS characteristics. This paper could be the initiation for build-up therapies of RA to focus on pathognomonic parameters for RA FLS.
Signalling pathways are integrated in various compounds or signalling molecule-related FLS pathogenesis. Assessment or prioritization of the potential translational value of such targets and compounds still needs to be substantially improved.
Introduction
The goal of treatment for rheumatoid arthritis (RA) is remission for early RA and low disease activity for long-standing disease. 1-3 Most current treatments for RA, including non-steroidal anti-inflammatory drugs (NSAIDs), synthetic or biological disease-modifying antirheumatic drugs (DMARDs), and glucocorticoids, are targeted to eliminate inflammation rapidly. This is because inflammation is established as the driving force for the clinical symptoms, joint damage, disability, and comorbidity in RA. 4,5 The biological DMARDs are specifically developed to target inflammatory cytokines and their receptors such as tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-6 receptor (IL-6R), interleukin-17 (IL-17), B cells (rituximab), or T cells (abatacept). Moreover, they are highly efficient in decrement of disease activity in about 75% to 80% of the RA patients. 5,6 However, there is still a requirement for development of new therapies for RA because 20% to 25% of patients do not reach low disease activity. Recent guidelines from the American College of Rheumatology (ACR) recommend an initial use of methotrexate and then adding or switching to other conventional synthetic DMARDs (csDMARDs) in patients with insufficient improvement in disease activity. 2 The European League Against Rheumatism (EULAR) recommends administration of biological DMARDs in patients with high titres of autoantibodies, early joint damage on radiography, and high disease activity with the previous therapy and after failure of the first treatment cycle. 1 It is clear that joint damage can occur if inflammation persists despite these reasonably successful treatments.
In RA, there occurs a massive cellular influx of immune cells such as T lymphocytes, macrophage-like synoviocytes, and fibroblastic synoviocytes (FLS) to the synovium. Their activation, proliferation, and differentiation contribute remarkably to synovial inflammation. 7 Moreover, hyperplasia of FLS is one of the major contributors of this synovial inflammation. It could be explained by the imbalance between apoptosis and proliferation of existing FLS by environmental and somatic mutation, besides increased differentiation of mesenchymal stem cells to FLS. 8,9 These FLS migrate to the inflammatory site in the joint and form hyperplastic synovial lining containing activated FLS and macrophages called the pannus. Additionally, these activated FLS play many roles in the joints of patients with RA. They can increase the expression of adhesion molecules and activate several signalling pathways such as nuclear factor kappa-B (NF-κB), mitogen-activated protein kinases (MAPK), and transcription factor activator protein-1 (AP-1) in early RA. 10-12 This is caused by the response to not only the pro-inflammatory environment of the immune cells, but also to autoantibodies, mechanical stimulus, and citrullination, which induces autoimmune diseases such as RA and activates the calcium channels in FLS directly. 13 This is strong evidence that FLS autonomously contribute to RA pathogenesis by driving joint inflammation and destruction. Moreover, FLS migrate, attach to, and invade the cartilage, produce matrix metalloproteinase (MMP), express receptor activator of NF-κB ligand (RANKL), and can be resistant to apoptosis by tumour-like transformation with epigenetic changes in joint destruction. 14-16 These molecular mechanisms of activated FLS are not fully understood and it is difficult to evaluate their degree of contribution to active RA or refractory RA or to current treatment strategies. Nevertheless, the current evidence indicates FLS as strong potential targets for the treatment of RA.
To understand RA, various signalling mechanisms and targets of FLS have been investigated to study cellular homeostasis and clinical implications. In this review, we summarize the novel therapeutic targets and valuable treatments in RA pathogenesis. In view of the applications of various antibodies such as TNF- α, IL-1β, and IL-6 receptor as treatments for RA, the establishment of alternative targets may be an expanded therapeutic market for RA. On the other hand, the modulation of cell fate involves tissue homeostasis and structural modification. The identification of new therapeutic strategies on RA involves the study of modulation of proliferation or apoptosis, resistance to apoptosis, and tumour cell-like features of FLS in RA. 17-19 The regulation of FLS involves the various molecules mentioned below. This section will discuss the proliferative and apoptotic signalling agents of FLS in RA. Beneficial effects of various agents on FLS may attenuate FLS hyperplasia, joint destruction, and pain. Here, we describe our current knowledge of FLS signalling and review target molecules and potential molecules before approval for RA treatment and depict articles in accordance with scope of the mode of action in FLS as proliferation, apoptosis, inflammatory activity, migration, and invasion.
Proliferative and apoptotic modulation of RA FLS
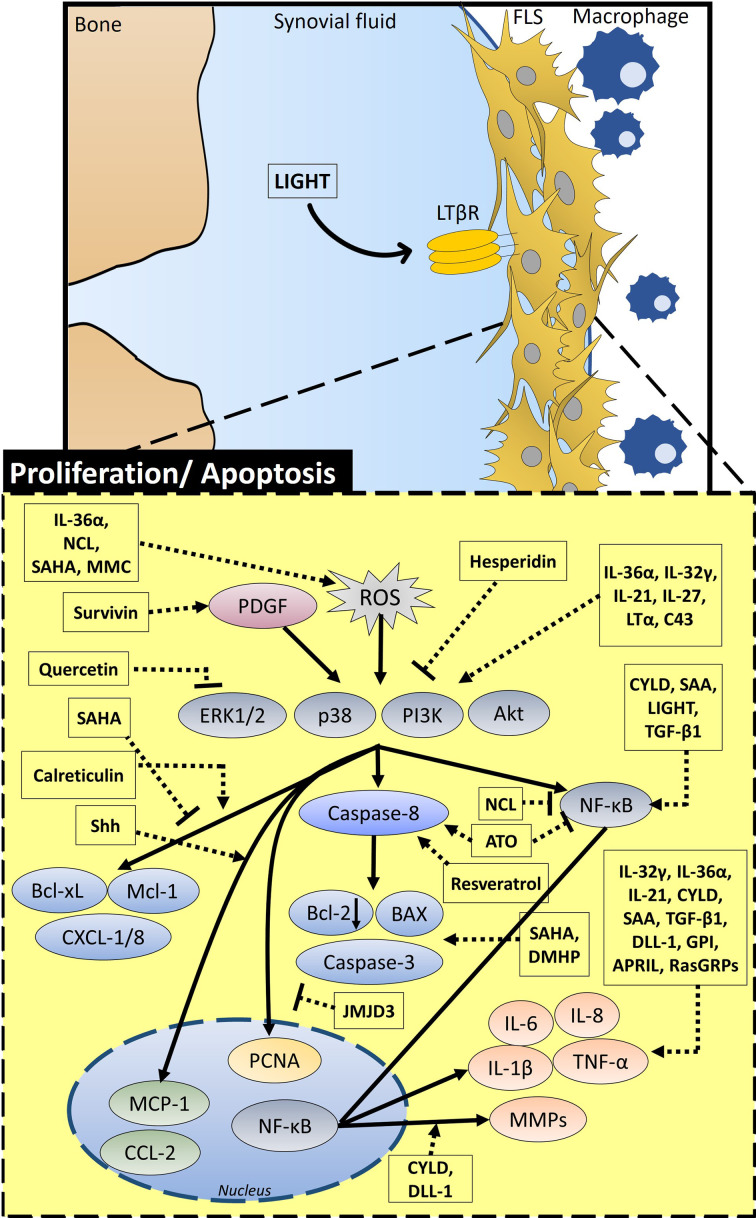
FLS of RA forms pannus with accumulated FLS, which is the result of hyperplasia of FLS. In this section, we will describe factors or molecules related to apoptosis or proliferation of FLS to control excessive multiplication of FLS and schematic summary, represented in Figure 1 and represented mode of action and working dose of molecules in Table I.
Fig. 1.
Schematic diagram of the overall signalling mechanism and related factors of proliferative and apoptotic modulation in the fibroblastic synoviocytes (FLS) of rheumatoid arthritis (RA). Proliferation and apoptosis in FLS are regulated by various signalling factors. IL-36α, NCL, SAHA, and MMC increase reactive oxygen species (ROS) and survivin activates platelet-derived growth factor (PDGF) signalling. PDGF and ROS affect extracellular signal‑regulated protein kinase 1/2 (ERK1/2), p38, phosphoinositide 3-kinase (PI3K), and protein kinase B (Akt) signalling. SAHA inhibits B-cell lymphoma-extra large (Bcl-xL) and myeloid cell leukemia-1 (Mcl-1) expression. NCL inhibits nuclear factor kappa-B (NF-κB). Quercetin and hesperidin inhibit extracellular signal-regulated kinase (ERK)/PI3K/Akt signalling, whereas IL-36α, IL-32γ, IL-21, IL-27, LTα, and C43 activate this signalling. While calreticulin stimulates Bcl-xL, Mcl-1, and C-X-C motif chemokine ligand (CXCL)-1/8 through this signalling, ATO and resveratrol enhance caspase-8 activity, and CYLD, SAA, LIGHT, and TGF-β1 activate NF-κB signalling. In the nucleus, Shh activates MCP-1 and CYLD and DLL-1 increase MMPs and IL-1β through NF-κB signalling. GPI, APRIL, and RasGRPs increase inflammatory factors such as TNF-α and IL-8. JMJD3 inhibits the activity of PCNA in the nucleus. SAHA and DMHP enhance expression of Bcl-2-associated X protein (BAX) and caspase-3, and they also attenuate Bcl-2 expression. LIGHT, lymphotoxin-like, herpes simplex virus glycoprotein D, a receptor expressed by T lymphocytes; LTα, lymphotoxin α; APRIL, a proliferation-inducing ligand; Shh, Sonic hedgehog; GPI, glucose-6-phosphate isomerase; IL, interleukin; JMJD3, Jumonji C family of histone demethylases; CYLD, cylindromatosis; RasGRPs, Ras guanine nucleotide-releasing proteins; TGF-β1, transforming growth factor-β1; SAA, serum amyloid A; DLL-1, δ like Notch ligand 1; C43, compound 43; ATO, arsenic trioxide; DMHP, 7,3'-dimethoxy hesperetin; NCL, niclosamide; MMC, mitomycin C; MCP-1, monocyte chemoattractant protein-1; SAHA, suberoylanilide hydroxamic acid; PCNA, proliferating cell nuclear antigen.
Table I.
Summary of molecular mechanism of fibroblastic synoviocytes (proliferation and apoptosis).
| Molecules | Mode of action | Species | Dose | Ref | |
|---|---|---|---|---|---|
| In vivo | In vitro | ||||
| Endogenous factors | |||||
| LIGHT | Enhanced the proliferation of FLS, expression of ICAM-1, MCP-1, IL-8, MIP-1α, and NF-κB translocation | RA-FLS | 10 ng/ml | 20 | |
| APRIL | Produced IL-6, TNF-α, IL-1β, and enhanced FLS proliferation | RA-FLS, Rat adjuvant-induced arthritis (AA) model | 30 to 300 ng/ml | 21,22 | |
| Shh signalling | Mediated the proliferation and migration through MAPK/ERK pathway | RA-FLS | 1, 10 μΜ | 23 | |
| GPI | Stimulated the secretion of TNF-α and IL-1β | RA-FLS, arthritic synovial tissues from RA patients | 1 to 10 μg/ml | 24 | |
| Survivin | Promoted proliferation | RA-FLS | 25 | ||
| JMJD3 | Activated proliferation and migration | RA-FLS, CIA mice | 26 | ||
| CYLD | Enhanced cell growth and cytokine production | RA-FLS | 27 | ||
| RasGRPs | Enhanced cell motility and IL-6 production | RA-FLS, CIA mice | 28 | ||
| TGF-β1 | Activated NF-κB, AP-1, migration, and invasion | RA-FLS, SF from RA patients | 1 to 100 ng/ml | 29,30 | |
| SAA | Promoted migration, angiogenesis through MMP-2/9, activation of NF-κB, and cytokine production | RA-FLS, RA synovial/SCID mouse | 50 μg/ml | 10 to 50 μg/ml | 31,32 |
| DLL-1 | Suppressed IL-6 and MMP-3 | 33 | |||
| Calreticulin | Induced Bcl-xL, Mcl-1 through PI3K/Akt and STAT3 pathways | RA-FLS | 34 | ||
| Cytokines | |||||
| LTα | Activation of MAPK, ERK1/2, p38, PI3K/Akt pathway, NF-κB translocation, IL-6/8, and MMP-3 | RA-FLS | 0.5 nM | 35 | |
| IL-21 | Stimulated the proliferation and secretion of TNF-α and IL-6 through ERK1/2, PI3K/Akt, and STAT3 | RA-FLS | 1 to 100 ng/ml | 36 | |
| IL-32γ | Enhanced expression of IL-6 and IL-8 through activation of ERK1/2 | RA-FLS | 50 to 100 ng/ml | 37 | |
| IL-27 | Induced expression of adhesion molecules, inflammatory cytokines, and activated inflammatory signalling pathways | RA-FLS | 10 to 100 ng/ml | 38 | |
| IL-36α | Activated p38 MAPK signalling and pro-inflammatory cytokines | RA/Murine FLS, IL-36R-deficient FLS | 39 | ||
| Synthetic compounds | |||||
| C43 | Inhibited proliferation, inflammation, and bone injury | RA-FLS, SIA mice, and CIA mice | 6 to 30 mg/kg | 30 μM | 40,41 |
| ATO | Induced apoptosis through caspase signalling | RA-FLS, CIA rats | 1 to 6 mg/kg | 0.1 to 8 μM | 42,43 |
| DMHP | Induced apoptosis through enhancing BAX and caspase-3 | RA-FLS, AA rats | 20 to 150 mg/kg | 2.5 to 20 μM | 44 |
| NCL | Reduced E-selectin, ICAM-1, and VCAM-1 and inhibited migration and invasion | RA-FLS, RA patients | 1,000 mg/day | 20 to 100 nmol/l | 45,46 |
| SAHA | Induced apoptosis through enhancing caspase-3 and ROS | RA-FLS | 5 μM | 47 | |
| Natural compounds | |||||
| Resveratrol | Mediated apoptosis and inhibited IL-1β, MMP-3, and phosphorylated Akt | RA-FLS, RA patients | 1 g/person | 6.25 to 50 μM | 48,49 |
| Hesperidin | Down-regulated TNF-α and reduced MMPs | RA-FLS, AIA and CIA mice | 20 to 150 mg/kg | 2.5 to 20 μM | 50,51 |
| MMC | Induced apoptosis through ROS production | RA-FLS | 10 to 100 μg/ml | 52 | |
| Quercetin | Enhanced apoptosis through inhibition of PI3K/Akt pathway | RA-FLS | 200 μM | 53 | |
APRIL, A proliferation-inducing ligand; ATO, arsenic trioxide; Bcl-xL, B-cell lymphoma-extra large; C43, compound 43; CYLD, cylindromatosis; DLL-1, delta like canonical notch ligand 1; DMHP, 7,3'-dimethoxy hesperetin; ERK1/2, extracellular signal‑regulated protein kinase 1/2; GPI, glucose 6-phosphate isomerase; IL-21, interleukin-21; IL-27, interleukin-27; IL-32γ, interleukin-32γ; IL-36α, interleukin-36α; JMJD3, Jumonji C family of histone demethylases; LIGHT, lymphotoxin-like herpes simplex virus glycoprotein D, a receptor expressed by T lymphocytes; LTα, lymphotoxin α; Mcl-1, myeloid cell leukemia-1; MMC, mitomycin C; NCL, niclosamide; RasGRPs, Ras guanine nucleotide-releasing proteins; Ref, reference; SAA, serum amyloid A; SAHA, suberoylanilide hydroxamic acid; Shh signaling, Sonic hedgehog signaling; STAT3, signal transducer and activator of transcription 3; TGF-β1, transforming growth factor-β1.
Endogenous factors
Lymphotoxin-like herpes simplex virus glycoprotein D, a receptor expressed by T lymphocytes
The expression level of lymphotoxin-like herpes simplex virus glycoprotein D, a receptor expressed by T lymphocytes (LIGHT) is upregulated in both the synovial fluid (SF) and the synovium. 20,54,55 LIGHT significantly enhances the proliferation of FLS and induces the expression of adhesion molecules such as the intercellular adhesion molecule-1 (ICAM-1) and various cytokines such as the monocyte chemoattractant protein-1, IL-8, macrophage inflammatory protein (MIP)-1α, and NF-κB translocation through the lymphotoxin β receptor. 20 Moreover, LIGHT also stimulates macrophage-mediated osteoclastogenesis in the SF. A higher concentration of LIGHT is revealed in RA SF compared with that in the OA SF. 54
A proliferation-inducing ligand
High level of A proliferation-inducing ligand (APRIL) is detected in RA serum and in the adjuvant-induced arthritis (AA) synovium of rat model. 21,22 APRIL stimulates the FLS to produce various cytokines including APRIL itself. 21 APRIL-stimulated T or B cells also enhance the FLS proliferation in a co-culture system. 22
Sonic hedgehog signalling
Sonic hedgehog (Shh) signalling is involved in various cell functions such as proliferation, differentiation, and embryonic development. 56,57 Higher expression of Shh messenger RNA (mRNA) in the RA synovium than that in the control synovium and treatment with cyclopamine (a specific inhibitor of Shh signalling) on FLS results in cell cycle arrest. 58 Currently, it is reported to mediate the proliferation and migration of FLS by the MAPK/extracellular signal-regulated kinase (ERK) pathway in RA. 23 Two Shh inhibitors, sonidegib and vismodegib, have received Food and Drug Administration (FDA) approval for basal cell carcinoma. 59
Glucose-6-phosphate isomerase
The biochemical role of glucose-6-phosphate isomerase (GPI) is the isomerization of glucose-6-phosphate to fructose 6-phosphate. GPI plays various roles in cell growth and motility and has been robustly studied for its pathological roles in tumour proliferation. 60-62 More recently, the pathophysiological role of GPI has been revealed in RA. The level of GPI is increased in RA FLS and it also stimulates the secretion of cytokines such as TNF-α and IL-1β in FLS. 24
Survivin
Survivin is considered a proto-oncogene and is involved in joint destruction in RA. 63-65 Enhanced expression of wild type and splice variant type 2B of survivin is demonstrated in the RA synovium. 65 Extracellular survivin is also increased in the SF of RA. 25 Platelet-derived growth factor (PDGF)-dependent survivin 2B expression and subsequent promotion of FLS proliferation suggest that survivin 2B plays an emerging role in RA.
Jumonji C family of histone demethylases
The expression of Jumonji C family of histone demethylases (JMJD3) is low in normal tissue; its expression is stimulated by various stress conditions such as amino acid deprivation and hypoxia. 66,67 The enhanced expression of JMJD3 in RA FLS activates proliferation and migration. 26
Cylindromatosis
Cylindromatosis (CYLD) is a condition involving multiple tumours of the skin appendages. 68,69 The gene of CYLD, a tumour suppressor, is associated with deactivation of the NF-κB signalling pathway. 27,70,71 CYLD suppression enhances cell growth and cytokine production in RA FLS. 27
Ras guanine nucleotide-releasing proteins
Ras guanine nucleotide-releasing proteins (RasGRPs) are identified in the FLS of RA. The overexpression of RasGRP2 enhances cell motility and IL-6 production, whereas the knockdown of RasGRP2 attenuates pannus formation in an experimental arthritis model. 28
Transforming growth factor-β1
Transforming growth factor (TGF)-β1 is expressed in the synovium of patients with RA. It enhances the DNA-binding activities of NF-κB and AP-1, 29,30,72 and promotes migration and invasion by activating Smad2/3 in the RA FLS. 73
Serum amyloid A
Serum amyloid A (SAA) is identified as a biomarker for acute phase inflammation in RA. 74,75 It promotes the migration of FLS and angiogenesis by induction of MMP-2 and MMP-9 in the synovium; thus, it supports the proliferation of synovium and formation of pannus. 31 SAA is enhanced in RA synovium, 76 and stimulates the transcriptional activation of NF-κB and the expression of IL-6 and IL-8 with the involvement of receptor for advanced glycation end-products (RAGE). 32 The SAA/RAGE/NF-κB signalling process is involved in the pathogenesis of RA. 32
δ like canonical Notch ligand 1
Blocking the δ like canonical Notch ligand 1 (DLL-1) protein improves arthritis in collagen-induced arthritis (CIA) mouse model and suppressed IL-6 and MMP-3. 33 Although the ameliorating effect of DLL-1 blocking on inflammatory cytokines is partial, DLL-1 has proved to be a new target for joint inflammation treatment.
Calreticulin
Calreticulin is known as the calcium-binding endoplasmic reticulum (ER) resident chaperone. Extracellular calreticulin is involved in apoptotic cell clearance as a recognition ligand. 77 Extracellular application of recombinant calreticulin inhibits inflammation-mediated bone resorption. 78 Although the intra-/extracellular functions of calreticulin are diverse, it is also considered as a biomarker of juvenile idiopathic arthritis. 34 In addition, enhanced expression of calreticulin is revealed in RA synovium, and it induces the expression of B-cell lymphoma-extra large (Bcl-xL) and myeloid cell leukaemia-1 (Mcl-1) proteins through the phosphoinositide 3-kinase (PI3K)/protein kinase B (PKB or Akt) and signal transducer and activator of transcription 3 (STAT3) pathways. 79
Cytokines
Lymphotoxin α
Lymphotoxin α (LTα), previously known as tumour necrosis factor-β (TNF-β), is a pro-inflammatory cytokine produced by T lymphocytes and is very similar to TNF-α. 35,80 LTα possesses high affinity for both TNF receptor 1 (TNFR1) and TNFR2. 81 It mediates the activation of MAPKs extracellular signal‑regulated protein kinase 1/2 (ERK1/2), p38, the PI3K/Akt pathway, NF-κB translocation, and the secretion of cytokines such as IL-6, IL-8, and MMP-3. 35 Being homologous to the cytokine TNF-α, LTα is also a stimulant of RA FLS as well as lymphocytes such as macrophages. However, the subcutaneous injection of pateclizumab, an anti-LTα antibody, does not show any clinical improvements in RA symptoms and signs, while adalimumab, a TNF-α inhibitor, demonstrates the clinical efficacy in RA patients with poor response to csDMARDs. 82
IL-21
Increased serum IL-21 levels and enhanced expression of IL-21 receptors in the synovium of RA patients are associated with the pathology of RA. 83,84 The treatment of IL-21-activated signalling pathways such as ERK1/2, PI3K/Akt, and STAT3 subsequently stimulate the proliferation and secretion of cytokines in RA FLS. 36 Recently, the first in-human phase 2 trial with recombinant anti-IL-21 monoclonal Ab has shown to be well tolerated in RA patients. 85
IL-32γ
IL-32γ is identified in the monocytes of active RA SF. 37 Stimulation of IL-32γ enhances the expression of IL-6 and IL-8 in RA FLS. Phosphorylated ERK1/2 is also involved in RA, and inhibition of ERK1/2 in RA FLS attenuates IL-32γ-induced IL-6 and IL-8 mRNA expressions. 86 It also mediates proinflammatory cytokines through the activation of ERK1/2.
IL-27
Increased level of IL-27 is observed in RA patients and IL-27 receptor is expressed in FLS. Stimulation of IL-27 induces the expression of adhesion molecules, release of inflammatory chemokines such as the C-C motif chemokine ligand 2 (CCL2), C-X-C motif chemokine ligand 9 (CXCL9), and CXCL10, and activates inflammatory signalling pathways such as STAT1, JAK-2, Akt, PI3K, and c-Jun N-terminal kinase (JNK) signalling in RA FLS. 38,87
IL-36α
Increased expression of IL-36α, an IL-1 family member, is identified in the synovium of patients with inflammatory arthritis such as RA and psoriatic arthritis. 88 As an inflammatory signalling mediator, IL-36α stimulation activates p38-MAPK signalling and enhances the production of proinflammatory cytokines in FLS. 39 This study of IL-36α receptor-depleted condition demonstrates that the IL-36α receptor provides a link between FLS and plasma or B cells in synovium. 39
Synthetic compounds
Compound 43
Compound 43 (C43) is an agonist of the formylpeptide receptor identified in various cells, such as endothelial cells 89 and fibroblasts, 90 and has been discovered as the receptor for the tripeptide N-formylmethionyl-leucyl-phenylalanine (fMLF). 91 C43 inhibits inflammation and bone injury, and decreases FLS proliferation and joint damage in RA. 40,41
Arsenic trioxide
Arsenic trioxide (ATO, As2O3) has been studied with reference to cellular apoptosis, and has been approved by the FDA for the treatment of acute promyelocytic leukaemia. ATO shows a beneficial effect for some solid tumours and haematological malignancies. 42,92 The action of ATO is also effective against the pathogenesis of RA by inducing apoptosis of FLS through the activation of caspase signalling and subsequently rebuilding the synovial tissue. 43 Although the molecular mechanism of ATO is poorly understood in RA, more recently ATO has been shown to improve RA symptoms by regulating autophagic signalling in combination with vitamin D. 42
7,3'-dimethoxy hesperetin
7,3'-dimethoxy hesperetin (DMHP) is a derivative of the bioflavonoid compound hesperidin. 44 It induces FLS apoptosis through enhanced expression of BAX (Bcl-2-associated X protein) and caspase-3 mRNA and caspase-3 activity in an experimental adjuvant arthritis model. 44
Niclosamide
Niclosamide (NCL) is known as a multifunctional agent with anti-inflammatory, antitumour, and antioxidative properties, 45,93-95 and is used for therapy of tapeworm infection. 96 Moreover, its role in the suppression of cell viability and ROS production through the mitochondrial-Akt signalling pathways has also been investigated. 45,97 A phase 2 clinical trial of NCL for RA was conducted as an adjuvant treatment along with administration of etanercept, and it showed a reduction of disease activity with marked decrease of E-selectin, ICAM-1, and vascular cell adhesion molecule-1 (VCAM-1). 46
Suberoylanilide hydroxamic acid
Suberoylanilide hydroxamic acid (SAHA, vorinostat), a class I histone deacetylase (HDAC) inhibitor, is an anticancer agent 98,99 and was approved by the FDA for treatment of cutaneous T cell lymphoma. 100 It induces the apoptosis of RA FLS through the involvement of enhanced caspase-3 activity and ROS production. 47
Natural compounds
Resveratrol
Polyphenol resveratrol, trans-3,5,4'-trihydroxystilbene, is an antioxidant abundant in red wines and displays a positive effect on cardiac protection. 101,102 Resveratrol mediates cell apoptosis through the modulation of mitochondrial signalling in RA FLS. It is a different strategy to reduce synovial hyperplasia. 102 Currently, it is reported that resveratrol inhibits inflammatory cytokine IL-1β, MMP-3, and phosphorylated Akt expression. 48 Moreover, a clinical trial has shown the efficacy of resveratrol as an adjuvant therapy to the conventional RA treatment. 49
Hesperidin
Hesperidin, a bioflavonoid, has revealed various protective roles in cognition, heart function, and inflammation. 103-105 Oral administration of hesperidin suppresses the clinical scores of RA patients. 50 More recently, hesperidin has shown an anti-inflammatory effect on FLS and reduces the polarization of macrophages in antigen-induced arthritis mouse model. 51
Mitomycin C
Mitomycin C (MMC) is an antibiotic and anti-tumour agent. 106,107 It has also been studied as an apoptotic agent of fibroblasts. 108-110 Treatment with MMC induces apoptosis of RA FLS through the production of ROS and disruption of the mitochondrial membrane potential. 52
Quercetin
Quercetin (3,3',4',5,7-pentahydroxyflavone) is a polyphenolic flavonoid and antioxidant in the human diet. 111,112 The treatment of quercetin enhances the apoptosis of RA FLS through the inhibition of the PI3K/Akt pathway. 53
Inflammatory activity, migration, and invasion
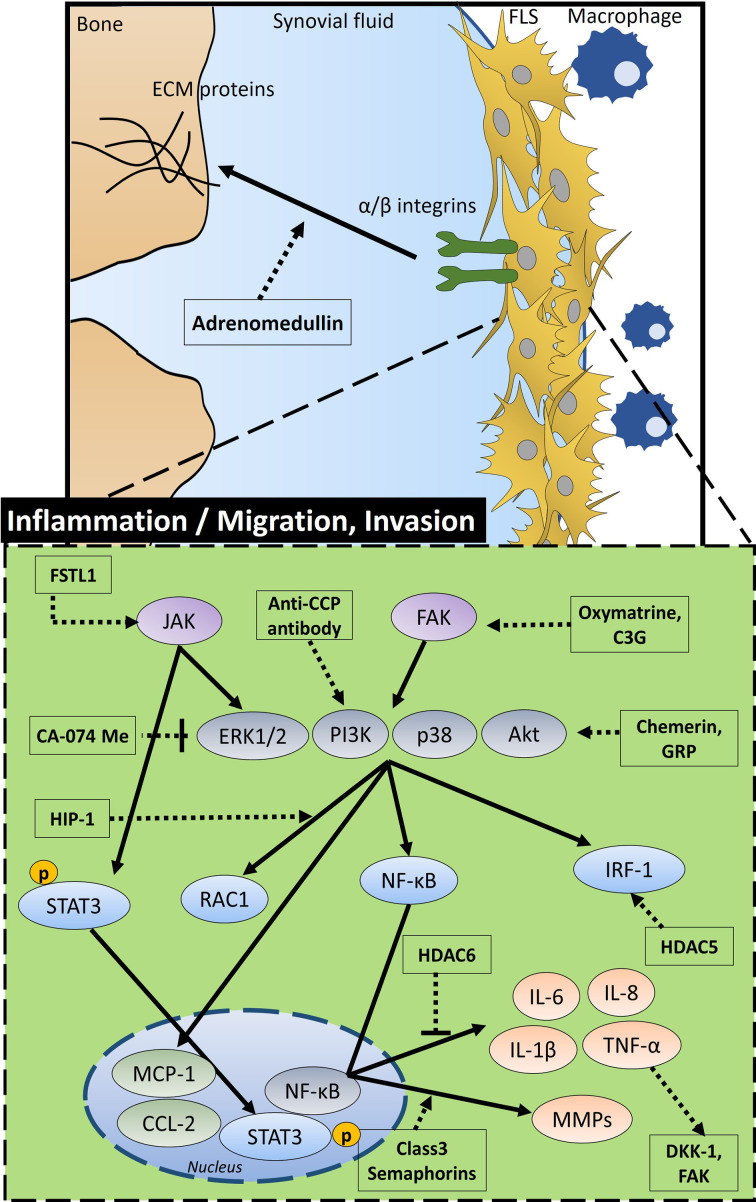
A characteristic inflamed synovium possesses dynamic FLS that reveals migration and invasive properties. This section focuses on the modulation of inflammation and migration of FLS and signalling molecules or enzymes on RA pathogenesis. A schematic diagram of migration and invasive properties is shown in Figure 2, and a summary of the mode of action and working dose of molecules is given in Table II.
Fig. 2.
Schematic diagram of the overall signalling mechanism and related factors of inflammatory activity, migration, and invasion in the fibroblastic synoviocytes (FLS) of rheumatoid arthritis (RA) FSTL1 stimulates JAK signalling. Oxymatrine and C3G enhance FAK signalling. Anti-CCP antibody activates PI3K. JAK induces activation of extracellular signal‑regulated protein kinase 1/2 (ERK1/2) and pSTAT3, FAK activates phosphoinositide 3-kinase (PI3K), p38, and protein kinase B (Akt) signalling. Chemerin and GRP activate PI3K/Akt signalling, but CA-074Me inhibits this signalling. HIP-1 activates RAC1 and HDAC5 activates interferon regulatory factor 1 (IRF-1) through PI3K/Akt signalling. HDAC6 inhibits inflammatory factors such as interleukin (IL)-6 and tumour necrosis factor-α (TNF-α) through nuclear factor kappa-B (NF-κB) transcription in the nucleus, whereas class 3 semaphorins increase levels of matrix metalloproteinases (MMPs). In addition, increased TNF-α enhances DKK-1 and FAK. This signalling is involved in the inflammation, migration, and invasion of FLS. C3G, cyanidin-3-glucoside; DKK-1, Dickkopf-1; FAK: integrin-related focal adhesion kinase; pSTAT3: phosphorylated signal transducer and activator of transcription 3; GRP, gastrin-releasing peptide; HIP-1, Huntingtin-interacting protein-1; HDAC, histone deacetylase; FSTL1, follistatin-like protein 1; ECM proteins, extracellular matrix proteins; anti-CCP, anti-cyclic citrullinated peptide.
Table II.
Summary of molecular mechanisms of fibroblastic synoviocytes (inflammation, migration, and invasion).
| Molecules | Mode of action | Species | Dose | Ref | |
|---|---|---|---|---|---|
| In vivo | in vitro | ||||
| Endogenous factors | |||||
| Chemerin | Activation of cytokine production, MMP-3 expression, and FLS migration | RA-FLS | 10 to 50 μM | 113 | |
| DKK-1 and FAK | Enhancement of FLS migration | RA-FLS | 114 | ||
| GRP | Enhancement of FLS invasion through Akt activation | RA-FLS | 10 μM | 115 | |
| Class 3 semaphorins | Activation of RAC1 and increasing effect on RA-FLS invasion | RA-FLS | 10 μM | 116 | |
| HIP-1 | Enhancement of FLS migration | RA-FLS | 117 | ||
| Adrenomedullin | Increase of FLS adhesion | RA-FLS | 100 nM | 118 | |
| HDAC | Increase of IL-6 and IL-1β expression | RA-FLS, CIA mice | 119 | ||
| FSTL1 | Enhancement of MMP expression and invasion of FLS | RA-FLS | 1 to 5 μg/ml | 120 | |
| Cadherin-11 | Enhancement of FLS adhesion and proliferation | RA-FLS | 121,122 | ||
| Anti-CCP antibody | Increase of FLS migration through PI3K activation | RA-FLS | 1 μg/ml | 123 | |
| Synthetic compounds | |||||
| CA-074Me | Inhibition of cathepsin B with decrease of MMP-2, F-actin, and phosphorylation of p38 MAPK/JNK | RA-FLS | 10 μM | 124 | |
| Natural compounds | |||||
| C3G | Inhibition of LPS-induced IL-6 and IL-1β production | RA-FLS, CIA mice | 50 mg/kg | 10 to 40 μM | 125 |
| Oxymatrine | Protection of joint destruction | RA-FLS, CIA mice | 100 mg/kg | 10 to 100 μM | 126 |
C3G, cyanidin-3-glucoside; CIA, collagen-induced arthritis; DKK-1, Dickkopf-1; FAK, focal adhesion kinase; FLS, fibroblastic synoviocytes; FSTL1, follistatin-like protein 1; GRP, gastrin-releasing peptide; HDAC, histone deacetylase; HIP-1, Huntingtin-interacting protein-1; IL, interleukin; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinases; MMP, matrix metalloproteinase; PI3K, phosphoinositide 3-kinase; RA, rheumatoid arthritis.
Endogenous factors
Chemerin
Chemerin is an agonist of chemokine-like receptor 1 (known as ChemR23) and identified in macrophages and dendritic cells. 127,128 The enhanced expressions of chemerin and its receptor ChemR23 are revealed in the RA synovium and related to disease severity. 113,129 Chemerin activates cytokine production, MMP-3 expression, and enhances FLS migration through the involvement of the p38-MAPK and Akt pathways. 113
Dickkopf-1 and integrin-related focal adhesion kinase
Stimulation by TNF-α causes the FLS-induced activation of Dickkopf-1 (DKK-1) and integrin-related focal adhesion kinase (FAK), and enhances migration of these FLS. 114 High levels of FAK, p-JNK, paxillin, and cell division control protein 42 (cdc42) expression are reported in the FLS migration machinery.
Gastrin-releasing peptide
Gastrin-releasing peptide (GRP) and its receptor GRPR are involved in inflammation processes such as gastritis and sepsis. 130 Activation of GRP enhances FLS invasion through Akt activation whereas RC-3095, the GRPR antagonist reduces it in a RA mice model. 115
Class 3 semaphorins
The semaphorins are implicated in autoimmune diseases such as RA and in the migration of immune cells and FLS. 116,131 Type-specific expression of semaphorin reveals the severity of RA. 116,132
Huntingtin-interacting protein 1
Huntingtin-interacting protein 1 (HIP-1) gene is identified using DNA sequencing by a phenotype-driven strategy in rats and patients with RA; it is involved in the enhanced invasiveness of RA FLS. 117 HIP-1 deficient human RA FLS decreased their invasion by nearly 50%, 133 suggesting that HIP-1 can be used for deteriorated joints in RA patients.
Adrenomedullin
Adrenomedullin is a secreted peptide from the FLS and is associated with the pathogenesis of RA. 118,134 Its stimulation mediates the adhesion of FLS by association of extracellular matrix proteins, integrin-α2 and -β1. 135
Histone deacetylase
The role of HDAC in RA FLS is not yet clear. Although the HDAC inhibitor SAHA mediates apoptosis in RA FLS, 47 the stimulation of inflammatory cytokines such as IL-1β and TNF-α suppresses the expression of HDAC5 in RA FLS. 136 In addition, application of HDAC6 inhibitor tubastatin A suppresses synovial inflammation and protects joint damage in CIA mice. 119
Follistatin-like protein 1
Follistatin-like protein 1 (FSTL1) is identified in RA synovium 137 and enhanced in the early stage of CIA in mice. 138 It is also involved in the progression of osteoclasts. 139 Its mechanism of pathogenesis in RA involves NF-κB, MAPK, and JAK/STAT3 signalling pathways. 120 MicroRNA-27a-targeted FSTL1 inhibits the migration and invasion of RA FLS. 140
Cadherin-11
Cadherin-11 is an adhesion molecule involved in various functions of the FLS. Cadherin-11-deficient FLS display diminished migration and invasion to cartilage under stimulation of serum or PDGF. 141 Cadherin-11 is selectively expressed on the FLS and supports the lining layer of the synovium via mediating FLS-to-FLS adhesion. 121 Knockdown of cadherin-11 reduces the IL-1β-induced proliferation of FLS. 122
Anti-cyclic citrullinated peptide antibody
The serum of RA patients is positive for anti-cyclic citrullinated peptide (anti-CCP). 142 Anti-CCP is used as a serological marker to diagnose RA. 143 Recently, it has been reported that FLS migration is increased by stimulation of anti-CCP polyclonal antibody through activating PI3K. 123
Synthetic compounds
Cathepsin B inhibitor, CA-074 Me
Cathepsin B, a proteinase, displays a higher activity in RA synovium. 144 Application of its inhibitor, CA-074 Me, inhibits invasion signalling through the reduced expression of MMP-2 mRNA, F-actin protein, and phosphorylation of P38-MAPK/JNK in FLS. 124
Natural compounds
Cyanidin-3-glucoside
Cyanidin-3-glucoside (C3G) is the most distributed anthocyanin compound in the flavonoid family. Being an antioxidant, it is studied for its inhibitory role on inflammation. 145 Treatment with C3G inhibits LPS-induced cytokine production in FLS and attenuates RA severity in the CIA mouse model. 125
Oxymatrine
Oxymatrine, a quinolizidine alkaloid, is an extract from the roots of Sophora flavescens. 126 It has demonstrated an inhibitory role in breast cancer cell migration and invasion, 146 an anti-inflammatory and antioxidant role, 147 and a protective effect on amyloid beta-induced neurotoxicity. 148 From the massive study conducted on oximatrine, it is found to be involved in the protection of joint destruction in RA. 126
Future perspectives and clinical strategies
Current medications, focused on inflammation and immunity, can influence FLS in RA. A therapeutic dose of methotrexate, the first choice for initial RA treatment, may attenuate the effects of PDGF and IL-1β on tumour suppressor expression and inhibit the proliferation and migration of FLS. 149,150 Hydroxychloroquine, a csDMARD, can also sensitize FLS to Fas-mediated apoptosis. 151 TNF-α inhibitors can influence FLS apoptosis via phosphoinositide-3-kinase Akt signal transduction and proliferation via the NF-κB pathway. 152,153 IL-6 and IL-6R inhibitors can also function well, since IL-6 is reported to promote proliferation of FLS 154 and facilitate angioneogenesis for pannus formation. 155 It is reported that IL-17 impairs FLS apoptosis through activation of autophagy 156 and enhances proliferation of FLS via STAT3 activation. 157 Thus, IL-17 inhibitors can attenuate the contribution of FLS in RA. Janus kinase inhibitors, which are targeted synthetic DMARDs (sDMARDs), may inhibit the FAK-mediated activation and invasion of FLS in RA. 158 Rituximab, a CD20 monoclonal antibody, might inhibit cellular adhesion and the production of MMP by FLS through LIGHT up-regulated B-cell depletion. 159 Abatacept, a cytotoxic T-lymphocyte-associated antigen 4-Ig that antagonizes CD28-medicated T cell activation, inhibits the FLS migration and expression of MMPs by inhibiting the MAPK pathway. 160 Although the influences of these medications on RA FLS have been reported, it is not known whether or not they can sufficiently control the RA FLS under therapeutic dose.
Research has been focusing on the greatest unmet needs of novel targeted therapies, including research of novel combination therapies for patients with refractory RA to available therapies, since the patients with remission are less than half of RA patients and there is no cure yet. 161 Current available therapies for RA including various synthetic and biological DMARDs, glucocorticoids, and their combination therapies should be used carefully due to hazards such as increased risk of infection and malignancy. Thus to minimize systemic adverse events from immune suppression and increase the response to these current treatments, more specific target molecules that have a weaker effect on immune cells may be needed. In this respect, molecules that regulate synoviocyte signalling may be one of the more efficient therapeutic targets for RA, and we review these here. Add-on or combination therapies with synoviocyte-targeting therapies may increase their treatment efficacy and decrease systemic adverse events by reducing dose of current therapies. For example, NCL, as mentioned earlier, in combination with etanercept increases the proportion of patients achieving an ACR 20% response (ACR20), ACR50 response, and ACR70 response, and improves tender joint count, swollen joint count, and disease activity score of 28 joints (DAS-28) in RA patients who show an inadequate response to etanercept. 46 Interestingly, this treatment does not reduce CRP and ESR compared to placebo, 46 and this suggests that adding NCL can improve the treatment response without having a certain effect on systemic inflammation. In this regard, GPI is also prudently suggested as one of the best prospective targets, although this treatment option is in the early stages since GPI could be involved in the pathogenesis of RA with joint-specific inflammation as an autoantigen. 162 GPI has also shown to be correlated with disease activity such as DAS-28, swollen and tender joint count, and GPI levels decreased in response to infliximab treatment in RA patients. 163
In this review, we have tried to highlight the unmet need to target FLS as one of the novel therapies for RA. Several therapeutic approaches may have a weak anti-inflammatory effect or no effect at all. However, it is clear that inflammation is at the heart of the pathogenesis of RA. In this respect, innovative strategies are required to target inflammation and pathognomonic parameters for RA FLS concurrently. This approach will provide us with a wide perspective to identify the appropriate target from multiple signalling of RA.
Author contributions
M. Ji: Conceptualized and designed the study, Collected the information, Prepared and revised the manuscript critically for intellectual content.
H. J. Ryu: Conceptualized and designed the study, Collected the information, Supervised the manuscript writing, Approved the final version of the manuscript.
J. H. Hong: Conceptualized and designed the study, Collected the information, Revised and supervised the manuscript writing, Approved the final version of the manuscript.
H. J. Ryu and J. H. Hong contributed equally to this work as corresponding authors.
Funding statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT; [NRF-2019R1F1A1046785]), and a grant from the Gachon University Gil Medical Center project [FRD 2018-07]. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
The authors declare that they have no competing interest with the contents of this article.
Acknowledgements
The authors thank Gachon University and Gil Medical Center for their technical supports.
Ethical review statement
This study does not include any studies with human participants or animals performed by any of the authors.
© 2021 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- 1. Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh JA, Saag KG, Bridges SL, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68(1):1–26. [DOI] [PubMed] [Google Scholar]
- 3. Smolen JS, Landewe RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. [DOI] [PubMed] [Google Scholar]
- 4. Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. The Lancet. 2007;370(9602):1861–1874. [DOI] [PubMed] [Google Scholar]
- 5. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. The Lancet. 2016;388(10055):2023–2038. [DOI] [PubMed] [Google Scholar]
- 6. Aga A-B, Lie E, Uhlig T, et al. Time trends in disease activity, response and remission rates in rheumatoid arthritis during the past decade: results from the NOR-DMARD study 2000-2010. Ann Rheum Dis. 2015;74(2):381–388. [DOI] [PubMed] [Google Scholar]
- 7. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–2219. [DOI] [PubMed] [Google Scholar]
- 8. Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9(1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. You S, Koh JH, Leng L, Kim W-U, Bucala R. The tumor-like phenotype of rheumatoid synovium: molecular profiling and prospects for precision medicine. Arthritis Rheumatol. 2018;70(5):637–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lefevre S, Meier FMP, Neumann E, Muller-Ladner U. Role of synovial fibroblasts in rheumatoid arthritis. Curr Pharm Des. 2014;21(2):130–141. [DOI] [PubMed] [Google Scholar]
- 11. Veale DJ, Orr C, Fearon U. Cellular and molecular perspectives in rheumatoid arthritis. Semin Immunopathol. 2017;39(4):343–354. [DOI] [PubMed] [Google Scholar]
- 12. Sweeney SE, Firestein GS. Rheumatoid arthritis: regulation of synovial inflammation. Int J Biochem Cell Biol. 2004;36(3):372–378. [DOI] [PubMed] [Google Scholar]
- 13. Bustamante MF, Garcia-Carbonell R, Whisenant KD, Guma M. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Korb-Pap A, Bertrand J, Sherwood J, Pap T. Stable activation of fibroblasts in rheumatic arthritis-causes and consequences. Rheumatology. 2016;55(suppl 2):ii64–ii67. [DOI] [PubMed] [Google Scholar]
- 15. Gravallese EM. Bone destruction in arthritis. Ann Rheum Dis. 2002;61(Supplement 2):84ii–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233(1):233–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pope RM. Apoptosis as a therapeutic tool in rheumatoid arthritis. Nat Rev Immunol. 2002;2(7):527–535. [DOI] [PubMed] [Google Scholar]
- 18. Liu H, Pope RM. The role of apoptosis in rheumatoid arthritis. Curr Opin Pharmacol. 2003;3(3):317–322. [DOI] [PubMed] [Google Scholar]
- 19. Baier A, Meineckel I, Gay S, Pap T. Apoptosis in rheumatoid arthritis. Curr Opin Rheumatol. 2003;15(3):274–279. [DOI] [PubMed] [Google Scholar]
- 20. Ishida S, Yamane S, Ochi T, Nakano S, Mori T, Juji T. Light induces cell proliferation and inflammatory responses of rheumatoid arthritis synovial fibroblasts via lymphotoxin beta receptor. J Rheumatol. 2008;35(6):960–968. [PubMed] [Google Scholar]
- 21. Nagatani K, Itoh K, Nakajima K, et al. Rheumatoid arthritis fibroblast-like synoviocytes express BCMA and are stimulated by April. Arthritis Rheum. 2007;56(11):3554–3563. [DOI] [PubMed] [Google Scholar]
- 22. Chang Y, Jia X, Sun X, et al. APRIL promotes proliferation, secretion and invasion of fibroblast-like synoviocyte from rats with adjuvant induced arthritis. Mol Immunol. 2015;64(1):90–98. [DOI] [PubMed] [Google Scholar]
- 23. Liu F, Feng XX, Zhu SL, et al. Sonic Hedgehog Signaling Pathway Mediates Proliferation and Migration of Fibroblast-Like Synoviocytes in Rheumatoid Arthritis via MAPK/ERK Signaling Pathway. Front Immunol. 2018;9:2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zong M, Lu T, Fan S, et al. Glucose-6-phosphate isomerase promotes the proliferation and inhibits the apoptosis in fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Res Ther. 2015;17(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ahn JK, Oh J-M, Lee J, et al. Increased extracellular survivin in the synovial fluid of rheumatoid arthritis patients: fibroblast-like synoviocytes as a potential source of extracellular survivin. Inflammation. 2010;33(6):381–388. [DOI] [PubMed] [Google Scholar]
- 26. Jia W, Wu W, Yang D, et al. Histone demethylase JMJD3 regulates fibroblast-like synoviocyte-mediated proliferation and joint destruction in rheumatoid arthritis. FASEB J. 2018;32(7):4031–4042. [DOI] [PubMed] [Google Scholar]
- 27. Zhang L-M, Zhou J-J, Luo C-L. CYLD suppression enhances the pro-inflammatory effects and hyperproliferation of rheumatoid arthritis fibroblast-like synoviocytes by enhancing NF-κB activation. Arthritis Res Ther. 2018;20(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakamura H, Shimamura S, Yasuda S, et al. Ectopic RasGRP2 (CalDAG-GEFI) expression in rheumatoid synovium contributes to the development of destructive arthritis. Ann Rheum Dis. 2018;77(12):1765–1772. [DOI] [PubMed] [Google Scholar]
- 29. Cheon H, Yu S-J, Yoo DH, Chae IJ, Song GG, Sohn J. Increased expression of pro-inflammatory cytokines and metalloproteinase-1 by TGF-beta1 in synovial fibroblasts from rheumatoid arthritis and normal individuals. Clin Exp Immunol. 2002;127(3):547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cho M-L, Min S-Y, Chang S-H, et al. Transforming growth factor beta 1(TGF-beta1) down-regulates TNFalpha-induced RANTES production in rheumatoid synovial fibroblasts through NF-kappaB-mediated transcriptional repression. Immunol Lett. 2006;105(2):159–166. [DOI] [PubMed] [Google Scholar]
- 31. Connolly M, Marrelli A, Blades M, et al. Acute serum amyloid A induces migration, angiogenesis, and inflammation in synovial cells in vitro and in a human rheumatoid arthritis/SCID mouse chimera model. J Immunol. 2010;184(11):6427–6437. [DOI] [PubMed] [Google Scholar]
- 32. Okamoto H, Katagiri Y, Kiire A, Momohara S, Kamatani N. Serum amyloid A activates nuclear factor-kappaB in rheumatoid synovial fibroblasts through binding to receptor of advanced glycation end-products. J Rheumatol. 2008;35(5):752–756. [PubMed] [Google Scholar]
- 33. Sekine C, Nanki T, Yagita H. Macrophage-derived delta-like protein 1 enhances interleukin-6 and matrix metalloproteinase 3 production by fibroblast-like synoviocytes in mice with collagen-induced arthritis. Arthritis Rheumatol. 2014;66(10):2751–2761. [DOI] [PubMed] [Google Scholar]
- 34. Hashaad NI, Fawzy RM, Elazem AAA, Youssef MI. Serum calreticulin as a novel biomarker of juvenile idiopathic arthritis disease activity. Eur J Rheumatol. 2017;4(1):19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Calmon-Hamaty F, Combe B, Hahne M, Morel J. Lymphotoxin α stimulates proliferation and pro-inflammatory cytokine secretion of rheumatoid arthritis synovial fibroblasts. Cytokine. 2011;53(2):207–214. [DOI] [PubMed] [Google Scholar]
- 36. Xing R, Yang L, Jin Y, et al. Interleukin-21 induces proliferation and proinflammatory cytokine profile of fibroblast-like synoviocytes of patients with rheumatoid arthritis. Scand J Immunol. 2016;83(1):64–71. [DOI] [PubMed] [Google Scholar]
- 37. Kim Y-G, Lee C-K, Oh JS, Kim S-H, Kim K-A, Yoo B. Effect of interleukin-32gamma on differentiation of osteoclasts from CD14+ monocytes. Arthritis Rheum. 2010;62(2):515–523. [DOI] [PubMed] [Google Scholar]
- 38. Wong CK, Chen DP, Tam LS, Li EK, Yin YB, Lam CWK. Effects of inflammatory cytokine IL-27 on the activation of fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Res Ther. 2010;12(4):R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schmitt V, Hahn M, Kästele V, et al. Interleukin-36 receptor mediates the crosstalk between plasma cells and synovial fibroblasts. Eur J Immunol. 2017;47(12):2101–2112. [DOI] [PubMed] [Google Scholar]
- 40. Kao W, Gu R, Jia Y, et al. A formyl peptide receptor agonist suppresses inflammation and bone damage in arthritis. Br J Pharmacol. 2014;171(17):4087–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Odobasic D, Jia Y, Kao W, et al. Formyl peptide receptor activation inhibits the expansion of effector T cells and synovial fibroblasts and attenuates joint injury in models of rheumatoid arthritis. Int Immunopharmacol. 2018;61:140–149. [DOI] [PubMed] [Google Scholar]
- 42. Yin J, Wang L, Wang Y, Shen H, Wang X, Wu L. Curcumin reverses oxaliplatin resistance in human colorectal cancer via regulation of TGF-β/Smad2/3 signaling pathway. Onco Targets Ther. 2019;12:3893–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mei Y, Zheng Y, Wang H, et al. Arsenic trioxide induces apoptosis of fibroblast-like synoviocytes and represents antiarthritis effect in experimental model of rheumatoid arthritis. J Rheumatol. 2011;38(1):36–43. [DOI] [PubMed] [Google Scholar]
- 44. Li R, Cai L, Xie X-feng, Peng L, Li J. 7,3'-dimethoxy hesperetin induces apoptosis of fibroblast-like synoviocytes in rats with adjuvant arthritis through caspase 3 activation. Phytother Res. 2010;24(12):1850–1856. [DOI] [PubMed] [Google Scholar]
- 45. Liang L, Huang M, Xiao Y, et al. Inhibitory effects of niclosamide on inflammation and migration of fibroblast-like synoviocytes from patients with rheumatoid arthritis. Inflamm Res. 2015;64(3-4):225–233. [DOI] [PubMed] [Google Scholar]
- 46. Al-Gareeb AIA, Gorial FI, Mahmood AS. Niclosamide as an adjuvant to etanercept in treatment patients with active rheumatoid arthritis: an 8-week randomized controlled pilot study. Clin Rheumatol. 2018;37(10):2633–2641. [DOI] [PubMed] [Google Scholar]
- 47. Chen H, Pan J, Wang J-D, Liao Q-M, Xia X-R. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, induces apoptosis in rheumatoid arthritis fibroblast-like synoviocytes. Inflammation. 2016;39(1):39–46. [DOI] [PubMed] [Google Scholar]
- 48. Tian J, Chen J-wei, Gao J-sheng, Li L, Xie X. Resveratrol inhibits TNF-α-induced IL-1β, MMP-3 production in human rheumatoid arthritis fibroblast-like synoviocytes via modulation of PI3kinase/Akt pathway. Rheumatol Int. 2013;33(7):1829–1835. [DOI] [PubMed] [Google Scholar]
- 49. Khojah HM, Ahmed S, Abdel-Rahman MS, Elhakeim EH. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: a clinical study. Clin Rheumatol. 2018;37(8):2035–2042. [DOI] [PubMed] [Google Scholar]
- 50. Kawaguchi K, Maruyama H, Kometani T, Kumazawa Y. Suppression of collagen-induced arthritis by oral administration of the citrus flavonoid hesperidin. Planta Med. 2006;72(5):477–479. [DOI] [PubMed] [Google Scholar]
- 51. Qi W, Lin C, Fan K, et al. Hesperidin inhibits synovial cell inflammation and macrophage polarization through suppression of the PI3K/Akt pathway in complete Freund's adjuvant-induced arthritis in mice. Chem Biol Interact. 2019;306:19–28. [DOI] [PubMed] [Google Scholar]
- 52. Yan C, Kong D, Ge D, et al. Mitomycin C induces apoptosis in rheumatoid arthritis fibroblast-like synoviocytes via a mitochondrial-mediated pathway. Cell Physiol Biochem. 2015;35(3):1125–1136. [DOI] [PubMed] [Google Scholar]
- 53. Pan F, Zhu L, Lv H, Pei C. Quercetin promotes the apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis by upregulating lncRNA MALAT1. Int J Mol Med. 2016;38(5):1507–1514. [DOI] [PubMed] [Google Scholar]
- 54. Sabokbar A, Afrough S, Mahoney DJ, Uchihara Y, Swales C, Athanasou NA. Role of light in the pathogenesis of joint destruction in rheumatoid arthritis. WJEM. 2017;7(2):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jayadev C, Hulley P, Swales C, et al. Synovial fluid fingerprinting in end-stage knee osteoarthritis: a novel biomarker concept. Bone Joint Res. 2020;9(9):623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Goetz JA, Suber LM, Zeng X, Robbins DJ. Sonic hedgehog as a mediator of long-range signaling. Bioessays. 2002;24(2):157–165. [DOI] [PubMed] [Google Scholar]
- 57. Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22(18):2454–2472. [DOI] [PubMed] [Google Scholar]
- 58. Wang M, Zhu S, Peng W, et al. Sonic hedgehog signaling drives proliferation of synoviocytes in rheumatoid arthritis: a possible novel therapeutic target. J Immunol Res. 2014;2014(5):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rimkus TK, Carpenter RL, Qasem S, Chan M, Lo H-W. Targeting the sonic hedgehog signaling pathway: review of smoothened and Gli inhibitors. Cancers. 2016;8(2):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Niinaka Y, Paku S, Haga A, Watanabe H, Raz A. Expression and secretion of neuroleukin/phosphohexose isomerase/maturation factor as autocrine motility factor by tumor cells. Cancer Res. 1998;58(12):2667–2674. [PubMed] [Google Scholar]
- 61. Tsutsumi S, Hogan V, Nabi IR, Raz A. Overexpression of the autocrine motility factor/phosphoglucose isomerase induces transformation and survival of NIH-3T3 fibroblasts. Cancer Res. 2003;63(1):242–249. [PubMed] [Google Scholar]
- 62. Kim J-W, Dang CV. Multifaceted roles of glycolytic enzymes. Trends Biochem Sci. 2005;30(3):142–150. [DOI] [PubMed] [Google Scholar]
- 63. Svensson B, Hafström I, Forslind K, Albertsson K, Tarkowski A, Bokarewa M. Increased expression of proto-oncogene survivin predicts joint destruction and persistent disease activity in early rheumatoid arthritis. Ann Med. 2010;42(1):45–54. [DOI] [PubMed] [Google Scholar]
- 64. Fukuda S, Pelus LM, Survivin PLM. Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther. 2006;5(5):1087–1098. [DOI] [PubMed] [Google Scholar]
- 65. Mokuda S, Miyazaki T, Ito Y, et al. The proto-oncogene survivin splice variant 2B is induced by PDGF and leads to cell proliferation in rheumatoid arthritis fibroblast-like synoviocytes. Sci Rep. 2015;5(1):9795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shan J, Fu L, Balasubramanian MN, Anthony T, Kilberg MS. ATF4-dependent regulation of the JMJD3 gene during amino acid deprivation can be rescued in Atf4-deficient cells by inhibition of deacetylation. J Biol Chem. 2012;287(43):36393–36403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee H-Y, Choi K, Oh H, Park Y-K, Park H. HIF-1-dependent induction of Jumonji domain-containing protein (JMJD) 3 under hypoxic conditions. Mol Cells. 2014;37(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bignell GR, Warren W, Seal S, et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25(2):160–165. [DOI] [PubMed] [Google Scholar]
- 69. Greenwood E. Tumour suppressors - Cylindromatosis: cause and treatment. Nat Rev Cancer. 2003;3(10):716–717. [Google Scholar]
- 70. Zhang J, Stirling B, Temmerman ST, et al. Impaired regulation of NF-kappaB and increased susceptibility to colitis-associated tumorigenesis in CYLD-deficient mice. J Clin Invest. 2006;116(11):3042–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Urbanik T, Köhler BC, Boger RJ, et al. Down-regulation of CYLD as a trigger for NF-κB activation and a mechanism of apoptotic resistance in hepatocellular carcinoma cells. Int J Oncol. 2011;38(1):121–131. [PubMed] [Google Scholar]
- 72. Yang J, Zhuang Y, Liu J. Upregulation of microRNA‑590 in rheumatoid arthritis promotes apoptosis of bone cells through transforming growth factor‑β1/phosphoinositide 3‑kinase/Akt signaling. Int J Mol Med. 2019;43(5):2212–2220. [DOI] [PubMed] [Google Scholar]
- 73. Zhu D, Zhao J, Lou A, et al. Transforming growth factor β1 promotes fibroblast-like synoviocytes migration and invasion via TGF-β1/Smad signaling in rheumatoid arthritis. Mol Cell Biochem. 2019;459(1-2):141–150. [DOI] [PubMed] [Google Scholar]
- 74. Nys G, Cobraiville G, Servais A-C, Malaise MG, de Seny D, Fillet M. Targeted proteomics reveals serum amyloid A variants and alarmins S100A8-S100A9 as key plasma biomarkers of rheumatoid arthritis. Talanta. 2019;204(6):507–517. [DOI] [PubMed] [Google Scholar]
- 75. de Seny D, Fillet M, Meuwis M-A, et al. Discovery of new rheumatoid arthritis biomarkers using the surface-enhanced laser desorption/ionization time-of-flight mass spectrometry ProteinChip approach. Arthritis Rheum. 2005;52(12):3801–3812. [DOI] [PubMed] [Google Scholar]
- 76. O'Hara R, Murphy EP, Whitehead AS, FitzGerald O, Bresnihan B. Acute-phase serum amyloid A production by rheumatoid arthritis synovial tissue. Arthritis Res. 2000;2(2):142–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gardai SJ, McPhillips KA, Frasch SC, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123(2):321–334. [DOI] [PubMed] [Google Scholar]
- 78. Fischer CR, Mikami M, Minematsu H, et al. Calreticulin inhibits inflammation-induced osteoclastogenesis and bone resorption. J Orthop Res. 2017;35(12):2658–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jiao Y, Ding H, Huang S, et al. Bcl-Xl and Mcl-1 upregulation by calreticulin promotes apoptosis resistance of fibroblast-like synoviocytes via activation of PI3K/Akt and STAT3 pathways in rheumatoid arthritis. Clin Exp Rheumatol. 2018;36(5):841–849. [PubMed] [Google Scholar]
- 80. Calmon-Hamaty F, Combe B, Hahne M, Morel J. Lymphotoxin α revisited: general features and implications in rheumatoid arthritis. Arthritis Res Ther. 2011;13(4):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Medvedev AE, Espevik T, Ranges G, Sundan A. Distinct roles of the two tumor necrosis factor (TNF) receptors in modulating TNF and lymphotoxin alpha effects. J Biol Chem. 1996;271(16):9778–9784. [DOI] [PubMed] [Google Scholar]
- 82. Kennedy WP, Simon JA, Offutt C, et al. Efficacy and safety of pateclizumab (anti-lymphotoxin-α) compared to adalimumab in rheumatoid arthritis: a head-to-head phase 2 randomized controlled study (the ALTARA study). Arthritis Res Ther. 2014;16(5):467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Spolski R, Leonard WJ. The yin and yang of interleukin-21 in allergy, autoimmunity and cancer. Curr Opin Immunol. 2008;20(3):295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jüngel A, Distler JHW, Kurowska-Stolarska M, et al. Expression of interleukin-21 receptor, but not interleukin-21, in synovial fibroblasts and synovial macrophages of patients with rheumatoid arthritis. Arthritis & Rheumatism. 2004;50(5):1468–1476. [DOI] [PubMed] [Google Scholar]
- 85. Ignatenko S, Skrumsager BK, Mouritzen U. Safety, PK, and PD of recombinant anti-interleukin-21 monoclonal antibody in a first-in-human trial. CP. 2016;54(04):243–252. [DOI] [PubMed] [Google Scholar]
- 86. Kim Y-G, Lee C-K, Kim S-H, Cho W-S, Mun SH, Yoo B. Interleukin-32gamma enhances the production of IL-6 and IL-8 in fibroblast-like synoviocytes via ERK1/2 activation. J Clin Immunol. 2010;30(2):260–267. [DOI] [PubMed] [Google Scholar]
- 87. González-Chávez SA, Pacheco-Tena C, Quiñonez-Flores CM, Espino-Solis GP, Burrola-De Anda JI, Muñoz-Morales PM. Positive transcriptional response on inflammation and joint remodelling influenced by physical exercise in proteoglycan-induced arthritis: an animal study. Bone Joint Res. 2020;9(1):36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Frey S, Derer A, Messbacher M-E, et al. The novel cytokine interleukin-36α is expressed in psoriatic and rheumatoid arthritis synovium. Ann Rheum Dis. 2013;72(9):1569–1574. [DOI] [PubMed] [Google Scholar]
- 89. Heo SC, Kwon YW, Jang IH, et al. WKYMVm-induced activation of formyl peptide receptor 2 stimulates ischemic neovasculogenesis by promoting homing of endothelial colony-forming cells. Stem Cells. 2014;32(3):779–790. [DOI] [PubMed] [Google Scholar]
- 90. VanCompernolle SE, Clark KL, Rummel KA, Todd SC. Expression and function of formyl peptide receptors on human fibroblast cells. J Immunol. 2003;171(4):2050–2056. [DOI] [PubMed] [Google Scholar]
- 91. Boulay F, Tardif M, Brouchon L, Vignais P. Synthesis and use of a novel N-formyl peptide derivative to isolate a human N-formyl peptide receptor cDNA. Biochem Biophys Res Commun. 1990;168(3):1103–1109. [DOI] [PubMed] [Google Scholar]
- 92. Hoonjan M, Jadhav V, Bhatt P. Arsenic trioxide: insights into its evolution to an anticancer agent. J Biol Inorg Chem. 2018;23(3):313–329. [DOI] [PubMed] [Google Scholar]
- 93. Jin Y, Lu Z, Ding K, et al. Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: inactivation of the NF-kappaB pathway and generation of reactive oxygen species. Cancer Res. 2010;70(6):2516–2527. [DOI] [PubMed] [Google Scholar]
- 94. Lee SL, Son A-R, Ahn J, Song J-Y. Niclosamide enhances ROS-mediated cell death through c-Jun activation. Biomed Pharmacother. 2014;68(5):619–624. [DOI] [PubMed] [Google Scholar]
- 95. Li X, Yang Y, Sun G, et al. Promising targets and drugs in rheumatoid arthritis: a module-based and cumulatively scoring approach. Bone Joint Res. 2020;9(8):501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pearson RD, Hewlett EL. Niclosamide therapy for tapeworm infections. Ann Intern Med. 1985;102(4):550–551. [DOI] [PubMed] [Google Scholar]
- 97. Huang M, Zeng S, Qiu Q, et al. Niclosamide induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes. Int Immunopharmacol. 2016;31(12):45–49. [DOI] [PubMed] [Google Scholar]
- 98. Garcia-Manero G, Yang H, Bueso-Ramos C, et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood. 2008;111(3):1060–1066. [DOI] [PubMed] [Google Scholar]
- 99. Chen S, Zhao Y, Gou WF, Zhao S, Takano Y, Zheng HC. The anti-tumor effects and molecular mechanisms of suberoylanilide hydroxamic acid (SAHA) on the aggressive phenotypes of ovarian carcinoma cells. PLoS One. 2013;8(11):e79781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Xu J, Sun J, Wang P, Ma X, Li S. Pendant HDAC inhibitor SAHA derivatised polymer as a novel prodrug micellar carrier for anticancer drugs. J Drug Target. 2018;26(5-6):448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Soleas GJ, Diamandis EP, Goldberg DM. Wine as a biological fluid: history, production, and role in disease prevention. J Clin Lab Anal. 1997;11(5):287–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Byun HS, Song JK, Kim Y-R, et al. Caspase-8 has an essential role in resveratrol-induced apoptosis of rheumatoid fibroblast-like synoviocytes. Rheumatology. 2008;47(3):301–308. [DOI] [PubMed] [Google Scholar]
- 103. Wang D, Liu L, Zhu X, Wu W, Wang Y. Hesperidin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress in a mouse model of Alzheimer’s disease. Cell Mol Neurobiol. 2014;34(8):1209–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Agrawal YO, Sharma PK, Shrivastava B, et al. Hesperidin produces cardioprotective activity via PPAR-γ pathway in ischemic heart disease model in diabetic rats. PLoS One. 2014;9(11):e111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fu Z, Chen Z, Xie Q, Lei H, Xiang S. Hesperidin protects against IL-1β-induced inflammation in human osteoarthritis chondrocytes. Exp Ther Med. 2018;16(4):3721–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sartorelli AC. The role of mitomycin antibiotics in the chemotherapy of solid tumors. Biochem Pharmacol. 1986;35(1):67–69. [DOI] [PubMed] [Google Scholar]
- 107. Galm U, Hager MH, Van Lanen SG, Ju J, Thorson JS, Shen B. Antitumor antibiotics: bleomycin, enediynes, and mitomycin. Chem Rev. 2005;105(2):739–758. [DOI] [PubMed] [Google Scholar]
- 108. Chou S-F, Chang S-W, Chuang J-L. Mitomycin C upregulates IL-8 and MCP-1 chemokine expression via mitogen-activated protein kinases in corneal fibroblasts. Invest Ophthalmol Vis Sci. 2007;48(5):2009–2016. [DOI] [PubMed] [Google Scholar]
- 109. Sun Y, Wang L-X, Wang L, et al. A comparison of the effectiveness of mitomycin C and 5-fluorouracil in the prevention of peridural adhesion after laminectomy. J Neurosurg Spine. 2007;7(4):423–428. [DOI] [PubMed] [Google Scholar]
- 110. Shi K, Wang D, Cao X, Ge Y. Endoplasmic reticulum stress signaling is involved in mitomycin C (MMC)-induced apoptosis in human fibroblasts via PERK pathway. PLoS One. 2013;8(3):e59330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lamson DW, Brignall MS. Antioxidants and cancer, part 3: quercetin. Altern Med Rev. 2000;5(3):196–208. [PubMed] [Google Scholar]
- 112. Coskun O, Kanter M, Korkmaz A, Oter S, Quercetin OS. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol Res. 2005;51(2):117–123. [DOI] [PubMed] [Google Scholar]
- 113. Kaneko K, Miyabe Y, Takayasu A, et al. Chemerin activates fibroblast-like synoviocytes in patients with rheumatoid arthritis. Arthritis Res Ther. 2011;13(5):R158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Choe J-Y, Hun Kim J, Park K-Y, Choi C-H, Kim S-K. Activation of dickkopf-1 and focal adhesion kinase pathway by tumour necrosis factor α induces enhanced migration of fibroblast-like synoviocytes in rheumatoid arthritis. Rheumatology. 2016;55(5):928–938. [DOI] [PubMed] [Google Scholar]
- 115. Clarimundo VS, Farinon M, Pedó RT, et al. Gastrin-releasing peptide and its receptor increase arthritis fibroblast-like synoviocytes invasiveness through activating the PI3K/Akt pathway. Peptides. 2017;95:57–61. [DOI] [PubMed] [Google Scholar]
- 116. Tang MW, Malvar Fernández B, Newsom SP, et al. Class 3 semaphorins modulate the invasive capacity of rheumatoid arthritis fibroblast-like synoviocytes. Rheumatology. 2018;57(5):909–920. [DOI] [PubMed] [Google Scholar]
- 117. Laragione T, Brenner M, Lahiri A, Gao E, Harris C, Gulko PS. Huntingtin-Interacting protein 1 (HIP1) regulates arthritis severity and synovial fibroblast invasiveness by altering PDGFR and Rac1 signalling. Ann Rheum Dis. 2018;77(11):1627–1635. [DOI] [PubMed] [Google Scholar]
- 118. Chosa E, Hamada H, Kitamura K, Eto T, Tajima N. Increased plasma and joint tissue adrenomedullin concentrations in patients with rheumatoid arthritis compared to those with osteoarthritis. J Rheumatol. 2003;30(12):2553–2556. [PubMed] [Google Scholar]
- 119. Lee J, Hong EC, Jeong H, et al. A novel histone deacetylase 6-selective inhibitor suppresses synovial inflammation and joint destruction in a collagen antibody-induced arthritis mouse model. Int J Rheum Dis. 2015;18(5):514–523. [DOI] [PubMed] [Google Scholar]
- 120. Ni S, Li C, Xu N, et al. Follistatin-like protein 1 induction of matrix metalloproteinase 1, 3 and 13 gene expression in rheumatoid arthritis synoviocytes requires MAPK, JAK/STAT3 and NF-κB pathways. J Cell Physiol. 2018;234(1):454–463. [DOI] [PubMed] [Google Scholar]
- 121. Chang SK, Gu Z, Brenner MB. Fibroblast-like synoviocytes in inflammatory arthritis pathology: the emerging role of cadherin-11. Immunol Rev. 2010;233(1):256–266. [DOI] [PubMed] [Google Scholar]
- 122. Yoshioka R, Kita Y, Nagahira A, et al. Quantitative analysis of cadherin-11 and β-catenin signalling during proliferation of rheumatoid arthritis-derived synovial fibroblast cells. J Pharm Pharmacol. 2015;67(8):1075–1082. [DOI] [PubMed] [Google Scholar]
- 123. Sun M, Rethi B, Krishnamurthy A, et al. Anticitrullinated protein antibodies facilitate migration of synovial tissue-derived fibroblasts. Ann Rheum Dis. 2019;78(12):1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tong B, Wan B, Wei Z, et al. Role of cathepsin B in regulating migration and invasion of fibroblast-like synoviocytes into inflamed tissue from patients with rheumatoid arthritis. Clin Exp Immunol. 2014;177(3):586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sun Y, Li L. Cyanidin-3-glucoside inhibits inflammatory activities in human fibroblast-like synoviocytes and in mice with collagen-induced arthritis. Clin Exp Pharmacol Physiol. 2018;45(10):1038–1045. [DOI] [PubMed] [Google Scholar]
- 126. Liang J, Chang B, Huang M, et al. Oxymatrine prevents synovial inflammation and migration via blocking NF-κB activation in rheumatoid fibroblast-like synoviocytes. Int Immunopharmacol. 2018;55:105–111. [DOI] [PubMed] [Google Scholar]
- 127. Wittamer V, Franssen J-D, Vulcano M, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198(7):977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Parolini S, Santoro A, Marcenaro E, et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood. 2007;109(9):3625–3632. [DOI] [PubMed] [Google Scholar]
- 129. Ha Y-J, Kang E-J, Song J-S, Park Y-B, Lee S-K, Choi ST. Plasma chemerin levels in rheumatoid arthritis are correlated with disease activity rather than obesity. Joint Bone Spine. 2014;81(2):189–190. [DOI] [PubMed] [Google Scholar]
- 130. Petronilho F, Danielski LG, Roesler R, Schwartsmann G, Dal-Pizzol F. Gastrin-releasing peptide as a molecular target for inflammatory diseases: an update. Inflamm Allergy Drug Targets. 2013;12(3):172–177. [DOI] [PubMed] [Google Scholar]
- 131. Nishide M, Kumanogoh A. The role of semaphorins in immune responses and autoimmune rheumatic diseases. Nat Rev Rheumatol. 2018;14(1):19–31. [DOI] [PubMed] [Google Scholar]
- 132. Garcia S. Role of semaphorins in Immunopathologies and rheumatic diseases. Int J Mol Sci. 2019;20(2):374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ai R, Laragione T, Hammaker D, et al. Comprehensive epigenetic landscape of rheumatoid arthritis fibroblast-like synoviocytes. Nat Commun. 2018;9(1):1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Yudoh K, Matsuno H, Kimura T. Plasma adrenomedullin in rheumatoid arthritis compared with other rheumatic diseases. Arthritis Rheum. 1999;42(6):1297–1298. [DOI] [PubMed] [Google Scholar]
- 135. Ah Kioon M-D, Asensio C, Ea H-K, Uzan B, Cohen-Solal M, Lioté F. Adrenomedullin increases fibroblast-like synoviocyte adhesion to extracellular matrix proteins by upregulating integrin activation. Arthritis Res Ther. 2010;12(5):R190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Angiolilli C, Grabiec AM, Ferguson BS, et al. Inflammatory cytokines epigenetically regulate rheumatoid arthritis fibroblast-like synoviocyte activation by suppressing HDAC5 expression. Ann Rheum Dis. 2016;75(2):430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Tanaka M, Ozaki S, Osakada F, Mori K, Okubo M, Nakao K. Cloning of follistatin-related protein as a novel autoantigen in systemic rheumatic diseases. Int Immunol. 1998;10(9):1305–1314. [DOI] [PubMed] [Google Scholar]
- 138. Clutter SD, Wilson DC, Marinov AD, Hirsch R. Follistatin-Like protein 1 promotes arthritis by up-regulating IFN-gamma. J Immunol. 2009;182(1):234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kim H-J, Kang WY, Seong SJ, Kim S-Y, Lim M-S, Yoon Y-R. Follistatin-like 1 promotes osteoclast formation via RANKL-mediated NF-κB activation and M-CSF-induced precursor proliferation. Cell Signal. 2016;28(9):1137–1144. [DOI] [PubMed] [Google Scholar]
- 140. Shi D-L, Shi G-R, Xie J, Du X-Z, Yang H. MicroRNA-27a Inhibits Cell Migration and Invasion of Fibroblast-Like Synoviocytes by Targeting Follistatin-Like Protein 1 in Rheumatoid Arthritis. Mol Cells. 2016;39(8):611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Lee DM, Kiener HP, Agarwal SK, et al. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315(5814):1006–1010. [DOI] [PubMed] [Google Scholar]
- 142. Kroot EJ, de Jong BA, van Leeuwen MA, et al. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2000;43(8):1831–1835. [DOI] [PubMed] [Google Scholar]
- 143. Inanc N, Dalkilic E, Kamali S, et al. Anti-CCP antibodies in rheumatoid arthritis and psoriatic arthritis. Clin Rheumatol. 2007;26(1):17–23. [DOI] [PubMed] [Google Scholar]
- 144. Mishiro T, Nakano S, Takahara S, et al. Relationship between cathepsin B and thrombin in rheumatoid arthritis. J Rheumatol. 2004;31(7):1265–1273. [PubMed] [Google Scholar]
- 145. Sukprasansap M, Chanvorachote P, Tencomnao T. Cyanidin-3-glucoside activates Nrf2-antioxidant response element and protects against glutamate-induced oxidative and endoplasmic reticulum stress in HT22 hippocampal neuronal cells. BMC Complement Med Ther. 2020;20(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 146. Guo L, Yang T. Oxymatrine inhibits the proliferation and invasion of breast cancer cells via the PI3K pathway. Cancer Manag Res. 2019;11:10499–10508. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 147. Lan X, Zhao J, Zhang Y, Chen Y, Liu Y, Xu F. Oxymatrine exerts organ- and tissue-protective effects by regulating inflammation, oxidative stress, apoptosis, and fibrosis: from bench to bedside. Pharmacol Res. 2020;151:104541. [DOI] [PubMed] [Google Scholar]
- 148. Dong P, Ji X, Han W, Han H. Oxymatrine attenuates amyloid beta 42 (Aβ1-42)-induced neurotoxicity in primary neuronal cells and memory impairment in rats. Can J Physiol Pharmacol. 2019;97(2):99–106. [DOI] [PubMed] [Google Scholar]
- 149. Bergström B, Carlsten H, Ekwall A-KH. Methotrexate inhibits effects of platelet-derived growth factor and interleukin-1β on rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Res Ther. 2018;20(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ma J-D, Jing J, Wang J-W, et al. A novel function of artesunate on inhibiting migration and invasion of fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Res Ther. 2019;21(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kim W-U, Yoo S-A, Min S-Y, et al. Hydroxychloroquine potentiates Fas-mediated apoptosis of rheumatoid synoviocytes. Clin Exp Immunol. 2006;144(3):503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Chen Q, Casali B, Pattacini L, Boiardi L, Salvarani C. Tumor necrosis factor-alpha protects synovial cells from nitric oxide induced apoptosis through phosphoinositide 3-kinase Akt signal transduction. J Rheumatol. 2006;33(6):1061–1068. [PubMed] [Google Scholar]
- 153. Fujisawa K, Aono H, Hasunuma T, Yamamoto K, Mita S, Nishioka K. Activation of transcription factor NF-kappa B in human synovial cells in response to tumor necrosis factor alpha. Arthritis Rheum. 1996;39(2):197–203. [DOI] [PubMed] [Google Scholar]
- 154. Nishimoto N, Ito A, Ono M, et al. IL-6 inhibits the proliferation of fibroblastic synovial cells from rheumatoid arthritis patients in the presence of soluble IL-6 receptor. Int Immunol. 2000;12(2):187–193. [DOI] [PubMed] [Google Scholar]
- 155. Hashizume M, Hayakawa N, Suzuki M, Mihara M. IL-6/sIL-6R trans-signalling, but not TNF-alpha induced angiogenesis in a HUVEC and synovial cell co-culture system. Rheumatol Int. 2009;29(12):1449–1454. [DOI] [PubMed] [Google Scholar]
- 156. Nascimbeni AC, Fanin M, Angelini C, Sandri M. Autophagy dysregulation in Danon disease. Cell Death Dis. 2017;8(1):e2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Chang L, Feng X, Gao W. Proliferation of rheumatoid arthritis fibroblast-like synoviocytes is enhanced by IL-17-mediated autophagy through STAT3 activation. Connect Tissue Res. 2019;60(4):358–366. [DOI] [PubMed] [Google Scholar]
- 158. Karonitsch T, Beckmann D, Dalwigk K, et al. Targeted inhibition of Janus kinases abates interfon gamma-induced invasive behaviour of fibroblast-like synoviocytes. Rheumatology. 2018;57(3):572–577. [DOI] [PubMed] [Google Scholar]
- 159. Kang YM, Kim SY, Kang JH, et al. Light up-regulated on B lymphocytes and monocytes in rheumatoid arthritis mediates cellular adhesion and metalloproteinase production by synoviocytes. Arthritis Rheum. 2007;56(4):1106–1117. [DOI] [PubMed] [Google Scholar]
- 160. Zou Q-F, Li L, Han Q-R, Wang Y-J, Wang X-B. Abatacept alleviates rheumatoid arthritis development by inhibiting migration of fibroblast-like synoviocytes via MAPK pathway. Eur Rev Med Pharmacol Sci. 2019;23(7):3105–3111. [DOI] [PubMed] [Google Scholar]
- 161. Winthrop KL, Weinblatt ME, Bathon J, et al. Unmet need in rheumatology: reports from the targeted therapies meeting 2019. Ann Rheum Dis. 2020;79(1):88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Matsumoto I, Kurata I, Ohyama A, et al. Revisit of autoimmunity to glucose-6-phosphate isomerase in experimental and rheumatoid arthritis. Mod Rheumatol. 2020;30(2):232–238. [DOI] [PubMed] [Google Scholar]
- 163. Xu J, Zhang X-Y, Li R, et al. Glucose-6-phosphate isomerase is associated with disease activity and declines in response to infliximab treatment in rheumatoid arthritis. Chin Med J. 2020;133(8):886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]