Abstract
Aims
Rheumatoid arthritis (RA), which mainly results from fibroblast-like synoviocyte (FLS) dysfunction, is related to oxidative stress. Advanced oxidation protein products (AOPPs), which are proinflammatory mediators and a novel biomarker of oxidative stress, have been observed to accumulate significantly in the serum of RA patients. Here, we present the first investigation of the effects of AOPPs on RA-FLSs and the signalling pathway involved in AOPP-induced inflammatory responses and invasive behaviour.
Methods
We used different concentrations of AOPPs (50 to 200 µg/ml) to treat RA-FLSs. Cell migration and invasion and the expression levels of tumour necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), matrix metalloproteinase-3 (MMP-3), and MMP-13 were investigated. Western blot and immunofluorescence were used to analyze nuclear factor-κB (NF-κB) activation.
Results
AOPPs promoted RA-FLS migration and invasion in vitro and significantly induced the messenger RNA (mRNA) and protein expression of TNF-α, IL-6, MMP-3, and MMP-13 in dose- and time-dependent manners. Moreover, AOPPs markedly activated the phosphorylation of nuclear factor-κB (NF-κB) p65 protein, which triggered inhibitory kappa B-alpha (IκBα) degradation, NF-κB p65 protein phosphorylation, and NF-κB p65 translocation into the nucleus. Furthermore, treatment with a neutralizing antibody specific to receptor for advanced glycation end products (RAGE) significantly suppressed aggressive behaviour and inflammation, decreased TNF-α, IL-6, MMP-3, and MMP-13 expression, and blocked AOPP-induced NF-κB pathway activation.
Conclusion
The results indicate that AOPPs can enhance aggressive behaviour and the inflammatory response in RA-FLSs via the RAGE–NF-κB pathway. These results present AOPPs as a new class of potentially important mediators of progressive disease in RA patients.
Cite this article: Bone Joint Res 2021;10(4):259–268.
Keywords: Rheumatoid arthritis, Advanced oxidation protein products, Fibroblast-like synoviocyte, Receptor for advanced glycation end products
Article focus
This study aims to investigate the relationship between advanced oxidation protein products (AOPPs), their potential receptors, and the nuclear factor-κB (NF-κB) signalling pathway in rheumatoid arthritis fibroblast-like synoviocytes (RA-FLSs).
Key messages
AOPPs act as a novel class of proinflammatory compounds in RA patients, and receptor for advanced glycation end products (RAGE) is a potential receptor.
FLSs are the main contributors to the formation of an invasive pannus in RA pathogenesis.
AOPPs promoted RA-FLS migration and invasion, as well as increasing tumour necrosis factor alpha (TNF-α), interleukin 6 (IL-6), matrix metalloproteinase-3 (MMP-3), and MMP-13 expression, and NF-κB pathway activation. However, blockade of RAGE significantly suppressed aggressive behaviour and inflammatory response.
Strengths and limitations
This study provides new insights into the molecular basis underlying AOPP-induced RA-FLS activation, potentially leading to novel potential targets for the treatment of RA.
Further studies in animal models may be required to verify our in vitro finding for AOPPs in the pathogenesis of RA.
Introduction
Rheumatoid arthritis (RA) is a multisystem, chronic, relapsing immunoinflammatory disease with predominant synovial proliferation and destruction of the articular cartilage. 1,2 It is the most common inflammatory arthritis, affecting approximately 1% to 2% of the general population worldwide. 3,4 The exact aetiology of RA remains unknown, but there is increasing evidence to indicate that reactive oxidative stress plays a significant role in the pathogenesis of RA. 5 Oxidative stress results from an imbalance between the peroxidation and antioxidant defense systems, followed by excessive reactive oxygen species (ROS) accumulation. Excess ROS can cause the degeneration of lipids, DNA, and proteins, inducing structural and functional changes in vivo. 6,7 Many reports have confirmed that oxidative stress amplifies inflammation and destroys cartilage in the joints of arthritic animals and RA patients. 8-11 Specifically, advanced oxidation protein products (AOPPs), which are formed by the reaction between chlorinated oxidants (HOCl/OCl-) and proteins, have been widely reported to contribute to the pathogenesis of chronic kidney diseases and other diseases. 12 In these reports, AOPPs were believed not only to be sensitive markers of oxidative stress but also to participate in the regulation of cell functions; AOPPs may contribute to the inflammatory response. 13-15 Baskol et al 6 found that serum myeloperoxidase (MPO) activity and AOPP, 3,4-Methylenedioxyamphetamine (MDA), and thiol levels were higher in an RA patient group than the control group, and only the AOPP level significantly differed between the active and inactive stages. Therefore, the authors believed that AOPPs act as a novel class of proinflammatory compounds in RA patients. In our previous study, we also demonstrated that the AOPP level increased in RA patients and was closely related to the disease activity of RA. 16 Whether the accumulation of AOPPs in patients with RA accelerates disease progression remains unknown.
Fibroblast-like synoviocytes (FLSs) play a pivotal role in RA pathogenesis. Synovial hyperplasia is a hallmark of RA and the main contributor to the formation of an invasive pannus. 17-20 The degree of synovial hyperplasia correlates with the severity of cartilage erosion, because RA-FLSs can not only invade the extracellular matrix (ECM) but also secrete multiple inflammatory cytokines into the synovial fluid, such as interleukin-6 (IL-6), interleukin 1 beta (IL-1β), tumour necrosis factor alpha (TNF-α), and matrix metalloproteinases (MMPs), which destroy the cartilage and bone and exacerbate joint damage. 21-23 However, little is known about the effects of AOPPs on RA-FLSs, and the mechanism by which AOPPs influence cellular function is poorly understood.
Previous studies have shown that the transmembrane receptors of AOPPs include receptor of advanced glycation end products (RAGE), CD36, and SR class A. 24-26 RAGE is a multiligand member of the immunoglobulin superfamily of cell-surface molecules. 27 RAGE and the accumulation of its ligands, namely, AGEs, amyloid fibrils, S100/calgranulins, and AOPPs, are implicated in several pathological conditions, such as diabetes, immune/inflammatory disorders, dialysis-related amyloidosis (DRA), and cancer. 24,25,28-33 Moreover, it has been demonstrated that human synovial tissue and synovial fibroblasts constitutively express the receptor for AGEs (RAGE), the role of which in the activation of FLSs contributes to increasing joint inflammation and upregulating corrosion. 28 Chen et al 34 also demonstrated that AGEs increased the invasiveness of RA-FLSs in a RAGE-dependent manner, as confirmed by the inhibitory effect of an anti-RAGE antibody. However, it is unclear whether AOPPs influence the function of RA-FLSs by binding to RAGE.
Nuclear factor-κB (NF-κB) molecules are a family of ubiquitously expressed transcription factors involved in immunity, stress responses, inflammatory diseases, cell proliferation, and cell death. 35-38 The mechanisms underlying NF-κB activation are complex. In the inactive state, NF-κB is largely located in the cytoplasm in complexes with IκB proteins. Following stimulation by an extracellular stimulus, IκB is rapidly phosphorylated and degraded, allowing the release of NF-κB p65 and its subsequent translocation into the nucleus. Accumulated evidence suggests that NF-κB activation participates in the pathogenesis of RA by mediating the synthesis of chemokines (IL-8), cytokines (IL-1β, IL-6, and TNF-α), prostaglandin E2 (PGE2), and MMPs, which cause further cartilage degradation and inflammation of the synovial membrane (synovitis). 38,39 Chen et al 34 also showed that AGEs induced an inflammatory response via RAGE–NF-κB pathway activation. AOPPs are structurally similar to AGEs and show similar biological activities. 40 Here, we hypothesized that AOPPs contribute to the propagation of tissue destruction in RA via the pathway activation process used by AGEs.
Therefore, the purpose of this study was to investigate the potential effects of AOPPs on RA-FLSs and to clarify whether AOPPs induce tissue destruction in RA via RAGE–NF-κB pathway activation.
Methods
AOPP preparation
AOPP-human serum albumin was prepared in vitro by incubation of human serum albumin (HSA) (Sigma-Aldrich, USA) with 200 mM HOCl (Fluke, Switzerland) for 30 minutes in the absence of free amino acids/carbohydrates/lipids to exclude the formation of AGE-like structures as previously described. 24,41 The preparation was dialyzed against phosphate-buffered saline (PBS) at 4°C overnight to remove any endotoxin contamination, and endotoxin levels were determined with an amebocyte lysate assay kit (Sigma Chemical) and found to be below 0.025 EU/ml.
RA-FLS culture and treatment
Synovial cells were isolated and cultured. FLSs were prepared from synovial tissue collected from RA patients undergoing arthroplasty surgery. The present study was approved by the Ethics Committee of Nafang Hospital, Southern Medical University in Guangdong, China (NFEC-20120201). Written informed consent was obtained from all patients. Synovial tissue samples from five RA patients were dissociated mechanically, washed in cold sterile PBS, and digested with 150 mg/ml dispase II (Roche, Switzerland) for four hours at 37°C with gentle agitation. 42
RA-FLSs were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 U/ml penicillin (Gibco), and 100 mg/ml streptomycin (Gibco) in a 37°C incubator containing a 5% CO2-enriched atmosphere. Cells from passages 3 to 8 were used in all experiments.
During most experiments, RA-FLSs (> 85% confluent) were cultured in DMEM supplemented with 1% serum for 12 hours before treatment with different doses of AOPP-HSA (50, 100, and 200 µg/ml), unmodified HSA (100 µg/ml), or a control (medium alone).
Cell migration and invasion assays
RA-FLS migration was determined using Transwell chambers with 8 μm pores (Corning, USA). 42 Briefly, 2 × 104 cells in 200 μl of 1% FBS DMEM were added in triplicate to the upper chambers of Transwell plates, and the lower chambers were filled with 800 µl of 10% FBS DMEM alone (control) or in combination with 100 µg/ml AOPP-HSA or unmodified HSA (100 µg/ml). After culturing at 37°C for 48 hours, the non-migrated cells were removed from the upper surface with cotton swabs, and the cells that invaded the matrix were fixed with 100% methanol for 30 minutes at room temperature, washed with PBS, and stained with 0.1% crystal violet (Merck Millipore, USA) solution for 30 minutes at room temperature. The number of cells that migrated through the membrane to the lower surface was counted in five representative microscopic fields (200× magnification).
Cell invasion was determined using Matrigel invasion chambers (BD Biosciences, USA) according to the manufacturer’s instructions. The upper chambers were freshly coated with Matrigel, and medium was added to the lower chamber as described above.
The blocking test was performed as follows: RA-FLSs were pretreated with neutralizing antibodies specific for RAGE (R&D Systems, 10 µg/ml) for one hour, and followed by treatment with AOPP-HSA (100 µg/ml) for 48 hours. Then, cell migration and invasion were detected.
Enzyme-linked immunosorbent assay (measurement of IL-6, TNFα, MMP-3, and MMP-13)
RA-FLSs were stimulated with the indicated concentration of AOPP-HSA (50, 100, or 200 µg/ml) for 48 hours or treated with 100 µg/ml AOPP-HSA for the indicated time (6, 12, 24, or 48 hours). Then, cell supernatants were collected and centrifuged at 12,000 g for 15 minutes. Supernatants were stored at -80°C until use. The levels of IL-6, TNFα (Boster, China), MMP-3, and MMP-13 (R&D Systems, USA) in the supernatant were quantified using enzyme-linked immunoabsorbent assay (ELISA) kits according to the manufacturer’s protocol. The optical density (OD) was measured at 450 nm with a spectrophotometric plate reader.
In a blocking test, RA-FLSs were pretreated with or without a neutralizing anti-RAGE antibody (10 µg/ml) for one hour, followed by treatment with AOPP-HSA (100 µg/ml) for 48 hours. The levels of IL-6, TNFα, MMP-3, and MMP-13 were detected. All experiments were performed in triplicate.
Real-time polymerase chain reaction (measurement of IL-6, TNFα, MMP-3, and MMP-13)
After stimulation as described for the ELISAs, total RNA was extracted from lysed cells using TRIzol reagent (Invitrogen, USA). One microgram of total RNA sample with a 260/280 ratio of 1.8 to 2.0 was used for reverse transcription (RT) using a PrimeScript RT reagent kit with gDNA Eraser (Takara Biotechnology, China) according to the manufacturer’s protocol. Sequences for the primers used are listed in Table I. Each specific primer was optimized for the concentration, and the resulting complementary DNA (cDNA) was then polymerase chain reaction (PCR)-amplified using a SYBR Premix Ex Taq II kit (Takara Biotechnology) according to the manufacturer’s instructions. Real-time PCR was carried out using the Applied Roche480 Real-Time PCR System (ABI, USA) with 40 cycles of denaturation at 95°C for 30 seconds, annealing at 95°C for five seconds, and extension at 60°C for 34 seconds. Relative quantification of the expression of each gene was calculated after normalization to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression using the comparative Ct method. The results are shown as the percentage change in expression with respect to the control (medium alone) for all samples.
Table I.
Primers of targeted genes.
| Gene | Sense | Sequence (5’→3’) | Size, bp |
|---|---|---|---|
| IL-6 | F | CTGGTCTTTTGGAGTTTGAG | 344 |
| R | TTTCTGACCAGAAGAAGGAA | ||
| TNF-α | F | GAGGCCAAGCCCTGGTATG | 91 |
| R | CGGGCCGATTGATCTCAGC | ||
| MMP-3 | F | ATGAAGAGTCTTCCAATCCTACTGT | 488 |
| R | CATTATATCAGCCTCTCCTTCATAC | ||
| MMP-13 | F | TCCTGATGTGGGTGAATACAATG | 184 |
| R | GCCATCGTGAAGTCTGGTAAAAT | ||
| GAPDH | F | GGAGCGAGATCCCTCCAAAAT | 191 |
| R | GGCTGTTGTCATACTTCTCATGG |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IL-6, interleukin-6; MMP, matrix metalloproteinase; TNF-α, tumour necrosis factor alpha.
The blocking test was also performed according to the above steps.
Western blot and immunofluorescence analyses of NF-κB p65
First, western blotting was performed to examine NF-κB activities. RA-FLSs were stimulated with the indicated concentration of AOPP-HSA (50, 100, or 200 µg/ml) for three hours or treated with 100 µg/ml AOPP-HSA for the indicated time (one, three, or six hours). Then, the cultured cells were lysed for protein extraction as described previously. The protein samples were subjected to SDS-PAGE before being transferred to polyvinylidene fluoride (PVDF) membranes (Merck Millipore). The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline-Tween (TBST) at room temperature for one hour and incubated overnight at 4°C with primary antibodies. The primary antibodies included rabbit anti-phospho-p65, rabbit anti-p65, and rabbit anti-IκBα (Cell Signalling Technology, USA, 1:1,000). Next, the membranes were washed three times with TBST and then incubated with a secondary antibody coupled to horseradish peroxidase. The proteins were visualized with an enhanced chemiluminescent system (Pierce Chemical, USA), and the band densitometry was analyzed with Quantity One software (Bio-Rad, USA). GAPDH was used as an internal control.
We also tested the activation of NF-κB via immunofluorescence. 24 RA-FLSs were stimulated with AOPP-HSA (100 µg/ml) for three hours. RA-FLSs were washed with PBS and fixed in 4% formaldehyde for 30 minutes at room temperature. After permeabilization with 1% Triton X-100 for ten minutes, the cells were blocked with PBS containing 5% bovine serum albumin for 30 minutes at room temperature, and immunofluorescence staining was performed using a specific rabbit monoclonal antibody against NF-κB p65 (Cell Signalling Technology, 1:500), followed by staining with fluoresceine isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulins (Bioworld Technology, USA, 1:1,000). The slides were counterstained with 4',6-diamidino-2-phenylindole (DAPI). Finally, coverslips were mounted on the slides, and the fluorescence was visualized using a fluorescence microscope.
In addition, after pretreatment with neutralizing antibodies for RAGE followed by AOPP-HSA (100 µg/ml) for three hours, the activation of NF-κB was tested by western blot and immunofluorescence analyses.
Statistical analysis
All data were analyzed using SPSS 13.0 software (SPSS, USA). The data are expressed as the mean and standard deviation (SD), and differences were analyzed using one-way analysis of variance (ANOVA) and an SNK post hoc test. A p-value < 0.05 was considered statistically significant.
Results
AOPPs promote the migration and invasion of RA-FLSs in vitro
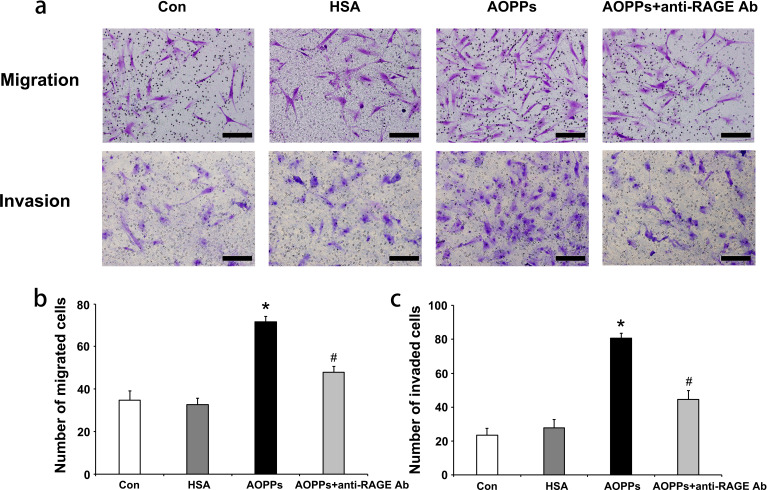
Initially, a Transwell assay was performed to determine the roles of AOPP-HSA in regulating the migration and invasion of RA-FLSs (Figure 1a). The number of migrated cells was higher when cells were cultured with 100 µg/ml AOPP-HSA for 48 hours than with the control treatment (medium alone) or unmodified HSA (Figure 1b) (migrated cell number: control group, 34.67 (SD 4.51); HSA group, 32.67 (SD 2.06); AOPP group, 71.67 (SD 2.52)). In the blocking test, AOPP-induced migration could be largely blocked by treatment with antibodies for RAGE (Figure 1b) (AOPPs + anti-RAGE Ab group, 48.01 (SD 2.65)).
Fig. 1.
a) Advanced oxidation protein products (AOPPs) promote the migration and invasion of rheumatoid arthritis fibroblast-like synoviocytes (RA-FLSs) in vitro. After RA-FLSs were incubated with medium alone (control), native human serum albumin (HSA), or AOPP-HSA (100 μg/ml) for 48 hours, cell migration and cell invasion were measured. In the blocking test, RA-FLSs were pretreated with a neutralizing anti-receptor for advanced glycation end products (RAGE) antibody (10 µg/ml) for one hour, followed by treatment with AOPP-HSA (100 µg/ml) for 48 hours. b) and c) The migrated and invaded cells were imaged (200× magnification) and counted in five random fields for each treatment (n = 3). Data are presented as the mean and standard deviation of triplicates. Scale bar, 50 µm. *p < 0.05 versus medium alone (control). #p < 0.05 versus the AOPP group. Con, control.
The ability to invade cartilage is a critical pathogenic behaviour of rheumatoid synoviocytes. Therefore, we also examined the role of AOPPs in RA-FLS invasion using Matrigel-coated Transwell membranes (Figure 1). The Transwell assay showed that compared with control or unmodified HSA treatment, AOPP treatment increased the invasion of RA-FLSs (Figure 1c) (control group, 22.33 (SD 4.16); HSA group, 27.67 (SD 5.03); AOPP group, 80.67 (SD 3.06)). However, AOPP-induced invasion could be substantially suppressed by a RAGE blocking antibody (Figure 1c) (AOPPs + anti RAGE Ab group, 44.67 (SD 5.03)).
AOPP-HSA upregulated the migration and invasion of RA-FLSs in vitro, suggesting that AOPP-HSA might be an important factor for mediating the invasive capacity of RA-FLSs. These effects could be substantially blocked by antibodies against RAGE, indicating that the invasive processes were dependent on RAGE.
AOPPs promote IL-6, TNFα, and MMP production and secretion by RA-FLSs
First, to test whether AOPPs could induce proinflammatory factor and MMP production in RA-FLSs, we analyzed the supernatant of RA-FLSs cultured with AOPPs by ELISA. The secretion of IL-6, TNF-α, MMP-3, and MMP-13 by RA-FLSs in the AOPP group was higher than that by RA-FLSs in the control group and occurred in a dose-dependent manner (Supplementary Figures ba to bd). Although the expression of cytokines was slightly lower in the 200 µg/ml AOPP group than the 100 µg/ml AOPP group, it was significantly higher than in the control group. Likewise, the secretion of IL-6, TNF-α, MMP-3, and MMP-13 increased 12 to 48 hours after 100 µg/ml AOPP-HSA treatment (Supplementary Figures be to bh). The protein secretion of IL-6, TNF-α, MMP-3, and MMP-13 could be obviously blocked by pretreatment with antibodies against RAGE (Supplementary Figures bi to bl).
Second, we further detected the messenger RNA (mRNA) levels of cytokines and MMPs after AOPP challenged. IL-6, TNF-α, MMP-3, and MMP-13 production was significantly enhanced in dose-dependent (Supplementary Figures ca and cb) and time-dependent manners (Supplementary Figures cc and cd). Consistent with the ELISA results, AOPP-HSA at 100 µg/ml exerted the maximal effect. The mRNA expression of IL-6, TNF-α, MMP-3, and MMP-13 could be also blocked by a RAGE blocking antibody (Supplementary Figures ce and cf).
No significant differences were found in protein secretion or mRNA expression between the unmodified HSA and control groups, suggesting that the overexpression of cytokines and MMPs was associated with advanced oxidation of HSA. The above data indicate that RA-FLSs can be stimulated by AOPP-HSA to secrete and produce cytokines and MMPs at both the protein and gene levels, respectively, which may be involved in the pathological progression of RA and possibly mediated by RAGE.
AOPP activates NF-κB pathways in RA-FLSs
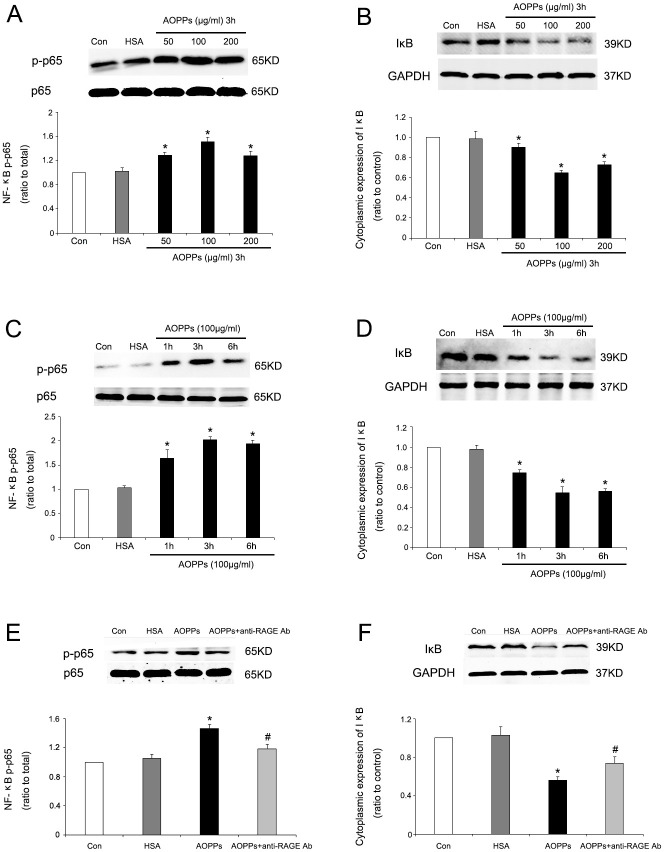
We further investigated the involvement of the NF-κB signalling pathway in AOPP-HSA-induced inflammatory responses in RA-FLSs. Two important steps before NF-κB activation are IκB degradation in the cytoplasm and NF-κB p65 translocation into the nucleus.
First, western blotting was performed to examine NF-κB activities. Treatment with AOPP-HSA at different doses (50, 100, or 200 µg/ml) for three hours increased the phosphorylation of NF-κB p65 (Figure 2a) and decreased the IκBα protein level (Figure 2b) in a dose-dependent manner. AOPP-HSA at 100 µg/ml exerted the maximal effect. Moreover, treatment with AOPP-HSA (100 µg/ml) for the indicated times (one, three, or six hours) markedly induced the phosphorylation of NF-κB p65 (Figure 2c) and degradation of the IκBα protein (Figure 2d) in a time-dependent manner. The maximum effect was achieved at three hours. To examine the potential mediators of NF-κB pathways, we also used a neutralizing antibody specific to RAGE to investigate its role. Pretreatment with antibodies against RAGE effectively suppressed AOPP-HSA (100 µg/ml)-induced phosphorylation of NF-κB p65 (Figure 2e) and decreased the IκBα protein level (Figure 2f).
Fig. 2.
Advanced oxidation protein product-human serum albumin (AOPP-HSA)-induced nuclear factor-κB (NF-κB) activation in rheumatoid arthritis fibroblast-like synoviocytes (RA-FLSs). a) and b) Cultured RA-FLSs were incubated with the indicated concentrations of AOPP-HSA, medium alone (control), or native HSA for three hours. c) and d) Cultured RA-FLSs were incubated for the indicated times with AOPP-HSA (100 μg/ml), medium alone (control), or native HSA (100 μg/ml). Decreasing expression of IκBα and increasing expression of phosphorylated NF-κB p65 occurred in dose-dependent (a) and b)) and time-dependent (c) and d)) manners. e) and f) Cultured RA-FLSs were incubated with medium alone (control), native HSA, AOPP-HSA (100 μg/ml), or AOPP-HSA (100 μg/ml) with a neutralizing antibody against receptor for advanced glycation end products (RAGE) (anti-RAGE Ab, 10 µg/ml) for three hours. Pretreatment with the antibody against RAGE effectively suppressed NF-κB activation. Data are presented as the mean and standard deviation of triplicates. *p < 0.05 versus medium alone (control). #p < 0.05 versus the AOPP group. Con, control; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IκB, inhibitory kappa B.
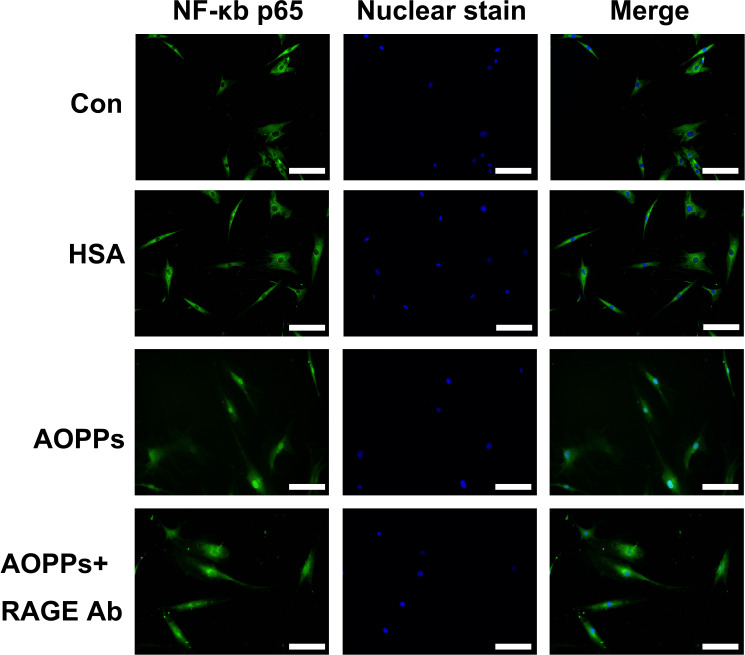
Next, we examined NF-κB p65 translocation into the nucleus. We performed immunofluorescence microscopy to assess the nuclear translocation of NF-κB. As shown in Figure 3, immunofluorescence staining showed the translocation of p65 into the nucleus of RA-FLSs after treatment with AOPP-HSA (100 µg/ml) for three hours; however, p65 remained in the cytoplasm in the cells in the control group and unmodified HSA group. NF-κB p65 translocation into the nucleus was also decreased after pretreatment with antibodies against RAGE.
Fig. 3.
NF-κB p65 translocation was detected by immunofluorescence and 4′,6-diamidino-2-phenylindole (DAPI) staining. Fluorescence micrographs with staining for nuclear factor-κB (NF-κB) p65 (green) and nuclei (blue) after rheumatoid arthritis fibroblast-like synoviocytes (RA-FLSs) were treated with advanced oxidation protein product-human serum albumin (AOPP-HSA) (100 μg/ml), medium alone (control), or native HSA for three hours. Magnification 400×. White scale bars represent 50 µm. Representative images of three independent experiments were shown. Con, control; RAGE, receptor for advanced glycation end products.
These data suggest that AOPP-HSA can induce the phosphorylation of p65, which leads to the nuclear translocation of p65 and ultimately activates NF-κB in RA-FLSs. These effects were associated with RAGE.
Discussion
The pathogenesis of RA is multifactorial, and recent research has indicated that highly reactive oxidative stress is involved in the pathogenesis of this disease. 5,8-11 AOPPs have recently been identified as members of oxidated protein compounds and been found accumulated in RA patients. 6,16 Our previous studies found that the concentration of plasma AOPP in RA patients was 305.44 µmol/l (SD 20.44), while the physiological concentration of plasma AOPP in healthy people was 102.55 µmol/l (SD 10.23). 16 Consistent with our findings, Baskol et al 6 found that there was a significant difference between concentrations of AOPPs in active and inactive stages of RA patients, thereby further indicating the importance of AOPPs in the pathogenesis of RA. However, the effects of AOPPs on FLSs are still largely unknown.
The present study first demonstrated that AOPPs bound to RAGE and triggered downstream signal transduction by NF-κB, resulting in the overexpression of TNF-α, IL-6, MMP-3, and MMP-13 at both protein and gene levels. This overexpression promoted the migration and invasion of RA-FLSs in vitro. AOPPs, but not native HSA, activated RAGE-dependent signals, suggesting that the triggering effect was due to AOPPs and not a property of HSA.
Proinflammatory cytokines, such as IL-6 and TNF-α, are central to the damaging cascade that can activate RA-FLSs to release cytokines/chemokines, ROS, and other mediators that likely contribute to sustaining inflammatory responses. In addition, these cytokines can further trigger the production of MMPs and result in heightened migration and invasion by RA-FLSs. MMPs, which are synthesized in the articular joints by synovial cells and chondrocytes, are considered to be crucial mediators of RA pathology. 20-22 MMP-3 and MMP-13 play pivotal roles in the destruction of the bone and degradation of various components of the cartilage, and baseline levels of MMP-3 serve as a biomarker of progressive RA joint damage. Therefore, we suspect that AOPPs participate in the progression of RA by inducing the release of inflammatory cytokines and promoting joint damage.
In the present study, the important finding is that AOPPs activated RA-FLSs by interacting with RAGE. RAGE is a transmembrane receptor in the immunoglobulin superfamily that is capable of binding to a broad repertoire of ligands, including AOPPs. It has been demonstrated that human synovial tissue and synovial fibroblasts constitutively express a RAGE. 27,28,43,44 However, the relationship between AOPPs and RAGE is unclear. AOPPs are similar to AGEs in structure and biological activities. 40 AGEs bound to RAGE have been implicated in inflammatory responses and ROS production in human umbilical vein endothelial cells (HUVECs), osteocyte-like cells, and other cells, and shown to result in several pathological conditions. 44-47 Hou et al 48 identified AGE-RAGE-synoviocyte interactions in the chronic inflammatory processes observed in dialysis-related amyloidosis (DRA). Consistent with the above studies, we found that AOPPs work on RA-FLSs mainly by combining with RAGE. A neutralizing antibody specific for RAGE effectively reversed AGE-induced inflammatory responses and MMP production in RA-FLSs, indicating RAGE's important role in the effects of AOPPs on synoviocytes. Our study provides evidence that AOPPs act as functional ligands for RAGE in RA-FLSs. In addition, these observations support the hypothesis that AOPPs bound to RAGE trigger downstream signalling that transduces signals leading to the propagation of inflammation.
In addition, targeting NF-κB in vivo can suppress experimental arthritis in several animal models of inflammatory arthritis. 49,50 Therefore, we observed the effect of AOPPs on NF-κB activation. In this study, increased phosphorylation of p65 was observed in RA-FLSs after treatment with AOPPs for one to six hours. Consistent with the increased phosphorylation of p65, degradation of IκBα also occurred. We further used immunofluorescence staining to show the translocation of p65 into the nucleus of RA-FLSs treated with AOPPs. However, when RAGE was blocked, reduced phosphorylation and nuclear translocation of p65 were observed. Therefore, we produced strong evidence to indicate that AOPPs may be involved in the activation of NF-κB in RA-FLSs via RAGE. However, Roman-Blas and Jimenez 51 reviewed many studies to examine the contribution of NF-κB-related pathways to the pathogenesis of osteoarthritis and RA, and great efforts have been devoted to pharmacological studies on the modulation of NF-κB pathways. 52-54 Moreover, other reports have explored whether p38 mitogen-activated protein kinase (p38 MAPK) and the c-Jun N-terminal kinase pathway also participate in inflammation in FLSs. 55-57 Therefore, further studies will be needed to identify NF-κB inhibitors and determine whether other transcription factors are involved in the AOPP-induced effects on RA-FLSs.
In summary, we identified that AOPPs induce invasion and proinflammatory changes in RA-FLSs by activating NF-κB via RAGE in vitro. These results present AOPPs as a new class and potentially important mediators of progressive disease in RA patients. Given their connection with RAGE, AOPPs may be a central binding factor, indicating the possibility for the development of new therapeutic strategies to block RAGE-AOPP interactions or the use of a soluble form of this receptor.
Author contributions
A. Lou: Designed the study, Executed the experiments, Wrote the manuscript.
L. Wang: Principal investigator for the project, Provided training for the lab members, Managed logistics and finances for the lab operations, Assisted with designing the study and executing the experiments, Wrote the manuscript.
W. Lai: Assisted with designing the study and executing the experiments, Wrote the manuscript.
D. Zhu: Assisted with designing the study and executing the experiments, Wrote the manuscript.
W. Wu: Assisted with designing the study and executing the experiments, Wrote the manuscript.
Z. Wang: Assisted with designing the study and executing the experiments.
Z. Cai: Assisted with designing the study and executing the experiments.
M. Yang: Principal investigator for the project, Provided funding, advice, and mentorship, Assisted with designing the study and executing the experiments, Wrote the manuscript.
A. Lou, L. Wang, W. Lai, D. Zhu and W. Wu contributed equally to this work.
Funding statement
This work was supported by National Natural Science Foundation of China (82072409, 81771747, 81702196), Natural Science Foundation of Guangdong Province (2019A1515012020), and Medical Scientific Research Foundation of Guangdong Province (A2017133). The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Ethical review statement
All approved experiments were performed in accordance with relevant guidelines and regulations of the Ethics Committee of Southern Medical University (Guangzhou, China, NFEC-20120201).
Supplementary material
Figures showing the secretion and mRNA expression levels of IL-6, TNFα, and MMPs.
References
- 1. Miossec P. Rheumatoid arthritis: still a chronic disease. Lancet. 2013;381(9870):884–886. [DOI] [PubMed] [Google Scholar]
- 2. Scott DL, Wolfe F, Huizinga TWJ. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–1108. [DOI] [PubMed] [Google Scholar]
- 3. Jin S, Li M, Fang Y, et al. Chinese Registry of rheumatoid arthritis (CREDIT): II. prevalence and risk factors of major comorbidities in Chinese patients with rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bellucci E, Terenzi R, La Paglia GMC, et al. One year in review 2016: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34(5):793–801. [PubMed] [Google Scholar]
- 5. García-González A, Gaxiola-Robles R, Zenteno-Savín T. Oxidative stress in patients with rheumatoid arthritis. Rev Invest Clin. 2015;67(1):46–53. [PubMed] [Google Scholar]
- 6. Baskol G, Demir H, Baskol M, et al. Investigation of protein oxidation and lipid peroxidation in patients with rheumatoid arthritis. Cell Biochem Funct. 2006;24(4):307–311. [DOI] [PubMed] [Google Scholar]
- 7. Ryan BJ, Nissim A, Winyard PG. Oxidative post-translational modifications and their involvement in the pathogenesis of autoimmune diseases. Redox Biol. 2014;2(2):715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Portal-Núñez S, Ardura JA, Lozano D, et al. Parathyroid hormone-related protein exhibits antioxidant features in osteoblastic cells through its N-terminal and osteostatin domains. Bone Joint Res. 2018;7(1):58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hsu C-C, Lin C-L, Jou I-M, Wang P-H, Lee J-S. The protective role of nitric oxide-dependent innate immunosuppression in the early stage of cartilage damage in rats: role of nitric oxide in cartilage damage. Bone Joint Res. 2017;6(4):253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Datta S, Kundu S, Ghosh P, De S, Ghosh A, Chatterjee M. Correlation of oxidant status with oxidative tissue damage in patients with rheumatoid arthritis. Clin Rheumatol. 2014;33(11):1557–1564. [DOI] [PubMed] [Google Scholar]
- 11. Zhou L, Feng J-T, Zhang L, Kuang Y. Clinical significance of serum total oxidant/antioxidant status for the disease activity in active rheumatoid arthritis. Int J Clin Exp Pathol. 2017;10(8):8895–8900. [PMC free article] [PubMed] [Google Scholar]
- 12. Witko-Sarsat V, Friedlander M, Nguyen Khoa T, et al. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. 1998;161(5):2524–2532. [PubMed] [Google Scholar]
- 13. Zhou LL, Hou FF, Wang GB, et al. Accumulation of advanced oxidation protein products induces podocyte apoptosis and deletion through NADPH-dependent mechanisms. Kidney Int. 2009;76(11):1148–1160. [DOI] [PubMed] [Google Scholar]
- 14. Zhou QG, Zhou M, Lou AJ, Xie D, Hou FF. Advanced oxidation protein products induce inflammatory response and insulin resistance in cultured adipocytes via induction of endoplasmic reticulum stress. Cell Physiol Biochem. 2010;26(4-5):775–786. [DOI] [PubMed] [Google Scholar]
- 15. Yu C, Huang D, Wang K, et al. Advanced oxidation protein products induce apoptosis, and upregulate sclerostin and RANKL expression, in osteocytic MLO-Y4 cells via JNK/p38 MAPK activation. Mol Med Rep. 2017;15(2):543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lou AJ, WR W, Cai XY, et al. Levels of oxidative stress and bone turnover markers in patients with rheumatoid arthritis complicated with low bone mineral density. Chinese Journal of Osteoporosis. 2016;22(3):102–106. [Google Scholar]
- 17. Huber LC, Distler O, Tarner I, Gay RE, Gay S, Pap T. Synovial fibroblasts: key players in rheumatoid arthritis. Rheumatology. 2006;45(6):669–675. [DOI] [PubMed] [Google Scholar]
- 18. Bartok B, Firestein GS. Fibroblast-Like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233(1):233–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scherer HU, Häupl T, Burmester GR. The etiology of rheumatoid arthritis. J Autoimmun. 2020;110:102400. [DOI] [PubMed] [Google Scholar]
- 20. Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9(1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tolboom TCA, Pieterman E, van der Laan WH, et al. Invasive properties of fibroblast-like synoviocytes: correlation with growth characteristics and expression of MMP-1, MMP-3, and MMP-10. Ann Rheum Dis. 2002;61(11):975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Noss EH, Brenner MB. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol Rev. 2008;223(1):252–270. [DOI] [PubMed] [Google Scholar]
- 23. Brondello J-M, Djouad F, Jorgensen C. Where to stand with stromal cells and chronic synovitis in rheumatoid arthritis? Cells. 2019;8(10):E1257:1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guo ZJ, Niu HX, Hou FF, et al. Advanced oxidation protein products activate vascular endothelial cells via a RAGE-mediated signaling pathway. Antioxid Redox Signal. 2008;10(10):1699–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou LL, Cao W, Xie C, et al. The receptor of advanced glycation end products plays a central role in advanced oxidation protein products-induced podocyte apoptosis. Kidney Int. 2012;82(7):759–770. [DOI] [PubMed] [Google Scholar]
- 26. Shi J, Sun S, Liao Y, et al. Advanced oxidation protein products induce G1 phase arrest in intestinal epithelial cells via a RAGE/CD36-JNK-p27kip1 mediated pathway. Redox Biol. 2019;25:101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Drinda S, Franke S, Rüster M, et al. Identification of the receptor for advanced glycation end products in synovial tissue of patients with rheumatoid arthritis. Rheumatol Int. 2005;25(6):411–413. [DOI] [PubMed] [Google Scholar]
- 28. Steenvoorden MMC, Toes REM, Ronday HK, Huizinga TWJ, Degroot J. Rage activation induces invasiveness of RA fibroblast-like synoviocytes in vitro. Clin Exp Rheumatol. 2007;25(5):740–742. [PubMed] [Google Scholar]
- 29. Ferrante E, Vazzana N, Santilli F, et al. Determinants of thromboxane biosynthesis in rheumatoid arthritis: role of RAGE and oxidant stress. Free Radical Biology and Medicine. 2010;49(5):857–864. [DOI] [PubMed] [Google Scholar]
- 30. Kislinger T, Tanji N, Wendt T, et al. Receptor for advanced glycation end products mediates inflammation and enhanced expression of tissue factor in vasculature of diabetic apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2001;21(6):905–910. [DOI] [PubMed] [Google Scholar]
- 31. Harja E, Bu D-xiu, Hudson BI, et al. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE-/- mice. J Clin Invest. 2008;118(1):183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li H, Chen J, Li B, Fang X. The protective effects of dulaglutide against advanced glycation end products (AGEs)-induced degradation of type Ⅱ collagen and aggrecan in human SW1353 chondrocytes. Chem Biol Interact. 2020;322:108968. [DOI] [PubMed] [Google Scholar]
- 33. Yu H, Ye W-bin, Zhong Z-ming, Ding R-ting, Chen J-ting. Effect of advanced oxidation protein products on articular cartilage and synovium in a rabbit osteoarthritis model. Orthop Surg. 2015;7(2):161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen Y-J, Chan D-C, Chiang C-K, et al. Advanced glycation end-products induced VEGF production and inflammatory responses in human synoviocytes via RAGE-NF-κB pathway activation. J Orthop Res. 2016;34(5):791–800. [DOI] [PubMed] [Google Scholar]
- 35. Bierhaus A, Schiekofer S, Schwaninger M, et al. Diabetes-Associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50(12):2792–2808. [DOI] [PubMed] [Google Scholar]
- 36. Hagar H, Husain S, Fadda LM, Attia NM, Attia MMA, Ali HM. Inhibition of NF-κB and the oxidative stress -dependent caspase-3 apoptotic pathway by betaine supplementation attenuates hepatic injury mediated by cisplatin in rats. Pharmacol Rep. 2019;71(6):1025–1033. [DOI] [PubMed] [Google Scholar]
- 37. Okamoto H, Yoshio T, Kaneko H, Yamanaka H. Inhibition of NF-kappaB signaling by fasudil as a potential therapeutic strategy for rheumatoid arthritis. Arthritis Rheum. 2010;62(1):82–92. [DOI] [PubMed] [Google Scholar]
- 38. Ding Q-H, Ye C-Y, Chen E-M, Zhang W, Wang X-H. Emodin ameliorates cartilage degradation in osteoarthritis by inhibiting NF-κB and Wnt/β-catenin signaling in-vitro and in-vivo. Int Immunopharmacol. 2018;61:222–230. [DOI] [PubMed] [Google Scholar]
- 39. Okazaki Y, Sawada T, Nagatani K, et al. Effect of nuclear factor-kappaB inhibition on rheumatoid fibroblast-like synoviocytes and collagen induced arthritis. J Rheumatol. 2005;32(8):1440–1447. [PubMed] [Google Scholar]
- 40. Kalousová M, Skrha J, Zima T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol Res. 2002;51(6):597–604. [PubMed] [Google Scholar]
- 41. Witko-Sarsat V, Gausson V, Nguyen A-T, et al. AOPP-induced activation of human neutrophil and monocyte oxidative metabolism: a potential target for N-acetylcysteine treatment in dialysis patients. Kidney Int. 2003;64(1):82–91. [DOI] [PubMed] [Google Scholar]
- 42. Zhu D, Zhao J, Lou A, et al. Transforming growth factor β1 promotes fibroblast-like synoviocytes migration and invasion via TGF-β1/Smad signaling in rheumatoid arthritis. Mol Cell Biochem. 2019;459(1-2):141–150. [DOI] [PubMed] [Google Scholar]
- 43. Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108(7):949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Prasad K, Tiwari S. Therapeutic interventions for advanced Glycation-End products and its receptor- mediated cardiovascular disease. Curr Pharm Des. 2017;23(6):937–943. [DOI] [PubMed] [Google Scholar]
- 45. Verzijl N, DeGroot J, Ben ZC, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46(1):114–123. [DOI] [PubMed] [Google Scholar]
- 46. Ozawa J, Kaneguchi A, Minamimoto K, Tanaka R, Kito N, Moriyama H. Accumulation of advanced-glycation end products (AGEs) accelerates arthrogenic joint contracture in immobilized rat knee. J Orthop Res. 2018;36(3):854–863. [DOI] [PubMed] [Google Scholar]
- 47. Chen Y-H, Chen Z-W, Li H-M, Yan X-F, Feng B. AGE/RAGE-Induced Emp release via the NOX-Derived ROS pathway. J Diabetes Res. 2018;2018:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hou FF, Jiang JP, Guo JQ, et al. Receptor for advanced glycation end products on human synovial fibroblasts: role in the pathogenesis of dialysis-related amyloidosis. J Am Soc Nephrol. 2002;13(5):1296–1306. [DOI] [PubMed] [Google Scholar]
- 49. Okazaki Y, Sawada T, Nagatani K, et al. Effect of nuclear factor-kappaB inhibition on rheumatoid fibroblast-like synoviocytes and collagen induced arthritis. J Rheumatol. 2005;32(8):1440–1447. [PubMed] [Google Scholar]
- 50. Jin Y, Chen X, Gao ZY, Liu K, Hou Y, Zheng J. The role of miR-320a and IL-1β in human chondrocyte degradation. Bone Joint Res. 2017;6(4):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roman-Blas JA, Jimenez SA. Nf-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14(9):839–848. [DOI] [PubMed] [Google Scholar]
- 52. Okamoto H, Yoshio T, Kaneko H, Yamanaka H. Inhibition of NF-kappaB signaling by fasudil as a potential therapeutic strategy for rheumatoid arthritis. Arthritis Rheum. 2010;62(1):82–92. [DOI] [PubMed] [Google Scholar]
- 53. Okazaki Y, Sawada T, Nagatani K, et al. Effect of nuclear factor-kappaB inhibition on rheumatoid fibroblast-like synoviocytes and collagen induced arthritis. J Rheumatol. 2005;32(8):1440–1447. [PubMed] [Google Scholar]
- 54. Jimi E, Aoki K, Saito H, et al. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med. 2004;10(6):617–624. [DOI] [PubMed] [Google Scholar]
- 55. Schett G, Zwerina J, Firestein G. The p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid arthritis. Ann Rheum Dis. 2008;67(7):909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hammaker DR, Boyle DL, Chabaud-Riou M, Firestein GS. Regulation of c-Jun N-terminal kinase by MEKK-2 and mitogen-activated protein kinase kinase kinases in rheumatoid arthritis. J Immunol. 2004;172(3):1612–1618. [DOI] [PubMed] [Google Scholar]
- 57. Mavropoulos A, Orfanidou T, Liaskos C, et al. p38 mitogen-activated protein kinase (p38 MAPK)-mediated autoimmunity: lessons to learn from ANCA vasculitis and pemphigus vulgaris. Autoimmun Rev. 2013;12(5):580–590. [DOI] [PubMed] [Google Scholar]