Abstract
Objective
To determine the prognostic role of big endothelin-1 (ET-1) in left ventricular non-compaction cardiomyopathy (LVNC).
Methods
We prospectively enrolled patients whose LVNC was diagnosed by cardiac MRI and who had big ET-1 data available. Primary end point was a composite of all-cause mortality, heart transplantation, sustained ventricular tachycardia/fibrillation and implanted cardioverter defibrillator discharge. Secondary end point was cardiac death or heart transplantation.
Results
Altogether, 203 patients (median age 44 years; 70.9% male) were divided into high-level (≥0.42 pmol/L) and low-level (<0.42 pmol/L) big ET-1 groups according to the median value of plasma big ET-1 levels. Ln big ET-1 was positively associated with Ln N-terminal pro-brain natriuretic peptide, left ventricular diameter, but negatively related to age and Ln left ventricular ejection fraction. Median follow-up was 1.9 years (IQR 0.9–3.1 years). Kaplan-Meier analysis showed that, compared with patients with low levels of big ET-1, those with high levels were at greater risk for meeting both primary (p<0.001) and secondary (p<0.001) end points. The C-statistic estimation of Ln big ET-1 for predicting the primary outcome was 0.755 (95% CI 0.685 to 0.824, p<0.001). After adjusting for confounding factors, Ln big ET-1 was identified as an independent predictor of the composite primary outcome (HR 1.83, 95% CI 1.27 to 2.62, p=0.001) and secondary outcome (HR 1.93, 95% CI 1.32 to 2.83, p=0.001).
Conclusions
Plasma big ET-1 may be a valuable index to predict the clinical adverse outcomes in patients with LVNC.
Keywords: myocardial disease
Introduction
Left ventricular non-compaction cardiomyopathy (LVNC) is a heterogeneous myocardial disorder characterised by prominent trabeculae, intratrabecular recesses and a left ventricular myocardium with distinct compacted and non-compacted layers.1 LVNC was classified as a distinct cardiomyopathy by the American Heart Association,2 whereas the European Society of Cardiology included it as an unclassified cardiomyopathy.3 LVNC, which can occur at any age, has various clinical presentations, ranging from an asymptomatic condition to congestive heart failure, arrhythmia to systemic embolism or even sudden cardiac death (SCD).4–6 Although the awareness of LVNC has increased in recent years, quantitative data about risk stratification and prognostication remain scarce. Hence, it is vital to identify novel and useful predictors for adverse events to improve the prognosis of LVNC.
Big endothelin-1 (ET-1), the precursor protein of ET-1, can provide the same quantities of biologic importance as ET-1 and has a higher concentration and longer half-life time in the peripheral circulation.7 8 Previous studies, however, have identified big ET-1 as a risk factor for a poor prognosis in patients with atrial fibrillation (AF)9 or coronary artery disease (CAD).10 11 In addition, several significant risk factors associated with disease progression or mortality in patients with LVNC have been reported, including cardiac dysfunction, arrhythmias, neuromuscular disorders, positive genotype, N-terminal pro-brain natriuretic peptide (NT-pro-BNP) and late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR).12–14 We found no investigations in the literature, though, that addressed the prognostic power of big ET-1 in patients with LVNC.
The present study aimed to determine the prognostic ability of big ET-1 in patients with LVNC and to verify the possibility that an elevated plasma level of big ET-1 is an independent predictor of adverse outcomes in patients with LVNC. We hypothesised that, as a novel biomarker, it could allow for improved prognostic risk stratification of LVNC.
Materials and methods
Study population
Patients with left ventricular abnormal trabeculations were prospectively recruited at Fuwai Hospital in Beijing between January 2014 and June 2019. LVNC was diagnosed based on the CMR criteria proposed by Petersen et al in 2005.15 The main criterion was an end-diastolic ratio between non-compacted and compacted myocardium of >2.3. Among them, patients with plasma big ET-1 data were included in this analysis (online supplemental figure 1). Baseline information of all subjects was collected, including demographic features, vital signs on admission, alcohol intake, smoking habits, medical history, family history and CMR features. Informed consent was obtained from all patients.
heartjnl-2020-317059supp001.pdf (12.6KB, pdf)
Laboratory measurements of big ET-1
Peripheral venous blood was collected into ethylenediaminetetraacetic acid-treated tubes for measuring plasma big ET-1 levels. The big ET-1 concentrations were quantified using the Big Endothelin-1 ELISA Kit (NO. BI-20082H; Biomedica, Wien, Austria), following the standard protocol.
Cardiac evaluation
CMR imaging was performed using a 1.5-T scanner (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany). The endocardial and epicardial contours of left ventricular myocardium were manually traced at end-diastole and end-systole on short-axis b-SSFP cine images. Cardiac volumetric parameters, including left ventricular end-diastolic volume (LVEDV) and left ventricular end-systolic volume (LVESV), were automatically generated. Left ventricular ejection fraction (LVEF) was calculated by following formula: LVEF (%) = (LVEDV – LVESV)/LVEDV*100%. Images of LGE were obtained 10–15 min after intravenous administration of gadolinium-diethylenetriamine pentaacetic acid (Magnevist, Schering AG, Berlin, Germany) at a dose of 0.2 mmol/kg.
Follow-up and outcomes
Follow-up data were obtained from the patient’s clinic history or by telephone interviews. The study was closed in July 2019. The primary end point was a composite of all-cause mortality, heart transplantation, the sustained ventricular tachycardia (VT) and ventricular fibrillation (VF), implanted cardioverter defibrillator discharge or systemic thromboembolism. Cardiac death or heart transplantation was regarded as a composite secondary end point. Cardiac death included SCD and heart-failure-related death. SCD was defined as rapid, and unexpected death due to a cardiac event occurring within 1 hour after symptom onset. Heart-failure-related death was defined as death occurring in the context of long-term cardiac decompensation with disease progression over the preceding year.
Statistical analysis
The study population was divided into high and low big ET-1 level groups according to the median value of plasma big ET-1. Continuous variables, including normally distributed data and skewed data, are expressed as means±SD and medians (25th–75th percentiles). They were compared using the unpaired Student t-test and the Mann-Whitney U test, respectively. Categorical variables were expressed as frequencies and percentages and were compared using Fisher’s test. The levels of big ET-1, NT-pro-BNP, LVEDV, left ventricular end-diastolic diameter (LVEDD), LVEF were converted into natural logarithmic transformations for regression analyses, since these parameters did not conform to normal distribution. Association between plasma big ET-1 levels and other clinical variables were tested using linear regression analysis. Survival curves were depicted according to the Kaplan-Meier method, and comparisons were performed using log-rank tests. To test the associations between variables and outcomes, Cox proportional hazards regression models were applied. Several variables were included in the univariate Cox regression models: age, sex, mean arterial pressure, previous systemic embolisation, family history of SCD, New York Heart Association (NYHA) functional class III/IV, CAD, AF, hypertension, Ln big ET-1 (or a dichotomous variable of big ET-1), Ln NT-pro-BNP, Ln LVEF, Ln LVEDV, LVEDD and LGE. The multivariate Cox regression models included age, sex and variables with p≤0.1 based on the univariate analysis to evaluate the independent effect of big ET-1 on outcomes. Both univariable and multivariable Cox proportional hazard regression models were used to calculate the HR and 95% CI. Statistical analyses were performed using SPSS software (V.22.0; IBM, Armonk, New York, USA) and the R software (V.3.6.1; R Foundation for Statistical Computing). All statistical tests were two-tailed, with p<0.05 considered to indicate statistical significance.
Patient and public involvement statement
The patients and public were not involved in designing of, recruitment to, or conduct of the present study.
Results
Baseline characteristics
Altogether, 203 patients with big ET-1 data were followed up and included in this analysis (online supplemental figure 1), and 153 (75.4%) patients were regarded as dilated subtype of LVNC. The median age of the patients was 44 years (IQR 29–58), and 144 (70.9%) of the patients were male (table 1). Of the participants, 15.3% suffered systemic thromboembolism previously, 6.4% had SCD family history, 11.3% had CAD, 13.3% had AF, 56.7% were in NYHA functional class III/IV, 70.4% were found LGE on CMR and 5.0% have received an implantable cardioverter-defibrillator or cardiac resynchronisation therapy with defibrillator. The distribution of big ET-1 was showed in a histogram (online supplemental figure 2), indicating that big ET-1 was a continuous non-normal distribution variable with the median concentration of 0.42 pmol/L (IQR 0.23–0.81 pmol/L). Based on this value, the patients were divided into two groups: those with high big ET-1 levels (≥0.42 pmol/L, n=103, 50.7%) and those with low big ET-1 levels (<0.42 pmol/L, n=100, 49.3%). For these two groups, there were several variables with significant differences, including the male (p=0.032), previous systemic thromboembolism (p=0.014), NYHA functional class III/IV (p<0.001), big ET-1 level (p<0.001), NT-pro-BNP (p<0.001), LVEF (p<0.001), LVEDV (p<0.001), LVEDD (p<0.001) and LGE (p=0.001) (table 1). There was no statistically significant difference in CAD (p=0.884) or AF (p=0.172) between the two groups. Linear regression analysis indicated that Ln big ET-1 was positively associated with Ln NT-pro-BNP (p<0.001), Ln LVEDV (p<0.001) and LVEDD (p<0.001), but negatively related to age (p=0.046) and Ln LVEF (p<0.001) (online supplemental table 1).
Table 1.
Baseline characteristics of participants
| Parameters | All patients (n=203) |
Big ET-1 <0.42 pmol/L group (n=100) |
Big ET-1 ≥0.42 pmol/L group (n=103) |
P value |
| Age (year) | 44 (29–58) | 47 (33–60) | 42 (25–56) | 0.150 |
| Male, n (%) | 144 (70.9) | 64 (64.0) | 80 (77.7) | 0.032 |
| Unexplained syncope, n (%) | 25 (12.3) | 12 (12.0) | 13 (12.6) | 0.893 |
| Mean arterial pressure (mm Hg) | 85 (77–94) | 88 (78–98) | 83 (77–90) | 0.075 |
| Previous systemic thromboembolism, n (%) | 31 (15.3) | 9 (9.0) | 22 (21.4) | 0.014 |
| Current smoking, n (%) | 92 (45.3) | 45 (45.0) | 47 (45.6) | 0.928 |
| Current drinking, n (%) | 84 (41.4) | 41 (41.0) | 43 (41.7) | 0.914 |
| Cardiomyopathy family history, n (%) | 37 (18.2) | 17 (17.0) | 20 (19.4) | 0.656 |
| SCD family history, n (%) | 13 (6.4) | 3 (3.0) | 10 (9.7) | 0.051 |
| NYHA functional class III/IV, n (%) | 115 (56.7) | 37 (37.0) | 78 (75.7) | <0.001 |
| Coronary artery disease, n (%) | 23 (11.3) | 11 (11.0) | 12 (11.7) | 0.884 |
| Congenital heart disease, n (%) | 8 (3.9) | 5 (5.0) | 3 (2.9) | 0.494 |
| Hypertension, n (%) | 54 (26.6) | 31 (31.0) | 23 (22.3) | 0.162 |
| Diabetes mellitus, n (%) | 22 (10.8) | 8 (8.0) | 14 (13.6) | 0.200 |
| Atrial fibrillation, n (%) | 27 (13.3) | 10 (10.0) | 17 (16.5) | 0.172 |
| Sustained VT/VF, n (%) | 6 (3.0) | 2 (2.0) | 4 (3.9) | 0.683 |
| NT-pro-BNP (fmol/mL) | 1424.0 (376.0–2521.3) | 540.6 (99.2–1943.5) | 2290.0 (1160.0–3945.0) | <0.001 |
| CMR | ||||
| LVEF (%) | 25 (17–41) | 33 (21–46) | 20 (15–32) | <0.001 |
| LVEDV (ml) | 240.4 (174.2–317.0) | 195.6 (156.7–264.3) | 289.5 (216.3–387.4) | <0.001 |
| LVEDD (mm) | 67.8±11.7 | 63.7±10.2 | 71.8±11.6 | <0.001 |
| LGE, n (%) | 143 (70.4) | 60 (60.0) | 83 (80.6) | 0.001 |
| ICD implantation, n (%) | 5 (2.5) | 2 (2.0) | 3 (2.9) | 1.000 |
| CRT-D implantation, n (%) | 5 (2.5) | 2 (2.0) | 3 (2.9) | 1.000 |
Bold and italic values are statistically significant (p<0.05).
Big ET-1, big endothelin-1; CMR, cardiac magnetic resonance; CRT-D, cardiac resynchronization therapy-defibrillation; ICD, implantable cardioverter-defibrillator; LGE, late gadolinium enhancement; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; NT-pro-BNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; SCD, sudden cardiac death; VT/VF, ventricular tachycardia/ventricular fibrillation.
heartjnl-2020-317059supp002.pdf (217.6KB, pdf)
heartjnl-2020-317059supp003.pdf (26.3KB, pdf)
Clinical outcomes
The median follow-up time was 1.9 years (IQR 0.9–3.1 years). In all, 7 (3.3%) patients were lost to follow-up. The occurrence of adverse events during the follow-up is shown in table 2. Compared with the low-level big ET-1 group, all-cause mortality (24.3% vs 2.0%, Log-rank p<0.001), heart-failure-related death (20.4% vs 2.0%, Log-rank p<0.001), heart transplantation (15.5% vs 3.0%, Log-rank p=0.001), and SCD (3.9% vs 0%, Log-rank p=0.030) occurred more often in the big ET-1 high-level group. In addition, during the follow-up period, one patient implanted a left ventricular assist device before heart transplantation, four patients were performed an implantable cardioverter-defibrillator and three patients had cardiac resynchronisation therapy with defibrillator.
Table 2.
End-point events during the follow-up according to the big ET-1 level
| Outcomes | All patients (n=203) |
Big ET-1 <0.42 pmol/L group (n=100) |
Big ET-1 ≥0.42 pmol/L group (n=103) |
Log-rank P value |
| Primary outcome (composite) | 57 (28.1) | 10 (10.0) | 47 (45.6) | <0.001 |
| All-cause mortality | 27 (13.3) | 2 (2.0) | 25 (24.3) | <0.001 |
| HF-related death | 23 (11.3) | 2 (2.0) | 21 (20.4) | <0.001 |
| SCD | 4 (2.0) | 0 (0) | 4 (3.9) | 0.030 |
| Heart transplantation | 19 (9.4) | 3 (3.0) | 16 (15.5) | 0.001 |
| Sustained VT/VF | 2 (1.0) | 2 (2.0) | 0 (0) | 0.204 |
| ICD discharge | 2 (1.0) | 1 (1.0) | 1 (1.0) | 0.871 |
| Systemic thromboembolism | 7 (3.4) | 2 (2.0) | 5 (4.9) | 0.112 |
| Secondary outcome | ||||
| Cardiac death or heart transplantation | 46 (22.7) | 5 (5.0) | 41 (39.8) | <0.001 |
Bold and italic values are statistically significant (p<0.05).
Big ET-1, big endothelin 1; HF, heart failure; ICD, implantable cardioverter defibrillator; SCD, sudden cardiac death; VT/VF, ventricular tachycardia/ventricular fibrillation.
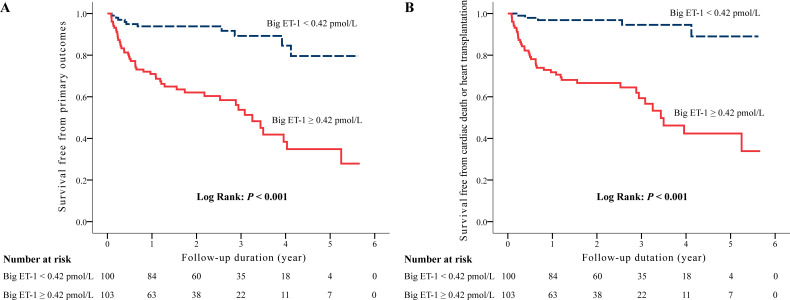
Kaplan-Meier analysis showed that patients with a high level of big ET-1 were at higher risk for meeting both the primary end point (Log-rank p<0.001) (figure 1A) and the secondary end point (Log-rank p<0.001) (figure 1B). Further analysis revealed that a high level of big ET-1 was associated with an increased risk for heart-failure-related death (Log-rank p<0.001) (online supplemental figure 3A) and heart transplantation (Log-rank p=0.001) (online supplemental figure 3C), but not for systemic embolism (Log-rank p=0.112) (online supplemental figure 3D), compared with those who had a low level of big ET-1. It was probably an elevated risk for SCD in the high-level big ET-1 group (Log-rank p=0.030) (online supplemental figure 3B).
Figure 1.
Kaplan-Meier curves of survival free from the primary outcome (A), and the secondary outcome (B) divided by the median value of plasma big endothlin-1 levels. The p values were calculated by the Log-rank test.
heartjnl-2020-317059supp004.pdf (412.9KB, pdf)
The C-statistic estimation of Ln big ET-1 for predicting the primary outcome was 0.755 (95% CI 0.685 to 0.824, p<0.001). After adjusting for age, male sex, previous systemic thromboembolism, SCD family history, NYHA functional class III/IV, Ln NT-pro-BNP, Ln LVEF, Ln LVEDV, LVEDD and LGE, the Cox proportional hazard regression analysis revealed that Ln big ET-1 was an adverse predictor of both the primary outcome (HR 1.83, 95% CI 1.27 to 2.62, p=0.001) and secondary outcome (HR 1.93, 95% CI 1.32 to 2.83, p=0.001) (table 3). The C-statistic estimations for the multivariate Cox proportional hazards model predicting the primary outcome were as follows: 0.816 (95% CI 0.759 to 0.873) for the model without Ln big ET-1 vs 0.828 (95% CI 0.770 to 0.886) for the model with Ln big ET-1. When plasma big ET-1 levels regarded as a binary variable, the high level of big ET-1 (≥0.42 pmol/L) was also an independent risk factor for the composite primary end point (HR 2.30, 95% CI 1.06 to 4.98, p=0.035) and secondary end point (HR 3.71, 95% CI 1.27 to 10.83, p=0.017) (online supplemental table 2).
Table 3.
Cox regression analysis of risk factors for primary and secondary outcomes
| Variables | Primary outcomes | Secondary outcomes | ||||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| Male | 1.19 | 0.66 to 2.14 | 0.573 | 0.54 | 0.26 to 1.14 | 0.105 | 1.33 | 0.68 to 2.63 | 0.409 | 0.54 | 0.23 to 1.28 | 0.163 |
| Age | 1.01 | 0.99 to 1.02 | 0.556 | 0.99 | 0.97 to 1.01 | 0.206 | 1.00 | 0.98 to 1.02 | 0.958 | 0.98 | 0.96 to 1.00 | 0.046 |
| Previous systemic thromboembolism | 2.48 | 1.38 to 4.49 | 0.003 | 1.15 | 0.55 to 2.41 | 0.721 | 3.01 | 1.60 to 5.64 | 0.001 | 1.56 | 0.70 to 3.49 | 0.283 |
| SCD family history | 3.13 | 1.48 to 6.63 | 0.003 | 1.77 | 0.72 to 4.37 | 0.217 | 3.39 | 1.51 to 7.61 | 0.003 | 1.84 | 0.67 to 5.02 | 0.235 |
| NYHA functional class III/IV | 4.11 | 2.12 to 7.94 | <0.001 | 1.16 | 0.52 to 2.59 | 0.723 | 5.37 | 2.40 to 12.02 | <0.001 | 1.45 | 0.53 to 4.00 | 0.474 |
| Ln Big ET-1* | 2.67 | 2.04 to 3.49 | <0.001 | 1.83 | 1.27 to 2.62 | 0.001 | 3.08 | 2.31 to 4.11 | <0.001 | 1.93 | 1.32 to 2.83 | 0.001 |
| Ln NT-pro-BNP | 2.03 | 1.59 to 2.60 | <0.001 | 1.65 | 1.22 to 2.24 | 0.001 | 2.33 | 1.74 to 3.12 | <0.001 | 1.94 | 1.35 to 2.79 | <0.001 |
| Ln LVEF | 0.21 | 0.12 to 0.38 | <0.001 | 0.72 | 0.30 to 1.75 | 0.472 | 0.18 | 0.09 to 0.36 | <0.001 | 0.91 | 0.33 to 2.50 | 0.859 |
| Ln LVEDV | 4.09 | 2.12 to 7.91 | <0.001 | 0.39 | 0.07 to 2.16 | 0.278 | 6.18 | 2.92 to 13.06 | <0.001 | 0.55 | 0.06 to 4.67 | 0.580 |
| LVEDD | 1.07 | 1.04 to 1.10 | <0.001 | 1.05 | 0.98 to 1.11 | 0.152 | 1.08 | 1.05 to 1.11 | <0.001 | 1.04 | 0.97 to 1.12 | 0.278 |
| LGE | 6.96 | 2.52 to 19.28 | <0.001 | 5.43 | 1.74 to 16.91 | 0.003 | 11.46 | 2.77 to 47.35 | 0.001 | 8.20 | 1.76 to 38.09 | 0.007 |
Bold and italic values are statistically significant (p<0.05).
*Big ET-1 levels were regarded as a continuous variable.
Big ET-1, big endothelin-1; LGE, late gadolinium enhancement; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricle ejection fraction; LVEF, left ventricle ejection fraction; NT-pro-BNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; SCD, sudden cardiac death.
heartjnl-2020-317059supp005.pdf (30.9KB, pdf)
Discussion
To the best of our knowledge, this study has uniquely evaluated the prognostic significance of big ET-1 in patients with LVNC and shown that plasma big ET-1 is an independent risk factor for poor outcomes. This high-risk biomarker was associated with increased adverse cardiovascular events, especially cardiac death and heart transplantation. LGE, reflecting myocardial fibrosis and left ventricular enlargement and dysfunction, was related to an increased presence of big ET-1.
The role of traditional predictors (eg, left ventricular enlargement16 17 or dysfunction,17 18 the presence of myocardial fibrosis,19 20 increased NT-pro-BNP levels21) has been confirmed in risk stratification studies of LVNC populations. Big ET-1, a 39-amino acid precursor of ET-18 and a reliable biomarker of cardiovascular stress,22 is mainly produced by vascular endothelial and smooth muscle cells and cardiomyocytes.23 In recent years, the impact of plasma big ET-1 on adverse outcomes has been investigated in patient cohorts with AF9 and CAD.10 11 However, as big ET-1 was independently associated with the adverse end points, patients with LVNC with CAD or AF were not excluded from this cohort, which might have affected the outcome.
Our study revealed that plasma big ET-1 is a valuable tool for risk stratification in patients with LVNC as well. Multivariate Cox regression analysis showed that big ET-1 was independently associated with the left ventricular size and function in terms of predicting adverse outcomes. Furthermore, a big ET-1 level of ≥0.42 pmol/L was significantly related to an increased incidence of major adverse cardiovascular events, suggesting that a high level of big ET-1 could be a potential indicator of a patient’s prognosis.
The study showed that a high level of big ET-1 (≥0.42 pmol/L) contributed to an elevated risk of major adverse cardiovascular events, especially heart-failure-related death and heart transplantation. Patients with high big ET-1 levels seemed to be a higher risk for SCD with a statistical difference, but the number of SCD cases was small. A previous study revealed that high big ET-1 levels were related to the development of a sustained VT and VF, and the recurrence of various arrhythmias.24 The present study, however, did not find a significant difference in the incidence of sustained VT or VF between the high-level and low-level big ET-1 groups. It is probably due to the fact that even asymptomatic patients with LVNC are at risk for life-threatening arrhythmia or SCD.25 Consequently, the relation between big ET-1 levels and SCD or a sustained VT or VF needs to be evaluated further in long-term follow-up studies with a large sample.
In this study, high level big ET-1 (≥0.42 pmol/L) was positively associated with cardiac fibrosis identified by LGE, which is a surrogate of CMR-diagnosed myocardial fibrosis.15 Patients in the high-level big ET-1 group had a higher rate of LGE, indicating a higher risk for cardiac fibrosis. Nucifora et al determined that myocardial fibrosis is related to clinical disease severity and LV systolic dysfunction in patients with isolated LVNC.20 After adjusting for LGE in a multivariate Cox proportional hazard regression model, big ET-1 remained an independent predictor of adverse outcomes in patients with LVNC.
Several mechanisms might explain why elevated big ET-1 levels are related to unfavourable prognoses in patients with LVNC. Myocardial ischaemia may play an essential role in the pathogenesis of LVNC.1 During embryonic heart development, an overrepresentation is plausible for pathogenesis of coronary anomalies.26 Intramural perfusion could be adversely affected by the prominent trabeculations and intratrabecular recesses, particularly in the subendocardium, causing subendocardial ischaemia.1 In addition, compression of intramural coronary vessels by the thickened myocardium may be a factor supporting myocardial ischaemia,27 which, in conjunction with hypoxia and vascular wall stress, could result in endothelial cell injury in coronary vessels, followed by overexpression of ET-1.28 At the same time, ET-1, as a potent vasoconstrictor, mitogen and proinflammatory mediator, may aggravate myocardial ischaemia.29 Chronic ischaemia, probably resulted from hypertrophy or insufficient vascular supply of the trabeculations, may cause endocardial and subendocardial fibrosis.30 Other causes of endocardial and subendocardial fibrosis could be abnormally increased focal intraventricular pressure and immaturity of endocardial cells or subendocardial cells.30 The presence of postischaemic myocardial dysfunction is mostly associated with adverse outcomes of patients with LVNC.1 Endocardial and subendocardial fibrosis may lead to diastolic dysfunction, restrictive filling pattern of the left ventricle and consecutive heart failure.30 These potential mechanisms may explain why patients with high levels of big ET-1 have poor outcomes.
This study has some limitations that should be acknowledged. First, because of its small sample size and short follow-up, its results must be verified by a study with a larger sample size and longer-term follow-up. Second, information about the specific ratio of non-compacted/compacted myocardium and the number of non-compacted segments was unavailable, so the relationship between big ET-1 and these variables were not assessed in our study. Third, the incidences of the arrhythmias, SCD and systemic thromboses were relatively low among the studied patients. Patients with LVNC with CAD or AF were not excluded from the cohort. The impact between big ET-1 and such events remains to be evaluated further. Finally, genetic data were not available so far, and the relation between genetic background and big ET-1 levels remains unknown.
Conclusion
The plasma big ET-1 level is proposed as a novel prognostic indicator of adverse outcomes for patients with LVNC. It can be used to help develop a risk stratification protocol for patients with LVNC and then its specific management.
Key messages.
What is already known on this subject?
Plasma big endothlin-1 (ET-1) is an established biomarker of cardiac dysfunction, which is major feature of left ventricular non-compaction cardiomyopathy (LVNC). However, the prognostic role of big ET-1 in LVNC is unclear.
What might this study add?
This study, for the first time, demonstrated that plasma big ET-1 was related to cardiac dysfunction and may be an independent predictor of adverse outcomes in patients with LVNC.
How might this impact on clinical practice?
It is convenient and inexpensive to detect plasma big ET-1 in routine practice. It could help us develop a risk stratification protocol for patients with LVNC and then its specific management.
Footnotes
Contributors: PF and TT: conception and preparation of the manuscript. PF, K-QY, FL and TT: management of the patient. PF, YZ, Y-TL, P-PL and Q-YZ: patient’s data collection and statistical analysis. PF, Y-HL, TT and X-LZ: revising the manuscript. All authors contributed and agreed with the content of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding: This work was supported by CAMS Innovation Fund for Medical Sciences (2016-I2M-1–002), PUMC Youth Fund and the Fundamental Research Funds for the Central Universities (3332018058), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2019XK320057), the National Key Research and Development Program of China (2016YFC1300100) and the Postgraduate Education and Teaching Reform Project of Peking Union Medical College (10023201900203).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study was approved by the ethics committee of Fuwai Hospital and was conducted in accordance with the Declaration of Helsinki.
References
- 1. Towbin JA, Lorts A, Jefferies JL. Left ventricular non-compaction cardiomyopathy. Lancet 2015;386:813–25. 10.1016/S0140-6736(14)61282-4 [DOI] [PubMed] [Google Scholar]
- 2. Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American heart association scientific statement from the Council on clinical cardiology, heart failure and transplantation Committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and Council on epidemiology and prevention. Circulation 2006;113:1807–16. 10.1161/CIRCULATIONAHA.106.174287 [DOI] [PubMed] [Google Scholar]
- 3. Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society of cardiology Working group on myocardial and pericardial diseases. Eur Heart J 2008;29:270–6. 10.1093/eurheartj/ehm342 [DOI] [PubMed] [Google Scholar]
- 4. Towbin JA. Left ventricular noncompaction: a new form of heart failure. Heart Fail Clin 2010;6:453–69. 10.1016/j.hfc.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 5. Hussein A, Karimianpour A, Collier P, et al. Isolated noncompaction of the left ventricle in adults. J Am Coll Cardiol 2015;66:578–85. 10.1016/j.jacc.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 6. Stanton C, Bruce C, Connolly H, et al. Isolated left ventricular noncompaction syndrome. Am J Cardiol 2009;104:1135–8. 10.1016/j.amjcard.2009.05.062 [DOI] [PubMed] [Google Scholar]
- 7. Wexberg P, Pacher R, Rödler S, et al. Intimal hyperplasia and coronary flow reserve after heart transplantation: association with big endothelin-1. J Heart Lung Transplant 2002;21:1257–63. 10.1016/S1053-2498(02)00464-3 [DOI] [PubMed] [Google Scholar]
- 8. Hemsén A, Ahlborg G, Ottosson-Seeberger A, et al. Metabolism of big endothelin-1 (1-38) and (22-38) in the human circulation in relation to production of endothelin-1 (1-21). Regul Pept 1995;55:287–97. 10.1016/0167-0115(94)00119-I [DOI] [PubMed] [Google Scholar]
- 9. Wu S, Yang Y-M, Zhu J, et al. The association between plasma big endothelin-1 levels at admission and long-term outcomes in patients with atrial fibrillation. Atherosclerosis 2018;272:1–7. 10.1016/j.atherosclerosis.2018.02.034 [DOI] [PubMed] [Google Scholar]
- 10. Zhou B-Y, Guo Y-L, Wu N-Q, et al. Plasma big endothelin-1 levels at admission and future cardiovascular outcomes: a cohort study in patients with stable coronary artery disease. Int J Cardiol 2017;230:76–9. 10.1016/j.ijcard.2016.12.082 [DOI] [PubMed] [Google Scholar]
- 11. Olivier A, Girerd N, Michel JB, et al. Combined baseline and one-month changes in big endothelin-1 and brain natriuretic peptide plasma concentrations predict clinical outcomes in patients with left ventricular dysfunction after acute myocardial infarction: insights from the eplerenone post-acute myocardial infarction heart failure efficacy and survival study (EPHESUS) study. Int J Cardiol 2017;241:344–50. 10.1016/j.ijcard.2017.02.018 [DOI] [PubMed] [Google Scholar]
- 12. Rigopoulos AG, Noutsias M. Prognostic risk stratification in left ventricular noncompaction: still a long way to go. Cardiology 2019;142:220–2. 10.1159/000500322 [DOI] [PubMed] [Google Scholar]
- 13. Brescia ST, Rossano JW, Pignatelli R, et al. Mortality and sudden death in pediatric left ventricular noncompaction in a tertiary referral center. Circulation 2013;127:2202–8. 10.1161/CIRCULATIONAHA.113.002511 [DOI] [PubMed] [Google Scholar]
- 14. Hirono K, Hata Y, Miyao N, et al. Left ventricular noncompaction and congenital heart disease increases the risk of congestive heart failure. J Clin Med 2020;9:785. 10.3390/jcm9030785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petersen SE, Selvanayagam JB, Wiesmann F, et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol 2005;46:101–5. 10.1016/j.jacc.2005.03.045 [DOI] [PubMed] [Google Scholar]
- 16. Tian T, Liu Y, Gao L, et al. Isolated left ventricular noncompaction: clinical profile and prognosis in 106 adult patients. Heart Vessels 2014;29:645–52. 10.1007/s00380-013-0409-z [DOI] [PubMed] [Google Scholar]
- 17. Aras D, Tufekcioglu O, Ergun K, et al. Clinical features of isolated ventricular noncompaction in adults long-term clinical course, echocardiographic properties, and predictors of left ventricular failure. J Card Fail 2006;12:726–33. 10.1016/j.cardfail.2006.08.002 [DOI] [PubMed] [Google Scholar]
- 18. Habib G, Charron P, Eicher J-C, et al. Isolated left ventricular non-compaction in adults: clinical and echocardiographic features in 105 patients. results from a French registry. Eur J Heart Fail 2011;13:177–85. 10.1093/eurjhf/hfq225 [DOI] [PubMed] [Google Scholar]
- 19. Grigoratos C, Barison A, Ivanov A, et al. Meta-Analysis of the Prognostic Role of Late Gadolinium Enhancement and Global Systolic Impairment in Left Ventricular Noncompaction. JACC Cardiovasc Imaging 2019;12:2141–51. 10.1016/j.jcmg.2018.12.029 [DOI] [PubMed] [Google Scholar]
- 20. Nucifora G, Aquaro GD, Pingitore A, et al. Myocardial fibrosis in isolated left ventricular non-compaction and its relation to disease severity. Eur J Heart Fail 2011;13:170–6. 10.1093/eurjhf/hfq222 [DOI] [PubMed] [Google Scholar]
- 21. Stämpfli SF, Erhart L, Hagenbuch N, et al. Prognostic power of NT-proBNP in left ventricular non-compaction cardiomyopathy. Int J Cardiol 2017;236:321–7. 10.1016/j.ijcard.2017.02.064 [DOI] [PubMed] [Google Scholar]
- 22. Matsubara TJ, Fujiu K. Endothelin-1 and atrial cardiomyopathy. Int Heart J 2019;60:238–40. 10.1536/ihj.19-039 [DOI] [PubMed] [Google Scholar]
- 23. Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol 2001;41:851–76. 10.1146/annurev.pharmtox.41.1.851 [DOI] [PubMed] [Google Scholar]
- 24. Szûcs A, Róka A, Soós P, et al. Effect of incessant ventricular tachyarrhythmias on serum endothelin and big-endothelin levels. J Cardiovasc Pharmacol 2004;44 Suppl 1:S402–6. 10.1097/01.fjc.0000166292.72324.30 [DOI] [PubMed] [Google Scholar]
- 25. Finsterer J, Stöllberger C, Towbin JA. Left ventricular noncompaction cardiomyopathy: cardiac, neuromuscular, and genetic factors. Nat Rev Cardiol 2017;14:224–37. 10.1038/nrcardio.2016.207 [DOI] [PubMed] [Google Scholar]
- 26. Mattsson G, Baroudi A, Tawfiq H, et al. Left ventricular non-compaction cardiomyopathy with coronary artery anomaly complicated by ventricular tachycardia. BMC Cardiovasc Disord 2017;17:263. 10.1186/s12872-017-0699-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sen-Chowdhry S, McKenna WJ. Left ventricular noncompaction and cardiomyopathy: cause, contributor, or epiphenomenon? Curr Opin Cardiol 2008;23:171–5. 10.1097/HCO.0b013e3282fdc939 [DOI] [PubMed] [Google Scholar]
- 28. Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med 1999;340:115–26. 10.1056/NEJM199901143400207 [DOI] [PubMed] [Google Scholar]
- 29. Abraham D, Dashwood M. Endothelin--role in vascular disease. Rheumatology 2008;47 Suppl 5:v23–4. 10.1093/rheumatology/ken282 [DOI] [PubMed] [Google Scholar]
- 30. Stöllberger C, Finsterer J. Understanding left ventricular hypertrabeculation/noncompaction: pathomorphologic findings and prognostic impact of neuromuscular comorbidities. Expert Rev Cardiovasc Ther 2019;17:95–109. 10.1080/14779072.2019.1561280 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2020-317059supp001.pdf (12.6KB, pdf)
heartjnl-2020-317059supp002.pdf (217.6KB, pdf)
heartjnl-2020-317059supp003.pdf (26.3KB, pdf)
heartjnl-2020-317059supp004.pdf (412.9KB, pdf)
heartjnl-2020-317059supp005.pdf (30.9KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.