Abstract
Objective:
The purpose of our study is to examine whether serial measurements of serum sodium values after diagnosis identify a higher-risk subset of patients with heart failure with preserved ejection fraction.
Methods:
We identified 50,932 subjects with HFpEF with 759,577 recorded sNa measurements (mean age 72±11 years) using a validated algorithm in the VA national database from 2002 to 2012. We examined the association of repeated measures of sNa with mortality using a multivariable Cox proportional hazards model.
Results:
After a median follow-up of 2.9 years (IQR: 1.2–5.4), 19,011 deaths occurred. After adjusting for age, sex, race, BMI, glomerular filtration rate, potassium, coronary artery disease, hypertension, hyperlipidemia, atrial fibrillation, pulmonary disease, diabetes, anemia, and medications, we found J-shaped associations of serum sodium with mortality. HRs for all-cause mortality were 2.48 (95% CI: 2.38–2.60) for the sNA 115.00–133.99 category; and 1.40 (95% CI: 1.35–1.46) for the sNA 143.00–175.00 category compared to the 137.01–140.99 category (ref). We used generalized estimating equation-based negative binomial regression to compute the incidence density ratios (IDR) to examine days hospitalized for heart failure and for all causes. There were a total of 1,275,614 days of all-cause hospitalization and 104,006 days of heart-failure hospitalization. The IDRs for the lowest sNA group were 2.03 (95% CI: 1.90–2.18) for all-cause hospitalization and 1.73 (95% CI: 1.39–2.16) for heart-failure hospitalization.
Conclusions:
Our findings suggest that monitoring of serum sodium values during longitudinal follow-up can identify HFpEF patients at risk of adverse outcomes.
Keywords: heart failure with preserved ejection fraction, serum sodium, outcomes, mortality, hospitalization, heart failure hospitalization
INTRODUCTION
Low serum sodium concentration (sNa), or hyponatremia (<135mmol/L), is highly prevalent in heart failure patients (1,2). It results from multiple factors that impair water excretion as well as from elevated levels of neurohormones, especially arginine vasopressin (AVP) (3). Both hyponatremia and elevated AVP levels are associated with more severe heart failure (4). Other causes of low serum sodium include use of diuretics, volume depletion, kidney failure, and liver disease. Although patients with heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF) may have different clinical characteristics, they are comparable in mortality, which may be as high as 29% at 1 year and 65% at 5 years for patients with HFpEF (1, 5–9). Few therapies have been found to be effective in HFpEF, and the quality of the evidence is moderate at best (10,11).
Several clinical studies, mostly in HFrEF, have found that low sNa is a powerful prognostic factor of mortality and re-hospitalization in patients with heart failure (1,12). A few studies with relatively small sample sizes, or meta-analyses, have found significant associations between sNa and outcomes in HFpEF (13–16). A major drawback of these studies is that they used threshold values of sNa or assumed linearity in the relation between sNa and outcomes. A recent study from our group demonstrated that the serum sodium value recorded around the time of diagnosis was significantly associated with adverse outcomes in HFpEF (17). An important question that has not been sufficiently answered is whether measuring serum sodium concentration longitudinally after diagnosis of HFpEF identifies patients at higher risk of adverse outcomes. Thus, the purpose of our study is to examine whether serum sodium concentration over time is associated with all-cause mortality, heart failure hospitalization and all-cause hospitalization in a national cohort of patients with HFpEF during long-term follow-up.
METHODS
All study procedures were approved by the Institutional Review Board of the VA Boston Healthcare System.
Cohort selection and validation
We utilized a previously developed and validated algorithm to curate a cohort of HFpEF from the national Veterans Affairs patient database from 01/01/2002 to 12/31/2012 (18). Briefly, the algorithm utilized natural language processing to extract all recorded left ventricular ejection fraction values to ensure that ejection fractions were > 50% and also included variables such as diuretic use and natriuretic peptide values to ensure the presence of clinical heart failure. Validation through chart review based on the Framingham criteria for HFpEF (9) demonstrated that our algorithm had a positive predictive value of 96% and a negative predictive value of 87% (18).
Exposure
The primary exposure for all analyses was time-varying serum sodium, which was updated at the time of each new measurement (2002–2013), to allow for examination of the short-term effect of sodium as it changes over time. The first sodium measurement was on or within 30 days after the diagnosis of HFpEF. For inpatient hospitalizations, the last serum sodium value measured during the hospitalization was selected as it was assumed to be most stable. If multiple measurements were recorded within the same week, the average value and the last date were used. If a patient was hospitalized within two days of an outpatient diagnosis of HFpEF, the initial sodium value was taken on or within 30 days after discharge to ensure a stable sodium value. Individual serum sodium measurements < 115 and >180 mmol/L were excluded from the analysis.
Outcome
The outcomes of our study were all-cause mortality, number of days hospitalized for all causes per year, and number of days hospitalized for heart failure per year. Patients were followed through 2013, with each outcome assessed within the interval from current sodium measurement until the next measurement, death, or end of follow-up (censoring). All-cause mortality was verified using the Veterans Health Administration Vital Status File, which includes data from Centers for Medicare & Medicaid Services (CMS), and the Social Security Administration. Hospitalization outcomes were captured from the VA database as well as data from CMS. HF hospitalizations were defined as having a principal diagnosis of heart failure (ICD9 code of 428.xx). Patients with any hospitalization > 180 days were excluded from the hospitalization outcome analyses as they were likely part of hospice or long-term nursing home care.
Covariates
We extracted demographic, laboratory, and medication information from the VA national patient care databases which include clinical notes, laboratory values, medications, discharge summaries and are linked to the databases of the Centers for Medicare and Medicaid Services (CMS). Time-varying covariates were updated at the time of each sodium measurement. Demographic covariates included age, sex, race, and body mass index. Race (self-reported) was recorded as white/Caucasian, black, or other, due to low numbers in the other categories. We used the standard World Health Organization principal cut-off points of body mass index (BMI) as listed in Table 1 (19). Laboratory measurements included serum potassium (mEq/L) extracted from the same chemistry panel as the serum sodium measurement, and estimated glomerular filtration rate (eGFR), which was calculated using the chronic kidney disease epidemiology collaboration formula (20).
Table 1.
Baseline characteristics of 50,932 participants with heart failure with preserved ejection fraction from 2002 to 2012
| HFpEF cohort (n=50,932) |
|
|---|---|
| Age (years) | 72.2 (11.3) |
| Men (%) | 96.4 |
| Race (%) | |
| White | 84.6 |
| Black | 13.7 |
| Other | 1.7 |
| Body mass index (kg/m2) (%) | |
| Underweight (< 18.5) | 4.6 |
| Normal (18.5–24.9) | 16.9 |
| Overweight (25.0–29.9) | 26.4 |
| Obese (30.0–34.9) | 22.6 |
| Morbidly obese (≥ 35.0) | 29.5 |
| Co-morbid conditions (%) | |
| Hypertension | 89.6 |
| Hyperlipidemia | 66.5 |
| Coronary artery disease | 46.5 |
| Atrial fibrillation | 26.5 |
| Diabetes Mellitus | 55.7 |
| COPD | 48.4 |
| Anemia | 37.8 |
| Laboratory tests | |
| eGFR (mL/min/1.73 m2) | 60.6 (24.7) |
| Serum Potassium (mEq/L) | 4.22 (0.5) |
| Medications (%) | |
| ACE/ARB | 67.6 |
| Beta Blocker | 65.7 |
| Calcium Channel Blocker | 44.6 |
Column percentages are shown. Continuous variables are represented as mean ± standard deviation. Categorical variables are represented as frequency and percentage. Missing values are as follows: eGFR = 780, serum potassium=40, race=2,883. COPD = chronic obstructive pulmonary disease; ACE=Angiotensin-converting enzyme; ARB=Angiotensin receptor blocker; eGFR = estimated glomerular filtration rate. eGFR (estimated glomerular filtration rate) was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
Co-morbid medical conditions were extracted from the VA electronic healthcare system requiring ICD-9 codes from one inpatient or two outpatient records prior to each sodium measurement: hypertension (401–405.99, 437.2), hyperlipidemia (272.x), coronary artery disease (including myocardial infarction and history of percutaneous coronary intervention or coronary artery bypass graft) (410, 411, 414.0x, 429.2x, 429.5x, 440.x, 429.7x, V45.82), atrial fibrillation (427.31), diabetes mellitus type 2 (250.x, 362.0, 357.2, 366.41, 249, 790.2–791.6, V45.85, V53.91, V65.46), chronic obstructive pulmonary disease (490–496, 510, 781.5), and anemia (280–285.xx). Medications, including angiotensin converting enzyme inhibitors and angiotensin receptor blockers, beta blockers, and calcium channel blockers were extracted from prescriptions in the VA healthcare system or CMS, and assessed whether each sodium measurement fell between the first and last prescription dates.
Statistical analysis
For the primary analysis, we analyzed repeated serum sodium measures after HF diagnosis as a continuous variable. Cubic spline plots were created to examine sodium’s non-linear relationship with mortality and hospitalization outcomes, fitting a Cox proportional hazard model with the counting process approach (19) and generalized estimating equation (GEE) based negative binomial models, respectively. We developed SAS macros to fit cubic spline plots, specifying 6 knots which were equally distributed across the range of data, 3 degrees of freedom, and a reference sodium value of 139.00 mmol/dL, as that was the median value amongst all measurements in the cohort. Predicted values were calculated for the reference sodium value. Serum sodium values less than 128mmol/L (1st percentile) and greater than 146mmol/L (99th percentile) were winsorized for the plots due to instability and low frequency among extreme values.
In secondary analyses, we categorized time-varying sodium values based on percentiles and generally established clinical ranges as follows: 115.00–133.99 (<10th percentile), 134.00–137.00 (10–25th percentile), 137.01–140.99 (25.01–74.99th percentile), 141.00–142.99 (75–90th percentile), and 143.00–175.00 (>90th percentile) mmol/L. (Only 1,237 subjects had sodium values > 150 mmol/L). The reference group was selected as the 137.01–140.99 mmol/L category because the center of the distribution fell in this range and it contained values that are considered clinically normal. These categories were used for all proceeding regression analyses. In addition, we also conducted analyses using clinical cut-points for serum sodium values as follows: 115–129.9, 130–135, 135.01–145, and > 145 mmol/L, using the 135.01–145 mmol/L range as the reference category. These results are shown in Supplemental tables 1 and 2.
Person-observation-years were counted as the time (in years) that each subject was observed from the current to subsequent sodium measurement. Each subject could contribute person-observation-years to multiple sodium categories as their sodium values changed over time. We used a Cox proportional hazard regression model to compute the hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) for all-cause mortality by serum sodium category. As expected, the association between sodium and mortality did not meet the proportional hazards assumption, which led us to construct a piecewise Cox proportional hazard model, evaluating short-term mortality (first 30 days) and long-term mortality (after 30 days), to account for clinical instability and thus different hazard rates during the initial 30 day period following heart failure diagnosis. Further assessment of hazards showed no meaningful deviations of non-proportionality. Subjects entered the analysis at the time of their first sodium measurement, using a counting process approach (21), with person-time for each repeated measure interval accruing from the current sodium measurement to the next sodium measurement, death, or end of follow-up (12/31/2013). P-values were calculated for each model to assess non-linearity. Crude mortality rates were also calculated.
For hospitalizations outcomes, we calculated incidence density ratios (IDRs) using generalized estimating equation-based negative binomial regression models with AR(1) correlation structure. Incidence density was calculated as the total number of days hospitalized per year divided by the total observed time per year (in years). P-values were calculated for each model to assess non-linearity. Crude Poisson hospitalization rates were also calculated.
We constructed our models starting with crude models, then age-adjusted, parsimonious (age, gender, race), and finally multivariable adjusted. Multivariable models adjusted for age, sex, race, body mass index (kg/m2), eGFR (mL/min/1.73 m2), serum potassium, and history of prevalent medical conditions including coronary artery disease, hypertension, hyperlipidemia, atrial fibrillation, chronic obstructive pulmonary disease, diabetes mellitus type II, anemia, as well as medications including angiotensin converting enzyme inhibitor/angiotensin receptor blocker prescription, beta blocker prescription, and calcium channel blocker prescription. Covariates were selected based on a priori knowledge of risk factors and examination of confounders (p<0.25). No automated variable selection process was used (e.g. stepwise). Observation periods (from one sodium measure to the next) that did not have sufficient covariate information were dropped from the analysis.
We completed all analysis using SAS Enterprise Guide 7.1 (SAS institute Inc, Cary, NC). All p values presented were two-tailed and we considered an alpha level of < 0.05 to be statistically significant.
RESULTS
The final cohort for this study included 50,932 subjects with 759,577 repeated serum sodium measurements. Patients had a median of 11 (IQR: 5–20) serum sodium measurements over follow-up. Participant baseline characteristics, including demographics, clinical variables, medications and co-morbid conditions are listed in Table 1. In the overall cohort, 97% of the subjects were men and 85% were white. Those in the lowest sNA category had a higher prevalence of chronic obstructive pulmonary disease and anemia.
Associations of outcomes with sodium as continuous variable
When serum sodium was analyzed as a continuous variable, a J-shaped association was observed between serum sodium values and all-cause mortality, number of days hospitalized per year for any cause, and number of days hospitalized per year for heart failure (test for nonlinear trend, all p<.0001) (Figure 1). The predicted cumulative incidence of death was 53.3% for the reference sodium level of 139.00 mmol/dl at five years.
Figure 1.
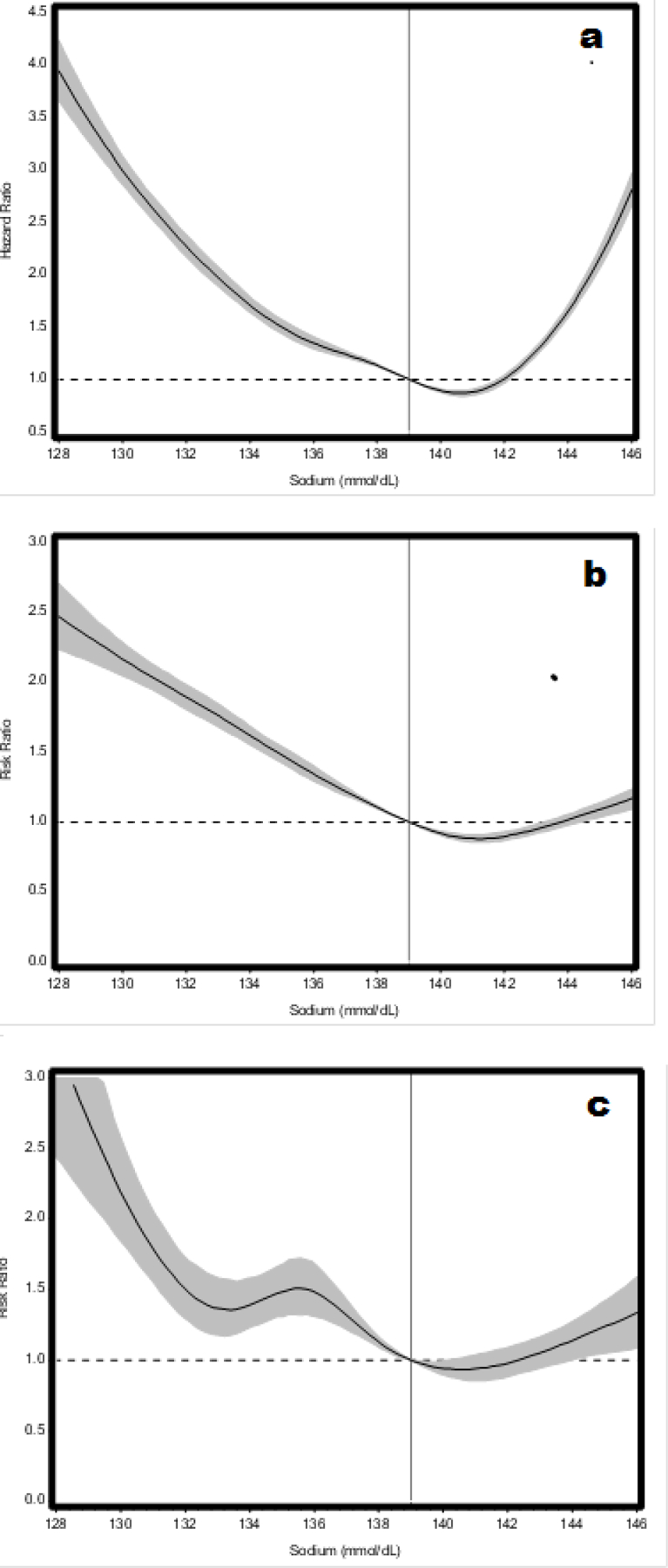
Cubic spline plots showing multivariable adjusted models for the association of serum sodium as a continuous variable and a) mortality b) number of days hospitalized per year for any cause, and c) number of days hospitalized per year for heart failure. These multivariable models were adjusted for age, sex, race, body mass index, estimated glomerular filtration rate, serum potassium, hypertension, hyperlipidemia, coronary artery disease, atrial fibrillation, diabetes mellitus type II, chronic obstructive pulmonary disease, anemia, angiotensin converting enzyme inhibitor/angiotensin receptor blocker prescription, beta blocker prescription, and calcium channel blocker prescription. Shaded areas denote 95% confidence intervals for the ratios and the vertical line marks the reference value (139.00mmol/dL).
Figure 1a demonstrates the relationship between serum sodium values and mortality 30 days post-diagnosis using a Cox proportional hazard model (test for non-linear trend, p<.0001).
Figure 1b demonstrates the relationship between serum sodium values and number of days hospitalized for any cause per year using a generalized estimating equation based negative binomial model (test for non-linear trend, p<.0001).
Figure 1c demonstrates the relationship between serum sodium values and number of days hospitalized for heart failure per year using a generalized estimating equation based negative binomial model (test for non-linear trend, p<.0001).
All-cause mortality
After a median follow-up of 2.9 years (IQR: 1.2–5.4), 19,011 deaths occurred. As shown in Table 2, multivariable analysis demonstrated significantly increased risk of 30-day mortality in lower and higher sNa groups compared to the referent group of sNa 137.01–140.99 (increases in risk were 96% in sNA 115.00–133.99 category, 47% in sNa 134.00–137.00 category, and 146% in sNA 143.00–175.00 category respectively).
Table 2.
Hazard ratios (95% confidence intervals) of mortality by current serum sodium level (mmol/L) in 50,932 participants with heart failure with preserved ejection fraction (HFpEF)
| Short-term (30-day) mortality | ||||||
|---|---|---|---|---|---|---|
| 115.00–133.99 | 134.00–137.00 | 137.01–140.99 | 141.00–142.99 | 143.00–175.00 | p trend | |
| Person Observations | 7,318 | 20,895 | 29,928 | 14,711 | 9,525 | |
| Deaths | 320 | 424 | 419 | 223 | 403 | |
| Person-Observation-years | 261.25 | 852.89 | 1,294.66 | 655.97 | 411.11 | |
| Deaths per 100 POYs | 122.49 | 49.71 | 32.36 | 34.00 | 98.03 | |
| Crude model | 3.37 (2.85–3.99) | 1.50 (1.29–1.74) | 1.00 (ref) | 1.03 (0.85–1.23) | 2.49 (2.12–2.92) | <0.0001 |
| Age adjusted model | 3.45 (2.92–4.07) | 1.54 (1.32–1.79) | 1.00 (ref) | 0.99 (0.83–1.19) | 2.35 (2.00–2.75) | <0.0001 |
| Multivariable modelǂ | 2.96 (2.48–3.53) | 1.47 (1.25–1.71) | 1.00 (ref) | 1.02 (0.85–1.24) | 2.46 (2.08–2.90) | <0.0001 |
| Long-term (>30 day) mortality | ||||||
| Person Observations | 51,865 | 179,463 | 272,156 | 137,405 | 85,410 | |
| Deaths | 3,371 | 7,189 | 8,767 | 4,538 | 4,579 | |
| Person-Observation-years | 8,838.89 | 39,633.90 | 70,704.47 | 38,380.01 | 23,460.67 | |
| Deaths per 100 POYs | 38.14 | 18.14 | 12.40 | 11.82 | 19.52 | |
| Crude model | 2.79 (2.68–2.91) | 1.43 (1.38–1.48) | 1.00 (ref) | 0.95 (0.92–0.99) | 1.45 (1.39–1.50) | <0.0001 |
| Age adjusted model | 2.90 (2.78–3.02) | 1.48 (1.44–1.53) | 1.00 (ref) | 0.92 (0.88–0.95) | 1.35 (1.30–1.40) | <0.0001 |
| Multivariable modelǂ | 2.48 (2.38–2.60) | 1.38 (1.34–1.43) | 1.00 (ref) | 0.95 (0.91–0.98) | 1.40 (1.35–1.46) | <0.0001 |
The 137.01–140.99 mmol/L is the reference serum sodium category. Missing values are as follows: eGFR = 780, serum potassium=40, race=2,883. POY= person-observation years.
Adjusted for age, sex, race, body mass index, estimated glomerular filtration rate, serum potassium, hypertension, hyperlipidemia, coronary artery disease, atrial fibrillation, diabetes mellitus type II, chronic obstructive pulmonary disease, anemia, angiotensin converting enzyme inhibitor/angiotensin receptor blocker prescription, beta blocker prescription, and calcium channel blocker prescription.
Hazard ratios for long-term mortality beyond 30 days in the multivariable adjusted model were 2.48 (95% CI: 2.38–2.60) for the sNA 115.00–133.99 category; 1.38 (95% CI: 1.34–1.43) for the sNA 134.00–137.00 category; 0.95 (95% CI: 0.91–0.98) for the sNA 141.00–142.99 category; and 1.40 (95% CI: 1.35–1.46) for the sNA 143.00–175.00 category compared to the 137.01–140.99 category (ref), p trend <0.0001 (Table 2). Results for the clinical cutoffs of serum sodium (115–129.9, 130–135, 135.01–145, and > 145 mmol/L) showed a similar trend. The hazard ratio for those in the 115–129.99 mmol/L sodium category was 3.50 (95% CI 3.25–3.77), and 1.73 (1.67–1.79) for serum sodium 130–135mmol/L compared to the reference category (serum sodium 135.01–145) (Suppl Table 1).
Number of days of all-cause hospitalization
During the follow-up period, there was a total of 1,275,614 days (3,492 person-years) in-hospital for all causes in the entire cohort. Information on the modeling of all-cause hospitalizations by serum sodium category is shown in table 3. In the multivariable adjusted model, the incidence density ratios and 95% CIs for all-cause hospitalization were 2.03 (95% CI: 1.90–2.18) for the sNA 115.00–133.99 category; 1.37 (95% CI: 1.30–1.44) for the sNA 134.00–137.00 category; 0.91 (95% CI: 0.86–0.96) for the sNA 141.00–142.99 category and 1.00 (95% CI: 0.94–1.06) for the sNA 143.00–175.00 category compared to the 137.01–140.99 category (ref) (Table 3).
Table 3.
Incidence density ratios (95% confidence intervals) of number of days of hospitalization by categories of current serum sodium (mmol/L) in participants with heart failure with ejection fraction (n=50,162*)
| All-cause hospitalization | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 115.00–133.99 | 134.00–137.00 | 137.01–140.99 | 141.00–142.99 | 143.00–175.00 | p trend | |||||
| Person Observations | 52,594 | 180,231 | 273,793 | 138,483 | 86,329 | |||||
| Number of days hospitalized | 123,738 | 337,860 | 443,990 | 219,419 | 150,607 | |||||
| Person-Observation-years | 8,860.70 | 39,648.51 | 70,754.61 | 38,424.21 | 23,513.82 | |||||
| Mean number of days hospitalized/100 POY | 1,396.5 | 852.1 | 627.5 | 571.0 | 640.5 | |||||
| Crude IDR | 2.20 (2.06–2.34) | 1.42 (1.35–1.49) | 1.00 (ref) | 0.91 (0.86–0.96) | 1.01 (0.95–1.07) | <.0001 | ||||
| Age adjusted IDR | 2.22 (2.09–2.37) | 1.42 (1.35–1.50) | 1.00 (ref) | 0.90 (0.85–0.95) | 1.00 (0.94–1.06) | <.0001 | ||||
| Multivariable modelǂ | 2.03 (1.90–2.18) | 1.37 (1.30–1.44) | 1.00 (ref) | 0.91 (0.86–0.96) | 1.00 (0.94–1.06) | <.0001 | ||||
| Heart failure hospitalization | ||||||||||
| Person Observations | 52,594 | 180,231 | 273,793 | 138,483 | 86,329 | |||||
| Number of days hospitalized | 8,945 | 24,974 | 36,847 | 18,865 | 14,375 | |||||
| Person-Observation-years | 8,860.70 | 39,648.51 | 70,754.61 | 38,424.21 | 23,513.82 | |||||
| Mean number of days hospitalized/100 POY | 100.95 | 62.99 | 52.08 | 49.10 | 61.13 | |||||
| Crude IDR | 1.85 (1.50–2.29) | 1.37 (1.16–1.61) | 1.00 (ref) | 0.93 (0.80–1.09) | 1.38 (1.18–1.63) | <.0001 | ||||
| Age adjusted IDR | 1.88 (1.55–2.28) | 1.42 (1.20–1.68) | 1.00 (ref) | 0.90 (0.78–1.04) | 1.32 (1.12–1.57) | <.0001 | ||||
| Multivariable modelǂ | 1.73 (1.39–2.16) | 1.33 (1.09–1.61) | 1.00 (ref) | 0.83 (0.70–0.98) | 1.09 (0.92–1.29) | <.0001 | ||||
The 138–140.99 mmol/L is the reference serum sodium category. Missing values are as follows: eGFR = 773, serum potassium=40, race=2,861. POY= person-observation years. IDR = incidence density ratio. There were 731,430 serum sodium measurements during the follow-up period (728,776 used in the model because 2,654 were excluded because they died or were censored on the same day as their sodium measurement).
Sample size is 50,162 because 770 people with any length of stay > 180 days were excluded.
Adjusted for age, sex, race, body mass index, estimated glomerular filtration rate, serum potassium, hypertension, hyperlipidemia, coronary artery disease, atrial fibrillation, diabetes mellitus type II, chronic obstructive pulmonary disease, anemia, angiotensin converting enzyme inhibitor/angiotensin receptor blocker prescription, beta blocker prescription, and calcium channel blocker prescription.
Number of days hospitalized for heart failure
There was a total of 104,006 days (285 person-years) in-hospital for heart-failure for all participants. Information on the modeling of heart failure hospitalizations by serum sodium category is shown in Table 3. In the multivariable adjusted model, the incidence density ratios and 95% CIs for heart failure hospitalization were 1.73 (95% CI: 1.39–2.16) for the sNA 115.00–133.99 category; 1.33 (95% CI: 1.09–1.61) for the sNA 134.00–137.00 category; 0.83 (95% CI: 0.70–0.98) for the sNA 141.00–142.99 category and 1.09 (95% CI: 0.92–1.29) for the sNA 143.00–175.00 category compared to the 137.01–140.99 category (ref) (Table 3).
Results for the clinical cutoffs of serum sodium (115–129.9, 130–135, 135.01–145, and > 145 mmol/L) showed a similar trend for all cause hospitalizations and heart failure hospitalizations. The incidence density ratio for those in the 115–129.99 mmol/L sodium category was 1.80 (95% CI 1.53–2.11), and 1.33 (1.17–1.50) for serum sodium 130–135mmol/L compared to the reference category (serum sodium 135.01–145) (Suppl Table 2) for all cause hospitalization. For heart failure hospitalization, the incidence density ratio for those in the 115–129.99 mmol/L sodium category was 2.90 (95% CI 2.01–4.19), and 1.33 (1.12–1.58) for serum sodium 130–135mmol/L compared to the reference category (serum sodium 135.01–145) (Suppl Table 2).
Discussion
In this well-curated national cohort of HFpEF, the first and largest study of serial sodium measurements after diagnosis of HFpEF to our knowledge, we found a J-shaped association between repeated serum sodium measurements and all-cause mortality and numbers of days of all-cause and heart failure hospitalizations. Our findings suggest that serum sodium could be used as a measure of risk during longitudinal follow-up of HFpEF.
Prior studies conducted in HFrEF or unclassified heart failure patients have found similar results. For example, in a study conducted in patients with end-stage heart failure, sNa< 137 meq/L was associated with a 50% lower median short-term survival (22). In a post- hoc analysis from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME–CHF), HFrEF patients in the lowest quintile of sNa (132–135 meq/L) had a greater risk of mortality in-hospital and at 60 days post-discharge ( 23). A recent study of 500 hospitalized patients with HFrEF found that those with low sNA had increased one-year mortality ( 24). Another study of unclassified heart failure (n=4295) found that among those with persistent hyponatremia, those with sNA < 130 mmol/L had a 68% higher risk of 30 day readmission or death on discharge (25). Konishi et al found that new-onset hyponatremia during an acute hospitalization for heart failure was associated with an increase in 1-year mortality and cardiac events (26). In contrast to these findings, in the Korea Acute Heart Failure registry of 2888 subjects, those with persistent low serum sodium and normal serum sodium on admission showed no significant difference in clinical outcomes on follow-up (27).
In contrast to these prior studies that included only HFrEF or all classes of heart failure, a few studies have examined the relation between sNA and outcomes in HFpEF. One study which examined sNa as a continuous variable in 405 HFpEF patients demonstrated a negative relation between sNa and cardiovascular mortality and rehospitalizations after 27 months of follow-up (13). Other studies examined the relation of sNa above and below a threshold value with outcomes. For example, Rosinaru and colleagues showed that sNa < 136 mEq/L was associated with 87% higher risk of mortality amongst 358 HFpEF patients who survived a first hospitalization for HF during 7-year follow up, (15) The Meta-Analysis Global Group in Chronic heart failure (MAGGIC) group analyzed serum sodium as a continuous variable and found a linear increase in the risk of all-cause mortality at 3 years up to a sNa level of 140 mmol/L(16). Bavishi and colleagues showed that in 2,704 ambulatory HFpEF patients treated in Veterans Affairs clinics, sNa ≤ 135 mEq/L was associated with all-cause mortality (HR 1.40, 95% CI 1.12–1.75) but not with all-cause hospitalization at 2-year follow-up (14). While these findings are in the same direction as ours, our study demonstrated the non-linear association of each value of sodium measured during a long follow-up period after diagnosis of HFpEF with the three major clinical outcomes of mortality, and all-cause and heart failure hospitalizations. A recent publication from our group showed that a single sodium value measured around the time of diagnosis of HFpEF was significantly associated with adverse outcomes in HFpEF. The current study demonstrates higher hazard ratios of repeated measures of serum sodium compared to baseline values. For e.g., hazard ratio for all-cause mortality (30 days post-diagnosis) in the lowest baseline sodium group (115.00–134.99 mmol/L) was 1.36 (95% C.I. 1.28–1.44) while the ratio in the lowest group of repeated measures of sodium in this analysis was 2.48 (95% C.I. 2.38–2.60). The current study also shows that a low serum sodium value at any time in the natural history of HFpEF is strongly associated with adverse outcomes. This suggests that repeated measures of serum sodium over time, which are often obtained in the clinical setting as part of a chemistry panel blood test, may potentially serve as a prognosticator of adverse outcomes in patients with HFpEF.
In the present study, we also found increased mortality in the sNA 143.00–175.0 mmol/L group both at 30-days post-diagnosis and at long-term follow-up. This is likely due to volume depletion, or unreplaced water loss (which may occur from gastrointestinal loss, urinary loss or skin loss). Also, hypernatremia is a common problem among the elderly who may have an impaired thirst mechanism. Hypernatremia has been noted to be an independent risk factor for mortality, thus this finding was not unexpected (28).
Heart failure patients with hyponatremia have increased activation of the renin-angiotensin-aldosterone system, upregulation of the sympathetic nervous system and excessive arginine vasopressin (AVP) release (29). The degree of neurohormonal activation is correlated with the severity of heart failure (30). While excess AVP decreases water excretion, increased levels of angiotensin II stimulates thirst, thus leading to increased water intake and lower serum sodium concentration in heart failure (31). Further studies are needed to clarify the mechanisms of hyponatremia in patients with HFpEF in particular as it is likely multi-factorial.
It is not clearly established whether correcting serum sodium improves clinical outcomes in patients with HFpEF. In the Efficacy of Vasopressin Antagonism in Heart Failure Outcomes Study with Tolvaptan Trial (EVEREST), a vasopressin antagonist, Tolvaptan, improved body weight, serum sodium levels, and symptoms of heart failure but had no effect on all-cause mortality, cardiovascular mortality or heart failure hospitalizations in patients with HFrEF (32–34). Further studies, particularly clinical trials, are needed to determine correction of serum sodium impacts clinical outcomes.
Limitations of our study include residual and/or unmeasured confounding that could partially or completely explain observed findings. Our study population mainly consists of Caucasian male veterans. However, the pathophysiology of the association of serum sodium with clinical outcomes in HFpEF is unlikely to be different across populations. Clinical care outside of the VA healthcare system may not have been entirely captured in this cohort. To account for this, we included data from Centers for Medicare and Medicaid services, which included clinical outcomes for those older than 65 years. Another limitation is the specificity of the co-morbid conditions that are included as covariates included in the analysis. We attempted to increase the specificity of the clinical diagnosis by including one inpatient diagnosis code and two outpatient diagnosis codes for each condition. Additionally, we could not account for individual fluctuations in fluid status with changes in serum sodium, which may confound the association of serum sodium concentration with clinical outcomes.
Despite above limitations, our study has several strengths. First, to our knowledge, this is the first study to examine the association of repeated serum sodium measurements with clinical outcomes in patients with HFpEF. Second, we incorporated detailed covariates, laboratory and clinical measurements and co-morbid conditions into our analysis. These were recorded measurements in the VA healthcare system and adjudicated by two physicians. Third, the large sample size and number of repeated serum sodium measurements in our study allows for adequate power to examine modest associations. Fourth, most studies on serum sodium measurement and heart failure have evaluated short-term outcomes, whereas our study examined the association of serum sodium with mortality and hospitalization with a long follow-up duration.
CONCLUSION
Our findings suggest that low serum sodium measurement recorded at any time after HFpEF diagnosis is associated with a higher risk of all-cause mortality and greater number of days of all-cause and heart failure hospitalization in patients with HFpEF. Serum sodium levels over time may be a marker for severity of heart failure and a potential therapeutic target in patients with HFpEF. Further studies, including randomized trials, are needed to further elucidate these findings.
Supplementary Material
Acknowledgements
The authors would like to thank Constance Nelson for her help with the management of this study. This study was funded by a grant from Otsuka Pharmaceuticals to Dr. Joseph. This material is the result of work supported with resources and the use of facilities at the VA Boston Healthcare System.
Sources of Funding:
This study was funded by a grant from Otsuka Pharmaceuticals to Dr. Joseph.
Footnotes
Disclosures:
TF Imran: None; KE Kurgansky: None; YR Patel: None; AR Orkaby: None; RR McLean: None; Y-L Ho: None; K Cho: None; JM Gaziano: None; L Djousse: None; DR Gagnon: None; J. Joseph: Research grant support: National Heart, Lung, Blood Institute, Novartis, Otsuka, Amgen.
REFERENCES
- 1.Lu DY, Cheng HM, Cheng YL et al. Hyponatremia and worsening sodium levels are associated with long-term outcome in patients hospitalized for acute heart failure. J Am Heart Assoc 2016. March 23;5(3):e002668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gheorghiade M, Rossi JS, Cotts W, Shin DD, Hellkamp AS, Pina IL, Fonarow GC, DeMarco T, Pauly DF, Rogers J, DiSalvo TG, Butler J, Hare JM, Francis GS, Stough WG, O’Connor CM. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial. Arch Intern Med 2007;167:1998–2005. [DOI] [PubMed] [Google Scholar]
- 3.Oren RM. Hyponatremia in congestive heart failure. Am J Cardiol 2005;95:2B–7B. [DOI] [PubMed] [Google Scholar]
- 4.Leier CV, Dei Cas L, Metra M. Clinical relevance and management of the major electrolyte abnormalities in congestive heart failure: hyponatremia, hypokalemia, and hypomagnesemia. Am Heart J 1994; 128:564. [DOI] [PubMed] [Google Scholar]
- 5.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006; 355: 260–269. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: Prevalence, therapies, and outcomes. Circulation 2012; 126: 65–75. [DOI] [PubMed] [Google Scholar]
- 8.Adebe TB, Gebreyohannes EA, Tefera YG et al. Patients with HFpEF and HFrEF have different clinical characteristics but similar prognosis: a retrospective cohort study. BMC Cardiovasc Disord 2016. November 21; 16(1):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: The Framingham Study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 10.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70(6):776–803. [DOI] [PubMed] [Google Scholar]
- 11.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370(15):1383–92. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 12.Kajimoto K, Minami Y, Sato N et al. Serum sodium concentration, blood urea nitrogen, and outcomes in patients hospitalized for acute decompensated heart failure. Int J Cardiol 2016. November 1;222:195–201. [DOI] [PubMed] [Google Scholar]
- 13.Kusaka H, Sugiyama S, Yamamoto E et al. Low-normal serum sodium and heart failure related events in patients with heart failure with preserved left ventricular ejection fraction. Circ J 2016;80(2):411–7. [DOI] [PubMed] [Google Scholar]
- 14.Bavishi C, Ather S, Bambhroliya A, Jneid H, Virani SS, Bozkurt B, Deswal A. Prognostic significance of hyponatremia among ambulatory patients with heart failure and preserved and reduced ejection fractions. The American journal of cardiology 2014;113:1834–1838. [DOI] [PubMed] [Google Scholar]
- 15.Rusinaru D, Buiciuc O, Leborgne L, Slama M, Massy Z, Tribouilloy C. Relation of serum sodium level to long-term outcome after a first hospitalization for heart failure with preserved ejection fraction. The American journal of cardiology 2009;103:405–410. [DOI] [PubMed] [Google Scholar]
- 16.Rusinaru D, Tribouilloy C, Berry C, Richards AM, Whalley GA, Earle N, Poppe KK, Guazzi M, Macin SM, Komajda M, Doughty RN, Investigators M. Relationship of serum sodium concentration to mortality in a wide spectrum of heart failure patients with preserved and with reduced ejection fraction: An individual patient data meta-analysis(dagger): Meta-analysis global group in chronic heart failure (maggic). European journal of heart failure 2012;14:1139–1146. [DOI] [PubMed] [Google Scholar]
- 17.Patel YR, Kurgansky KE, Imran TF, Orkaby AR, McLean RR, Ho YL, Cho K, Gaziano JM, Djousse L, Gagnon DR, Joseph J. Prognostic significance of baseline serum sodium in heart failure with preserved ejection fraction. Journal of the American Heart Association 2018;7:e007529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel YR, Robbins JM, Kurgansky KE, Imran T, Orkaby AR, Mclean RR, Ho YL, Cho K, Gaziano JM, Djousse L, Gagnon DR, Joseph J. Development and validation of a heart failure with preserved ejection fraction cohort using electronic medical records. BMC Cardiovasc Disord 2018. June 28; 18(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. WHO Technical Report Series 894 Geneva: World Health Organization, 2000. [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sas/stat(r) 14.1 user’s guide. The phreg procedure Http://support.Sas.Com/documentation/cdl/en/statug/68162/html/default/viewer.Htm#statug_phreg_syntax17.Htm. Accessed on December 2. 2017.
- 22.Lee WH, Packer M. Prognostic importance of serum sodium concentration and its modification by converting-enzyme inhibition in patients with severe chronic heart failure. Circulation 1986; 73:257. [DOI] [PubMed] [Google Scholar]
- 23.Klein L, O’Connor CM, Leimberger JD, et al. Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study. Circulation 2005; 111:2454. [DOI] [PubMed] [Google Scholar]
- 24.Avc BK, Kucuk M, Muderrisoqlu H et al. Relation between serum sodium levels and clinical outcomes in turkish patients hospitalized for heart failure: a multicenter retrospective observational study. Anatol J Cardiol 2017. January;17(1):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donze JD, Beeler PE, Bates DW. Impact of hyponatremia correction on the risk for 30-day readmission and death in patients with congestive heart failure. Am J Med 2016. August;129(8):836–42. [DOI] [PubMed] [Google Scholar]
- 26.Konishi M, Haraguchi G, Ohigashi H, Sasaoka T, Yoshikawa S, Inagaki H, Ashikaga T, Isobe M. Progression of hyponatremia is associated with increased cardiac mortality in patients hospitalized for acute decompensated heart failure. J Card Fail 2012;18:620–625. [DOI] [PubMed] [Google Scholar]
- 27.Lee SE, Choi DJ, Yoon CH, Oh IY, Jeon ES, Kim JJ, Cho MC, Chae SC, Ryu KH, Oh BH; KorHF Registry. Improvement of hyponatraemia during hospitalization for acute heart failure is not associated with improvement of prognosis: an analysis from the Korean Heart Failure (KorHF) registry. Heart 2012;98:1798–1804. [DOI] [PubMed] [Google Scholar]
- 28.Lindner G, Funk GC, Schwarz C et al. Hypernatremia in the critically ill is an independent risk factor for mortality. Am J Kidney Dis 2007. December;50(6):952–7. [DOI] [PubMed] [Google Scholar]
- 29.Farmakis D, Filippatos G, Parissis J, Kremastinos DT, Gheorghiade M. Hyponatremia in heart failure. Heart Fail Rev 2009; 14: 59–63. [DOI] [PubMed] [Google Scholar]
- 30.Benedict CR, Johnstone DE, Weiner DH, et al. Relation of neurohumoral activation to clinical variables and degree of ventricular dysfunction: a report from the Registry of Studies of Left Ventricular Dysfunction. SOLVD Investigators. Coll Cardiol 1994; 23:1410. [DOI] [PubMed] [Google Scholar]
- 31.Verbrugge FH, Steels P, Grieten L, et al. Hyponatremia in acute decompensated heart failure: depletion versus dilution. J Am Coll Cardiol 2015; 65:480. [DOI] [PubMed] [Google Scholar]
- 32.Konstam MA, Gheorghiade M, Burnett JC Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007; 297:1319. [DOI] [PubMed] [Google Scholar]
- 33.Gheorghiade M, Konstam MA, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan Clinical Status Trials. JAMA 2007; 297: 1332–1343. [DOI] [PubMed] [Google Scholar]
- 34.Gheorghiade M, Gattis WA, O’Connor CM, Adams KF Jr, Elkayam U, Barbagelata A, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: A randomized controlled trial. JAMA 2004; 291: 1963–1971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


