Abstract
Low‐dose aspirin (LDA)–induced gastric mucosal injury always requires rigorous follow‐up while taking LDA, even in adolescents, and after a long time from the start of LDA.
Keywords: adolescent, aspirin, endoscopy, Fontan surgery, peptic ulcer
Low‐dose aspirin (LDA)–induced gastric mucosal injury always requires rigorous follow‐up while taking LDA, even in adolescents, and after a long time from the start of LDA.
1. INTRODUCTION
It is important to undergo more rigorous follow‐up regarding gastric status considering low‐dose aspirin (LDA)–induced gastric mucosal injury when an LDA regimen is accompanied by abdominal symptoms, even in adolescents, and after a long time from the start of LDA.
Fontan surgery is a palliative surgical method used in children with a univentricular heart. It involves diverting the venous blood from the inferior vena cava and the superior vena cava. 1 , 2 Although the short‐term prognosis of Fontan surgery is favorable, thromboembolism, heart failure, and sudden death are the three major causes of death. 3 Therefore, anticoagulant or antiplatelet drugs are commonly prescribed to prevent thromboembolism. 4 , 5 , 6 However, gastromucosal injury, such as gastrointestinal bleeding associated with low‐dose aspirin (LDA), remains a problem. 7 , 8 The risk of gastrointestinal bleeding due to LDA use is 5.5 times higher in Japan. 9
LDA‐induced gastric mucosal injury tends to occur in older people 8 and often develops within three months of oral administration. 10 Herein, we describe a case of an adolescent patient with LDA‐induced gastric mucosal injury that developed after a long time from the start of LDA after Fontan surgery.
2. CASE PRESENTATION
A 16‐year‐old girl visited our division with epigastric pain lasting three months without gastrointestinal bleeding. Immediately after birth, she had cyanosis and was diagnosed with pulmonary atresia (PA). In the first month of life, she underwent Blalock‐Taussig shunt surgery, followed by Glenn surgery at age one year and a Fontan procedure at age three years. After the second operation, she was prescribed LDA at a dose of 1 mg per body weight. After the Fontan procedure, her circulatory dynamics had been stable, and our hospital's pediatric cardiologist performed regular medical examinations. There were no gastrointestinal symptoms during the 15 years leading up to this visit, and there was no new stress load. She had been taking digoxin (0.25 mg/d), enalapril (5 mg/d), and aspirin (100 mg/d). On her initial visit to our division, her vital signs were normal as follows: body temperature 36.2°C, heart rate 88 beats per minute, and saturation of percutaneous oxygen 98% without the need for supplemental oxygen. Physical examination showed no abnormal findings in her chest, but there was tenderness in the epigastric region. Her height was 162 cm (standard deviation, 0.9 cm), and her weight was 51.3 kg (standard deviation, −0.1 kg). The patient's blood test data were as follows: white blood cell count 6400/μL, neutrophils 60.6%, hemoglobin level 16.1 g/dL, and platelet count 249 × 103/µL (Table 1). Prothrombin international normalized ratio was 1.12, and activated partial thromboplastin time activity was 82.2%. Serum C‐reactive protein level was 0.01 mg/dL, and liver and kidney function tests were in the normal range. N‐terminal fragment of pro‐B‐type natriuretic peptide level was in the normal range (<100 pg/mL). Plain chest and abdominal radiographs revealed no obvious abnormalities.
TABLE 1.
Blood test and biochemical test results at the first visit
| WBC | 6,400/μL | TP | 7.5 g/dL |
| Neu | 60.6% | Alb | 4.4 g/dL |
| Lym | 32.4% | AST | 19 U/L |
| Mo | 3.6% | ALT | 8 U/L |
| Hb | 16.1 g/dL | LDH | 196 U/L |
| Plt | 249 × 103/μL | BUN | 8.3 mg/dL |
| Cre | 0.6 mg/dL | ||
| Na | 136 mEq/L | ||
| PT‐INR | 1.12 | K | 4.1 mEq/L |
| APTT | 82.2% | Cl | 103 mEq/L |
| Fib | 288.1 mg/dL | CRP | 0.01 mg/dL |
| NT‐proBNP | 34.1 pg/mL |
Abbreviation: NT‐proBNP, N‐terminal fragment of pro‐B‐type natriuretic peptide.
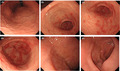
At this point, we performed esophagogastroduodenoscopy (EGD) considering an upper gastrointestinal disease, which revealed gastric mucosal redness and erosion, with irregular and small, shallow ulcers, primarily in the antrum (Figure 1A–D). There was no atrophy of gastric mucosa or portal hypertensive gastropathy. No findings of esophageal varices were found. There were also no abnormal findings on the anus side of the duodenum, including the portion of the duodenal bulb (Figure 1E–F). A gastric mucosal biopsy was not performed because of the risk of upper gastrointestinal bleeding when taking LDA. Contrast‐enhanced abdominal computed tomography showed the findings of chronic liver disease and mild splenomegaly, but no development of collateral circulation (Figure 2A–B). Since both a urinary anti‐Helicobacter pylori antibody test and fecal antigen Helicobacter pylori test revealed negative results, she was diagnosed with LDA‐induced gastric mucosal injury based on endoscopic findings. 11 The patient was prescribed esomeprazole (20 mg/day), ecabet sodium (2000 mg/day), and sodium alginate (3000 mg/d) with a continuation of LDA, and her abdominal symptoms disappeared immediately. A subsequent EGD, performed three months later, showed that a slight mucous membrane redness in the antrum remained, but the erosion had ceased, and the ulcer had disappeared (Figure 3A–F). Her mild epigastric pain recurred one month after the second EGD. Without performing another EGD due to her refusal, LDA was changed to warfarin (3 mg/d), and her symptoms resolved. Her gastrointestinal medication was stopped five months after she switched to warfarin, and there has been no epigastric pain for the past 18 months.
FIGURE 1.
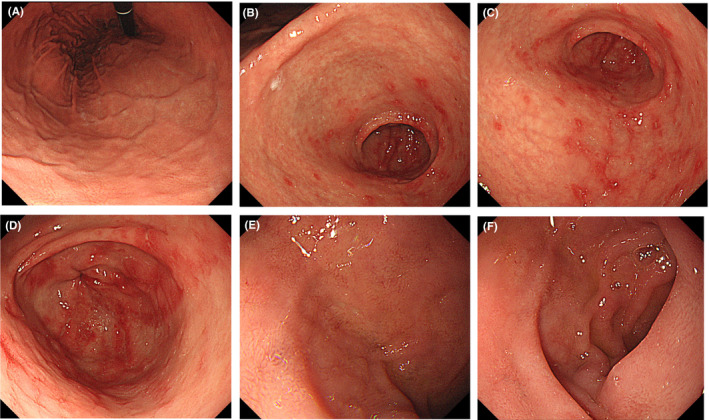
Gastric mucosal redness, erosion, and small ulcers in the antrum as revealed by esophagogastroduodenoscopy. A small ulcer was shallow and irregular in shape. (A) Cardiac zone, (B‐D) pyloric zone
FIGURE 2.
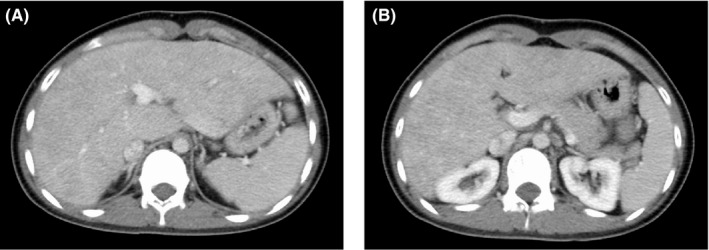
Contrast‐enhanced abdominal computed tomography showed the findings of chronic liver disease and mild splenomegaly, but no development of collateral circulation
FIGURE 3.
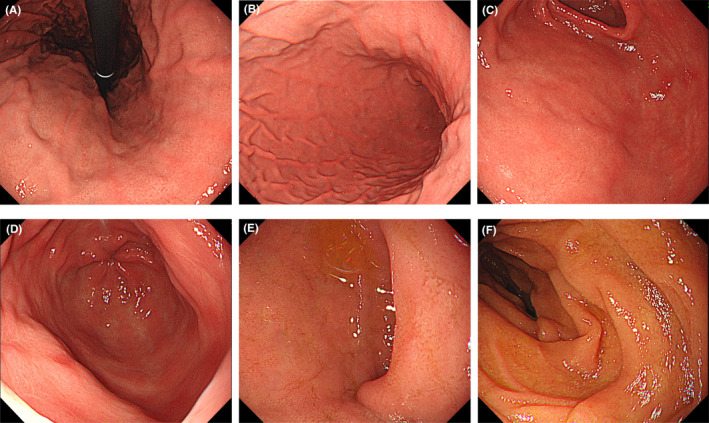
Three months after the first esophagogastroduodenoscopy, a second examination revealed that erosion had ceased, the ulcer had disappeared, and only slight redness remained. (A) Cardiac zone, (B‐D) pyloric zone
3. DISCUSSION
This case report describes a LDA‐induced gastric mucosal injury after Fontan surgery in an adolescent patient. To our knowledge, a case report of an adolescent with an LDA‐induced gastric mucosal injury after Fontan procedure is scarce in the literature. The present patient's course provides two clinical suggestions.
First, although LDA‐induced gastric mucosal injury tends to occur in elderly individuals, 8 the present patient was a 16‐year‐old girl. High‐risk factors for LDA‐induced gastric mucosal injury include older age, higher dose aspirin, history of ulcer, concomitant use of corticosteroids, a higher dose of nonsteroidal anti‐inflammatory drugs (NSAIDs), including the use of more than one NSAIDs, concomitant administration of anticoagulants, serious systemic disorders, concomitant infection with H pylori, cigarette smoking, and consumption of alcohol. 12 , 13 , 14 , 15 , 16 , 17 In contrast, there is also a report that no significant association exists between age and LDA‐induced gastric mucosal injury. 18 Several mechanisms of action of NSAIDs or LDA have been reported in a previous review. 19 The pathogenesis, related to the inhibition of cyclooxygenase (COX)‐1, includes reduced mucosal flow, reduced mucus and bicarbonate secretion, and impaired platelet aggregation. The pathogenic mechanisms involved in inhibiting COX‐2 include reduced angiogenesis and increased leukocyte adherence. The pathogenesis, associated with direct epithelial damage, involves acid back diffusion and impaired platelet aggregation. Aspirin is a more potent inhibitor of COX‐1 than COX‐2. 7 Considering that gastric mucosal blood flow, bicarbonate secretion, and platelet aggregation decline with age, it appears that older patients are more likely to suffer from LDA‐induced gastric mucosal injury than the young. The present case suggested that it is necessary to pay sufficient attention to gastric mucosal injury even in young patients during LDA administration.
Second, a gastric mucosal injury may develop even after a long time from the start of LDA. It has been reported LDA‐induced gastric mucosal injury often develops within three months of oral administration. 10 Exploring the literature for the onset of LDA‐induced gastric mucosal injury from the time of gastrointestinal bleeding, the risks for LDA‐induced gastrointestinal bleeding were found to be 7.6% (range, 6.0%–9.5%) within one month from the start of LDA oral administration, 7.3% (range, 4.0%–13.2%) within one to three months, 2.6% (range, 1.6%–4.1%) within three months to one year, and 2.5% (range, 1.8%–3.4%) after one year. 10 , 20 Although the reason for the frequent early onset of LDA‐induced gastric mucosal injury after the start of oral administration remains unknown, 21 , 22 the onset of LDA‐induced gastric mucosal injury may depend on the LDA dose. 23 , 24 In general, venous pressure becomes higher than usual after the Fontan procedure, including in the present case. 1 Chronic venous pressure increase due to congestion of the right heart system may have caused a state of congestive liver, resulting in increased portal vein pressure and decreased portal vein blood flow gradually over the years. With chronic congestion of the gastric mucosa due to the decrease in portal vein blood flow, LDA clearance in the gastric mucosa may decrease as the local LDA concentration increases. This may be a reason for the development of LDA‐induced gastric mucosal injury 15 years after the start of LDA administration in our patient.
In contrast, the onset of LDA‐induced gastric mucosal injury may be independent of the dose of LDA. 25 In this opinion, the reason for LDA‐induced gastric mucosal injury may not be due to the increased concentration of LDA by the decreased local clearance, but because of the decreased gastric mucosal blood flow after the Fontan procedure. The incidence of gastrointestinal bleeding due to antiplatelet therapy with LDA after Fontan surgery is reportedly approximately 5%, 26 and LDA‐induced gastric mucosal injury is an important issue. Ultimately, there has been almost no manuscript as detailed case reports of LDA‐induced gastric mucosal injury caused due to aspirin after Fontan surgery. Therefore, it is necessary to examine this complication by accumulating further cases.
In conclusion, it is important to undergo more rigorous follow‐up regarding gastric status considering LDA‐induced gastric mucosal injury when an LDA regimen is accompanied by abdominal symptoms, even in adolescents, and after a long time from the start of LDA.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. There was no external funding in the preparation of this manuscript.
AUTHOR CONTRIBUTIONS
Dr Kakiuchi and Dr Kumamoto: involved in patient care as well as the drafting, review, and revision of the initial manuscript. Dr Koji: involved in patient care as well as the review and revision of the initial manuscript. Dr Matsuo: involved in patient care and project administration as well as the review and revision of the initial manuscript. All authors approved the final manuscript submission and agreed to be accountable for all aspects of the study.
ETHICAL APPROVAL
Ethical review and approval of the study on human participants in accordance with the local legislation and institutional requirements were not required in this case. Written informed consent to publish this case was provided by the patient herself and the patient's legal guardian/next of kin.
ACKNOWLEDGMENTS
The authors thank the patient's family for providing consent and granting permission to draft and publish this case report. We acknowledge Saga University Hospital medical stuff related to these patients in the preparation of this manuscript. Published with written consent of the patient.
Kakiuchi T, Kumamoto T, Koji A, Matsuo M. Low‐dose aspirin‐induced gastric mucosal injury after Fontan surgery in an adolescent. Clin Case Rep. 2021;9:2460–2464. 10.1002/ccr3.4070
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kreutzer G, Galíndez E, Bono H, De Palma C, Laura JP. An operation for the correction of tricuspid atresia. J Thorac Cardiovasc Surg. 1973;66:613‐621. [PubMed] [Google Scholar]
- 3. Khairy P, Fernandes SM, Mayer JE Jr, et al. Long‐term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117:85‐92. [DOI] [PubMed] [Google Scholar]
- 4. Potter BJ, Leong‐Sit P, Fernandes SM, et al. Effect of aspirin and warfarin therapy on thromboembolic events in patients with univentricular hearts and Fontan palliation. Int J Cardiol. 2013;168:3940‐3943. [DOI] [PubMed] [Google Scholar]
- 5. Alsaied T, Alsidawi S, Allen CC, Faircloth J, Palumbo JS, Veldtman GR. Strategies for thromboprophylaxis in Fontan circulation: a meta‐analysis. Heart. 2015;101:1731‐1737. [DOI] [PubMed] [Google Scholar]
- 6. Ooi YK, Ligon RA, Kelleman M, et al. Thromboprophylaxis strategies for children with single‐ventricle circulations (superior or total cavo‐pulmonary connections) after stent implantation. Cardiol Young. 2019;29:877‐884. [DOI] [PubMed] [Google Scholar]
- 7. Iwamoto J, Saito Y, Honda A, Matsuzaki Y. Clinical features of gastroduodenal injury associated with long‐term low‐dose aspirin therapy. World J Gastroenterol. 2013;19:1673‐1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shiotani A, Manabe N, Kamada T, Fujimura Y, Sakakibara T, Haruma K. Risk and preventive factors of low‐dose aspirin‐induced gastroduodenal injuries: a comprehensive review. J Gastroenterol Hepatol. 2012;27(suppl 3):8‐12. [DOI] [PubMed] [Google Scholar]
- 9. Sakamoto C, Sugano K, Ota S, et al. Case‐control study on the association of upper gastrointestinal bleeding and nonsteroidal anti‐inflammatory drugs in Japan. Eur J Clin Pharmacol. 2006;62:765‐772. [DOI] [PubMed] [Google Scholar]
- 10. Lanas A, García‐Rodríguez LA, Arroyo MT, et al. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo‐oxygenase‐2 inhibitors, traditional non‐aspirin non‐steroidal anti‐inflammatory drugs, aspirin and combinations. Gut. 2006;55:1731‐1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Graham DY. Nonsteroidal anti‐inflammatory drugs, Helicobacter pylori, and ulcers: where we stand. Am J Gastroenterol. 1996;91:2080‐2086. [PubMed] [Google Scholar]
- 12. Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340:1888‐1899. [DOI] [PubMed] [Google Scholar]
- 13. Lanas A, Scheiman J. Low‐dose aspirin and upper gastrointestinal damage: epidemiology, prevention and treatment. Curr Med Res Opin. 2007;23:163‐173. [DOI] [PubMed] [Google Scholar]
- 14. Yeomans ND, Lanas AI, Talley NJ, et al. Prevalence and incidence of gastroduodenal ulcers during treatment with vascular protective doses of aspirin. Aliment Pharmacol Ther. 2005;22:795‐801. [DOI] [PubMed] [Google Scholar]
- 15. Hart J, Hawkey CJ, Lanas A, et al. Predictors of gastroduodenal erosions in patients taking low‐dose aspirin. Aliment Pharmacol Ther. 2010;31:143‐149. [DOI] [PubMed] [Google Scholar]
- 16. Lanas A, Fuentes J, Benito R, Serrano P, Bajador E, Sáinz R. Helicobacter pylori increases the risk of upper gastrointestinal bleeding in patients taking low‐dose aspirin. Aliment Pharmacol Ther. 2002;16:779‐786. [DOI] [PubMed] [Google Scholar]
- 17. Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non‐steroidal anti‐inflammatory drugs in peptic‐ulcer disease: a meta‐analysis. Lancet. 2002;359:14‐22. [DOI] [PubMed] [Google Scholar]
- 18. Shiotani A, Sakakibara T, Yamanaka Y, et al. Upper gastrointestinal ulcer in Japanese patients taking low‐dose aspirin. J Gastroenterol. 2009;44:126‐131. [DOI] [PubMed] [Google Scholar]
- 19. Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn't the stomach digest itself? Physiol Rev. 2008;88:1547‐1565. [DOI] [PubMed] [Google Scholar]
- 20. Hernández‐Díaz S, Rodríguez LA. Association between nonsteroidal anti‐inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Arch Intern Med. 2000;160:2093‐2099. [DOI] [PubMed] [Google Scholar]
- 21. Buttgereit F, Burmester GR, Simon LS. Gastrointestinal toxic side effects of nonsteroidal anti‐inflammatory drugs and cyclooxygenase‐2‐specific inhibitors. Am J Med. 2001;110(suppl 3A):13s‐19s. [DOI] [PubMed] [Google Scholar]
- 22. Leandro G, Pilotto A, Franceschi M, Bertin T, Lichino E, Di Mario F. Prevention of acute NSAID‐related gastroduodenal damage: a meta‐analysis of controlled clinical trials. Dig Dis Sci. 2001;46:1924‐1936. [DOI] [PubMed] [Google Scholar]
- 23. García Rodríguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non‐steroidal anti‐inflammatory drugs. Lancet. 1994;343:769‐772. [DOI] [PubMed] [Google Scholar]
- 24. Serrano P, Lanas A, Arroyo MT, Ferreira IJ. Risk of upper gastrointestinal bleeding in patients taking low‐dose aspirin for the prevention of cardiovascular diseases. Aliment Pharmacol Ther. 2002;16:1945‐1953. [DOI] [PubMed] [Google Scholar]
- 25. Derry S, Loke YK. Risk of gastrointestinal haemorrhage with long term use of aspirin: meta‐analysis. BMJ. 2000;321:1183‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Monagle P, Cochrane A, Roberts R, et al. A multicenter, randomized trial comparing heparin/warfarin and acetylsalicylic acid as primary thromboprophylaxis for 2 years after the Fontan procedure in children. J Am Coll Cardiol. 2011;58:645‐651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


