Abstract
Case reports of CAPA emerged. In most of the reports, the predominant species is Aspergillus fumigatus. Uncommon species are less reported. Due to poor clinical outcome with Aspergillus terreus, the increasing reports with this agent require attention.
Keywords: aspergillosis, aspergillus, Aspergillusterreus, CAPA, COVID‐19, ICU, SARS‐CoV‐2
Case reports of CAPA emerged. In most of the reports, the predominant species is Aspergillus fumigatus. Uncommon species are less reported. Due to poor clinical outcome with Aspergillus terreus, the increasing reports with this agent require attention.
1. INTRODUCTION
We present a fatal case of COVID‐19‐associated pulmonary aspergillosis (CAPA) caused by Aspergillus terreus in an immunocompetent patient. Due to the poor clinical outcome with A. terreus independent of the type of antifungal therapy, the increasing reports with this agent requires special attention.
China reported an outbreak of COVID‐19 in December 2019. This entity has rapidly become a global threat, with high mortality and morbidity rates worldwide. 1 Secondary fungal infections are increasingly being reported in patients with COVID‐19. Invasive pulmonary aspergillosis (IPA) with Aspergillus spp. occurs primarily in patients with immunodeficiency. 2 Risk factors for IPA are well described in immunocompromised patients. On the other hand, patients with some severe viral pneumonia such as COVID‐19 are prone to secondary complications like IPA in the absence of immunocompromising condition, which is termed COVID‐19 Associated Pulmonary Aspergillosis (CAPA). 3 Here, we present a fatal case of CAPA caused by Aspergillus terreus in an immunocompetent patient.
2. CASE PRESENTATION
In October 2020, a 66‐year‐old immunocompetent woman with fever (38.5°C), myalgia, dry cough, and dyspnea and oxygen saturation (SpO2) of 87% on room air was admitted to the emergency department of Taleghani hospital, Tehran, Iran. Laboratory results showed white blood cells: 8500/µL (normal range: 4000‐11 000), hemoglobin: 10.5 mg/dL (normal range: 12.3‐15.3), platelets: 257 000/µL (normal range: 150 000‐450 000), creatinine: 0.9 mg/dL (normal range: 0.5‐1.3), C‐reactive protein: 26 mg/dL (normal range: up to 6), and real‐time reverse transcriptase polymerase chain reaction confirmed SARS‐CoV‐2 infection. Chest computed tomography scan was highly suggestive of COVID‐19‐induced pneumonia (Figure 1). Supportive oxygen therapy with the nasal cannula at 4‐5 liters per minute, and treatment with interferon beta 1a, 12 million units subcutaneously once in alternate day for five doses and dexamethasone 8 mg intravenously daily was administered from the first day of hospital admission (day 0). On day 5, the SpO2 was 88% and with relative improvement of dyspnea. On day 8, the patient was afebrile and SpO2 was 91%. On day 9, the interferon beta 1a was discontinued. Dexamethasone was continued until day 11 and then was stopped. Supportive oxygen therapy was continued. On day 15, the patient became tachypnic and SpO2 was 89%. With the suspicion of pulmonary embolism, the patient underwent chest MDCT scan and was negative for pulmonary embolism. On day 17, the SpO2 decreased to 77% with respiratory distress and the patient was intubated because of respiratory failure and progressive hypoxemia. Portable chest x‐ray was performed and revealed new progressive infiltration (Figure 2). Empirical antibiotic therapy consisting of meropenem 1gr every 8 hours and vancomycin 1 gr every 12 hours were started. Serum galactomannan antigen (GM) test with the Platelia Aspergillus ELISA kit (Bio‐Rad) was requested and was 4.15. According to the high level of serum GM, voriconazole 6 mg/kg every 12 hours on day one, and then, 4 mg/kg every 12 hours was added to the empiric antimicrobial regimen. Then, bronchoscopy and mini‐bronchoalveolar lavage (BAL) was performed and sent for microbiology evaluation. The BAL sample evaluated for both bacterial and fungal smear and culture. Bacterial smear and culture reported negative but the sample was cultured with colonies grew within 4 days that phenotypically was consistent with A. terreus. The fungal stain we used was lacto phenol cotton blue. Microscopy features classified as A. terreus (Figure 3A and 3B). Although the culture plate has 2 distinct growths, A. terreus and Aspergillus fumigatus, but because the colony count of A. fumigatus was low we ignored it. We isolated A. terreus as the predominant colony. Caspofungin was added to voriconazole therapy, until the MIC results were ready. We repeated the mini BAL sample for aspergillus culture and the result were the same. DNA was extracted from 4‐day‐old culture and stored. The DNA sequence matched that of A. terreus. The molecular results confirmed the diagnosis of pulmonary aspergillosis due to A. terreus.
FIGURE 1.

Thorax CT scan revealed diffuse bilateral ground glass opacities
FIGURE 2.
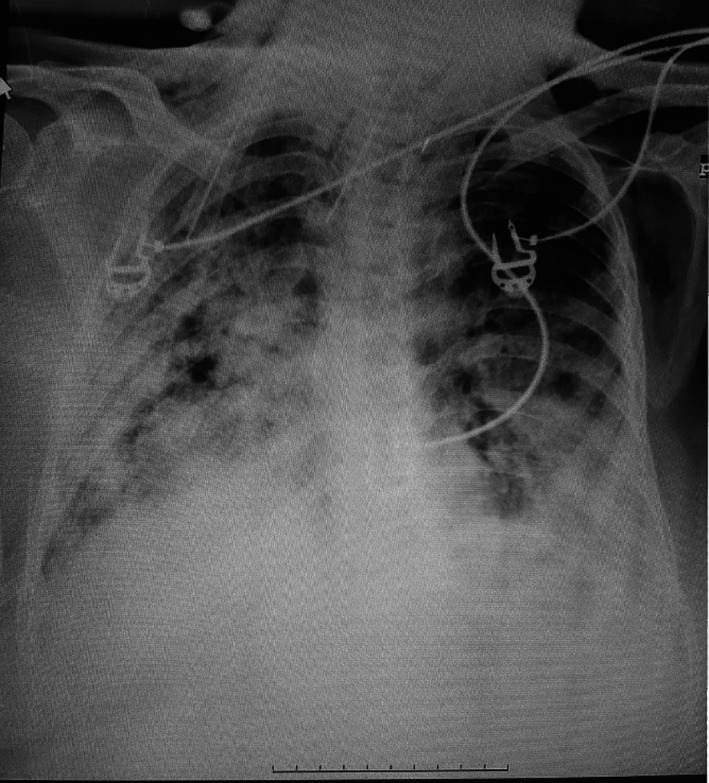
Chest x‐ray revealed bilateral progressive alveolar parenchymal opacification
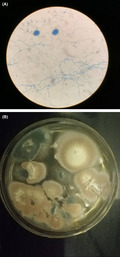
FIGURE 3.
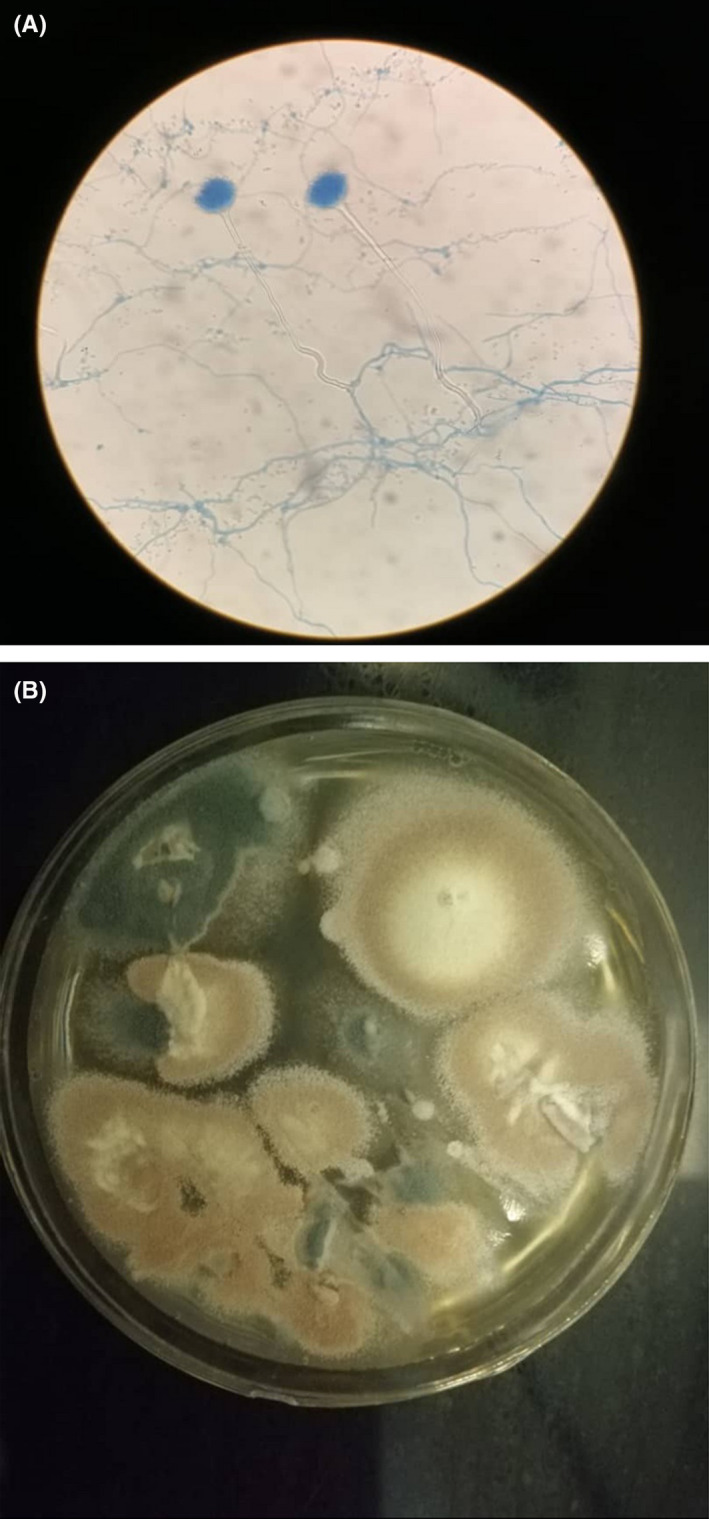
A, Microscopy features classified as Aspergillus terreus. B, Colonies phenotypically were consistent with A. terreus
Antifungal susceptibility tests were performed according to clinical and laboratory standard institute methods. 4 The MIC values were as follows: amphotericin B (≥16 μg/mL), itraconazole (1 μg/mL), and voriconazole (0.5 μg/mL). For echinocandins (including caspofungin), we use MEC (minimum effective concentration) instead of MIC and it was 1 μg/mL. Hence, phenotypic and molecular identification was A. terreus which was resistant to amphotericin B and caspofungin and sensitive to itraconazole and voriconazole. Despite antifungal therapy and in vitro susceptibility to voriconazole, the patient died due to respiratory failure on day 29.
3. DISCUSSION
We report a case of CAPA due to A. terreus, which occurred in a hospitalized immunocompetent patient in intensive care unit (ICU). It has been recognized that rates of IPA are high among patients in ICUs with severe influenza, even without other known risk factors for invasive fungal infections. Other severe viral pneumonias also may increase the risk of invasive aspergillosis. The incidence, outcomes, and pathophysiological mechanisms for CAPA in patients with critical illness are unknown. 5 , 6 Perhaps the development of CAPA is a result of the pathogen itself and some environmental factors. Structural lung damage caused by the virus may favor the development of concurrent invasive fungal infections. On the other hand, steroid use has emerged as a supportive treatment for COVID‐19 in guidelines, especially in severe cases. Its use disturbs the activity of alveolar macrophages and accelerates the growth of fungi. 7 Case reports of CAPA have been published till now, and the most common species was reported A. fumigatus. Koehler et al. described 5 cases of CAPA in 19 patients with severe COVID‐19. 3 Helleberg M et al. reported 2 cases of severe CAPA due to A. fumigatus. One of them had received steroids, and the other had not. Both of them received Voriconazole as antifungal therapy. 5 Arastehfar et al reviewed 35 cases of CAPA. The most common species was reported to be A. fumigatus. 8 Similar to our case, Abdallah et al reported a fatal case of CAPA due to A. terreus, that the patient received voriconazole. 9
Two points are of interest in the case we described: first, to our knowledge, this case is the first report from Iran. Second, this description highlights the increased risk of CAPA even with unusual species in patients without immunodeficiency, which is a matter of concern. The patient, we reported here had no well‐defined and classical risk factors for IPA; hence, COVID‐19 infection was the most probable etiology. HIV‐Ab test was nonreactive and nitroblue tetrazolium (NBT) test was negative. It is well known that the most common agent of invasive aspergillosis is A. fumigatus. Aspergillus terreus is an unusual emerging infection, and its incidence has increased recently with the high mortality rate in comparison to other Aspergillus species. On the other hand, A. terreus species complex can also affect immunocompetent patients with underlying lung diseases. 10 The remarkable note and challenging issue about this notoriously difficult species is that it is intrinsically resistant to amphotericin B and may even be resistant to azoles. 11 In our case, the species was resistant to amphotericin B but susceptible to voriconazole. It is well recognized that voriconazole is the first‐line therapy for IPA, but failure to treatment against A. terreus infection has been documented in 52.9% of cases. 12 This is a crucial point in clinical practice when selecting empiric antifungal therapy in critically ill patients with suspicion of CAPA pending culture results. So, uncommon species also should be considered when initiating antifungal therapy. Combination therapy with caspofungin may be helpful, 13 , 14 although there are limited data and clinical experience with echinocandins in the treatment of A. terreus species. 10 Our case revealed in vitro resistance to caspofungin. Clinical signs in CAPA are nonspecific and include fever, chest pain, cough, and hemoptysis. 15 GM antigen, the specific marker of invasive aspergillosis, is not as sensitive as in neutropenic patients, and detection of it contributes substantially to the diagnosis of probable IPA. 16 , 17 However, the serum GM level in our non‐neutropenic patient was significantly elevated and was compatible with microbiological findings. The level of GM in different species of Aspergillus can vary, and its level in A. terreus is considerably higher than that of A. fumigatus. 18 We repeated the microbiology evaluation two times, and both of them revealed A. terreus species. In addition to significantly elevated serum GM level, and typical COVID‐19 findings in chest CT Scan, these results supported the diagnosis of probable CAPA due to A. terreus. Because the patient was intubated and unstable, the tissue biopsy was not possible.
4. CONCLUSION
CAPA should always be considered even with uncommon species. Early clinical suspicion and timely appropriate antifungal therapy are also necessary. The incidence and outcomes for CAPA need further studies.
CONFLICT OF INTEREST
The authors declare that they have no competing interest.
AUTHOR CONTRIBUTIONS
S.A and A.H acquired data, analyzed, and interpreted the data. S.S and M.PT identified Aspergillus terreus. A.H wrote the first draft of the manuscript. S.A revised the manuscript. All authors have read and approved the final manuscript.
ETHICAL STATEMENT
This research was approved by the ethics committee of Shahid Beheshti University of Medical Sciences (Ethical code: IR.SBMU.MSP.REC.1399.739) and written informed consent was obtained from the patient.
ACKNOWLEDGMENTS
We would like to thank the Masih Daneshvari mycology laboratory, Tehran, Iran, for determining the isolate and MIC.
Abolghasemi S, Hakamifard A, Sharifynia S, Pourabdollah Toutkaboni M, Azhdari Tehrani H. Fatal invasive pulmonary aspergillosis in an immunocompetent patient with COVID‐19 due to Aspergillus terreus: A case study. Clin Case Rep. 2021;9:2414–2418. 10.1002/ccr3.4051
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this case report type article as no new data were created or analyzed in this study.
REFERENCES
- 1. VanArkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID‐19‐associated Pulmonary Aspergillosis. Am J RespirCrit Care Med. 2020;202(1):132‐135. 10.1164/rccm.202004-1038LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20(121):156‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koehler P, Cornely OA, Böttiger BW, et al. COVID‐19 associated pulmonary aspergillosis. Mycoses. 2020;63(6):528‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clinical and Laboratory Standards Institute . Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard. CLSI document M38–A2, 2nd ed; Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 5. Helleberg M, Steensen M, Arendrup MC. Invasive aspergillosis in patients with severe COVID‐19 pneumonia. Clin Microbiol Infect. 2021;27(1):147‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma A, Hofmeyr A, Bansal A, et al. COVID‐19 associated pulmonary aspergillosis (CAPA): An Australian case report. Med Mycol Case Rep. 2020;31:6‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Apostolopoulou A, EsquerGarrigos Z, Vijayvargiya P, Lerner AH, Farmakiotis D. Invasive pulmonary aspergillosis in patients with SARS‐CoV‐2 infection: a systematic review of the literature. Diagnostics. 2020;10(10):807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arastehfar A, Carvalho A, van de Veerdonk FL, et al. COVID‐19 Associated Pulmonary Aspergillsis (CAPA)‐from immunology to treatment. J Fungi (Basel). 2020;6(2):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abdalla S, Almaslamani MA, Hashim SM, Ibrahim AS, Omrani AS. Fatal coronavirus disease 2019‐associated pulmonary aspergillosis; a report of two cases and review of the literature. IDCases. 2020;1(22):e00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lass‐Flörl C. Treatment of infections due to Aspergillus terreus species complex. J Fungi. 2018;4(3):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pastor FJ, Guarro J. Treatment of Aspergillus terreus infections: a clinical problem not yet resolved. Int J Antimicrob Agents. 2014;44(4):281‐289. [DOI] [PubMed] [Google Scholar]
- 12. Steinbach WJ, Benjamin DK Jr, Kontoyiannis DP, et al. Infections due to Aspergillus terreus: a multicenter retrospective analysis of 83 cases. Clin Infect Dis. 2004;39(2):192‐198. [DOI] [PubMed] [Google Scholar]
- 13. Cooke FJ, Terpos E, Boyle J, Rahemtulla A, Rogers TR. Disseminated Aspergillus terreus infection arising from cutaneous inoculation treated with caspofungin. Clin Microbiol Infect. 2003;9(12):1238‐1241. [DOI] [PubMed] [Google Scholar]
- 14. Van Der Linden JW, Warris A, Verweij PE. Aspergillus species intrinsically resistant to antifungal agents. Med Mycol. 2011;49(suppl 1):S82‐S89. [DOI] [PubMed] [Google Scholar]
- 15. Reichenberger F, Habicht JM, Gratwohl A, Tamm M. Diagnosis and treatment of invasive pulmonary aspergillosis in neutropenic patients. Eur Respir J. 2002;19(4):743‐755. [DOI] [PubMed] [Google Scholar]
- 16. Van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID‐19 associated pulmonary aspergillosis. Am J RespirCrit Care Med. 2020;63(6):528‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duarte RF, Sánchez‐Ortega I, Cuesta I, et al. Serum galactomannan‐based early detection of invasive Aspergillosis in hematology patients receiving effective antimold prophylaxis. Clin Infect Dis. 2014;59:1696‐1702. [DOI] [PubMed] [Google Scholar]
- 18. Mennink‐Kersten MA, Ruegebrink D, Wasei N, Melchers WJ, Verweij PE. In vitro release by Aspergillus fumigatus of galactofuranose antigens, 1, 3‐β‐D‐glucan, and DNA, surrogate markers used for diagnosis of invasive aspergillosis. J Clin Microbiol. 2006;44(5):1711‐1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this case report type article as no new data were created or analyzed in this study.


