Abstract
Larotrectinib, a highly selective TRK inhibitor, was administered to a patient with rapidly progressing radioactive iodine‐refractory papillary NTRK3 fusion‐positive thyroid cancer. The patient achieved a durable (sustained for 11 months) complete response after 2 months of treatment and complete intracranial responses in metastatic brain lesions after 7 months of treatment.
Larotrectinib may provide a therapeutic route for patients with RAI‐R‐differentiated thyroid cancer who might otherwise have few treatment options.
Keywords: iodine uptake and iodine metabolism, thyroid cancer‐clinical, thyroid cancer‐genetics
1. INTRODUCTION
Differentiated thyroid cancer (DTC) is the most common type of thyroid cancer, comprising 85‐95% of all thyroid cancers; 1 , 2 , 3 from 1990 to 2013, the global age‐standardized incidence rate increased by 20%. 4 DTC is dominated by papillary (PTC) and follicular subtypes, and rarer variants include Hürthle cell and poorly differentiated thyroid cancers. 1
Surgical treatment with complete or partial thyroidectomy is central to the management of DTC, followed by administration of radioactive iodine (RAI). 5 , 6 For the majority of patients with DTC, the disease course is indolent and can be managed by these standard treatments. 6 However, 3‐20% of patients may develop distant metastatic disease, and around half of these become RAI‐refractory (RAI‐R); this presents a significant clinical challenge, with a 10‐year survival rate of less than 10%. 7 , 8 , 9 , 10 For these patients, sorafenib and lenvatinib are routinely used in real‐world practice and confer clinical benefit to the majority of patients; however, an unmet clinical need persists for those patients who progress despite treatment with these multikinase inhibitors (MKIs). 11 Further advances in genomic profiling have led to the discovery of other targetable mutations in thyroid cancer. 8 , 12
Recently, NTRK gene fusions, which lead to overexpression of chimeric TRK proteins, have been identified as primary oncogenic drivers across multiple cancer types—characterized as TRK fusion cancer—including thyroid cancer, in adult and pediatric patients. 13 Larotrectinib is a tyrosine kinase inhibitor and a highly selective inhibitor of NTRK1‐3, and has received approval as a tumor‐agnostic treatment for any solid tumor harboring an NTRK gene fusion based on the results from three basket trials, which included patients with TRK fusion thyroid cancer. 13 , 14
Detection of actionable genomic events can dramatically alter treatment plans for patients and may extend clinical benefit compared with standard of care. Larotrectinib presents a promising treatment strategy for patients who have exhausted all other treatment options and fulfills an unmet clinical need. 13 , 15 Here, we present the case of a 56‐year‐old woman with advanced RAI‐R PTC who progressed on both sorafenib and lenvatinib and then received larotrectinib following the detection of an NTRK gene fusion.
2. CASE PRESENTATION
A 56‐year‐old female with PTC was referred to the clinic following an incomplete surgical resection in November 2017. The pathology report showed multifocal classical PTC, with a larger focus of 2 cm. The TNM status for this patient was IVB. 16 After thyroid hormone withdrawal, the patient's thyroid stimulating hormone level was 144 mUI/mL, with positive thyroglobulin antibody levels of 1081 UI/mL and undetectable thyroglobulin levels one month after surgery in December 2017; RAI was subsequently administered at a dose of 130 mCi 131I. Following RAI treatment, the patient underwent a whole‐body scan, which showed uptake in the thyroid bed and mediastinum only—a concomitant computed tomography (CT) scan revealed persistent macroscopic disease in the patient's neck and mediastinum, and the presence of a single lung lesion larger than 15 mm in diameter. At this point, RAI‐R PTC was diagnosed.
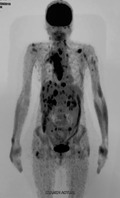
In April 2018, five months after initial referral to the clinic, the patient underwent a positron emission tomography (PET) scan with 2‐deoxy‐2‐[fluorine‐18] fluoro‐D‐glucose integrated with a CT scan (18F‐FDG PET/CT), which revealed multiple metastatic sites (Figure 1 and Figure 2). Lesions were identified in the neck, mediastinum, and several locations in soft and muscular tissues. The lesion previously identified in the left lung had grown to a size of 23 mm. In addition, the patient had uptake in multiple bones and a scalp lesion that was confirmed as a PTC metastasis via biopsy. The patient's disease was characterized as metastatic, rapidly progressive RAI‐R PTC.
FIGURE 1.
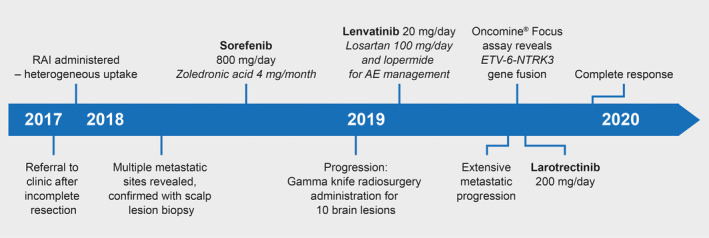
Treatment pathway of a patient with PTC who progressed on sorafenib and lenvatinib, and who achieved a complete response with larotrectinib treatment. AE, adverse event; PTC, papillary thyroid cancer; RAI, radioactive iodine
FIGURE 2.
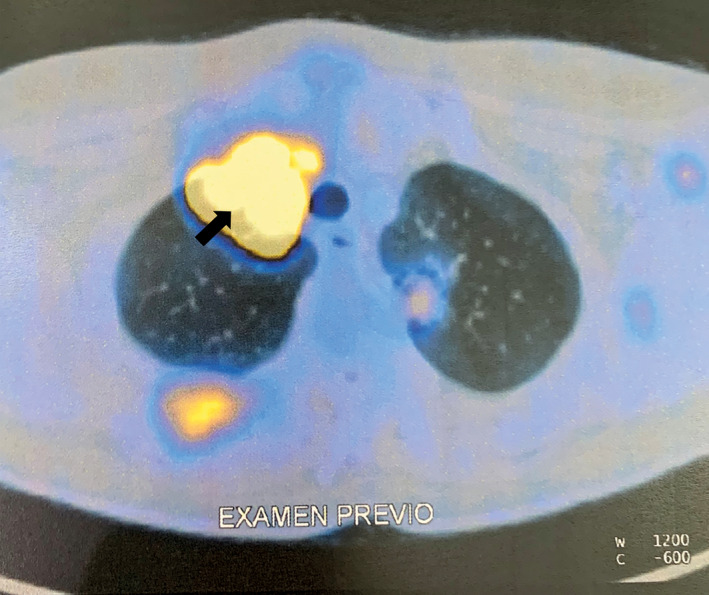
A diagnosis of RAI‐R thyroid cancer was based on the presence of multiple metastatic sites revealed with 18F‐FDG uptake in the first PET/CT scan conducted in April 2018. Diffuse lesions in soft and muscular tissues, multiple 18F‐FDG uptake in bones, and a large target lesion in the left lung were observed (arrow) (transverse view). 18 F‐FDG, 2‐deoxy‐2‐[fluorine‐18] fluoro‐D‐glucose; PET/CT, positron emission tomography/computed tomography; RAI‐R, radioiodine refractory
In August 2018, the patient was prescribed systemic therapy comprising zoledronic acid (4 mg/month; intravenous) combined with sorafenib (800 mg/day; oral). The sorafenib dose was subsequently reduced to 600 mg/day following development of grade 2 hand‐foot skin reaction; however, after 6 months (January 2019) on sorafenib treatment, disease progression was evident at multiple sites, including the development of metastases to the brain (progressive disease [PD] as per Response Evaluation Criteria in Solid Tumors [RECIST v1.1] 17 ). The patient subsequently underwent external beam radiotherapy (EBRT; gamma knife) for 10 separate brain lesions. Two months later, lenvatinib was initiated (20 mg/day) and was generally well tolerated—and the patient experienced grade 2 hypertension and grade 1 diarrhea, for which losartan (100 mg) and loperamide (4‐6 mg/day) were prescribed after each diarrheic episode. After five months of lenvatinib treatment, the patient underwent a further 18F‐FDG PET/CT scan, which revealed extensive metastatic progression: multiple lymph node metastases in the neck and mediastinum, multiple secondary lesions in subcutaneous tissues and muscles, a new target lesion in the right adrenal gland, and multiple metastases in the liver and right pleura (PD as per RECIST v1.1) (Figure 3). Despite prior gamma knife EBRT, most of the brain metastases had persisted.
FIGURE 3.
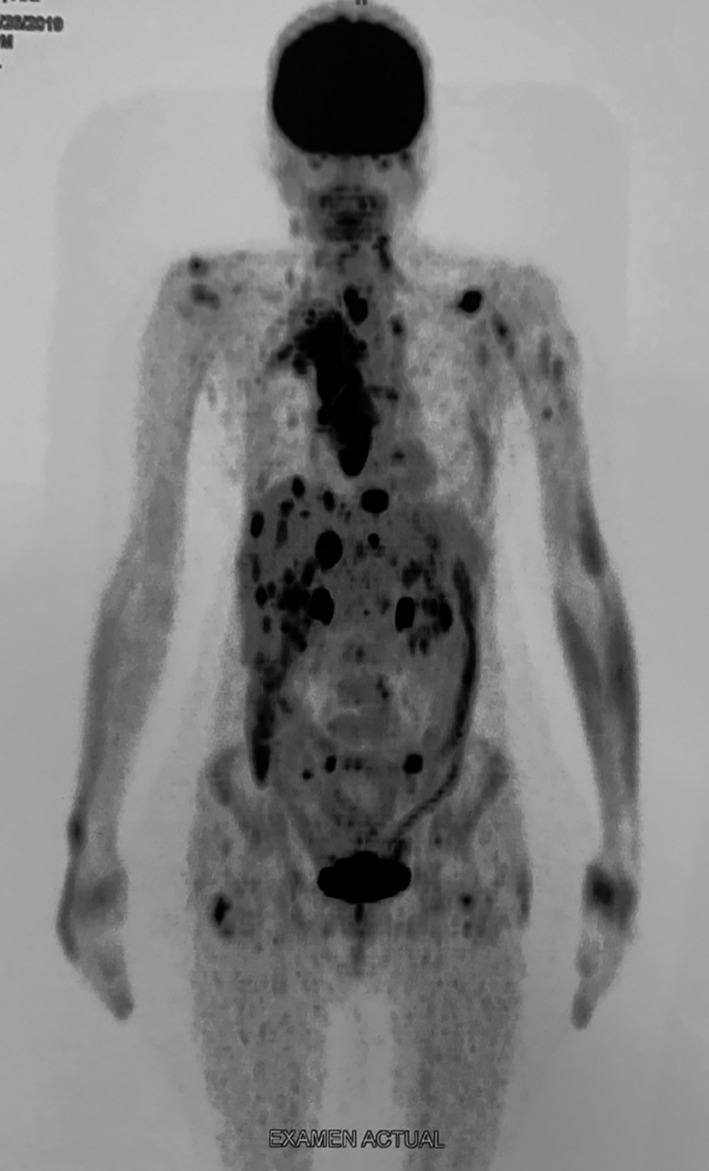
The extent of metastatic disease following treatment was determined by 18F‐FDG uptake in the PET/CT scan conducted in August 2019 after 6 months of sorafenib treatment (reduced to 600 mg/day) and 5 months of lenvatinib treatment (20 mg/day). Multiple lymph node metastases were observed in the neck and mediastinum, with multiple secondary lesions in subcutaneous tissues and muscles, the liver, adrenal gland, and right pleura were identified (coronal view). 18 F‐FDG, 2‐deoxy‐2‐[fluorine‐18] fluoro‐D‐glucose; PET/CT, positron emission tomography/computed tomography
As there were no further approved treatment options available, the decision was made to perform genomic sequencing to identify possible actionable molecular targets. The next‐generation sequencing assay, using the scalp lesion biopsy taken in April 2018, revealed the presence of an ETV6‐NTRK3 gene fusion. Larotrectinib was subsequently administered to the patient at a dose of 200 mg/day (100 mg administered twice daily), provided by Bayer as part of a compassionate use program in September 2019, almost two years after initial presentation. Larotrectinib was well tolerated—the patient experienced grade 1 fatigue and mild hepatic enzyme elevation was observed. After 4 weeks on larotrectinib treatment (October 2019), the patient underwent another 18F‐FDG PET/CT scan, which showed a near‐complete response: most of the lesions had disappeared, there was a decrease in volume in the metastatic lesion of the adrenal gland, and only the lung and neck lymph node metastases persisted (Figure 4). After 8 weeks on larotrectinib (November 2019), the patient underwent a further 18F‐FDG PET/CT scan, which showed a complete response—all target lesions had disappeared (Figure 5), and a magnetic resonance imaging scan performed after 7 months of larotrectinib treatment revealed that all brain metastases had disappeared (Figure 6). The complete response has been sustained after 11 months of treatment with larotrectinib, and the patient has returned to her normal quality of life. Additionally, the patient's thyroglobulin antibody levels had decreased from 1081 UI/mL at baseline to 400 UI/mL when measured after 7 months of larotrectinib treatment.
FIGURE 4.
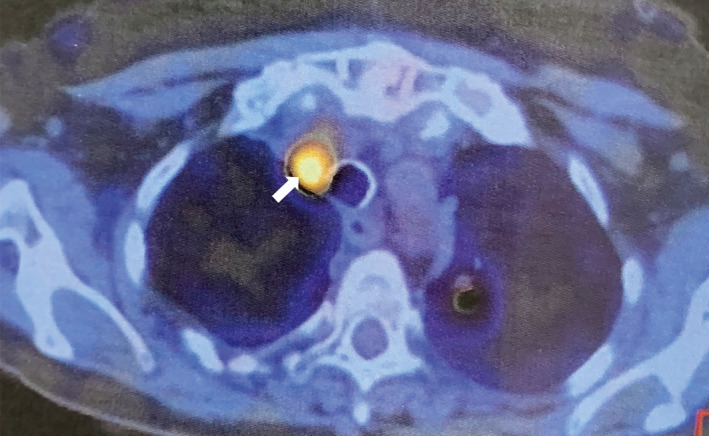
After 4 weeks on larotrectinib treatment, an 18F‐FDG PET/CT scan showed a near‐complete response—only neck lymph node and lung (arrowed) lesions persisted in October 2019 after 4 weeks of larotrectinib (200 mg/day) treatment (transverse view); most lesions had disappeared. 18 F‐FDG, 2‐deoxy‐2‐[fluorine‐18] fluoro‐D‐glucose; PET/CT, positron emission tomography/computed tomography
FIGURE 5.
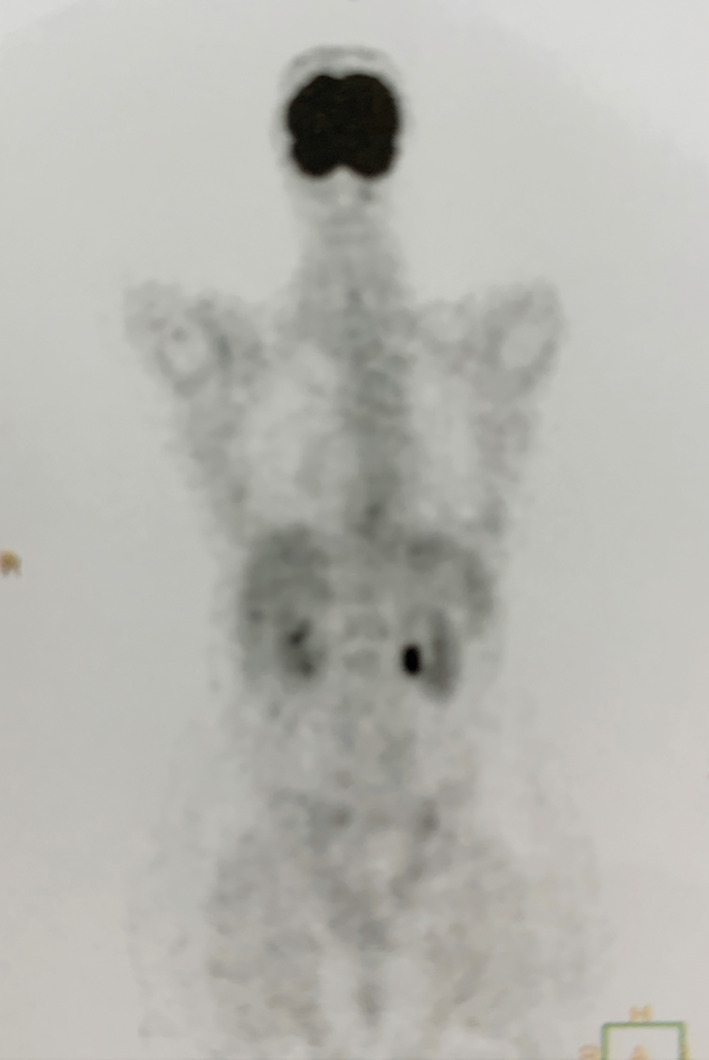
Complete response after 8 weeks of larotrectinib (200 mg/day) treatment in November 2019. No lesions with 18F‐FDG uptake were observed during the PET/CT scan. 18 F‐FDG, 2‐deoxy‐2‐[fluorine‐18] fluoro‐D‐glucose; PET/CT, positron emission tomography/computed tomography
FIGURE 6.
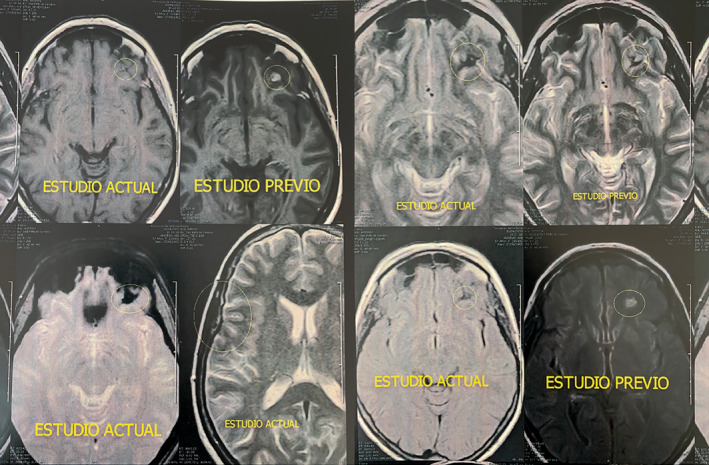
Intracranial response of metastatic lesions in the brain following larotrectinib (200 mg/day) treatment. MRI scans show substantial reductions in lesion sizes following 7 months of treatment. MRI, magnetic resonance imaging
3. DISCUSSION
A combination treatment approach involving surgery with or without thyroid hormones and RAI is a curative treatment approach for most patients with thyroid cancer, and typically provides an excellent prognosis. 1 , 5 However, this approach is unsuitable for patients who have metastatic disease or are RAI‐R, and it is this subset of patients who principally contribute to thyroid cancer‐related deaths. 8 , 10 An evolved understanding of the molecular mechanisms contributing to the development of DTC has recently ushered in a move toward targeted therapies. MKIs block kinase receptors involved in angiogenesis (vascular endothelial growth factor receptor and platelet‐derived growth factor receptor) and downstream cell survival and proliferation (fibroblast growth factor receptor and proto‐oncogene tyrosine‐protein kinase receptor Ret [RET]) via the RTK/BRAF/MAPK/ERK and PI3K/AKT/mTOR pathways. 18 MKIs, such as sorafenib and lenvatinib, are now used routinely in clinical practice for patients who are RAI‐R. 8 , 11 , 19 , 20 , 21 , 22 , 23 , 24 , 25 However, their toxicity and the small but significant subset of patients (including the patient described in this report) who do not benefit from MKIs highlight the need for more effective systemic therapies for RAI‐R DTC. 12 , 20 , 26 , 27 , 28 Patients who progressed on both first‐line and second‐line MKIs were, until recently, left with limited alternative treatment options. As ever, clinical trials provided experimental options and off‐label use of other MKIs, such as cabozantinib, pazopanib, sunitinib, donafenib, and apatinib, which may have been available depending on regional health system constraints. 29 , 30 If these were not available, the only feasible treatment option outside of best supportive care would have been to rechallenge with sorafenib or lenvatinib, despite disease progression.
The focus has now shifted toward the identification of specific molecular alterations, which can dramatically alter treatment pathways for patients with limited treatment options. 12 , 29 Detection of an actionable alteration with genomic profiling is mandatory for patients to be able to receive these matched targeted therapies. In the case described in this report, a comprehensive next‐generation sequencing assay was used to detect the presence of an ETV6‐NTRK3 gene fusion; 31 this unveiled an alternative treatment strategy as larotrectinib is approved for all solid tumors harboring an NTRK gene fusion. 32 This case illustrates the importance of genomic profiling—a patient who was initially left with very limited treatment options having progressed on both sorafenib and lenvatinib was ultimately able to achieve a durable complete response with larotrectinib. Of note, complete responses were also seen in the central nervous system, with the abolition of intracranial metastatic lesions (that had been persistent after EBRT) following larotrectinib treatment.
This patient not only achieved a complete response with larotrectinib after failing multiple lines of prior therapy, but was also able to undergo treatment with minimal toxicity—grade 1 fatigue and mild hepatic enzyme elevation have been the only adverse events observed so far. Larotrectinib is typically well tolerated, with few dose modifications required in an analysis of 260 patients. 14 This contrasts with sorafenib and lenvatinib; their use in thyroid cancer may be limited by a high frequency of adverse events. 11 , 19 , 20
Larotrectinib has been evaluated across 15 tumor types in 159 patients with TRK fusion cancer, and the overall response rate (ORR) to larotrectinib was reported as 79%. 14 In patients with TRK fusion thyroid cancer who have undergone multiple rounds of prior therapy, larotrectinib has elicited response rates of approximately 80% and a median progression‐free survival of 24.6 months across papillary, follicular, and anaplastic subtypes. 14 , 15 , 33 In addition, a recent study reported a patient with ETV6‐NTRK secretory thyroid carcinoma who had a long‐lasting response (21 months) to larotrectinib with complete resolution of the radiologically detectable tumor, and a patient with TPR‐NTRK1 PTC who had a 33% decrease in tumor burden and stable disease for 15 months. 34 NTRK gene fusions occur relatively frequently in thyroid cancer—in 2‐15% of adult patients, with the highest levels observed in post‐Chernobyl reactor accident cases, and in 2‐26% of pediatric patients with thyroid cancer. 34 , 35 , 36 , 37 , 38 , 39 , 40 A number of NTRK gene rearrangements have been observed in thyroid carcinomas, including ETV6‐NTRK3, TPR‐NTRK1, RBPMS‐NTRK3, SQSTM1‐NTRK1/3, and EML4‐NTRK3. 34 As demonstrated in this case, NTRK‐rearranged thyroid cancer is clinically aggressive with a high rate of metastatic spread, but with low mortality when treated with TRK inhibitor therapy. 34 Due to the prevalence and actionability of NTRK gene fusions, upfront molecular testing should be recommended for patients with advanced or metastatic RAI‐R DTC, if feasible. 41 , 42 , 43
Entrectinib, another recently developed TRK inhibitor, also targets NTRK1‐3, but also has activity against proto‐oncogene tyrosine‐protein kinase ROS1 (ROS1), anaplastic lymphoma kinase (ALK), Janus kinase 2 (JAK2), and activated CDC42 kinase 1 (TNK2). 44 , 45 At the time of publication, entrectinib had been evaluated in five patients with TRK fusion thyroid cancer, with a recorded response in one patient (ORR 20%). 45 Entrectinib has also demonstrated efficacy in a patient with thyroid cancer harboring a rare EZR‐ROS1 gene fusion, previously only observed in non‐small cell lung cancer. 46
Inhibitors targeting NTRK gene fusions, such as larotrectinib and entrectinib, are not the only drugs that target a specific genomic alteration in DTC. The MAPK pathway plays a key role in pathogenesis of DTC; oncogenic BRAF mutations are present in approximately 30‐70% of PTC cases, followed by RAS mutations in approximately 15‐20% of cases (40‐50% in follicular thyroid cancer), and RET gene fusions in approximately 5% of cases. 12 , 47 Like larotrectinib, novel therapeutic agents in DTC are often highly selective with few targets, in contrast with the relatively broad inhibitory profiles of sorafenib and lenvatinib. The combination BRAF/MEK inhibitor therapy dabrafenib with trametinib has received Food and Drug Administration (FDA) approval for anaplastic thyroid cancer that harbors a BRAF V600 mutation. 48 , 49 , 50 Dabrafenib and another BRAF V600 inhibitor, vemurafenib, have been investigated in phase 2 trials in DTC with some promising results, but neither are currently approved for DTC. 51 , 52
Selpercatinib (LOXO‐292) is a potent, highly selective RET inhibitor that targets both RET rearrangements and mutations. In a phase 1/2 study, selpercatinib demonstrated very promising efficacy with a tolerable safety profile in 26 patients with thyroid cancer of all histologies harboring RET alterations; based upon these results, the FDA has recently granted selpercatinib priority review status and a phase 3 trial is currently underway in medullary thyroid cancer (MTC) (NCT04211337). 53 Another highly selective RET inhibitor, pralsetinib (BLU‐667), has also demonstrated good efficacy with minimal toxicity in 60 patients with MTC and five patients with PTC harboring RET alterations in a phase 1/2 trial. 54
Highly selective inhibitors targeting oncogenic driver alterations in NTRK, RET, and BRAF seemingly open up several treatment pathways for patients with advanced RAI‐R DTC. However, these oncogenic drivers typically occur in a mutually exclusive manner in thyroid cancer, and so each of these highly selective drugs must be considered as an equivalent, but typically separate, treatment option for advanced RAI‐R DTC. 47 , 55 , 56
RAI‐R DTC is, by nature, an advanced cancer in which several common molecular targets have been identified and, therefore, is suitable for genomic profiling, yet the timing of genomic profiling within the treatment pathway is still a topic of debate. 57 Nevertheless, for this patient, conducting genomic profiling earlier in the treatment pathway would have enabled earlier identification of the NTRK gene fusion and administration of larotrectinib. Earlier genomic profiling at the time of initial disease progression in RAI‐R DTC would have precluded this patient from undergoing treatment with sorafenib and lenvatinib; these treatments were unnecessary despite them being the standard of care for progressive RAI‐R DTC that is not amenable to local treatments, until now. The timing of when and for which patients to perform genomic profiling in RAI‐R DTC is an ongoing debate.
Alterations in the RTK/BRAF/MAPK/ERK and PI3K/AKT/mTOR pathways, most notably BRAF and MEK alterations, have been found to underly the molecular mechanisms of RAI refractoriness. 47 , 58 Novel, single‐target kinase inhibitors of BRAF and MEK, such as vemurafenib, dabrafenib, and selumetinib, have had some success at reversing RAI refractoriness in DTC, and RAI resensitization is a very promising treatment strategy for RAI‐R DTC. 58 , 59 , 60 , 61 Whether inhibition of the TRK receptor, and the downstream RTK/BRAF/MAPK/ERK pathways, with larotrectinib is also able to reverse RAI refractoriness remains to be seen; this was going to be tested in the patient described in this case, but treatment was interrupted by the global COVID‐19 pandemic after confirmation of a complete response.
In summary, a patient with advanced RAI‐R DTC was able to achieve a complete response following larotrectinib treatment, including intracranial responses in metastatic brain lesions. The patient experienced disease progression on both sorafenib and lenvatinib, leaving limited treatment options, and so the decision was made to conduct comprehensive genomic profiling. The identification of an NTRK gene fusion, targetable with larotrectinib, dramatically altered the treatment pathway for this patient. NTRK gene fusions are an actionable alteration in DTC, and genomic profiling of RAI‐R DTC should be undertaken to improve the clinical course for these patients.
CONFLICT OF INTEREST
Fabián Pitoia discloses speaker bureau/expert testimony fees/honoraria from Bayer, Exelixis, Sanofi, Grupo Biotoscana (Representative of EISAI in Argentina), and Raffo Laboratory.
AUTHOR CONTRIBUTIONS
Dr Fabián Pitoia: contributed to the conception, collection/assembly of data, data analysis/interpretation, and writing of this case report.
ETHICS STATEMENT
Ethical committee approval was not required for this case report. Informed patient consent was provided and is available from the corresponding author.
ACKNOWLEDGMENTS
Medical writing support for the development of this case report was provided by Matthew Naylor, PhD, and Emily Clark, PhD, at OPEN Health Communications (London, UK), with financial support from Bayer. The next‐generation sequencing assay was procured from ThermoFisher: ThermoFisher Oncomine® Focus assay. This case report was published with written consent of the patient.
Pitoia F. Complete response to larotrectinib treatment in a patient with papillary thyroid cancer harboring an ETV6‐NTRK3 gene fusion. Clin Case Rep. 2021;9:1905–1912. 10.1002/ccr3.3900
DATA AVAILABILITY STATEMENT
The data that support the findings of this case report are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Fugazzola L, Elisei R, Fuhrer D, et al. 2019 European Thyroid Association Guidelines for the treatment and follow‐up of advanced radioiodine‐refractory thyroid cancer. Eur Thyroid J. 2019;8:227‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. James BC, Mitchell JM, Jeon HD, Vasilottos N, Grogan RH, Aschebrook‐Kilfoy B. An update in international trends in incidence rates of thyroid cancer, 1973–2007. Cancer Causes Control. 2018;29:465‐473. [DOI] [PubMed] [Google Scholar]
- 3. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317‐322. [DOI] [PubMed] [Google Scholar]
- 4. Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. 2020;16:17‐29. [DOI] [PubMed] [Google Scholar]
- 5. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388:2783‐2795. [DOI] [PubMed] [Google Scholar]
- 7. Nixon IJ, Whitcher MM, Palmer FL, et al. The impact of distant metastases at presentation on prognosis in patients with differentiated carcinoma of the thyroid gland. Thyroid. 2012;22:884‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmidt A, Iglesias L, Klain M, Pitoia F, Schlumberger MJ. Radioactive iodine‐refractory differentiated thyroid cancer: an uncommon but challenging situation. Arch Endocrinol Metab. 2017;61:81‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schlumberger M, Brose M, Elisei R, et al. Definition and management of radioactive iodine‐refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014;2:356‐358. [DOI] [PubMed] [Google Scholar]
- 10. Durante C, Haddy N, Baudin E, et al. Long‐term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892‐2899. [DOI] [PubMed] [Google Scholar]
- 11. Jerkovich F, García Falcone MG, Pitoia F. The experience of an Endocrinology Division on the use of tyrosine multikinase inhibitor therapy in patients with radioiodine‐resistant differentiated thyroid cancer. Endocrine. 2019;64:632‐638. [DOI] [PubMed] [Google Scholar]
- 12. Naoum GE, Morkos M, Kim B, Arafat W. Novel targeted therapies and immunotherapy for advanced thyroid cancers. Mol Cancer. 2018;17:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion‐positive cancers in adults and children. N Engl J Med. 2018;378:731‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hong DS, DuBois SG, Kummar S, et al. Larotrectinib in patients with TRK fusion‐positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21:531‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Waguespeck S, Drilon A, Farago AF, et al. Treatment of advanced TRK fusion thyroid cancer with larotrectinib. Eur Thyroid J. 2019;8:1‐127.30800635 [Google Scholar]
- 16. Tuttle M, Morris LF, Haugen BR, et al. Thyroid‐differentiated and anaplastic carcinoma (Chapter 73), 8th edn. New York, NY, USA: Springer International Publishing; 2017. [Google Scholar]
- 17. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 18. Grillo F, Florio T, Ferraù F, et al. Emerging multitarget tyrosine kinase inhibitors in the treatment of neuroendocrine neoplasms. Endocr Relat Cancer. 2018;25:R453‐R466. [DOI] [PubMed] [Google Scholar]
- 19. Fleeman N, Houten R, Chaplin M, et al. A systematic review of lenvatinib and sorafenib for treating progressive, locally advanced or metastatic, differentiated thyroid cancer after treatment with radioactive iodine. BMC Cancer. 2019;19:1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chrisoulidou A, Mandanas S, Margaritidou E, et al. Treatment compliance and severe adverse events limit the use of tyrosine kinase inhibitors in refractory thyroid cancer. Onco Targets Ther. 2015;8:2435‐2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine‐refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double‐blind, phase 3 trial. Lancet. 2014;384:319‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine‐refractory thyroid cancer. N Engl J Med. 2015;372:621‐630. [DOI] [PubMed] [Google Scholar]
- 23. Dacosta BSA,Adejoro O, Copher R, Chatterjee D, Joshi PR, Worden FP. Real‐world treatment patterns among patients initiating small molecule kinase inhibitor therapies for thyroid cancer in the United States. Adv Ther. 2019;36:896‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brose MS, Schlumbeger M, Jeffers M, Kappeler C, Meinhardt G, Peña CEA. Analysis of biomarkers and association with clinical outcomes in patients with differentiated thyroid cancer: Subanalysis of the sorafenib phase III DECISION trial. Clin Cancer Res. 2019;25:7370‐7380. [DOI] [PubMed] [Google Scholar]
- 25. Tahara M, Schlumberger M, Elisei R, et al. Exploratory analysis of biomarkers associated with clinical outcomes from the study of lenvatinib in differentiated cancer of the thyroid. Eur J Cancer. 2017;75:213‐221. [DOI] [PubMed] [Google Scholar]
- 26. Sugino K, Nagahama M, Kitagawa W, et al. Clinical factors related to the efficacy of tyrosine kinase inhibitor therapy in radioactive iodine refractory recurrent differentiated thyroid cancer patients. Endocr J. 2018;65:299‐306. [DOI] [PubMed] [Google Scholar]
- 27. Klein Hesselink EN, Steenvoorden D, Kapiteijn E, et al. Therapy of endocrine disease: response and toxicity of small‐molecule tyrosine kinase inhibitors in patients with thyroid carcinoma: a systematic review and meta‐analysis. Eur J Endocrinol. 2015;172:R215‐225. [DOI] [PubMed] [Google Scholar]
- 28. Kim SY, Kim S‐M, Chang H‐J, et al. SoLAT (Sorafenib Lenvatinib alternating treatment): a new treatment protocol with alternating Sorafenib and Lenvatinib for refractory thyroid Cancer. BMC Cancer. 2018;18:956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brose MS, Robinson B, Bermingham C, et al. A phase 3 (COSMIC‐311), randomized, double‐blind, placebo‐controlled study of cabozantinib in patients with radioiodine (RAI)‐refractory differentiated thyroid cancer (DTC) who have progressed after prior VEGFR‐targeted therapy. J Clin Oncol. 2019;37(15_suppl):TPS6097. [Google Scholar]
- 30. Lirov R, Worden FP, Cohen MS. The treatment of advanced thyroid cancer in the age of novel targeted therapies. Drugs. 2017;77:733‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. ThermoFisher: Oncomine Focus Assay. https://www.thermofisher.com/uk/en/home/clinical/preclinical‐companion‐diagnostic‐development/oncomine‐oncology/oncomine‐focus‐assay.html. Accessed April 8, 2020.
- 32. Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet. 2013;381:295‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Waguespeck SG, Drilon A, Farago AG, et al. Treatment of advanced TRK fusion thyroid cancer with larotrectinib. Budapest, Hungary: European Thyroid Association Annual Meeting; 2019. [Google Scholar]
- 34. Chu YH, Dias‐Santagata D, Farahani AA, et al. Clinicopathologic and molecular characterization of NTRK‐rearranged thyroid carcinoma (NRTC). Mod Pathol. 2020;33:2186‐2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pfeifer A, Rusinek D, Zebracka‐Gala J, et al. Novel TG‐FGFR1 and TRIM33‐NTRK1 transcript fusions in papillary thyroid carcinoma. Genes Chromosomes Cancer. 2019;58:558‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leeman‐Neill RJ, Kelly LM, Liu P, et al. ETV6‐NTRK3 is a common chromosomal rearrangement in radiation‐associated thyroid cancer. Cancer. 2014;120:799‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cordioli MI, Moraes L, Bastos AU, et al. Fusion oncogenes are the main genetic events found in sporadic papillary thyroid carcinomas from children. Thyroid. 2017;27:182‐188. [DOI] [PubMed] [Google Scholar]
- 38. Picarsic JL, Buryk MA, Ozolek J, et al. Molecular characterization of sporadic pediatric thyroid carcinoma with the DNA/RNA ThyroSeq v2 next‐generation sequencing assay. Pediatr Dev Pathol. 2016;19:115‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cocco E, Scaltriti M, Drilon A. NTRK fusion‐positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15:731‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pekova B, Sykorova V, Dvorakova S, et al. RET, NTRK, ALK, BRAF and MET fusions in a large cohort of pediatric papillary thyroid carcinomas. Thyroid. 2020. [DOI] [PubMed] [Google Scholar]
- 41. National Comprehensive Cancer Network . Thyroid cancer (V1.2020). https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf. Accessed July 7, 2020.
- 42. Schroader B, Kong S, Anderson S, Williamson T, Sireci A, Shields K. Current status of biomarker testing in historically rare, high‐unmet‐need tumors: soft tissue sarcomas and thyroid cancers. Expert Rev Anticancer Ther. 2019;19:929‐938. [DOI] [PubMed] [Google Scholar]
- 43. Pitoia F, Smulever A, Jerkovich F. Letter to the Editor: "Foundation OneTM Genomic Interrogation of Thyroid Cancers in Patients with Metastatic Disease Requiring Systemic Therapy”. J Clin Endocrinol Metab. 2020;105:dgaa421. [DOI] [PubMed] [Google Scholar]
- 44. Fujiwara T, Fukushi J, Yamamoto S, et al. Macrophage infiltration predicts a poor prognosis for human ewing sarcoma. Am J Pathol. 2011;179:1157‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Doebele RC, Drilon A, Paz‐Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion‐positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21:271‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu SV, Macke LA, Colton BS, et al. Response to entrectinib in differentiated thyroid cancer with a ROS1 fusion. JCO Precis Oncol. 2017;1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cancer Genome Atlas Research Network . Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600‐mutant anaplastic thyroid cancer. J Clin Oncol. 2018;36:7‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Taflinar (dabrafenib) Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/202806s010lbl.pdf. Accessed April 8, 2020.
- 50. Mekinist (trametinib) Prescribing Information. https://www.hcp.novartis.com/globalassets/eg‐plus‐assets7/taf‐mek/hcp/nsclc/global/mekinist.pdf?id=39913. Accessed April 8, 2020.
- 51. Brose MS, Cabanillas ME, Cohen EE, et al. Vemurafenib in patients with BRAF(V600E)‐positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non‐randomised, multicentre, open‐label, phase 2 trial. Lancet Oncol. 2016;17:1272‐1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Falchook GS, Millward M, Hong D, et al. BRAF inhibitor dabrafenib in patients with metastatic BRAF‐mutant thyroid cancer. Thyroid. 2015;25:71‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wirth L, Sherman E, Drilon A, et al. LBA93 ‐ Registrational results of LOXO‐292 in patients with RET‐altered thyroid cancers. Ann Oncol. 2019;30(Suppl_5):v851‐v934. [Google Scholar]
- 54. Taylor MH, Gainor JF, Hu MI‐N, et al. Activity and tolerability of BLU‐667, a highly potent and selective RET inhibitor, in patients with advanced RET‐altered thyroid cancers. J Clin Oncol. 2019;. 37(15_suppl):6018. [Google Scholar]
- 55. Solomon JP, Linkov I, Rosado A, et al. NTRK fusion detection across multiple assays and 33,997 cases: diagnostic implications and pitfalls. Mod Pathol. 2020;33:38‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colomer R., Mondejar R., Romero‐Laorden N., Alfranca A., Sanchez‐Madrid F., Quintela‐Fandino M. 2020. When should we order a next generation sequencing test in a patient with cancer? EClinicalMedicine 25. [DOI] [PMC free article] [PubMed]
- 58. Dunn LA, Sherman EJ, Baxi SS, et al. Vemurafenib redifferentiation of BRAF mutant, RAI‐refractory thyroid cancers. J Clin Endocrinol Metab. 2019;104:1417‐1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rothenberg SM, McFadden DG, Palmer EL, Daniels GH, Wirth LJ. Redifferentiation of iodine‐refractory BRAF V600E‐mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. 2015;21:1028‐1035. [DOI] [PubMed] [Google Scholar]
- 60. Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib‐enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jaber T, Waguespack SG, Cabanillas ME, et al. Targeted therapy in advanced thyroid cancer to resensitize tumors to radioactive iodine. J Clin Endocrinol Metab. 2018;103:3698‐3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this case report are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


