Abstract
Fever of unknown origin (FUO) is a diagnostic challenge. Anti‐N‐methyl‐D‐aspartate receptor encephalitis should be considered in children with FUO and new‐onset neurological symptoms without significant encephalopathy.
Keywords: encephalitis, infectious diseases, neurology, paediatrics and adolescent medicine
Fever of unknown origin (FUO) is a diagnostic challenge. Anti‐N‐methyl‐D‐aspartate receptor encephalitis should be considered in children with FUO and new‐onset neurological symptoms without significant encephalopathy.
![]()
1. INTRODUCTION
We describe a child with anti‐N‐methyl‐D‐aspartate receptor (NMDAR) encephalitis who had fever of unknown origin (FUO) without encephalopathy or behavioral change. The main neurological symptoms were tremor, truncal ataxia, and postural hypotension. Anti‐NMDAR antibodies were present in serum and cerebrospinal fluid. With intravenous immunoglobulin, she made a complete recovery.
Children and adolescents who have fever of unknown origin (FUO), defined as temperature more than 38 degrees Celsius for longer than 14 days, are usually prescribed empiric antimicrobial treatment while the underlying cause is being evaluated. 1 This involves obtaining a detailed travel and symptom history, a physical examination, and an array of investigations for an underlying infection, autoimmune disorder, oncologic or genetic etiology. Infections are the most common etiology, but an increasing number of noninfectious causes are now known. 2 To this list, we would add anti‐N‐methyl‐D‐aspartate (NMDAR) receptor encephalitis.
Anti‐NMDAR receptor encephalitis is a devastating disease. A short prodromal febrile illness usually precedes a fairly characteristic neurological syndrome involving psychiatric symptoms or mood change, encephalopathy, movement disorder, speech or language difficulties, and seizures. Some patients have autonomic dysfunction, respiratory insufficiency, or cerebellar symptoms. 3 , 4 We report a patient with anti‐NMDAR encephalitis who primarily had FUO without prominent neuropsychiatric symptoms or encephalopathy and had less typical disease manifestations.
2. CASE REPORT
This is a previously well 13‐year‐old girl who presented to the hospital with a 3‐day history of fever, headache, dizziness, nausea, and vomiting. Mild, terminal neck stiffness was the only significant finding when evaluated at the emergency room and she was duly admitted for suspected meningitis. A clear source of fever was not evident. There was no history of recent vaccination and no cough, coryza, sore throat, diarrhea, or dysuria. She had no lymphadenopathy, hepatosplenomegaly, or chest signs. During her inpatient reviews, she was oriented to time, place and person, and exhibited no abnormal behavior. Neck stiffness was not observed, and the neurological examination was otherwise unremarkable.
The fever persisted throughout her hospital stay (Figure 1) and was highest in the first week of admission (maximum temperature 39.8 degrees Celsius, with chills). She remained lucid throughout this period, with no clinical signs suggestive of meningitis. Bloods were drawn for complete blood count, urea and electrolytes, and C‐reactive protein. Infection studies included blood and urine cultures, blood smear for malaria parasite, and nasopharyngeal aspirates for respiratory pathogen identification. The initial management was conservative with oral analgesia and intravenous fluids to her address her presenting symptoms. As her fever persisted without a clear etiology, she was prescribed intravenous ceftriaxone 2 g on day 6 of hospitalization, and separate infectious disease, oncology, and rheumatology consults were obtained on day 7. A second panel of investigations undertaken for occult infections, autoimmune disorders, and malignancy (including carcinoid tumor and paraneoplastic syndromes) (Tables 1 and 2) returned negative. No cardiac thrombus or vegetations were evident on a transthoracic echocardiogram. Notably, blood inflammatory markers were not raised: erythrocyte sedimentation rate 22 mm/hr, C‐reactive protein <0.2 mg/L and procalcitonin <0.1 μg/L. A chest radiograph, and neck, abdominal, and pelvic ultrasound scans were unremarkable. Magnetic resonance imaging (MRI) of the brain showed no focal lesions or leptomeningeal enhancement although mucosal thickening was seen in the ethmoid and frontal sinuses, suggesting possible sinusitis.
FIGURE 1.
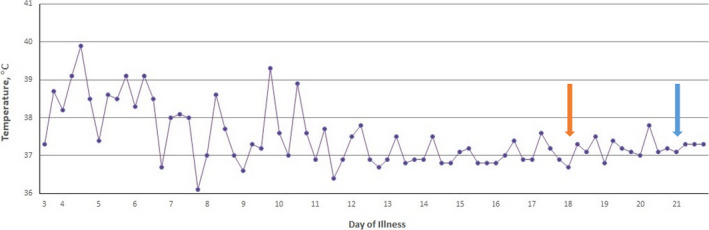
Temperature chart indicating fever trend in degree Celsius (oC). Orange arrow: development of postural hypotension with ataxia, administration of 1st dose of intravenous immunoglobulin, day 18 of illness. Blue arrow: hospital discharge on day 21 of illness
TABLE 1.
Investigations for infectious diseases
| Investigations | Result |
|---|---|
| Blood, CSF and urine cultures | Negative |
| Bacterial Antigen latex agglutinin from CSF | Negative |
| Enterovirus RNA from CSF | Not Detected |
| Malaria parasite on blood film | Not Detected |
| Tuberculosis T‐Spot reagent | Negative |
| Mycoplasma pneumonia Antigen and M pneumoniae serology titer | Negative |
| Antistreptolysin O titer | Negative |
| Widal Weil Felix Serology for paratyphi and typhi Antigen | Not Detected |
| EBV Capsid Antigen IgM Antibody | Not Detected |
|
Respiratory viral pathogens from Nasopharyngeal Aspirate (Influenzae A& B, Parainfluenzae, RSV, Coronavirus, Adenovirus, Metapneumovirus) |
Not Detected |
| Anti‐Chikungunya IgM Antibody IFA | Negative |
| Zika virus RNA PCR from urine | Not Detected |
| Dengue IgM and IgG | Negative |
| Dengue Virus Antigen | Negative |
| Adenovirus PCR | Not Detected |
| Parvovirus B19 PCR | Not Detected |
| HSV DNA from CSF | Not Detected |
| Enterovirus RNA from CSF | Not Detected |
| HHV 6 PCR | Not Detected |
All investigations were from serum samples unless reflected.
Abbreviations: CSF: cerebrospinal fluid, EBV: Ebstein Barr virus, HSV: herpes simplex virus, HHV: human papilloma virus, IgG: Immunoglobulin G, IgM: Immunoglobulin M, IFA: immune‐fluorescence assay, PCR: polymerase chain reaction, RSV: respiratory syncytial virus.
TABLE 2.
Investigations for noninfectious diseases
| Test | Result |
|---|---|
| Nerve conduction study | Normal amplitudes, latencies, and conduction velocities in the upper and lower limb motor and sensory nerves. |
| Antidouble stranded DNA antibody | 2.81 IU |
| ANA screen | Negative |
| C3 and C4 complement levels | 1.13 and 0.28 g/L |
| Chromogranin A | 75.4uG/L |
| 5‐OH Indole Acetic Acid (24‐hour urine) | 10.7 umol/L |
| 5‐HIAA, 24 hour | 11.7 umol/ day |
| Catecholamines and Metanephrines | Normal |
| CSF serine | 23 umol/L |
| CSF glycine | 11 umol/L |
| CSF/Plasma glycine ratio | 0.050 |
All investigations were from serum samples unless reflected.
Abbreviations: ANA: antinuclear antibody, HIAA: hydroxyindoleacetic acid.
As her fever abated on day 9 of hospitalization (day 12 of illness, Figure 1), new neurological signs emerged with a coarse tremor in the upper limb and an inability to perform tandem gait. She experienced light‐headedness, pallor, and cold peripheries associated with a postural drop in blood pressure (lowest reading 73/36 mm Hg, mean arterial blood pressure of 53 mm Hg), when attempting to walk or when attempting to stand from a seated position. These episodes took up to 30 minutes to resolve. Cerebral spinal fluid (CSF) analysis on day 16 of hospitalization showed elevated white blood cell count (56/mm3) and protein level (1.29 g/L), low glucose (2.0 mmol/L), and the presence of oligoclonal bands (isoelectric focusing). Polymerase chain reaction studies for enterovirus RNA and herpes simplex virus DNA in CSF were negative. Further imaging involving the MRI spine was normal, and nerve root enhancements were not demonstrated, and a nerve conduction study documented normal amplitudes, latencies, and conduction velocities in the upper and lower limb motor and sensory nerves. An electroencephalogram (EEG) was not performed in the absence of seizures or a significant change in mental status.
The clinical impression at the time was a Guillain‐Barrè/ Miller‐Fisher overlap syndrome due to the combination of autonomic dysfunction, ataxia, and elevated CSF protein levels, and she was given intravenous immunoglobulins 2 g/kg over 2 days. Her neurological symptoms and autonomic dysfunction gradually improved, hence a repeat lumbar puncture was not done.
At discharge on day 19 of hospitalization, she no longer had postural hypotension and displayed only mild tremors with mild unsteadiness on tandem gait. Several weeks later, her blood and CSF samples returned positive for anti‐NMDAR antibodies (1/10 titers, performed via a commercial fixed‐cell based assay (Euroimmun®). By this time, her neurological deficits symptoms have completely resolved and she has not experienced a relapse over a 4‐year follow‐up period with the pediatric neurology service.
3. DISCUSSION
Our patient is unique as prolonged fever was the main presenting symptom of anti‐NMDAR encephalitis as opposed to encephalopathy or psychosis. There was a case report of a 36‐year‐old woman with FUO and anti‐NMDAR encephalitis who also had a concurrent mood disorder and frank psychosis. 5 In another case report, a 21‐month‐old toddler who presented with prolonged fever and behavioral deterioration with features of an autism spectrum disorder was later diagnosed with anti‐NMDAR encephalitis. This patient also had a concurrent urinary tract infection and gastroenteritis. 6 Fever early in the course of anti‐NMDAR encephalitis is usually the result of a prodromal illness, commonly a respiratory illness or gastroenteritis, and precedes the neurological symptoms. 3 Persisting fever occurring later in the illness is more likely a result of temperature dysregulation from autonomic dysfunction as a feature of the encephalitis. 3 , 4 In our patient, the absence of concurrent symptoms of a prodromal illness and the presence of CSF oligoclonal bands, indicating an established process of central nervous system inflammation, would suggest that the prolonged fever was more likely a result of dysautonomia. The exaggerated manifestation and slow recovery from postural hypotension are also a unique form of dysautonomia not previously reported with anti‐NMDAR encephalitis (eg, hypertension, tachycardia, bradycardia, hypersalivation, and urinary incontinence). 3
Compared to adults with anti‐NMDAR encephalitis, children are more likely to present with seizures, prolonged dyskinesia, and cerebellar symptomatology. 3 , 4 , 7 Our patient had a combination of symptoms involving dysautonomia, limb tremors, and truncal ataxia, which are the least common features of the syndrome. These features, together with the elevated CSF protein level, led us to consider an inflammatory neuropathy (Guillain‐Barrè /Miller‐Fisher overlap syndrome) as the likely diagnosis. 8 As such, intravenous immunoglobulin (IVIg) was chosen as empiric immunotherapy. Our patient did not require second‐line immunotherapy such as rituximab or cyclophosphamide as she improved rapidly following IVIg therapy.
FUO can result from infectious, oncologic, autoimmune, neurologic, or genetic etiologies (Figure 2). As illustrated in the context of our patient, a young girl living in South East Asia, the diagnostic evaluation included regional infections from malaria, Dengue virus, typhoid, tuberculosis, and mycoplasma (Tables 1 and 2). Systemic lupus erythematosus, a common systemic autoimmune disorder in children, 1 may have neurological involvement. Apart from lymphoma and leukemia that are common childhood cancers and often associated with FUO, a carcinoid syndrome was also considered given the prominent dysautonomia. It is notable that the type of tumors associated with NMDAR encephalitis, that is, ovarian teratoma and neural crest tumors, 3 , 9 is not usually associated with FUO.
FIGURE 2.
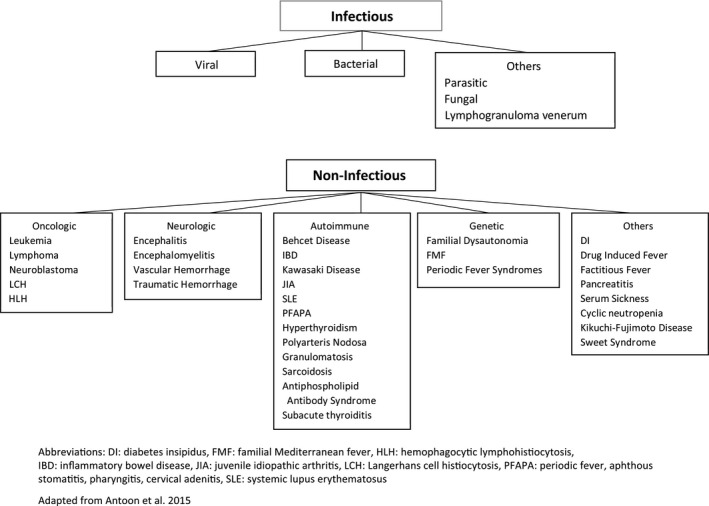
Common causes of fever of unknown origin in children. Abbreviations: DI: diabetes insipidus, FMF: familial Mediterranean fever, HLH: hemophagocytic lymphohistiocytosis, IBD: inflammatory bowel disease, JIA: juvenile idiopathic arthritis, LCH: Langerhans cell histiocytosis, PFAPA: periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis, SLE: systemic lupus erythematosus. Adapted from Antoon et al 2015
Although our case report highlights a rare presentation of anti‐NMDAR encephalitis presenting as FUO, we must interpret it in the context of its limitations. We did not perform an EEG in our patient. An EEG, often abnormal albeit nonspecific, may have been helpful to support a diagnosis of NMDAR encephalitis in our patient. Extreme delta brushes are a unique feature to NMDAR encephalitis although only seen in 11% of patients. 10
4. CONCLUSION
FUO is a diagnostic challenge for physicians who need to distinguish time‐sensitive and life‐threatening disorders from benign or self‐limiting etiologies. Anti‐NMDAR encephalitis may present with FUO as an incomplete or variant phenotype. Early recognition and treatment of anti‐NMDAR encephalitis will limit prolonged hospitalization and reduce the risk for neurodisability.
5. ETHICS STATEMENT
Our institution does not require ethical approval for case reports. There is no personal data information in this case report, and informed consent was received from the parents for using the patient's clinical data.
CONFLICT OF INTEREST
Dr Heng, Dr Lee, and Dr Thomas do not have any conflict of interests to declare and declare no competing interests.
AUTHOR CONTRIBUTIONS
Dr Heng conceptualized the case report and drafted the initial manuscript, coordinated and collected data, and reviewed and revised the manuscript. Dr Lee and Dr Thomas: critically reviewed and revised the manuscript. All authors: approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
ACKNOWLEDGMENTS
Published with written consent of the patient.
Heng KYC, Lee JH, Thomas T. Anti‐N‐Methyl‐D‐aspartate receptor encephalitis masquerading as fever of unknown origin. Clin Case Rep. 2021;9:2323–2327. 10.1002/ccr3.4025
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Antoon JW, Potisek NM, Lohr JA. Pediatric fever of unknown origin. Pediatrics Rev. 2015;36(9):380‐391; quiz 391. [DOI] [PubMed] [Google Scholar]
- 2. Marshall GS. Prolonged and recurrent fevers in children. J Infect. 2014;68(suppl 1):S83‐93. [DOI] [PubMed] [Google Scholar]
- 3. Dalmau J, Lancaster E, Martinez‐Hernandez E, Rosenfeld MR, Balice‐Gordon R. Clinical experience and laboratory investigations in patients with anti‐NMDAR encephalitis. Lancet Neurol. 2011;10(1):63‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long‐term outcome in patients with anti‐NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hur J. Fever of unknown origin: an unusual presentation of anti‐N‐methyl‐D‐aspartate receptor encephalitis. Infect Chemother. 2015;47(2):129‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khundakji Y, Masri A, Khuri‐Bulos N. Anti‐NMDA receptor encephalitis in a toddler: A diagnostic challenge. Int J Pediatr Adolesc Med. 2018;5(2):75‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Armangue T, Titulaer MJ, Málaga I, et al. Pediatric anti‐N‐methyl‐D‐aspartate receptor encephalitis‐clinical analysis and novel findings in a series of 20 patients. J Pediatr. 2013;162(4):850‐856.e852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Korinthenberg R, Trollmann R, Felderhoff‐Müser U, et al. Diagnosis and treatment of Guillain‐Barré Syndrome in childhood and adolescence: An evidence‐ and consensus‐based guideline. Eur J Paediatr Neurol. 2020;25:5‐16. [DOI] [PubMed] [Google Scholar]
- 9. Iizuka T, Sakai F, Ide T, et al. Anti‐NMDA receptor encephalitis in Japan: long‐term outcome without tumor removal. Neurology. 2008;70(7):504‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sonderen AV, Arends S, Tavy DLJ, et al. Predictive value of electroencephalography in anti‐NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry. 2018;89(10):1101‐1106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


