Abstract
Gastrointestinal metastasis could be considered in the differential diagnosis of melena in the patient with NSCLC history.
Keywords: gastrointestinal metastasis, NSCLC, small bowel
Gastrointestinal metastasis could be considered in the differential diagnosis of melena in the patient with NSCLC history.

1. INTRODUCTION
This study was about a 66‐year‐old man with small bowel metastasis of non‐small cell lung cancer (NSCLC). We described the rare NSCLC metachronous multiple gastrointestinal metastatic disease, which might be considered in the differential diagnosis of melena in the patient with NSCLC history.
Primary non‐small cell lung cancer (NSCLC) is the leading cause of cancer‐related death in South Korea. Primary NSCLC frequently metastasizes to the brain, liver, adrenal glands, and bones. 1 , 2 However, the incidence of gastrointestinal metastasis of NSCLC has been reported to be as low as 0.2%‐1.7%. 3 , 4 Till now, only a few studies about gastrointestinal metastasis of NSCLC have been reported 5 , 6 , 7 due to its paucity. The understanding of the clinical presentation of metastasis of primary NSCLC to the gastrointestinal tract is currently incomplete. This report describes a case of gastrointestinal metastasis of NSCLC, which was treated by surgical resection with adjuvant chemotherapy.
2. CASE PRESENTATION
A 66‐year‐old man presented to the thoracic surgery department for the surgical treatment of right upper lobe adenocarcinoma. The patient had a smoking history of 45 pack‐year. The adenocarcinoma was diagnosed by percutaneous core‐needle biopsy and F‐18‐fluoro‐positron emission tomography (FDG‐PET) showed no evidence of lymph node involvement or distant metastasis. He underwent right upper lobectomy with mediastinal lymph node dissection. Histopathological diagnosis was a poorly differentiated adenocarcinoma without visceral pleural invasion, lymphovascular invasion or regional lymph node metastasis, T1aN0M0. The patient had an uneventful clinical course and discharged without complication. The patient did not receive adjuvant therapy. Five months later, the patient was referred to emergency department because of melena and dizziness. It was thought that symptoms were expressed due to bleeding because hemoglobin was identified to be 6.8 g/dl. The esophagogastroduodenoscopy (EGDS) was performed and showed about 2cm sized polypoid lesion with oozing bleeding on the duodenal second portion (Figure 1). Biopsy of the duodenal lesion revealed a poorly differentiated adenocarcinoma. FDG‐PET showed no definite evidence of abnormal hypermetabolic lesion suggesting distant metastasis. Subsequent laparotomy was planned, and total pancreatectomy with partial gastrectomy, splenectomy, and small bowel resection was done. Partial gastrectomy was performed due to the presence of mass in the distal antrum. Pancreatic juice leak was observed at each pancreatico‐jejunal anastomotic stitch site, and the tissue was friable, resulting in total pancreatectomy conversion. Additional jejunal resection, ileal segmental resection, and end to end anastomosis were done. The length of total resected small bowel was 68 cm. Gross examination of resected specimen showed multiple polyp masses on the stomach and small intestine (duodenum, jejunum, and ileum). A total of 22 polyps (stomach: 1, duodenum: 8, jejunum to ileum: 13) were observed. These polyps were of various sizes ranging from 0.8 cm to 4.0 cm. The largest one measured 4.0 x 2.5 x 2.0 cm. Cut sections of the masses were whitish and solid. Histologically, the tumors were composed of poorly differentiated carcinoma with a predominant sheet‐like arrangement and poorly formed glands. Immunohistochemistry of the tumor showed positivity (>50%) for pan‐cytokeratin, EMA, vimentin, and focal positivity (<10%) for CK7. The tumor cells were negative for TTF‐1, CK20, LCA, HMB45, S100, CD31, and c‐KIT (Figure 2). Based on histological similarity and the results of immunohistochemistry, the final diagnosis was metastasis of lung adenocarcinoma. Palliative chemotherapy was done with pemetrexed and cisplatin. The patient received 3‐week cycle, total 3 cycles of pemetrexed 760 mg/m2, and cisplatin 60 mg/m2. On about 5 months later follow‐up, the patient showed no evidence of disease.
FIGURE 1.
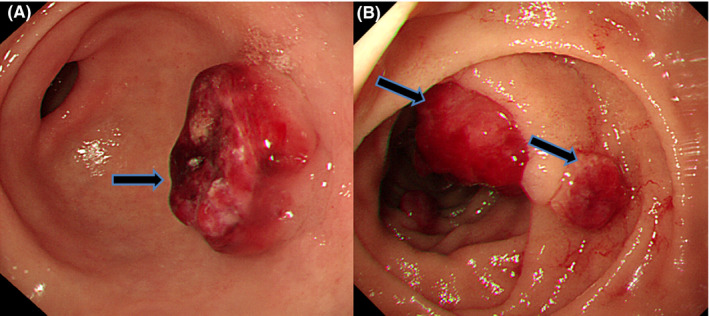
EGDS showed about 2 cm sized rubbery hard tumor with ulceration on the antrum (A, black arrow) and polypoid lesions with oozing bleeding on the duodenal second portion (B, black arrow)
FIGURE 2.
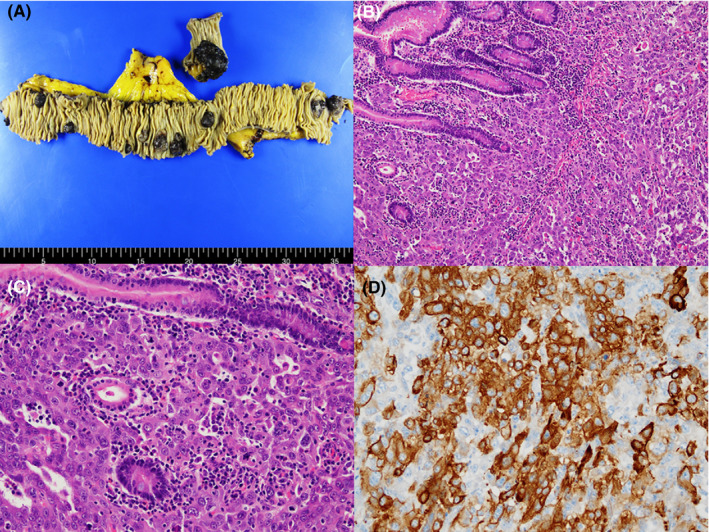
Gross picture of resected small intestine showing multiple polyps with erosion (A). Microscopic image of the duodenal polyp showing tumor‐forming solid and glandular pattern with marked nuclear pleomorphism similar to those of the primary lung cancer (H&E stain, ×100, B), (H&E stain, ×200, C). Tumor cells showing positivity for pan‐cytokeratin (immunohistochemistry, ×200, D)
3. DISCUSSION
A small bowel metastasis of NSCLC is very rare. The natural course of gastrointestinal metastasis with lung cancer remains unclear due to its paucity. Rossi et al described two patients with NSCLC and synchronous solitary small bowel metastasis who underwent small bowel resection, subsequent pulmonary lobectomy, and chemotherapy and were alive without evidence of disease. 8 FDG‐PET scan might have a diagnostic role in the detection of gastro intestinal metastasis of NSCLC. 8 , 9 Surgical resection might be the treatment choice. Goh et al reported a case series of 8 patients with gastrointestinal metastasis from NSCLC. 10 After resection of the metastasis, four of eight lived longer than six months. The role of chemotherapy could not be determined till now. Kant et al reported a patient who survived more than 4 years after undergoing palliative surgery, palliative radio‐ and chemotherapy. 11
Our case, which describes a patient who has NSCLC with metachronous multiple metastases to the small bowel, is unique because the previous case reports described occurence of NSCLC with synchronous solitary metastasis to the small bowel.
Our report could serve as reminder that gastrointestinal metastasis might be considered in the differential diagnosis of melena in the patient with NSCLC history although it is a very rare condition.
CONSENT
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
MKK: involved in designing the project and writing the manuscript. DKK: involved in collecting and creating the figures. WH and HYH: performed manuscript writing and editing. KHN: involved in pathologic advice. All authors have read and approved the final manuscript.
ACKNOWLEDGEMENTS
Published with written consent of the patient.
Kang DK, Kang MK, Heo W, Hwang Y‐H, Nam KH. Multiple small bowel metastasis of primary non‐small cell lung cancer. Clin Case Rep. 2021;9:1896–1898. 10.1002/ccr3.3821
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article.
REFERENCES
- 1. Auerbach O, Garfinkel L, Parks VR. Histologic type of lung cancer in relation to smoking habits, year of diagnosis and sites of metastases. Chest. 1975;67(4):382‐387. [DOI] [PubMed] [Google Scholar]
- 2. Hillers TK, Sauve MD, Guyatt GH. Analysis of published studies on the detection of extrathoracic metastases in patients presumed to have operable non‐small cell lung cancer. Thorax. 1994;49(1):14‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim SY, Ha HK, Park SW, et al. Gastrointestinal metastasis from primary lung cancer: CT findings and clinicopathologic features. AJR Am J Roentgenol. 2009;193(3):W197‐W201. [DOI] [PubMed] [Google Scholar]
- 4. Lee PC, Lo C, Lin MT, Liang JT, Lin BR. Role of surgical intervention in managing gastrointestinal metastases from lung cancer. World J Gastroenterol. 2011;17(38):4314‐4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Casella G, Di Bella C, Cambareri AR, et al. Gastric metastasis by lung small cell carcinoma. World J Gastroenterol. 2006;12(25):4096‐4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suzaki N, Hiraki A, Ueoka H, et al. Gastric perforation due to metastasis from adenocarcinoma of the lung. Anticancer Res. 2002;22(2B):1209‐1212. [PubMed] [Google Scholar]
- 7. Yamamoto M, Matsuzaki K, Kusumoto H, et al. Gastric metastasis from lung carcinoma. Case report. Hepatogastroenterology. 2002;49(44):363‐365. [PubMed] [Google Scholar]
- 8. Rossi G, Marchioni A, Romagnani E, et al. Primary lung cancer presenting with gastrointestinal tract involvement: clinicopathologic and immunohistochemical features in a series of 18 consecutive cases. J Thorac Oncol. 2007;2(2):115‐120. [PubMed] [Google Scholar]
- 9. Uesaka D, Demura Y, Umeda Y, et al. Nihon Kokyuki Gakkai Zasshi. 2006;44(12):899‐905. [PubMed] [Google Scholar]
- 10. Goh BK, Yeo AW, Koong HN, Ooi LL, Wong WK. Laparotomy for acute complications of gastrointestinal metastases from lung cancer: is it a worthwhile or futile effort? Surg Today. 2007;37(5):370‐374. [DOI] [PubMed] [Google Scholar]
- 11. Kant KM, Noordhoek Hegt V, Aerts JG. A patient with four‐year survival after nonsmall cell lung carcinoma with a solitary metachronous small bowel metastasis. J Oncol. 2010;2010:616130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


