Abstract
Purpose
To report a case of recalcitrant pseudomonas keratitis with a rare presentation of hyphaema.
Observation
A 45-year-old female was noted to have contact lens-related pseudomonas keratitis with hyphaema. The organism was refractory to multiple antibiotics and only responded to Tazocin eye drops.
Conclusion and Importance
Hyphaema is a rare presentation in bacterial keratitis and could represent infection with an especially virulent organism. Use of Aspirin could precipitate hyphaema in infective keratitis. Alternative antibiotic choices such as Tazocin, colistin, meropenem, and imipenem can be considered when standard therapy is ineffective for multidrug-resistant Pseudomonas keratitis.
Keywords: Pseudomonas keratitis, Hyphaema, Ophthalmic solutions
Introduction
Bacterial keratitis is a sight-threatening infection that is commonly associated with contact lens wear [1] and can lead to corneal perforation in 24–48 h [2]. Pseudomonas aeruginosa, a Gram-negative rod that is often found in water and soil, is a frequent cause of contact lens-related infective keratitis [3, 4]. Up to 30% of contact lens wearers have Gram-negative bacteria growth with 19% being culture positive for Pseudomonas, which is linked with severe anterior chamber reaction and larger infiltrates [5]. Increasing drug resistance in Pseudomonas keratitis makes treatment of this highly virulent and destructive organism difficult [6].
Although Pseudomonas keratitis is relatively common, associated spontaneous hyphaema is rare. Pseudomonas normally responds to intensive topical fluoroquinolones, cephalosporins, and aminoglycosides [7]. We report a case of recalcitrant pseudomonas keratitis with a large hyphaema on presentation.
Case Report
A 45-year-old Chinese female presented to the Emergency Department with a 4-day history of right eye redness, pain, and decreased vision. Her past ocular history was negative except for daily wear soft contact lens for 30 years. She reported good contact lens hygiene and no overnight wear. Her past medical history included hypertension, hyperlipidaemia, and ischaemic heart disease on Aspirin.
On presentation, her best-corrected visual acuity was hand movement in the right eye and 6/9 in the left eye. Intraocular pressure was 21 mm Hg in the right eye and 17 mm Hg in the left eye by applanation tonometry. Pupillary exam was normal with no relative afferent pupillary defect.
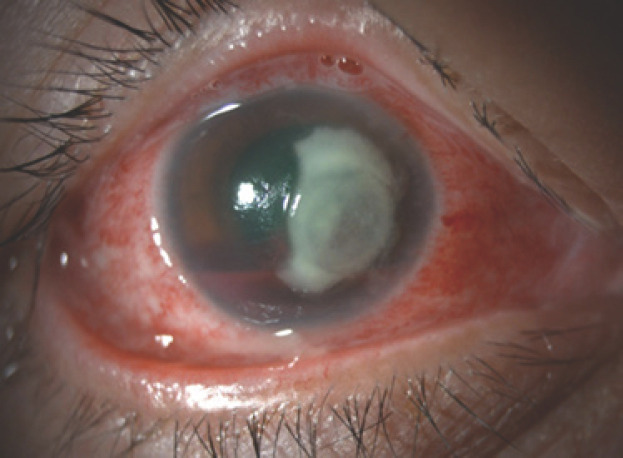
On slit lamp examination, there was a 7.2 × 6.2 mm yellow-white corneal infiltrate involving the visual axis in her right eye, with an accompanying epithelial defect, mild thinning, and corneal oedema (shown in Fig. 1). The anterior chamber was deep with a large, layered hyphaema that was 4 mm in height, with no visible hypopyon. There was superficial peripheral corneal pannus in both eyes consistent with long-term contact lens wear and no neovascularization of the iris. Since the posterior segment could not be visualized, a B-scan ultrasonography of the right eye confirmed a flat retina, no vitritis, and no intraocular tumour.
Fig. 1.
Anterior segment photograph of her right eye at presentation.
Corneal scrapings were performed and sent for Gram stain and fungal smear. The material was also inoculated on chocolate agar, blood agar, and Sabouraud's dextrose agar. The patient was admitted and started on intensive fortified cefazolin (50 mg/mL) and gentamicin (14 mg/mL) alternating every half hour. Atropine 1% three times a day and Refresh Plus preservative free every 2 h were also added. She was also continued on Aspirin for ischaemic heart disease. Corneal cultures showed heavy growth of Pseudomonas aeruginosa that was sensitive to ceftazidime, TazocinTM, gentamicin, amikacin, and ciprofloxacin.
Although her hyphaema resolved after 2 weeks, her central corneal ulcer did not show interval improvement. She was then switched to hourly moxifloxacin 0.5% eye drops, with cessation of cefazolin and gentamicin, and was also started on Pred Forte 1% eye drops 4 times a day. She was discharged and seen in the eye clinic subsequently. There was minimal improvement 1 week later. Oral doxycycline 100 mg two times a day was added.
Two weeks' post-discharge, there was still no improvement, so moxifloxacin was stopped and hourly ciprofloxacin 0.3% eye drops were started. Despite this, the central corneal infiltrate and ulcer remained unchanged (5.6 mm in height) and a small hypopyon developed.
Hourly Tazocin eye drops (Tazobactam 12 mg/mL and Piperacillin 1.5 mg/mL) were then started, 1 month after failing standard therapy with cefazolin, gentamicin, moxifloxacin, and ciprofloxacin. Pred Forte 1% eye drops were ceased when Tazocin was initiated and later restarted 4 times a day after a week of Tazocin eye drops. There was a good response to Tazocin eye drops with a decrease in central infiltrate size (3.6 mm in height) and resolution of the hypopyon. After a month on Tazocin eye drops, her cornea had re-epithelized and the involved area was scarred, with a quiet anterior chamber. The patient's keratitis took 2 months to resolve and she was left with residual scarring. Her visual acuity in the affected eye improved to 6/12-2, which was corrected to 6/6-2 with pinhole.
Discussion
Anterior chamber hyphaema is normally associated with ocular trauma. Other causes of anterior chamber haemorrhage include iris neovascularization, over-distension of vessels, blood derangement, fragile vessel walls, highly vascular tumours, juvenile xanthogranuloma, post-surgery, and can be idiopathic [8].
Anterior chamber haemorrhage due to microbial keratitis is rare and has been described in a few case reports. Hyphaema was reported as a complication in 1 of 300 bacterial keratitis cases studied [5]. It has also been described in Moraxella keratitis where hyperacute inflammation can cause hyphaema [9]. Finally, in a large case series on complications of microbial keratitis, spontaenous hyphaema was noted in 16 out of 458 patients. This was often associated with chronic corneal conditions and rubeosis iridis [10]. These hyphaemas had persisted longer, especially when a hypopyon was concurrent [10].
Spontaenous hyphaema in microbial keratitis was noted with Staph. aureus (4 cases), coagulase-negative staphylococcus (2 cases), Streptococcus pneumoniae (3 cases), ß-streptococcus (2 cases), Pseudomonas aeruginosa (2 cases), and Pseudomonas mesophilica (1 case). Two cases of microbial keratitis with hyphaema were culture negative. These cases were noted to be associated with rubeotic glaucoma (3 cases), uveitis (1 case), trauma (5 cases), and bullous keratopathy (5 cases). Other significant associations with hyphaema included advanced age (median age 68.5 years, p < 0.005), female sex (67%, p < 0.05), Gram-positive organisms (81%) and a presence of a hypopyon (75%). Of the 16 patients with hyphaema, 2 had diabetes, 1 was a chronic alcoholic, and 1 was on warfarin for pulmonary embolism [10].
This patient did not have significant risk factors for hyphaema, such as advanced age, trauma, a chronic corneal condition, or rubeosis. Her risk factors included Aspirin use and development of hypopyon [10]. Aspirin has been linked to spontaneous hyphaema development [11]; however, there are currently no reports of Aspirin use and hyphaema in infective keratitis. Only warfarin has been associated with hyphaema in infective keratitis [10]. Haemorrhagic complications in infective keratitis have been suggested to involve bacterial strains of moderate virulence and high phylogenicity due to high prevalence of a hypopyon [10], which our patient also developed later on. It is also suggested that haemorrhage might be due to bacterial proteases, streptokinases, and cytolytic toxins [10]. Our patient's hyphaema was likely due to her use of Aspirin and the virulence of her Pseudomonas infection. Fortunately, her hyphaema resolved in 2 weeks and did not cause complications such as peripheral anterior synechiae formation, corneal blood staining, or glaucoma.
Bacterial keratitis due to Pseudomonas aeruginosa is a serious and potentially blinding condition. The aggressive nature of the organism coupled with its evolving multidrug resistance is an important cause of ocular morbidity.
Besides the hyphaema, this patient had culture-positive Pseudomonas aeruginosa that was pan sensitive on testing, however, it behaved differently clinically and was refractory to most medications (cefazolin, gentamicin, moxifloxacin, and ciprofloxacin) except for Tazocin. There are a few studies on recalcitrant multidrug-resistant Pseudomonas keratitis that have responded to alternative antibiotic choices such as Tazocin [12], colistin [13], meropenem [13], and imipenem [14].
Chew et al. [12] described 3 cases that did not respond to various anti-microbials except for Tazocin, with no adverse side effects noted. Each case had good resolution with Tazocin after a month of instillation with a slow taper. These 3 cases also had pan-sensitivity on antibiotic sensitivity testing but showed much drug-resistance clinically. This is similar to our experience with this case. This disparity could be due to the amount of corneal drug penetration, increasing use of fluoroquinolones with an associated rise in resistance and as a result of different minimum inhibitory concentrations of the antibiotics in the cornea [12]. Treatment of pseudomonas keratitis is becoming increasingly challenging due to its evolving drug-resistance.
Conclusion
Hyphaema is a rare presentation in bacterial keratitis and could represent infection with an especially virulent organism. Use of Aspirin could precipitate hyphaema in infective keratitis. Alternative antibiotic choices such as Tazocin, colistin, meropenem, and imipenem can be considered when standard therapy is ineffective for multidrug-resistant Pseudomonas keratitis. More research into the safety and efficacy of these alternative antibiotics is especially crucial in light of the increasing prevalence of multidrug-resistant Pseudomonas keratitis.
Statement of Ethics
This study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The subject has given written informed consent to publish this case report and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding or grant support.
Author Contributions
A.C. and H.O. analysed and collected background data. A.C. drafted the manuscript. P.S. and B.C. revised the manuscript. All authors read and approved the final manuscript.
References
- 1.Schaefer F, Bruttin O, Zografos L, Guex-Crosier Y. Bacterial keratitis: a prospective clinical and microbiological study. Br J Ophthalmol. 2001 Jul;85((7)):842–7. doi: 10.1136/bjo.85.7.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostler H. Disease of the external eye and adnexa. Baltimore: Williams & Wilkins; 1993. Disease of the cornea; pp. pp. 137–252. [Google Scholar]
- 3.Fleiszig SM, Evans DJ. The pathogenesis of bacterial keratitis: studies with Pseudomonas aeruginosa. Clin Exp Optom. 2002 Sep;85((5)):271–8. doi: 10.1111/j.1444-0938.2002.tb03082.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang M, Smith WA, Duncan JK, Miller JM. Treatment of Pseudomonas keratitis by continuous infusion of topical antibiotics with the Morgan Lens. Cornea. 2017 May;36((5)):617–20. doi: 10.1097/ICO.0000000000001128. [DOI] [PubMed] [Google Scholar]
- 5.Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003 Jul;87((7)):834–8. doi: 10.1136/bjo.87.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sy A, Srinivasan M, Mascarenhas J, Lalitha P, Rajaraman R, Ravindran M, et al. Pseudomonas aeruginosa keratitis: outcomes and response to corticosteroid treatment. Invest Ophthalmol Vis Sci. 2012 Jan;53((1)):267–72. doi: 10.1167/iovs.11-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawla B, Agarwal P, Tandon R, Titiyal JS, Sharma N, Agarwal T, et al. In vitro susceptibility of bacterial keratitis isolates to fourth-generation fluoroquinolones. Eur J Ophthalmol. 2010 Mar-Apr;20((2)):300–5. doi: 10.1177/112067211002000207. [DOI] [PubMed] [Google Scholar]
- 8.McDonald CJ, Raafat A, Mills MJ, Rumble JA. Medical and surgical management of spontaneous hyphaema secondary to immune thrombocytopenia. Br J Ophthalmol. 1989 Nov;73((11)):922–5. doi: 10.1136/bjo.73.11.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barash A, Chou TY. Moraxella atlantae keratitis presenting with an infectious ring ulcer. Am J Ophthalmol Case Rep. 2017 Jun;7:62–5. doi: 10.1016/j.ajoc.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ormerod LD, Egan KM. Spontaneous hyphaema and corneal haemorrhage as complications of microbial keratitis. Br J Ophthalmol. 1987 Dec;71((12)):933–7. doi: 10.1136/bjo.71.12.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagnis A, Lai S, Iester M, Bacino L, Traverso CE. Spontaneous hyphaema in a patient on warfarin treatment. Br J Clin Pharmacol. 2008 Sep;66((3)):414–5. doi: 10.1111/j.1365-2125.2008.03233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chew FL, Soong TK, Shin HC, Samsudin A, Visvaraja S. Topical piperacillin/tazobactam for recalcitrant pseudomonas aeruginosa keratitis. J Ocul Pharmacol Ther. 2010 Apr;26((2)):219–22. doi: 10.1089/jop.2009.0077. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee S, Agrawal D. Multi-drug resistant Pseudomonas aeruginosa keratitis and its effective treatment with topical colistimethate. Indian J Ophthalmol. 2016 Feb;64((2)):153–7. doi: 10.4103/0301-4738.179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandes M, Vira D, Medikonda R, Kumar N. Extensively and pan-drug resistant Pseudomonas aeruginosa keratitis: clinical features, risk factors, and outcome. Graefes Arch Clin Exp Ophthalmol. 2016 Feb;254((2)):315–22. doi: 10.1007/s00417-015-3208-7. [DOI] [PubMed] [Google Scholar]