Abstract
Patients with complete concentric collapse of the redropalatal airway are excluded from unilateral hypoglossal nerve stimulation. This case report shows good control of OSA in a patient with CCC with a new bilateral hypoglossal nerve stimulator.
Keywords: bilateral stimulation, complete concentric collapse, hypoglossal nerve, surgical treatment of obstructive sleep apnea
Patients with complete concentric collapse of the redropalatal airway are excluded from unilateral hypoglossal nerve stimulation. This case report shows good control of OSA in a patient with CCC with a new bilateral hypoglossal nerve stimulator.

1. INTRODUCTION
Hypoglossal nerve stimulation (HNS) has gained acceptance as a valid means of reducing obstructive sleep apnea (OSA) severity in certain patients who are intolerant of CPAP or mandibular advancement splint (MAS) therapy. 5 Until now, unilateral HNS has been the only commercially available means to deliver this treatment. One of the exclusion criteria for use of this therapy is complete concentric collapse (CCC) of the velopharyngeal airway on sleep endoscopy (DISE) 1 , 2 During the pilot study of the first bilateral HN stimulator (The Genio System™ by Nyxoah), significant opening of the retropalatal airway was observed in some patients. 3 Patients with CCC were excluded from this study. However, in the second study of this device now underway (BETTER SLEEP ClinicalTrials.gov Identifier: NCT03763682), approximately one third of the patients will have CCC. Here, we present the first case from this study with CCC at the level of the soft palate. The Better Sleep Trial was granted Ethics Approval by the St. Vincent's Hospital Human Research Ethics Committee for all sites participating in the study.
2. CASE REPORT
The patient is a 56‐year‐old male with severe OSA and loud snoring. He was intolerant of CPAP. He had a BMI of 27, a Friedman tongue position 2, and Friedman grade 1 tonsils. Jaw thrust increased both the retropalatal and retrolingual airways.
Screening polysomnogram revealed an AHI of 33.9 (AASM 2014 Recommended Criteria), and the patient was consented for implantation. Pre‐implant DISE (using IV propofol) showed CCC as well as tongue‐base obstruction. The patient was implanted with the Genio™ Bilateral HN stimulator (Nyxoah SA, Belgium).
The device was activated at 6 weeks postimplant on a low‐power setting to facilitate acclimation to the device. At weekly visits, the energy levels were gradually increased within the patient's comfort levels. At 2 months, another DISE was performed as part of the Better Sleep Trial protocol. CCC was again evident, but when the device was activated, there was strong opening of the airway both behind the palate and tongue.
At 3 months postimplantation, the patient underwent overnight in laboratory PSG and titration. The results of the screening and 3 month PSG are presented in Figures 1 and 2. The AHI reduced from 33.6 to 1.6 events/hour as measured on a full‐night PSG and scored by an independent scorer. The ODI was reduced by 97%, and nadir SaO2 increased from 80% to 88%. Time with SaO2 < 90% decreased from 11.3 to 0.2 minutes. Daytime tiredness reduced, and the bed partner reported a marked change in snoring from very loud to soft. Sleep architecture was also improved with an increase in N2 sleep from 57% to 62%. While the patient did spend more time in supine sleep during the screening PSG (67.9 in the screening vs 18 minutes in the M3 PSG), the supine AHI was reduced from 72.5 to16 events per hour.
FIGURE 1.
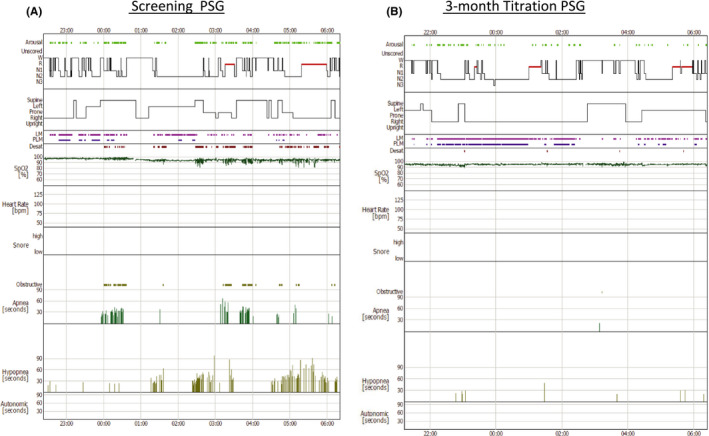
Screening and 3‐month PSG hypnograms. The left panel (A) is the screening PSG. The right panel (B) is the 3‐month titration study with bilateral hypoglossal nerve stimulation, showing improvement in OSA
FIGURE 2.
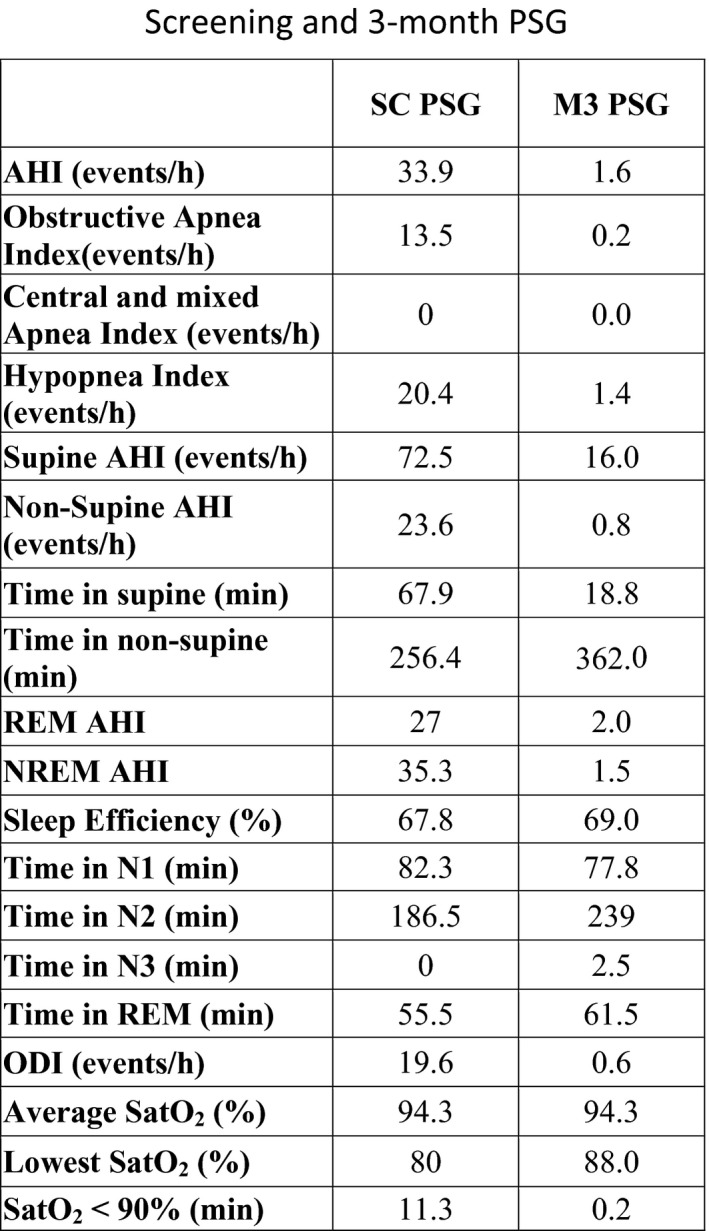
Screening and 3‐month titration PSG. The first column (SC PSG) shows the data obtained during the screening PSG. The second column (M3 PSG) shows the data obtained during the entire night of the 3‐month PSG
3. DISCUSSION
Exclusion of patients with CCC from HNS implantation arose following a paper by Van de Heyning et al in 2012, 1 supported by a paper from Venderveken et al in 2013. 2 The relevance of these findings to bilateral HNS is unknown, but is being tested for the first time in a prospective trial in the Better Sleep OSA trial. Recently, a small study showed that almost 50% of patients had EMG evidence of cross‐stimulation of the HN. 4 The PSG outcomes in patients with bilateral tongue protrusion were marginally better than those with unilateral protrusion, although these patients did not have CCC of the palate. The case reported herein is the first patient in the Better Sleep Trial, with confirmed CCC, to demonstrate successful control of OSA. One hypothesis for the effect on the palate of HNS is palatoglossal coupling (PGC). A possible mechanism of PGC is reflex contraction of the palatoglossus (PG) muscle in response to negative airway pressure, or anterior movement of the tongue causing reflex contraction of the PG muscle. PG is innervated by the pharyngeal branch of the vagus nerve and extends from the palatine aponeurosis to the tongue. If the tongue is significantly stiffened and moved anteriorly, contraction of the palatoglossus would be more likely to result in anterior movement of the soft palate. Another possible explanation is differences in palatoglossus anatomy (such as muscle length) between individuals. The pathophysiological mechanisms determining why bilateral HNS is successful in some patients with CCC, and indeed why PGC occurs in some patients and not others remain enigmatic.
4. CONCLUSION
CCC of the soft palate is an exclusion criterion for unilateral HNS, but it is unknown whether this is true of bilateral HNS. We present the first case in a new trial of bilateral HNS which demonstrates successful control of OSA in a patient with CCC. Further research needs to be done to look for anatomical features in patients which might predict the sort of response to bilateral HNS stimulation demonstrated by the patient in this case report, and it is hoped that the Better Sleep OSA trial might reveal this.
5. ETHICS APPROVAL
The Better Sleep Trial was granted ethics approval by the St. Vincent's Hospital Ethics Committee on Jan 10, 2019 (HREC 147/18).
CONFLICT OF INTEREST
Dr R. Lewis is the PI for the Better Sleep Trial and is a paid consultant for Nyxoah SA.
AUTHOR CONTRIBUTION
RL: final draft, collation of images. JL, CC: initial draft, tabulation of data. GR: contribution to discussion and methods.
ACKNOWLEDGMENTS
This case was part of the Better Sleep Trial funded by Nyxoah SA, Belgium (ClinicalTrials.gov Identifier: NCT03763682).
Lewis R, Le J, Czank C, Raux G. Control of OSA in a patient with CCC of soft palate using bilateral hypoglossal nerve stimulation. Clin Case Rep. 2021;9:2222–2224. 10.1002/ccr3.3990
REFERENCES
- 1. Van de Heyning P, Badr S, Baskin J, et al. Implanted upper airway stimulation device for obstructive sleep apnea. Laryngoscope. 2012;122:1626‐1633. [DOI] [PubMed] [Google Scholar]
- 2. Vanderveken O, Maurer J, Hohenhorst W, et al. Evaluation of drug‐induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med. 2013;09(05):433‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eastwood P, Barnes M, MacKay S, et al. Bilateral hyoglossal nerve stimulation for treatment of adult obstructive sleep apnea. Eur Respir J. 2020;55(1):1901320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heiser C, Vanderveken O, Edenharter G, Hofauer B. Cross motor innervation of the hypoglossal nerve—a pilot study of predictors for successful opening of the soft palate. Sleep and Breathing. 2020. 10.1007/s11325-020-02112-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Constantino A, Rinaldi V, Moffa A, et al. Hypoglossal nerve stimulation long‐term clinical outcomes: a systematic review and meta‐analysis. Sleep Breath. 2020;24(2):399‐411. 10.1007/211325-019-01923-2 [DOI] [PubMed] [Google Scholar]


