Abstract
In chronic polyarthritis, the presence of macroglossia with absence of rheumatoid factor and anti‐CCP antibodies may be suggestive of amyloid arthopathy. Clinical evaluation takes precedence over classification criteria.
Keywords: AL amyloidosis, macroglossia, multiple myeloma, rheumatoid arthritis
In chronic polyarthritis, the presence of macroglossia with absence of rheumatoid factor and anti‐CCP antibodies may be suggestive of amyloid arthopathy. Clinical evaluation takes precedence over classification criteria.

1. INTRODUCTION
Amyloid arthropathy can mimic inflammatory rheumatism and is often underdiagnosed. The presence of macroglossia should draw attention to chronic polyarthritis. We report a case of AL amyloidosis complicating multiple myeloma in a 50‐year‐old man with chronic peripheral polyarthralgia and macroglossia.
AL amyloidosis is related to extracellular deposition of free light chains (more rarely heavy chains) of monoclonal immunoglobulins produced by a lymphoplasmacytic population. It is secondary to multiple myeloma (MM) in 5%‐15% of cases and often underdiagnosed due to its polymorphic presentation. 1 The arthropathy secondary to amyloid depositions remains rare with a prevalence of 3% according to studies. 2 We report a case of AL amyloidosis complicating MM revealed by bilateral peripheral polyarthritis associated with macroglossia.
2. OBSERVATION
A 50‐year‐old man had come for consultation for hands and wrists bilateral joint pain that had been evolving since 2 years, affecting the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints, sometimes waking him up at night, not calmed at rest, with gradual increase in intensity. These symptoms occurred in a context of asthenia, anorexia, and weight loss. In his history, he reported repeated episodes of infectious lung disease.
The clinical examination at admission revealed a reducible deformation of the hands such as cubital gale of the fingers and flexion of the MCP and PIP (Figure 1A). There was no joint swelling or skin nodule in relation to the joints. The examination also found a painless macroglossia with indentations of the lateral edges (Figure 1B). This macroglossia was responsible for moderate dysphagia.
FIGURE 1.

Photographs of the patient showing characteristic deformation of the hand (A) and macroglossia (B)
The biological balance showed an increase in the sedimentation rate to 66 mm, a C‐reactive protein at 59 mg/L, and a normocytic anemia at 9 g/dL of hemoglobin. The corrected serum calcium was very high at 3.1 mmol/L with normal proteinemia (69 g/L) and serum creatinine was 82 μmol/L. With regard to immunological examinations, serum protein electrophoresis showed hypogammaglobulinemia at 6.35 g/L with the presence of a kappa light chain monoclonal band on the immunofixation of serum and urinary proteins (Figure 2). Total proteinuria was 7 g/24 h with high Bence Jones proteinuria. Furthermore, the tests for antinuclear, anti‐dsDNA, anti‐ENA, antifillagrine, anticitrullin antibodies, and rheumatoid factor were negative. Multiple myeloma has been suspected due to anemia, hypercalcemia, and significant proteinuria. These elements associated with the absence of monoclonal peak led us to carry out a sternal puncture and cytological examination highlighted dystrophic plasma cells infiltration at 10%.
FIGURE 2.

Serum protein electrophoresis showing absence of monoclonal peak with hypogammaglobulinemia (A). Presence of monoclonal band on the kappa light chain track to the immunofixation of serum (B) and urinary (C) proteins on agarose gel
X‐rays of the hands (Figure 3) and pelvis (Figure 4) showed diffuse bone demineralization without erosion or involvement of joint spaces.
FIGURE 3.

X‐ray of the right (D) and left (G) hand showing diffuse bone demineralization
FIGURE 4.

X‐ray of the pelvis showing diffuse bone demineralization
The diagnosis of light chains MM was retained in front of medullar plasmocytosis, hypercalcemia, anemia, bone demineralization, absence of monoclonal peak, and presence of kappa light chains at immunoelectrophoresis. Assuming an associated amyloidosis, a biopsy of the minor salivary glands was carried out and the histological examination found amorphous eosinophilic material, colored by red Congo (Figure 5) and taking on a birefringent aspect to polarized light. It was, therefore, an AL amyloidosis complicating a kappa light chain MM presenting with rheumatoid arthritis‐like and macroglossia.
FIGURE 5.
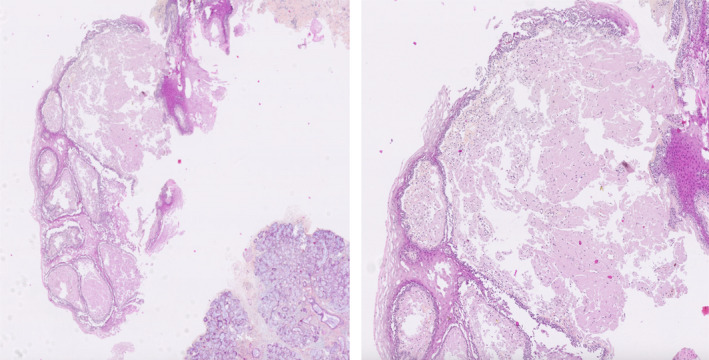
Histological section (Congo red stain and zoom): positivity of amyloid deposits to Congo red
Multidisciplinary care was planned. Our patient had given up the treatment, and 3 months later, he had deceased. The cause of death has not been determined.
3. DISCUSSION
The most common form of amyloidosis is AL form. The diagnosis is essentially anatomopathological by the identification of extracellular depositions colored in red Congo with dichroism and birefringence yellow‐green characteristic in polarized light. In our case, amyloidosis was confirmed by biopsy of the minor salivary glands. MM was diagnosed by the presence of medullar plasmocytosis associated with anemia, hypercalcemia, and bone involvement according to the criteria of the International Myeloma Working Group revised in 2014. 3
AL amyloidosis may mimic several systemic pathologies. Skin depositions may have a sclerosis‐like appearance. The damage to the salivary glands can manifest itself as a xerostomia evoking Sjögren's syndrome. We may also encounter joint deformations similar to rheumatoid arthritis (RA) as found in our case, secondary to synovial and periarticular infiltration. 4
Amyloid joint involvement during MM remains rare with a prevalence of 3% in the Fautrel et al series and 2%‐5% according to Gertz and Kyle. 2 , 5 Generally, AL amyloidosis arthropathy appears during the evolution of a known myeloma and exceptionally revealing, as in our case. The frequently encountered picture is that of a symmetrical bilateral polyarthritis with progressive installation and rheumatoid appearance. The flexor tendons damage of the fingers can cause a disabling bending attitude. An aspect of swelling or nodule secondary to amyloid infiltration of the soft tissues in regard to the joints can be found. 6 , 7 Axial involvement is possible, such as atlantoaxial luxation reported by Fautrel et al. 2 The quasi‐pathognomonic articular sign of amyloidosis is the epaulet aspect of the scapular belt, called shoulder pad sign. 8 Radiographic signs of amyloid arthropathies are often absent or nonspecific. When they exist, they consist of either bone erosions, or juxta‐articular geodes, or bone demineralization, or thickening of the soft tissues.
The articular forms of AL amyloidosis are very varied and may suggest a possible RA. Some semiological elements should not be overlooked, such as the presence of macroglossia and the absence of anticyclic citrullinated peptide antibodies. The peculiarity of this observation is the clinical predominance of chronic polyarthralgia. According to the literature, several cases of amyloid arthropathy have been mistakenly diagnosed with RA. 9 In a study by Prokaeva et al, 10 the incidence of amyloid arthropathy mimicking seronegative RA was 3.7% and the majority of patients were misdiagnosed initially. Clinical assessment takes precedence over classification criteria. The diagnosis of seronegative RA associated with AL amyloidosis had not been discussed as this association has only been described during AA amyloidosis. 11
Progressively, amyloid arthropathy is not associated with decreased survival, with the exception of concomitant heart or kidney damage. In the study by Prokaeva et al, 10 the median survival time in patients with isolated joint, bone, and soft tissue injuries was 70 months. In our observation, the progression of polyarthralgia was 2 years.
4. CONCLUSION
Amyloid arthropathy associated with MM remains rare and occasionally causes deforming peripheral polyarthritis simulating RA. The clinical presentation of this patient is similar to RA with the exception of the autoantibodies negativity and shows the importance of AL amyloidosis in differential diagnosis of chronic polyarthralgia. The search for macroglossia is essential in the face of any suspicion of systemic pathology, and biopsy plays an important role in supporting the diagnosis. This observation illustrates the importance of serum protein electrophoresis and calcemia in the face of any chronic polyarthralgia.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
RMFR, ER, and HAR: followed up the patient, collected the clinical data, and drafted the report. MR, VA, SNA: designed and critically revised the report. FR, HMDV, and MJDR: validated the report. All the authors have read and approved the final draft of the manuscript.
ETHICAL APPROVAL
This article does not contain any personal information that could identify the patient. The names and dates on the X‐ray films have been hidden. The authors have included only information necessary for scientific understanding.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Pasteur Institute of Madagascar for sending the biopsy piece to the Center of Pathological Morphology of Brussels for histological reading. Thanks to Mrs Fetraniaina Vololona RAMANOHARIJAONA who helped us with the English translation of this article. Published with written consent of the patient.
Randrianarisoa RMF, Rafanomezantsoa E, Razafindrazaka HA, et al. Light chain Amyloidosis (AL) associated with multiple myeloma revealed by peripheral bilateral polyarthritis: a case report. Clin Case Rep. 2021;9:2153–2157. 10.1002/ccr3.3968
DATA AVAILABILITY STATEMENT
HA Razafindrazaka who is the third author of this article took the photographs of the patient in the observation. These images can be published on request to the first author, RMF Randrianarisoa. No other data were used to support this study.
REFERENCES
- 1. Raubenheimer EJ, Dauth J, Pretorius FJ. Multiple myeloma and amyloidosis of the tongue. J Oral Pathol Med. 1988;17(9‐10):554‐559. [DOI] [PubMed] [Google Scholar]
- 2. Fautrel B, Fermand JP, Sibilia J, Nochy D, Rousselin B, Ravaud P. Amyloid arthropathy in the course of multiple myeloma. J Rheumatol. 2002;29(7):1473‐1481. [PubMed] [Google Scholar]
- 3. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538‐e548. [DOI] [PubMed] [Google Scholar]
- 4. Fujishima M, Komatsuda A, Imai H, Wakui H, Watanabe W, Sawada K. Amyloid arthropathy resembling seronegative rheumatoid arthritis in a patient with IgD‐kappa multiple myeloma. Intern Med. 2003;42(1):121‐124. [DOI] [PubMed] [Google Scholar]
- 5. Gertz MA, Kyle RA. Primary systemic amyloidosis‐ a diagnostic primer. Mayo Clin Proc. 1989;64(12):1505‐1519. [DOI] [PubMed] [Google Scholar]
- 6. Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337(13):898‐909. [DOI] [PubMed] [Google Scholar]
- 7. Lafforgue P, Senbel E, Figarella‐Branger D, et al. Systemic amyloidosis AL with temporal artery involvement revealing lymphoplasmatic malignancy in a man presenting as polymyalgia rheumatica. Ann Rheum Dis. 1993;52(2):158‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katz GA, Peter JB, Pearson CM, Adams WS. The shoulder pad sign‐ a diagnostic feature of amyloid arthropathy. N Engl J Med. 1973;288(7):354‐355. [DOI] [PubMed] [Google Scholar]
- 9. Elsaman AM, Radwan AR, Akmatov MK, et al. Amyloid arthropathy associated with multiple myeloma: a systematic analysis of 101 reported cases. Semin Arthritis Rheum. 2013;43(3):405‐412. [DOI] [PubMed] [Google Scholar]
- 10. Prokaeva T, Spencer B, Kaut M, et al. Soft tissue, joint, and bone manifestations of AL amyloidosis: clinical presentation, molecular features, and survival. Arthritis Rheum. 2007;56(11):3858‐3868. [DOI] [PubMed] [Google Scholar]
- 11. Hazenberg BP, van Rijswijk MH. Aspects cliniques de l'amylose AA. In: Grateau G, Benson MD, Delpech M, eds. Les Amyloses. Paris: Flammarion Médecine‐Sciences; 2000:377‐427. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
HA Razafindrazaka who is the third author of this article took the photographs of the patient in the observation. These images can be published on request to the first author, RMF Randrianarisoa. No other data were used to support this study.


