Abstract
Background/Purpose
90-day mortality is a key performance indicator for short-term perioperative outcome of hepatic resection (HR). Although many preoperative, intraoperative, and postoperative variables predict 90-day mortality following elective HR, only few are specific to hepatocellular carcinoma (HCC). This study aims to determine the predictors of 90-day mortality following elective HR for HCC.
Methods
We report a retrospective analysis of patients who underwent elective HR between January 1, 2007, and December 31, 2017. Health status, perioperative variables, and the presence of post-hepatectomy liver failure (PHLF) were studied. Cox's regression evaluated factors predicting 90-day mortality.
Results
Two hundred and forty-four patients diagnosed with HCC underwent HR; 102 (41.8%) underwent a major HR. The postoperative 90-day mortality rate was 5.3%. Multivariate analysis demonstrated that Child-Pugh score (p < 0.001), intraoperative blood loss (p = 0.013), the 50-50 criteria for PHLF (p < 0.001) on postoperative day 5, and peak serum bilirubin >119 µmol/L (p = 0.007) on postoperative day 3 predict 90-day mortality.
Conclusion
In patients with HCC undergoing HR, Child-Pugh score, intraoperative blood loss, the 50-50 criteria for PHLF on postoperative day 5, and peak serum bilirubin >119 µmol/L on postoperative day 3 predict 90-day mortality following elective HR for HCC.
Keywords: Hepatic resection, Hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is globally the fifth most commonly diagnosed malignancy in men and the ninth most commonly diagnosed in women [1]. It is also the fourth leading cause of cancer-related death worldwide [2]. Curative treatment for HCC is mainly surgical or thermal ablation. Given the limited donor liver pool for liver transplantation and the consensus that radiofrequency ablation (RFA) is not suitable for multiple or large lesions [3], hepatic resection (HR) is currently the mainstay treatment for HCC [4]. With advances in surgical technology and critical care, HR is considered a safe procedure. Over the past decades, perioperative mortality following elective HR has been 1–3% [5]. Oncologic outcomes of HR are comparable to those of combined transarterial chemoembolization (TACE) plus RFA, but procedure-related morbidity is lower for the TACE-RFA combination [6]. It is essential to know the variables predicting morbidity and mortality. Such knowledge can guide patient selection for surgery or impact decisions on alternative options.
Many studies report factors predicting morbidity and mortality following HR. However, these reports are heterogeneous. Authors included patients with metastatic liver disease or cholangiocarcinoma, reported variables predicting post-hepatectomy liver failure (PHLF) (and not mortality), or reported either 30-day mortality or in-hospital mortality [7, 8, 9, 10]. Due to such heterogeneity, it is unclear which variables impact 90-day mortality following elective HR for patients with HCC. Patients with HCC are unique when compared to other solid organ malignancies. In addition to the oncologic burden, HCC patients have an added burden of liver dysfunction. Hence, the outcomes of HR for other liver pathologies such as colorectal liver metastases are different from those for HCC.
Furthermore, many authors validate their data with existing scoring systems, which were either not designed exclusively for HR in the first place or derived from different patient profiles [11, 12, 13]. Hence there is a paucity of data with regard to variables predicting 90-day mortality following elective HR for HCC. Moreover, each hospital has its own unique management experience with different sets of inclusion criteria, which can impact outcomes. The available expertise, local resources, and compliance with staging systems can impact outcomes. In a local study reporting on 766 patients with HCC, it was shown that patients with Barcelona Clinic Liver Cancer stage C managed according to Hong Kong Liver Cancer recommendations have improved survival [14].
Indications of HR are evolving. Many patients who have not been surgical candidates in the recent past are now resected. Repeat liver resections, two-staged hepatectomy, associating liver partition and portal vein ligation for staged hepatectomy, and preoperative portal vein embolization (PVE) techniques have evolved. As such, more data are needed with regard to the surgical outcomes following elective HR for HCC [15]. Our study aims to evaluate factors that predict 90-day mortality after elective HR in patients with HCC.
Methods
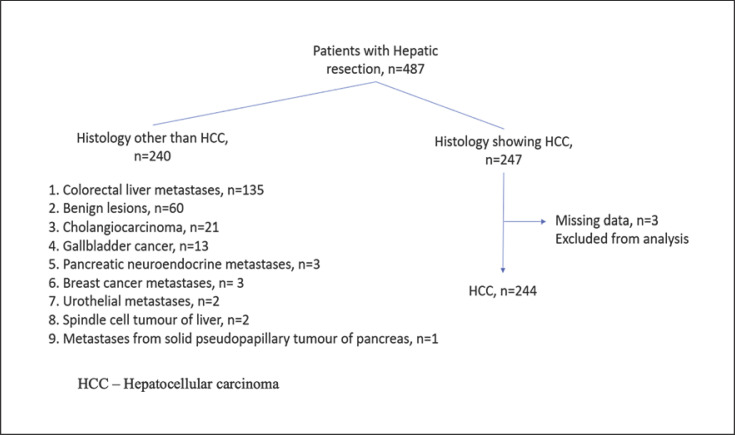
We report a retrospective cohort study of patients treated with elective HR for HCC at a university-affiliated hospital between January 1, 2007, and December 31, 2017. Locally, laparoscopic liver resection started in June 2006 [16]. We included patients treated with open or laparoscopic techniques and excluded patients having undergone synchronous resections of other organs such as the pancreas or lung. Patients with final histological results that were not proven to be HCC were also excluded. Figure 1 shows the patient inclusion process. Two hundred and forty-seven patients underwent HR for HCC. We excluded 3 patients with missing data from analysis.
Fig. 1.
Flow chart showing inclusion of study patients.
All patients underwent clinical, biochemical, and radiological evaluation before the decision for HR. Their demographic profiles, clinical data, and laboratory parameters were retrieved from electronic medical records. The demographic data collected included age, gender, Eastern Cooperative Oncology Group performance status, and American Society of Anesthesiology (ASA) physical status classification scores. Patient comorbidities and previous treatments such as TACE, PVE, and RFA were recorded. Complete blood count, serum albumin, liver function, renal function, hepatitis B and C status, serum α-fetoprotein, and Child-Pugh score were recorded.
A Child-Pugh score of 9 or above is a contraindication to HR, and our cohort included 1 patient with a Child-Pugh score of 9. All patients were reviewed by a multidisciplinary hepatobiliary tumor board and were deemed suitable for HR. The indocyanine green (ICG) dye retention time was calculated for patients planned for major HR. ICG retention >15% at 15 min was considered a contraindication to major HR. For minor HR, we adopt ICG retention >25% at 15 min as an exclusion criterion, with the exception of laparoscopic hepatic wedge resections.
All patients scheduled for trisectionectomy had their liver volume calculated as an adjunct to surgical planning. Hepatopancreaticobiliary consensus guidelines were used to determine the future liver remnant (FLR). Preoperative PVE was done on patients with inadequate FLR [17]. The FLR is calculated by contrast-enhanced computerized tomography (CT) scan. Once listed for surgery, patients were reviewed at the preadmission evaluation and counseling clinic by anesthetists and case managers. The patients were scored for ASA physical status classification and referred appropriately to the cardiology or respiratory unit for optimization of comorbidities. Selected patients were offered cardiopulmonary exercise testing, and we determined an anaerobic threshold of <11 mL/kg/min as a contraindication to HR [12]. The standard operating principles of both open and laparoscopic liver surgery at our unit were published previously [13]. We used the Brisbane 2000 terminology and classification system to define the type of resection [18]. HRs were classified as major or minor. Major hepatectomy was defined as the resection of ≥3 segments, and minor hepatectomy was defined as the resection of <3 segments.
The biochemical values of international normalized ratio, prothrombin time, and total serum bilirubin were recorded before the operation and on a postoperative day 5 for all patients. Peak serum bilirubin was recorded on postoperative day 3. Intraoperative data such as the type of HR, operative time, blood loss, volume of blood transfusion, conversion to open surgery, and surgical adjuncts, e.g., Pringle's maneuver, were documented. We do not have a policy for routine (active) Pringle's maneuver and reserve it for patients with intraoperative bleeding (reactive).
We reviewed the histological results for staging, presence of cirrhosis, vascular invasion, and R1 resection rate. We defined R1 resection as positive histologic margins or a tumor within 0.1 mm from the margin. Macrovascular invasion was determined by an intraoperative ultrasound scan and confirmed on histopathology showing direct tumor invasion into major hepatic veins or portal veins. We did not distinguish macrovascular invasion of the hepatic venous system from that of the portal venous system. Postoperative morbidity, 30-day mortality, and 90-day mortality were studied. The 50-50 criteria, peak serum bilirubin criteria, and International Study Group of Liver Surgery criteria defined PHLF [5, 19, 20]. We calculated various biochemical indices to study their impact on 90-day mortality.
The prognostic nutritional index, bilirubin-albumin ratio, platelet-albumin-bilirubin index, and neutrophil-to-lymphocyte ratio were calculated [13, 21, 22]. All patients were managed according to the local care pathway, and patients with any deviation were actively investigated, including a liberal policy for postoperative CT scanning of the abdomen and pelvis. We defined any volume of bile in the postoperative drainage tube at or after the third day or bile in the image-guided inserted percutaneous drainage catheter as a bile leak. The length of stay was calculated from surgery date to discharge date, with both dates inclusive. We report overall survival at 1 year, 3 years, and 5 years.
SPSS v.25 was used to perform the statistical analysis. A p value of <0.05 was considered as statistically significant. Patients' demographic and clinical characteristics were summarized descriptively. The Kaplan-Meier technique was used to estimate median overall survival after HR. Cox regression was carried out to evaluate the prognostic factors for 90-day mortality.
Results
Two hundred and forty-four patients diagnosed with HCC underwent elective HR. Table 1 shows the demographic and clinical profiles of the patients. The majority of the patients were male with underlying liver cirrhosis. Most patients had Child-Pugh class A cirrhosis (n = 223, 91.4%), and 1 patient had Child-Pugh class C cirrhosis. Around half of the patients were hepatitis B carriers, and over half of the patients had minor HR. Fifteen patients (6.1%) had TACE performed before surgery, and 8 patients (3.3%) had RFA performed. Four patients (1.6%) had PVE done to achieve hypertrophy of the FLR. Ninety-seven patients (39.8%) underwent laparoscopic HR, and 103 patients (42.2%) had major HR done. Two hundred and three patients had an ICG clearance test performed; 1 patient had an ICG retention of 35% and ultimately underwent laparoscopic hepatic wedge resection.
Table 1.
Demographic and clinical profile of the patients (N = 244)
| Gender (male), n (%) | 206 (84.4) |
| Median age (range), years | 67 (28–88) |
| Mean body mass index (range), kg/m2 | 23.5 (15.6–40.4) |
| ASA score ≥3, n (%) | 134 (54.9) |
| ECOG performance status ≥2, n (%) | 13 (5.3) |
| Diabetes mellitus, n (%) | 113 (46.7) |
| Hypertension, n (%) | 160 (65.6) |
| Hepatitis B carrier (positive for HBsAg), n (%) | 116 (47.5) |
| Alcohol consumption, n (%) | 48 (19.7) |
| Median albumin (range), g/L | 38.0 (22–48) |
| Median bilirubin (range), µmol/L | 15.5 (4–100) |
| Median creatinine (range), µmol/L | 82 (34–828) |
| Median prothrombin time (range), s | 13.3 (11.9–34.5) |
| Median platelet count (range), ×109/L | 202 (43.3–1,445) |
| Child-Pugh class, n (%) | |
| A | 223 (91.4) |
| B | 20 (8.2) |
| C | 1 (0.4) |
| ICG dye retention test | |
| Performed, n (%) | 203 (83.2) |
| Mean (range) (n = 203), % | 9.32 (1.8–35.0) |
| Liver parenchyma, n (%) | |
| Fatty | 43 (17.6) |
| Cirrhotic | 154 (63.1) |
| Normal | 47 (19.3) |
| Previous treatment, n (%) | |
| Transarterial chemoembolization | 15 (6.1) |
| Portal vein embolization | 4 (1.6) |
| Radiofrequency ablation | 8 (3.3) |
ASA, American Society of Anesthesiologists score; ECOG, Eastern Cooperative Oncology Group; ICG, indocyanine green.
Postoperative outcomes, including histopathological results, postoperative complications, and oncological outcomes, are reported in Table 2. The mean tumor diameter was 56.3 mm (range 1–200), and the mean number of tumors was 1.6 (range 1–11). Forty-six patients (18.9%) needed a postoperative CT scan of the abdomen and pelvis to evaluate for any deviation from the normal recovery process. The two most common postoperative complications were pleural effusion (n = 44, 18%) and ileus (n = 26, 10.7%). Eighty-five patients (34.8%) had at least one feature of liver decompensation (ascites, encephalopathy, bilirubin >31 µmol/L, or international normalized ratio >1.4) or required fresh frozen plasma transfusion. The postoperative 90-day mortality rate was 5.3%. Table 3 shows the results of a multivariate analysis of factors predicting 90-day mortality. Child-Pugh score (p < 0.001), intraoperative blood loss (p = 0.013), 50-50 criteria for PHLF (p < 0.001), and peak serum bilirubin >119 µmol/L (p = 0.007) predict 90-day mortality. Logistic regression further shows that Child-Pugh score (p < 0.001), 50-50 criteria for PHLF (p < 0.001), and peak serum bilirubin >119 µmol/L (p = 0.007) are significant.
Table 2.
Perioperative outcomes (N = 244)
| Operative variables | |
| Laparoscopic liver resection, n (%) | 97 (39.8) |
| Major liver resection, n (%) | 103 (42.2) |
| Median operation time (range), min | 280 (60–620) |
| Median blood loss (range), mL | 500 (20–3,900) |
| Mean duration of Pringle's maneuver (standard deviation), min | 5.2 (17.1) |
| Postoperative outcomes | |
| Median length of stay (range), days | 7 (1–150) |
| High dependency unit stay, n (%) | 224 (91.8) |
| Intensive care unit stay, n (%) | 47 (19.3) |
| Vasopressor support, n (%) | 11 (4.5) |
| Renal replacement therapy, n (%) | 2 (0.8) |
| Postoperative complications, n (%) | |
| Pneumonia | 21 (8.6) |
| Cardiac events (arrythmias and ischemic heart disease) | 18 (7.4) |
| Stroke/transient ischemic attack | 1 (0.4) |
| Acute kidney injury | 9 (3.7) |
| Urinary tract infection | 12 (4.9) |
| Deep vein thrombosis | - |
| Pulmonary embolism | 2 (0.8) |
| Superficial surgical site infection | 12 (4.9) |
| Pleural effusion | 44 (18) |
| Intra-abdominal abscess | 8 (3.3) |
| Bile leak | 5 (2.0) |
| Ileus | 26 (10.7) |
| Portal vein thrombosis | 4 (1.6) |
| Respiratory failure | 8 (3.3) |
| Ascites | 16 (6.6) |
| Encephalopathy | 8 (3.3) |
| PHLF, n (%) | |
| PHLF criterion 1: 50/50 − bilirubin >50 µmol/L, INR >1.7 | 7 (2.9) |
| PHLF criterion 2: peak serum bilirubin >119 µmol/L | 10 (4.1) |
| PHLF criterion 3: local laboratories: bilirubin >31 µmol/L, INR >1.4 | 26 (10.7) |
| PHLF (any of the above criteria) | 28 (11.5) |
| Mortality, n (%) | |
| 30-day mortality | 8 (3.28) |
| 90-day mortality | 13 (5.33) |
| Tumor characteristics (at final histology) | |
| Mean size (range), mm | 56.3 (1–200) |
| Mean number (range) | 1.6 (1–11) |
| Histopathology, n (%) | |
| Macrovascular invasion | 25 (10.2) |
| Microvascular invasion | 68 (27.9) |
| R1 resection | 14 (5.7) |
| Stage T1 | 119 (48.8) |
| Stage T2 | 72 (29.5) |
| Stage T3 | 44 (18) |
| Stage T4 | 9 (3.7) |
PHLF, post-hepatectomy liver failure; INR, international normalized ratio.
Table 3.
Multivariate analysis of factors predicting 90-day mortality after hepatectomy
| Unadjusted HR (95% CI) | Unadjusted p value | Adjusted HR (95% CI) | Adjusted p value | |
|---|---|---|---|---|
| Alkaline phosphatase (units/L) | 1.004 (1.001–1,007) |
0.004 | 1.002 (0.997–1.006) |
0.504 |
| Gamma-glutamyl transferase (units/L) | 1.003 (1.001–1.004) |
<0.001 | 1.001 (0.998–1.003) |
0.661 |
| Child-Pugh class B/C |
2.55 (1.44–4.54) |
0.001 |
3.44 (0.152–0.557) |
<0.001 |
| Platelet-albumin-bilirubin (PALBI)1 | 1.417 (1.025–1.959) |
0.035 | 1.203 (0.771–1.876) |
0.415 |
| Prognostic nutritional index | 0.948 (0.917–0.979) |
0.001 | 0.977 (0.929–1.027) |
0.358 |
| Bilirubin/albumin ratio (µmol/g) | 1.921 (1.23–2.983) |
0.004 | 0.747 (0.318–1.754) |
0.503 |
| Neutrophil-to-lymphocyte ratio | 1.056 (0.994–1.122) |
0.076 | 1.013 (0.916–1.120) |
0.799 |
| Model for End-Stage Liver Disease (MELD) | 1.055 (0.993–1.1210 |
0.083 | 1.014 (0.942–1.092) |
0.708 |
| Indocyanine green | 1.828 (0.916–3.650) |
0.087 | 1.546 (0.643–3.716) |
0.330 |
| Operation time | 1.003 (1.001–1.004) |
0.005 | 0.999 (0.997–1.002) |
0.670 |
| Blood loss |
1.392 (1.083–1.790) |
0.010 |
1.486 (1.088–2.031) |
0.013 |
| PHLF criterion 1: 50/50 − bilirubin >50 µmol/L, INR >1.7 |
7.166 (2.853–18.002) |
<0.001 |
8.628 (3.330–22.353) |
<0.001 |
| PHLF criterion 2: peak serum bilirubin >119 µmol/L |
3.910 (1.941–7.878) |
<0.001 |
2.963 (1.355–6.480) |
0.007 |
| PHLF criterion 3: local laboratories: bilirubin >31 µmol/L, INR >1.4 |
2.177 (1.235–3.835) |
0.007 | 0.966 (0.396–2.357) |
0.940 |
Bold type denotes significance. PHLF, post-hepatectomy liver failure.
PALBI = 2.02 × log10 bilirubin − 0.37 × (log10 bilirubin) 2 − 0.04 × albumin − 3.48 × log10 platelets + 1.01 × (log10 platelets) [23].
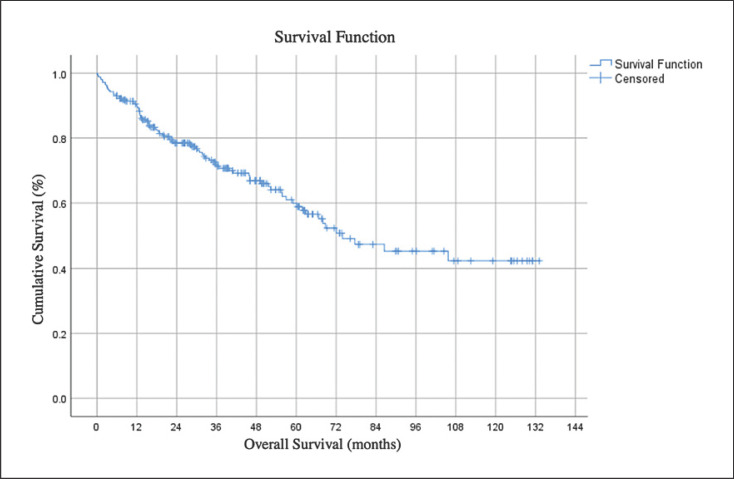
The 1-year, 3-year, and 5-year overall survival rate was 75.4% (n = 184), 46.3% (n = 113), and 23% (n = 56), respectively. The median survival time for all patients was 39 months (5–133). Figure 2 shows overall survival.
Fig. 2.
Survival time after hepatic resection.
Discussion
In our study, the Child-Pugh score, volume of intraoperative blood loss, 50-50 criteria for PHLF, and peak serum bilirubin >119 µmol/L predicted 90-day mortality. Liver function tests performed by serum biochemistry are sine qua non prior to HR. In patients with compromised liver function, treatment has to be tailored to ensure sufficient residual liver function, and in some instances, liver transplantation would be warranted.
A liver function test also forms the basis for calculating the Child-Pugh score. The Child-Pugh score is calculated based on variables that define the synthetic and metabolic activities of the liver. Hence, it is expected to be sensitive in predicting perioperative outcomes of patients with liver dysfunction. The score has been validated in hepatic and nonhepatic, elective, and emergency surgeries for short-term as well as long-term outcome prediction [23, 24, 25]. There is enough evidence that elective surgery is safe and feasible in patients with Child-Pugh class A cirrhosis who have preserved liver function [26, 27]. Child-Pugh class B and C patients are at high risk of perioperative outcomes, and some authors consider this as a relative contraindication to surgery. In a study including 273 patients, Sonohara et al. [26] reported clinical data on 235 patients who were all Child-Pugh class A. In a multicenter study including 253 patients with Child-Pugh class B cirrhosis from 14 international centers, Berardi et al. [28] have shown that postoperative morbidity following HR in these patients remains high (42.7%). The authors report treating 12,814 HCC patients with Child-Pugh class A (n = 11,983) and class B (n = 831) cirrhosis. Our rate of operating on Child-Pugh class B patients (8.2%) is higher than in this multicenter study (6.9%). Our report includes 1 patient with Child-Pugh class C cirrhosis, who had a wedge resection performed, and this illustrates that the patient selection process is individualized for each patient and pathology.
Besides serum biochemistry, liver function measured via ICG clearance is also reported by many studies to prognosticate posthepatic liver failure, and it is recommended that major HR be avoided for patients with ICG retention >15% at 15 min [29]. Our institution follows this guideline to exclude patients with poor ICG clearance from major HR. While our study did not show a significance of ICG clearance in predicting 90-day mortality, this could be due to its small sample size and should be interpreted in the context that the patients with poor ICG clearance were excluded from major HR.
Intraoperative bleeding and blood transfusion are essential considerations in surgical oncology and reported to impact both perioperative and oncologic outcomes [30, 31]. The threshold for transfusion of blood at a hemoglobin level <8 g/dL is widely adopted internationally. In a study including 1,222 consecutive liver resections for hepatobiliary diseases, Poon et al. [32] showed that reduced perioperative transfusion was contributory to improved outcomes. Martin et al. [33] showed that blood transfusion was independently associated with postoperative morbidity (OR = 4.18, 95% CI 2.18–8.02, p < 0.001) and 30-day mortality (OR 14.5, 95% CI 3.08–67.8, p = 0.001) following liver resection for all indications.
The impact of blood loss or blood transfusion on both short-term and long-term outcomes is multifactorial. Higher intraoperative blood loss could indicate major, difficult, or complicated resection with a greater likelihood of complications or a cirrhotic liver with underlying portal hypertension. Meticulous surgical and monitored anesthetic techniques reduce blood loss. Low central venous pressure anesthesia, vascular inflow occlusion, restrictive transfusion strategies, ultrasonic energy or stapling devices, hemostatic adjuncts, and communication between anesthetists and surgeons are essential considerations to reduce blood loss. In a study including 315 patients undergoing liver resection, Nonami et al. [29] reported that blood loss was independently associated with PHLF and mortality. This association is consistent with the fact that PHLF is a primary cause of death after HR despite improvements in surgical techniques and perioperative management [34].
There are many definitions of PHLF [5, 19, 20]. Hyder et al. [8] reported that 50-50 criteria and peak serum bilirubin >7 mg/dL did not predict 90-day mortality. However, this is likely due to a varied selection of patients with benign lesions (17%) and colorectal (39%) and noncolorectal (14%) liver metastases. Furthermore, they had only 1 patient who met the 50-50 criteria, and that patient survived. PHLF is an important outcome measure for patients undergoing HR; hence, many authors have attempted to study the role of inflammation-based indices in predicting PHLF. Inflammation-based scores are simple and easy to compute from existing biochemical investigations at no extra cost. Thus, they have recently gained attention. In a study including 3,064 patients from the National Surgical Quality Improvement Program, Andreatos et al. [35] showed that the albumin-bilirubin (ALBI) score was associated with PHLF (OR 1.57, 95% CI 1.08–2.27, p = 0.02) and predicted its severity (OR 3.06, 95% CI 1.50–6.23, p = 0.003) on multivariate analysis. The albumin-bilirubin score predicts PHLF and overall survival following HR for HCC [36]. We have previously reported that the preoperative neutrophil-to-lymphocyte ratio plus the platelet-to-lymphocyte ratio predict oncologic outcomes [13]. In our current study, we did not find any association with an inflammation-based score. This fact could be due to the cutoff values used or the small number of patients with 90-day mortality data in our study.
Our study has several limitations. Outcome data from a specialist unit and a single center should not be generalized to all demographic profiles. We did not report the causes of 90-day mortality and whether they were related to early tumor recurrence. We also did not report variables such as platelet count and all inflammation-based indices. Furthermore, due to the retrospective nature of the data, the cause-effect relationship cannot be determined.
In conclusion, the Child-Pugh score and volume of intraoperative blood loss predict 90-day mortality following elective HR in patients with HCC. Patients with PHLF defined according to the 50-50 criteria and peak serum bilirubin are also more likely to experience 90-day mortality. Our results serve to guide patient selection for surgical and nonsurgical approaches to the management of HCC. Less invasive approaches such as TACE and RFA or combination therapies should be explored for patients with liver dysfunction or predicted technically difficult resection with potential for blood loss.
Statement of Ethics
This study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The Institutional Review Board (IRB), National Healthcare Group (NHG) Domain Specific Review Board (DSRB), has approved a standing order database for liver resection, and all patients provided informed consent. The approval number is DSRB SDB TTSH/2017-00036.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was provided for this study.
Author Contributions
G.Y. Lei: data collection and interpretation; drafting of the manuscript. L. Shen: data analysis and reporting. S.P. Junnarkar, C.T. Huey, J. Low, and V.G. Shelat: data interpretation and critical revision of the manuscript.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015 Mar;136((5)):E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Global Burden of Disease Liver Cancer Collaboration et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017 Dec;3((12)):1683–91. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Panel of Experts in HCC-Design Clinical Trials et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008 May;100((10)):698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi SK, Eguchi S. Hepatic resection for hepatocellular carcinoma. Hepatoma Res. 2018;4((8)):50. [Google Scholar]
- 5.Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007 May;204((5)):854–62. doi: 10.1016/j.jamcollsurg.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 6.Gui CH, Baey S, D'cruz RT, Shelat VG. Trans-arterial chemoembolization + radiofrequency ablation versus surgical resection in hepatocellular carcinoma - A meta-analysis. Eur J Surg Oncol. 2020 May;46((5)):763–71. doi: 10.1016/j.ejso.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Simons JP, Ng SC, Hill JS, Shah SA, Bodnari A, Zhou Z, et al. In-hospital mortality for liver resection for metastases: a simple risk score. J Surg Res. 2009 Sep;156((1)):21–5. doi: 10.1016/j.jss.2009.03.073. [DOI] [PubMed] [Google Scholar]
- 8.Hyder O, Pulitano C, Firoozmand A, Dodson R, Wolfgang CL, Choti MA, et al. A risk model to predict 90-day mortality among patients undergoing hepatic resection. J Am Coll Surg. 2013 Jun;216((6)):1049–56. doi: 10.1016/j.jamcollsurg.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang CM, Yin WY, Su YC, Wei CK, Lee CH, Juang SY, et al. Preoperative risk score predicting 90-day mortality after liver resection in a population-based study. Medicine (Baltimore) 2014 Sep;93((12)):e59. doi: 10.1097/MD.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann K, Hinz U, Stravodimos C, Knoblich T, Schön MR, Büchler MW, et al. Risk assessment for liver resection. Surgery. 2018 Nov;164((5)):998–1005. doi: 10.1016/j.surg.2018.06.024. [DOI] [PubMed] [Google Scholar]
- 11.Knoblich T, Hinz U, Stravodimos C, Schön MR, Mehrabi A, Büchler MW, et al. Comparison of score-based prediction of 90-day mortality after liver resection. BMC Surg. 2020 Jan;20((1)):19. doi: 10.1186/s12893-020-0678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madhavan S, Shelat VG, Soong SL, Woon WW, Huey T, Chan YH, et al. Predicting morbidity of liver resection. Langenbecks Arch Surg. 2018 May;403((3)):359–69. doi: 10.1007/s00423-018-1656-3. [DOI] [PubMed] [Google Scholar]
- 13.Kabir T, Ye M, Mohd Noor NA, Woon W, Junnarkar SP, Shelat VG. Preoperative Neutrophil-to-Lymphocyte Ratio Plus Plateletto-Lymphocyte Ratio Predicts the Outcomes after Curative Resection for Hepatocellular Carcinoma. Int J Hepatol. 2019 Apr;2019:4239463. doi: 10.1155/2019/4239463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selby LK, Tay RX, Woon WW, Low JK, Bei W, Shelat VG, et al. Validity of the Barcelona Clinic Liver Cancer and Hong Kong Liver Cancer staging systems for hepatocellular carcinoma in Singapore. J Hepatobiliary Pancreat Sci. 2017 Mar;24((3)):143–52. doi: 10.1002/jhbp.423. [DOI] [PubMed] [Google Scholar]
- 15.Chan KS, Low JK, Shelat VG. Associated liver partition and portal vein ligation for staged hepatectomy: a review. Transl Gastroenterol Hepatol. 2017 Mar;24((3)):143–52. doi: 10.21037/tgh.2019.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang E, Kow AW, Chan CY, Liau KH, Ho CK. Starting a laparoscopic hepatectomy programme. Singapore Med J. 2009 Apr;50((4)):354–9. [PubMed] [Google Scholar]
- 17.Vauthey JN, Dixon E, Abdalla EK, Helton WS, Pawlik TM, Taouli B, American Hepato-Pancreato-Biliary Association. Society of Surgical Oncology. Society for Surgery of the Alimentary Tract et al. Pretreatment assessment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford) 2010 Jun;12((5)):289–99. doi: 10.1111/j.1477-2574.2010.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.IHPBA TCot Terminology of liver anatomy and resections. HPB (Oxford) 2000;2:333–9. doi: 10.1080/136518202760378489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005 Dec;242((6)):824–8. doi: 10.1097/01.sla.0000189131.90876.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011 May;149((5)):713–24. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008 Aug;32((8)):1757–62. doi: 10.1007/s00268-008-9552-6. [DOI] [PubMed] [Google Scholar]
- 22.Okamura Y, Ashida R, Ito T, Sugiura T, Mori K, Uesaka K. Preoperative neutrophil to lymphocyte ratio and prognostic nutritional index predict overall survival after hepatectomy for hepatocellular carcinoma. World J Surg. 2015 Jun;39((6)):1501–9. doi: 10.1007/s00268-015-2982-z. [DOI] [PubMed] [Google Scholar]
- 23.Farnsworth N, Fagan SP, Berger DH, Awad SS. Child-Turcotte-Pugh versus MELD score as a predictor of outcome after elective and emergent surgery in cirrhotic patients. Am J Surg. 2004 Nov;188((5)):580–3. doi: 10.1016/j.amjsurg.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 24.Jeffrey D, Wayne M, Gregory Y. Preoperative predictors of survival after resection of small hepatocellular carcinomas. Ann Surg. 2002 May;235((5)):722–30. doi: 10.1097/00000658-200205000-00015. discussion 730–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng Y, Qi X, Guo X. Child-Pugh Versus MELD Score for the Assessment of Prognosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis of Observational Studies. Medicine (Baltimore) 2016 Feb;95((8)):e2877. doi: 10.1097/MD.0000000000002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonohara F, Yamada S, Tanaka N, Suenaga M, Takami H, Hayashi M, et al. Perioperative and prognostic implication of albumin-bilirubin-TNM score in Child-Pugh class A hepatocellular carcinoma. Ann Gastroenterol Surg. 2018 Sep;3((1)):65–74. doi: 10.1002/ags3.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul JL, European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu. European Association for the Study of the Liver et al. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018 Jul;69((1)):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Berardi G, Morise Z, Sposito C, Igarashi K, Panetta V, Simonelli I, et al. Development of a nomogram to predict outcome after liver resection for hepatocellular carcinoma in Child-Pugh B cirrhosis. J Hepatol. 2020 Jan;72((1)):75–84. doi: 10.1016/j.jhep.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 29.Nonami T, Nakao A, Kurokawa T, Inagaki H, Matsushita Y, Sakamoto J, et al. Blood loss and ICG clearance as best prognostic markers of post-hepatectomy liver failure. Hepatogastroenterology. 1999 May-Jun;46((27)):1669–72. [PubMed] [Google Scholar]
- 30.Kim SY, Choi M, Hwang HK, Rho SY, Lee WJ, Kang CM. Intraoperative Transfusion is Independently Associated with a Worse Prognosis in Resected Pancreatic Cancer-a Retrospective Cohort Analysis. J Clin Med. 2020 Mar;9((3)):E689. doi: 10.3390/jcm9030689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischer D, Neb H, Choorapoikayil S, Zacharowski K, Meybohm P. Red blood cell transfusion and its alternatives in oncologic surgery-A critical evaluation. Crit Rev Oncol Hematol. 2019 Feb;134:1–9. doi: 10.1016/j.critrevonc.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1,222 consecutive patients from a prospective database. Ann Surg. 2004 Oct;240((4)):698–708. doi: 10.1097/01.sla.0000141195.66155.0c. discussion 708–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin AN, Kerwin MJ, Turrentine FE, Bauer TW, Adams RB, Stukenborg GJ, et al. Blood transfusion is an independent predictor of morbidity and mortality after hepatectomy. J Surg Res. 2016 Nov;206((1)):106–12. doi: 10.1016/j.jss.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schreckenbach T, Liese J, Bechstein WO, Moench C. Posthepatectomy liver failure. Dig Surg. 2012;29((1)):79–85. doi: 10.1159/000335741. [DOI] [PubMed] [Google Scholar]
- 35.Andreatos N, Amini N, Gani F, Margonis GA, Sasaki K, Thompson VM, et al. Albumin-Bilirubin Score: Predicting Short-Term Outcomes Including Bile Leak and Post-hepatectomy Liver Failure Following Hepatic Resection. J Gastrointest Surg. 2017 Feb;21((2)):238–48. doi: 10.1007/s11605-016-3246-4. [DOI] [PubMed] [Google Scholar]
- 36.Wang YY, Zhong JH, Su ZY, Huang JF, Lu SD, Xiang BD, et al. Albumin–bilirubin versus Child–Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg. 2016 May;103((6)):725–34. doi: 10.1002/bjs.10095. [DOI] [PubMed] [Google Scholar]