Abstract
Backgound
Even a minor iodine deficiency can result in adverse thyroidal health consequences while excess iodine intake can also result in thyroid function disorders. One source of iodine is seaweed which as a foodstuff is enjoying an increasing profile in Western countries. Apart from its potential involvement in thyroidal health, gaseous iodine released from seaweeds plays a significant role in influencing coastal climate through cloud formation.
Summary
Sources of dietary iodine, its assessment, recommended dietary intake, and consequences of iodine excess are outlined. The benefits and possible dangers of dietary intake of iodine-rich seaweed are described. Studies linking seaweed intake to breast cancer prevalence are discussed as is the role of gaseous iodine released from seaweeds influencing weather patterns and contributing to iodine intake in coastal populations.
Key Messages
Universal salt iodization remains the optimum method of achieving optimum iodine status. Promoting increased dietary iodine intake is recommended in young women, in early pregnancy, and in vegan and vegetarian diets. Even where iodine intake is enhanced, regular assessment of iodine status is necessary. Caution against consumption of brown seaweeds (kelps) is required as even small amounts can have antithyroid actions while product labelling may be insufficient. Gaseous iodine produced from seaweeds can have a significant effect on cloud formation and associated global warming/cooling. Increased overall iodine deposition through rainfall and apparent uptake in populations dwelling in seaweed-rich coastal regions may provide a partial natural remedy to global iodine deficits.
Keywords: Atmospheric iodine, Iodine, Iodine deficiency, Seaweed, Thyroid, Urinary iodine
Introduction
Iodine was joined with seaweed at birth as Courtois in 1811 in France searching on behalf of his emperor for sources of nitrates to use in gunpowder found that seaweed when burned gave off a vapour. In 1813, Gay Lussac named this vapour iodes, the Greek word for “violet coloured,” subsequently shown as a constituent of thyroxine (T4) and as an active goitre-preventing ingredient [1]. As iodine is not produced in the body, daily intake is required from external sources. This is especially true in the first weeks of life, as the developing foetus does not develop a functioning thyroid until about 18- to 20-week gestation during which time it is totally dependent on the mother to produce thyroid hormones necessary for growth and neuropsychological development [2]. Thus, maternal iodine intake is necessary for both mother and baby to thrive, and even mild iodine deficiency can lead to lower cognitive development [3]. The increased iodine requirement in pregnancy has led the WHO to recommend increasing iodine intake from 150 µg to 250 µg in pregnant mothers [4, 5]. Under iodine replete conditions, this requirement can usually be met from normal foodstuffs, particularly if salt is iodized. However, as salt iodization is not always mandatory, or even available, additional iodine supplementation may be required, and intake needs to be continuously monitored [6, 7]. While promoting increased dietary iodine intake is useful in correcting iodine deficiency, this cannot be an open-ended process as too much iodine can produce deleterious effects on thyroid function [8]. In the marine environment, seaweeds − marine algae, form the major supplier of iodine [9]. The iodine content of seaweed and its chemical form varies with different species but is generally greater in brown seaweeds (kelps) than the green or red varieties [9]. The use of seaweed in Asian cooking has been long established and is enjoying increasing popularity in the Western diet [10]. Iodine derived from seaweed intake can be taken up through a number of routes. Equally important is the role of seaweed-derived iodine in influencing climate cloud formation [11]. Iodine, particularly in seaweed-abundant coastal areas, acts as a powerful antioxidant [12] and may contribute via respiration to iodine intake in humans [13].
The objective of this communication is to review the association with thyroid function of seaweed-derived iodine intake, outlining the beneficial and in some case adverse consequences of this association. The influence on cloud formation and possible iodine intake of gaseous iodines released in seaweed-abundant coastal areas will also be examined.
Sources of Food Iodine Intake
Fish and crustacean of marine origin, seaweeds, and sea vegetables are the richest food sources of iodine. However, in many countries, particularly Northern Europe and Australasia, dairy products, including milk, cheese, yogurt, and eggs, are the main source, particularly where seafood, due to cost or availability, may not be favoured [4, 7]. As intake of fish or dairy products is discretionary, iodization of table salt with KI or KIO3 at a level of 20–40 mg/kg provides a useful method of achieving population cover [6]. Use of iodized table salt is only mandatory in some countries and is almost entirely absent in others such as the UK and Ireland [14, 15, 16]. In countries where salt iodization programmes have failed to achieve 20% population coverage, the WHO recommends iodine supplementation for pregnant women and infants aged <2 years [4, 6]. Deficient iodine intake in the Great Lakes area of the USA was reported in 1920 [17]. Iodization of table salt introduced in the 1920s greatly improved the situation. Goitre prevalence of 5% in WW1 recruits in the USA had declined to 0.06% in WW2 recruits [18]. In the 1960s, iodate, used as the so-called dough conditioner to speed up bread production in the USA, resulted in excessive iodine intake [19].
However, even in the absence of discretionary use of table salt, it must be remembered that salt sprinkled on food represents only approximately 40% of salt consumed [7] or even less (18%) according to UK figures [20], the balance being made up by salt-containing processed foods [15]. Introduction of iodized salt is often resisted on the mistaken grounds that its advocates favour increased salt consumption with associated risks of hypertension [21].
The use of iodized salt added to flour in breadmaking has become mandatory in both Australia and New Zealand with corresponding improvements in population iodine status [22, 23]. Another vector for increasing population iodine intake was the beneficial side effect of administering a bolus of iodine to cattle in a bid to improve fertility with resulting increases in dairy milk iodine content [24]. Staying with the agricultural experience, the practice of sanitizing milking machines with iodophor-containing disinfectants inadvertently contributed to increased milk iodine concentration [25]. Despite its benefits for increasing iodine intake, iodized salt does not provide a panacea for solving iodine deficiency. Many vulnerable groups where iodine requirement is increased such as pregnant mothers, their infants, vegans, or women of childbearing age continue to show deficits in iodine intake despite the availability of iodized salt [6, 7]. Many commercial supplements containing iodine are available, but there is no recommendation for taking supplements even in countries such as Ireland and the UK where universal salt iodization is not practiced [14].
Seaweed-Derived Iodine Intake
Although the concentration of iodine in seawater is relatively low (approx. 58 µg/L) [26], its massive abundance makes it the major source of iodine on the planet. Iodine in sea water is mainly present as I− and IO3, with I− predominating in surface waters while IO3 forms the greatest proportion in deep water [27]. Iodide taken up by seaweeds growing in shallow water, when uncovered and under stress at low tide, will react with atmospheric ozone (O3) to produce iodine (I2) [11, 12]. The thyroid gland concentrates iodide from the bloodstream by a factor of about 40 [28]. However, its iodide-concentrating ability is grossly inferior to that of seaweeds which can concentrate up to 30,000 times that of seawater [29]. The exact role for iodine in the life cycle of seaweeds remains unknown. It is speculated that it may offer protection against microbial attack [12]. The principal species of seaweeds are Phaeophyceae (brown), Rhodophyta (red), and Chlorophyta (green).
All of these seaweeds have the ability to concentrate iodine from seawater which is analogous to the human thyroid taking up iodine from the blood stream, except more efficiently. The iodine content of seaweeds is highly variable depending on factors such as species, area of the plant, stage of growth, season, and geographical location. In general, the iodine content of brown seaweeds is > red or green [30, 31]. In addition to their total iodine content, the bioavailability of the iodine and losses in cooking must be considered when determining iodine intake from seaweeds [31, 32, 34]. Table 1 shows a range of seaweed iodine content derived from publications cited in this review [31, 33, 35]. Seaweeds have been divided into commonly consumed varieties, sea lettuce, nori, wakame, kombu, dulse, and Irish moss and is intended to give a rough indication of potential iodine intake. The iodine content of individual species can be viewed in the cited publications. These publications refer to seaweeds sourced in different parts of the world, and the wide range of findings reflect this fact. The potential contribution of 1.0 g of dried seaweed to the achievement of the recommended nutrient intake of 150 μg is shown in Table 1. In the case of green or red seaweeds, 1.0 g might not be sufficient to provide 100% of the daily requirement, and up to 6.0 g is needed in some cases, while 1.0 g of brown seaweed would exceed the EFSA upper tolerable daily iodine limit of 600 μg [5] or even the 900–1,100 μg per day recommended for adults or pregnant women [6].
Table 1.
Range of iodine content µg/g DW of examples of the 3 main classes of edible seaweeds
| Classification and species | Common names | Iodine content, µg/g DW (range) | Grams of seaweed required to achieve daily RNI of 150 µg/day |
|---|---|---|---|
| Green algae (Chlorophyta) | |||
| Undaria pinnatifida | Wakame | 30–185 | 2.0–6.0 |
| Ulva lactuca | Sea lettuce | ||
| Ulva intestinalis | |||
| Red algae (Rhodophyta) | |||
| Palmaria | Dulse, dillisk | 20–200 | 0.3–3.0 |
| Porphyra | Nori | ||
| Alaria | Irish wakame | ||
| Chondrus crispus | Irish moss | ||
| Brown algae (Phaeophyceae) | |||
| Laminaria Ascophyllum Fucoids |
Kombu | 2,500–10,000 | 0.01–0.04 |
These values were extracted from publications cited in this review [35, 37, 39] and are intended to give an indication of possible iodine intake arising from consumption of these seaweeds. As shown in the final column, consuming 1.0 g DW of red or green seaweed might not always achieve a daily RNI of 150 µg while an equivalent amount of brown seaweed would greatly exceed the TUL of 600 µg [5] or even the 900–1,100 µg per day recommended for adults or pregnant women [6]. DW, dry weight; RNI, recommended nutrient intake; TUL, tolerable upper level.
Dried seaweed or seaweed extracts are often found in foodstuffs, and recommendations for safe levels of iodine vary from 20 mg/kg in Germany to 500 mg/kg in the USA and a maximum tolerable level of 100 mg/kg dried weight in Australia [35]. In the case of pregnant women and children, the recommendation is to limit brown seaweed to 1 serving per week. However, iodine content can be extremely variable depending on seaweed species, bioavailability, and losses in cooking [31, 32, 36]. Consuming seaweed alone or as a dietary supplement is unlikely to be harmful if taken occasionally (once or twice a week). However, regular intake of iodine-rich seaweeds such as kelps (Laminaria/Ascophyllum/fucoids) has the potential of exposure to excess iodine with possible adverse effects on thyroid function, particularly in those with pre-existing thyroid disorder, pregnant women, and neonates [37, 38]. The dietary preferences of vegetarians and vegans frequently result in iodine deficiency [38, 39]. Iodine-supplemented milk alternatives may be available, but as with other supplements consumed by vegans, seaweed additives made from brown seaweeds may contain excess iodine [25]. Use of iodized salt with or without appropriate supplements to achieve adult or pregnancy daily (150 or 250 μg) intakes in vegans and non-vegans alike is recommended [37, 38, 39]. Seaweed supplements are more problematic, but if used, it has been suggested that “nutrients frequently lacking in vegan and vegetarian diets should be routinely labelled on foods regularly consumed by these groups in order to highlight to those making these purchases” that excessive consumption may be injurious to human health [38].
Iodine Excess
Thyroid disorders arising from excess iodine intake including seaweed consumption can take the form of goitre, thyroid autoimmunity, hypothyroidism, or hyperthyroidism [8, 9, 40, 41, 42]. Patients with autonomously functioning nodules perhaps arising from a previous iodine deficiency state can develop a form of hyperthyroidism termed Jod-Basedow arising from excess iodine intake [8]. Excess iodine consumption can induce hypothyroidism or AITD [40, 41]. The cardiac anti-arrhythmic drug amiodarone contains 75-mg iodine per 200-mg tablet and can result in two different disorders, amiodarone-induced thyrotoxicosis (AIT 1), characterized by hyperthyroidism, or AIT 2, characterized by destructive thyroiditis, sometimes a combination of both [42]. Some authors have suggested that excessive seaweed consumption may itself result in chronic lymphocytic thyroiditis [43]. Iodide-induced goitre was first reported in the residents of the island of Hokkaido, Japan, termed “endemic coastal goitre” [44]. Iodization of table salt in Denmark showed a decrease in goitre and hyperthyroidism, mainly toxic nodular goitre, but a small increase in hypothyroidism [45]. The authors noted that even small systematic increase in iodine supply can significantly increase the risk of thyroid disease and emphasized the importance of increasing iodine intake to the level where iodine deficiency disorders are prevented and not higher [45]. An increased prevalence of papillary thyroid cancer has been reported in postpartum Japanese consuming daily seaweed dishes compared to those whose consumption was 2 days per week or less [46]. The mechanism through which seaweed or high iodine levels might promote thyroid cancer remains unclear, but a role for iodine in suppressing miR-422a and thereby facilitating tumourogenesis by upregulating MAPK1 has been proposed [47].
Not all excess iodine intake has damaging consequences. Administration of high-dose (approx. 100 mg) KI or KIO3 tablets is used for blocking the uptake of RAI in the event of a nuclear accident. It can be speculated that the consequences of RAI uptake and future thyroid cancers following the 2011 Fukushima accident would have been much worse in a population other than seaweed-consuming iodine replete Japanese [48].
Iodine Absorption Pathways
The principal pathway for iodine absorption is through the gut into the bloodstream from where it is carried to the thyroid and some extrathyroidal organs [49]. Absorption is facilitated by the sodium iodide symporter which appears to be downregulated in iodine excess [49]. Iodine can also be absorbed through the skin, and thyroid blockade has been reported in patients treated with povidone iodine during surgery [50]. Earlier reports have suggested that living near the sea confers advantages in terms of population iodine status. However, recent studies from the author's laboratory have shown that urinary iodine (UI) excretion in coastal areas did not differ substantially from their inland neighbours [13, 16]. The findings demonstrate that a higher UI was only observed in those communities living adjacent to an abundant seaweed growth and suggests that the higher concentration of atmospheric gaseous iodine of seaweed origin may have contributed to this iodine bonus. The increasing popularity of seaweed baths showed modest uptake of iodine probably through a mixture of skin absorption and respiration following such treatments [51]. The finding that iodine status was not significantly enhanced by coastal living alone was supported by findings in Portugal [52] of lower UI values in the Azores and Madeira islands compared to the Portuguese mainland. These mid-Atlantic islands have little seaweed growth. Lower UI values were reported on the island of Zakynthos and higher values on Chios than on mainland Greece [53, 54]. Chios in the Aegean is seaweed rich, while Zakynthos in the Ionian Sea has relatively little seaweed [53]. Similarly, low UI was reported from Hong Kong [55], a coastal area where adequate iodine intake might have been anticipated. However, the benefits of living beside seaweed-abundant shores are less clear in Tasmania, which in the past was iodine deficient despite being the location for giant kelp forests. These have been in decline in recent years, attributed to global warming [56]. Iodine deficiency persists in Tasmania but shows regional variation with highest values in the northeast [57]. Relative seaweed abundance was not noted.
Extrathyroidal Actions
Although the thyroid is the principal iodine-concentrating organ in the body, the ability to take up iodine also resides in other organs including the stomach, breast, and skin [49]. Thyroid and breast diseases predominantly affect females of a similar age range resulting in many studies on the coincidence of the two disorders [58]. The low rate of breast cancer reported in Japanese compared to Western women has been suggested to implicate iodine intake and specifically seaweed-derived iodine as a possible protective factor [29]. However, findings in Korean women have shown conflicting findings in that miyeok (Undaria pinnatifida) consumption was not significantly associated with breast cancer risk while gim (porphyria) was associated with decreased risk [59]. Although there is little epidemiological evidence of an association between seaweed consumption and breast cancer in Korea [59, 60], high iodine intake is a distinguishing mark of Japanese populations. Lower rates and increasing trends in mortality and incidence patterns of breast cancer and other disorders in Japanese women compared to their Western counterparts [61], attributed to environmental factors, have been observed in Asian immigrants to the USA [60]. An anti-tumour effect involving seaweed lowering of urokinase-type plasminogen activator receptor concentrations has been suggested in understanding the lower breast cancer rates reported in Japan [61]. A role for elemental iodine (I2) as distinct from iodide (I−) was suggested as an active protective factor against breast cancer while Aceves and colleagues [62] proposed the use of elemental iodine in the treatment of breast cancer.
Seaweeds can also entrap heavy metals including Al, Cd, Fe, and particularly arsenic (As) [63]. While inorganic As compounds are known to be carcinogenic, the majority of As in seaweeds is present as arsenosugars which are much less toxic. Of the brown seaweeds, Laminaria digitata had the highest inorganic As levels [63].
Assessment of Iodine Intake
The relative iodine sufficiency of populations is routinely examined by measuring UI excretion. Although assessment of UI provides a useful index of population iodine intake, the value obtained reflects recent dietary intake and is not useful for determining the long-term iodine status of an individual. Another possible pitfall in investigating iodine sufficiency is that population iodine status is commonly established by measuring urine samples from school-aged children. This can often yield misleading results when applied to adults, particularly pregnant women, as milk consumption, a major source of dietary iodine, is typically greater in school-aged children [7, 64].
An individual's ability to produce adequate levels of thyroid hormones is dependent on iodine stores built up and maintained over a period of time. For assessment of population iodine status, the WHO recommends a population median UI value of 100 µg/L for the general population and 150 µg/L for pregnant or lactating women [7]. Populations with median UI values <100 µg/L are judged deficient. The UI distribution (percentage of UI <20, 20–49, and 50–99 µg/L) is more informative than the percent UI <100 µg/L [7] with a recommendation that not >20% of samples should be <50 μg/L [8]. The accuracy of UI can be improved by collecting repeat samples to correct for intra-individual variation providing a more accurate indication of an estimated average requirement for iodine [7, 8].
Atmospheric Iodine
Another non-dietary role for iodine is the involvement of seaweed-generated iodine gas (I2) in influencing weather and global warming/cooling [65]. The origin of atmospheric gaseous iodine (I2) is believed to be the reaction of inorganic iodide (I−) present in seaweed with ozone present in the lower atmosphere [12]. This reaction is believed to be catalyzed by haloperoxidases and gives rise to iodine oxides (IO) and elemental Iodine (I2) [66]. When the tide recedes, seaweed is exposed to the atmosphere, desiccated, and becomes stressed. This stress involves contact with atmospheric O3 leading to the following reaction:
O3 + H+ + I− > HOI + O2,
H+ + HOI + I− > I2 + H2O.
This is a simplified version of the reactions involving iodide and other iodine oxides. O3 in the lower atmosphere (troposphere) is the product of the reaction of chemical pollutants with sunlight and is a strong reactive oxygen species whose removal by seaweed is beneficial to human respiratory health [67]. Different seaweeds grow at different tidal levels with the result that some will be uncovered by seawater for longer or shorter times which will also be determined by tidal conditions, high or low tides [11]. Both living and decaying seaweeds release iodine into the atmosphere but also into surrounding seawater thus contributing to global iodine fluxes [68]. Coastal seaweed-rich areas are responsible for most of this iodine although oceanic phytoplankton also play a role [11]. Iodine vapour condenses to form nuclear clusters which can coalesce to produce new particles leading to aerosol and cloud formation affecting climate change [11]. The clouds so formed provide a heat shield contributing to global cooling by partially offsetting global warming due to greenhouse gases. Gaseous iodine formed over a seaweed bed is often short lived and subject to photolysis by sunlight and dispersion by wind. Thus, the highest concentration of gaseous iodine is found over or close to the seaweed bed after dark on a calm night [13].
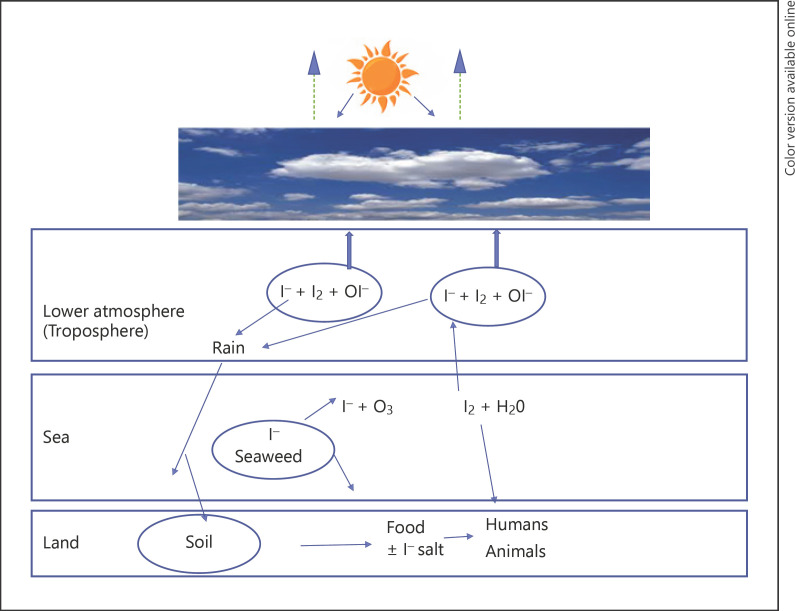
In addition to iodine released from seaweeds, it appears that iodine deposition as rain has increased in recent times. An increase in atmospheric iodine over the North Atlantic has been recently reported [69] as have a threefold increase in iodine deposition in Europe since 1950 [70]. Increasing emissions of pollutants O3 and NO2 react with seaweed-derived I− to produce I2 and iodine oxides including IONO2 which is very water soluble and thus deposited in rain [69]. The combination of atmospheric and deposited iodine will make a contribution to iodine flux. A diagram showing an overview of iodine/iodide uptake and deposition patterns is shown in Figure 1.
Fig. 1.
Iodide (I−) and iodine (I2) uptake and deposition. I− in seaweed reacts with O3 to produce I2 gas leading to atmospheric aerosol generation resulting in cloud formation which reflects sunlight partially offsetting global warming. Iodinated compound can be deposited in rain, and gaseous I2 can be taken up by respiration.
Conclusions
Although the ocean and its resident seaweed provide the greatest single store of iodine, consumption of seaweeds or seafoods in general do not for most populations provide the major dietary source of iodine. In most countries, this requirement is met through iodization of table salt, universal salt iodization. This is particularly important in at risk groups such as pregnant women, their neonates, females of childbearing age, or those such as vegans whose dietary preferences do not include iodine-containing foods. Although iodine supplied via seaweed consumption can rectify an iodine deficiency state, the species consumed needs to be monitored to avoid too much brown seaweeds (kelps) producing disorders of thyroid function. As well as dietary intake, volatile iodines such as gaseous iodine (I2) released from seaweeds and other marine algae have been shown to play a significant role in atmospheric aerosol generation leading to cloud formation in coastal areas, thereby partially offsetting global warming due to greenhouse gases. In addition, increased iodine deposition in rainfall and intake from seaweed-derived atmospheric gaseous iodine may help offset global iodine deficits.
Future Studies
Studies on population iodine status might be re-evaluated to determine the relevance of dwelling in proximity to abundant algal growths rather than simply coastal dwelling. The ability to take up by respiration or otherwise gaseous iodine released from seaweeds, living or dead, needs further elucidation using animal/human or in vitro studies. The extent of iodine release by oceanic algae blooms (phytoplankton) and their possible contribution to atmospheric iodine and quantitation of oceanic gases over algal blooms need to be undertaken.
Conflict of Interest Statement
The author has no conflicts of interest to declare.
Funding Sources
No current funding.
Acknowledgements
The author acknowledges the collaboration of Professor Colin O'Dowd, CCAPS, School of Physics, National University of Ireland, Galway, Ireland.
References
- 1.Kelly FC. Iodine in medicine and pharmacy since its discovery-1811–1961. Proc R Soc Med. 1961;54((10)):831–6. doi: 10.1177/003591576105401001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown RS. Minireview: developmental regulation of thyrotropin receptor gene expression in the fetal and newborn thyroid. Endocrinology. 2004;145((9)):4058–61. doi: 10.1210/en.2004-0458. [DOI] [PubMed] [Google Scholar]
- 3.Bath SC, Steer CD, Golding J, Emmett P, Rayman MP. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC) Lancet. 2013;382((9889)):331–7. doi: 10.1016/S0140-6736(13)60436-5. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization, United Nations Children's Fund, and the International Council for the Control of Iodine Deficiency Disorders . Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. 3rd ed. Geneva: World Health Organization; 2007. [Google Scholar]
- 5.EFSA summary of tolerable upper intake levels: version 4 (September 2018) 1 overview on tolerable upper intake levels as derived by the scientific committee on food (SCF) and the EFSA panel on dietetic products, nutrition and allergies (NDA)
- 6.Zimmermann MB, Andersson M. Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev. 2012;70((10)):553–70. doi: 10.1111/j.1753-4887.2012.00528.x. [DOI] [PubMed] [Google Scholar]
- 7.UNICEF 2018 guidance on the monitoring of salt iodization programmes and determination of population iodine status
- 8.Leung AM, Braverman LE. Consequences of excess iodine. Nat Rev Endocrinol. 2014 Mar;10((3)):136–42. doi: 10.1038/nrendo.2013.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mussig K. Iodine-induced toxic effects due to seaweed consumption. In: Preedy V, Burrow G, Watson R, editors. Comprehensive Handbook of Iodine. Academic Press; 2009. pp. p. 897–908. [Google Scholar]
- 10.Palmieri N, Forleo M. The potential of edible seaweed within the Western diet. A segmentation of Italian consumers. Int J Gastron Food Sci. 2020:100202. [Google Scholar]
- 11.O'Dowd CD, Hoffmann T. Coastal new particle formation: a review of the current state-of-the-art. Environ Chem. 2005;2:245–55. [Google Scholar]
- 12.Küpper FC, Carpenter LJ, McFiggans GB, Palmer CJ, Waite TJ, Boneberg EM, et al. Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. Proc Natl Acad Sci U S A. 2008 May 105;105((19)):6954–8. doi: 10.1073/pnas.0709959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smyth PP, Burns R, Huang RJ, Hoffman T, Mullan K, Graham U, et al. Does iodine gas released from seaweed contribute to dietary iodine intake? Environ Geochem Health. 2011 Aug;33((4)):389–97. doi: 10.1007/s10653-011-9384-4. [DOI] [PubMed] [Google Scholar]
- 14.Lazarus JH, Smyth PP. Iodine deficiency in the UK and Ireland. Lancet. 2008;372((9642)):888. doi: 10.1016/S0140-6736(08)61390-2. [DOI] [PubMed] [Google Scholar]
- 15.Bath SC, Combet E, Scully P, Zimmermann MB, Hampshire-Jones KH, Rayman MP. A multi-centre pilot study of iodine status in UK schoolchildren, aged 8–10 years. Eur J Nutr. 2016;55((6)):2001–9. doi: 10.1007/s00394-015-1014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smyth P, Burns R, Casey M, Mullan K, O'Herlihy C, O'Dowd C. Iodine status over two decades: influence of seaweed exposure. Ir Med J. 2016;109((6)):421. PMID: 27814438. [PubMed] [Google Scholar]
- 17.Marine D, Kimball OP. Prevention of simple goiter in man. Arch Intern Med (Chic) 1920;25((6)):661–72. [Google Scholar]
- 18.Oddie TH, Fisher DA, McConahey WM, Thompson CS. Iodine intake in the United States: a reassessment. J Clin Endocrinol Metab. 1970 May;30((5)):659–65. doi: 10.1210/jcem-30-5-659. [DOI] [PubMed] [Google Scholar]
- 19.Pearce EN. Iodine in pregnancy: Is salt iodization enough? J Clin Endocrinol Metab. 2008;93((7)):2466–8. doi: 10.1210/jc.2008-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National diet and nutrition survey assessment of salt intake from urinary sodium in adults (aged 19 to 64 years) in England, 2018/19. Public Health England
- 21.Tayie FA, Jourdan K. Hypertension, dietary salt restriction, and iodine deficiency among adults. Am J Hypertens. 2010;23((10)):1095–102. doi: 10.1038/ajh.2010.120. [DOI] [PubMed] [Google Scholar]
- 22.Jones E, McLean R, Davies B, Hawkins R, Meiklejohn E, Ma ZF, et al. Adequate iodine status in New Zealand school children post-fortification of bread with iodised salt. Nutrients. 2016;8((5)):298. doi: 10.3390/nu8050298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlton K, Probst Y, Kiene G. Dietary iodine intake of the Australian population after introduction of a mandatory iodine fortification programme. Nutrients. 2016 Nov;8((11)):701. doi: 10.3390/nu8110701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Brien D, Gleeson D, Jordan K. Iodine concentrations in milk. Irish J Agric Food Res. 2013;52:209–16. [Google Scholar]
- 25.Bath S, Hill S, Infante H, Elghul S, Nezianya C, Rayman MP. Iodine concentration of milk-alternative drinks available in the UK in comparison with cows' milk. Br J Nutr. 2017;118((7)):525–32. doi: 10.1017/S0007114517002136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuge R, Johnson CC. The geochemistry of iodine: a review. Environ Geochem Health. 1986;8((2)):31–54. doi: 10.1007/BF02311063. [DOI] [PubMed] [Google Scholar]
- 27.Truesdale VW. The biogeochemical effect of seaweeds upon close-to natural concentrations of dissolved iodate and iodide in seawater: preliminary study with Laminaria digitata and Fucus serratus. Estuar Coast Shelf Sci. 2008;78((1)):155–65. [Google Scholar]
- 28.Lazarus JH. The importance of iodine in public health. Environ Geochem Health. 2015;37((4)):605–18. doi: 10.1007/s10653-015-9681-4. [DOI] [PubMed] [Google Scholar]
- 29.Zava TT, Zava DT. Assessment of Japanese iodine intake based on seaweed consumption in Japan: a literature-based analysis. Thyroid Res. 2011;4:14. doi: 10.1186/1756-6614-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roleda MY, Skjermo J, Marfaing H, Jónsdóttir R, Rebours C, Gietl A, et al. Iodine content in bulk biomass of wild-harvested and cultivated edible seaweeds: inherent variations determine species-specific daily allowable consumption. Food Chem. 2018 Jul 15;254:333–9. doi: 10.1016/j.foodchem.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Teas J, Pino S, Critchley A, Braverman LE. Variability of iodine content in common commercially available edible seaweeds. Thyroid. 2005;14((10)):836–41. doi: 10.1089/thy.2004.14.836. [DOI] [PubMed] [Google Scholar]
- 32.Aquaron R, Delange F, Marchal P, Lognoné V, Ninane L. Bioavailability of seaweed iodine in human beings. Cell Mol Biol. 2002;48((5)):563–9. [PubMed] [Google Scholar]
- 33.Nitschke U, Stengel DB. A new HPLC method for the detection of iodine applied to natural samples of edible seaweeds and commercial seaweed food products. Food Chem. 2015;172:326–34. doi: 10.1016/j.foodchem.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 34.FSANZ . Survey of iodine levels in seaweed and seaweed containing products available in Australia. Food Standards Australia New Zealand; 2010. Available from: http://www.foodstandards.gov.au/scienceandeducation/monitoringandsurveillance/foodsurveillance/surveyofiodinelevels5369.cfm. [Google Scholar]
- 35.Yeh TS, Hung NH, Lin TC. Analysis of iodine content in seaweed by GC-ECD and estimation of iodine intake. J Food Drug Anal. 2014;22((2)):189–96. [Google Scholar]
- 36.Nitschke U, Stengel DB. Quantification of iodine loss in edible Irish seaweeds during processing. J Appl Phycol. 2016;28((6)):3527–33. [Google Scholar]
- 37.Bouga M, Combet E. Emergence of seaweed and seaweed-containing foods in the UK: focus on labeling, iodine content, toxicity and nutrition European Food Safety Authority (EFSA) Foods. 2015;4:240–53. doi: 10.3390/foods4020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sebastiani G, Herranz Barbero A, Borrás-Novell C, Alsina Casanova M, Aldecoa-Bilbao V, Andreu-Fernández V, et al. The effects of vegetarian and vegan diet during pregnancy on the health of mothers and offspring. Nutrients. 2019;11((3)):557. doi: 10.3390/nu11030557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eveleigh ER, Coneyworth LJ, Avery A, Welham SJM. Vegans, vegetarians, and omnivores: How does dietary choice influence iodine intake? A systematic review. Nutrients. 2020;12((6)):E1606. doi: 10.3390/nu12061606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Müssig K, Thamer C, Bares R, Lipp HP, Häring HU, Gallwitz B. Iodine-induced thyrotoxicosis after ingestion of kelp-containing tea. J Gen Intern Med. 2006;21((6)):C11–4. doi: 10.1111/j.1525-1497.2006.00416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farebrother J, Zimmermann MB, Andersson M. Excess iodine intake: sources, assessment, and effects on thyroid function. Ann N Y Acad Sci. 2019;1446((1)):44–65. doi: 10.1111/nyas.14041. [DOI] [PubMed] [Google Scholar]
- 42.Bartalena L, Bogazzi F, Chiovato L, Hubalewska-Dydejczyk A, Links TP, Vanderpump M. 2018 European Thyroid Association (ETA) guidelines for the management of amiodarone-associated thyroid dysfunction. Eur Thyroid J. 2018;7((2)):55–66. doi: 10.1159/000486957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inoue M, Taketani N, Sato T, Nakajima H. High incidence of chronic lymphocytic thyroiditis in apparently healthy school children: epidemiological and clinical study. Endocrinol Jpn. 1975;22((6)):483–8. doi: 10.1507/endocrj1954.22.483. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki H, Higuchi T, Sawa K, Ohtaki S, Horiuchi Y. “Endemic coast goitre” in Hokkaido, Japan. Acta Endocrinol. 1965;50((2)):161–76. [PubMed] [Google Scholar]
- 45.Laurberg P. DanThyr-20 years of iodine monitoring and research. Thyroid Int. 2015;2:1–25. [Google Scholar]
- 46.Michikawa T, Inoue M, Shimazu T, Sawada N, Iwasaki M, Sasazuki S, et al. Seaweed consumption and the risk of thyroid cancer in women: the Japan Public Health Center-based Prospective Study. Eur J Cancer Prev. 2012;21((3)):254–60. doi: 10.1097/CEJ.0b013e32834a8042. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Yang H, Si Y, Hu D, Yu Y, Zhang Y, et al. Iodine promotes tumorigenesis of thyroid cancer by suppressing miR-422a and up-regulating MAPK1. Cell Physiol Biochem. 2017;43((4)):1325–36. doi: 10.1159/000481844. [DOI] [PubMed] [Google Scholar]
- 48.Nagataki S. The average of dietary iodine intake due to the ingestion of seaweeds is 1.2 mg/day in Japan. Thyroid. 2008;18((6)):667–8. doi: 10.1089/thy.2007.0379. [DOI] [PubMed] [Google Scholar]
- 49.Pesce L, Kopp P. Iodide transport: implications for health and disease. Int J Pediatr Endocrinol. 2014;2014((1)):8. doi: 10.1186/1687-9856-2014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nobukuni K, Hayakawa N, Namba R, Ihara Y, Sato K, Takada H, et al. The influence of long-term treatment with povidone-iodine on thyroid function. Dermatology. 1997;195((Suppl 2)):69–72. doi: 10.1159/000246034. [DOI] [PubMed] [Google Scholar]
- 51.Westby T, Cadogan A, Duignan G. In vivo uptake of iodine from a Fucus serratus Linnaeus seaweed bath: Does volatile iodine contribute? Environ Geochem Health. 2018 Apr;40((2)):683–91. doi: 10.1007/s10653-017-0015-6. [DOI] [PubMed] [Google Scholar]
- 52.Limbert E, Prazeres S, São Pedro M, Madureira D, Miranda A, Ribeiro M, et al. Iodine intake in Portuguese pregnant women: results of a countrywide study. Eur J Endocrinol. 2010;163((4)):631–5. doi: 10.1530/EJE-10-0449. [DOI] [PubMed] [Google Scholar]
- 53.Koukkou EG, Ilias I, Mamalis I, Markou KB. Pregnant Greek women may have a higher prevalence of iodine deficiency than the general Greek population. Eur Thyroid J. 2017;6((1)):26–30. doi: 10.1159/000449285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giassa Τ, Mamali I, Gaki Ε, Kaltsas G, Kouraklis G, Markou ΚΒ, et al. Iodine intake and chronic autoimmune thyroiditis: a comparative study between coastal and mainland regions in Greece. Hormones. 2018;17((4)):565–71. doi: 10.1007/s42000-018-0057-x. [DOI] [PubMed] [Google Scholar]
- 55.Kung AW, Chan LW, Low LC, Robinson JD. Existence of iodine deficiency in Hong Kong: a coastal city in Southern China. Eur J Clin Nutr. 1996;50((8)):569–72. [PubMed] [Google Scholar]
- 56.Layton C, Coleman MA, Marzinelli EM, Steinberg PD, Swearer SE, Vergés A, et al. Kelp forest restoration in Australia. Front Mar Sci. 2020;74:1–12. [Google Scholar]
- 57.Guttikonda K, Burgess JR, Hynes K, Boyages S, Byth K, Parameswaran V. Recurrent iodine deficiency in Tasmania, Australia: a salutary lesson in sustainable iodine prophylaxis and its monitoring. J Clin Endocrinol Metab. 2002;87((6)):2809–15. doi: 10.1210/jcem.87.6.8600. [DOI] [PubMed] [Google Scholar]
- 58.Smyth PPA. The thyroid and breast cancer. Curr Opin Endocrinol Diabetes Obes. 2016;23 doi: 10.1097/MED.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 59.Yang YJ, Nam SJ, Kong G, Kim MK. A case-control study on seaweed consumption and the risk of breast cancer. Br J Nutr. May 2010;103((9)):1345–53. doi: 10.1017/S0007114509993242. [DOI] [PubMed] [Google Scholar]
- 60.Kim Z, Min SY, Yoon CS, Jung KW, Ko BS, Kang E, et al. The basic facts of Korean breast cancer in 2012: results from a nationwide survey and breast cancer registry database. J Breast Cancer. 2015 Jun;18((2)):103–11. doi: 10.4048/jbc.2015.18.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teas J, Vena S, Cone DL, Irhimeh M. The consumption of seaweed as a protective factor in the etiology of breast cancer: proof of principle. J Appl Phycol. 2013;25((3)):771–9. doi: 10.1007/s10811-012-9931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aceves C, Anguiano B, Delgado G. Is iodine a gatekeeper of the integrity of the mammary gland? J Mammary Gland Biol Neoplasia. 2005;10((2)):189–96. doi: 10.1007/s10911-005-5401-5. [DOI] [PubMed] [Google Scholar]
- 63.Cherry P, O'Hara C, Magee PJ, McSorley EM, Allsopp PJ. Risks and benefits of consuming edible seaweeds. Nutr Rev. May 2019;77((5)):307–29. doi: 10.1093/nutrit/nuy066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lazarus JH. Monitoring iodine nutritional status: adults or schoolchildren? J Endocrinol Invest. 2021 Feb;44((2)):383–5. doi: 10.1007/s40618-020-01313-6. [DOI] [PubMed] [Google Scholar]
- 65.O'Dowd CD, Aalto P, Hmeri K, Kulmala M, Hoffmann T. Aerosol formation: atmospheric particles from organic vapours. Nature. 2002;416((6880)):497–8. doi: 10.1038/416497a. [DOI] [PubMed] [Google Scholar]
- 66.Küpper FC, Schweigert N, Ar Gall E, Legendre J-M, Vilter H, Kloareg B. Iodine uptake in Laminariales involves extracellular, haloperoxidase-mediated oxidation of iodide. Planta. 1998;207((2)):163–71. [Google Scholar]
- 67.Zhang J, Wei W, Fang Z. Ozone pollution: a major health hazard worldwide frontiers in immunology. Front Immunol. 2019 Oct;10 doi: 10.3389/fimmu.2019.02518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nitschke U, Dixneuf S, Schmid M, Ruth AA, Stengel DB. Contribution of living and degrading kelp to coastal iodine fluxes. Mar Biol. 2015;162((9)):1727–38. [Google Scholar]
- 69.Cuevas CA, Maffezzoli N, Corella JP, Spolaor A, Vallelonga P, Kjær HA, et al. Rapid increase in atmospheric iodine levels in the North Atlantic since the mid-20th century. Nat Commun. 2018;9((1)):1452. doi: 10.1038/s41467-018-03756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Legrand M, McConnell JR, Preunkert S, Arienzo M, Chellman N, Nathan & Gleason K, et al. Alpine ice evidence of a three-fold increase in atmospheric iodine deposition since 1950 in Europe due to increasing oceanic emissions. Proc Natl Acad Sci U S A. 2018 Nov 27;115((48)):12136–41. doi: 10.1073/pnas.1809867115. [DOI] [PMC free article] [PubMed] [Google Scholar]