Abstract
We report a finding of a pigmented chorioretinal scar with acute retinal necrosis (ARN) caused by herpes simplex virus 2 (HSV-2) infection rather than toxoplasma, creating an initial diagnostic dilemma. A 53-year-old functionally monocular male presented with painless floaters and blurry vision in his seeing eye over a period of 4 days. An exam demonstrated anterior chamber (AC) reaction, vitritis, multifocal patches of whitening, and an occlusive retinal vasculitis. A superior pigmented chorioretinal scar with overlying contracted vitreous was noted in the periphery with no adjacent retinal whitening. The patient was treated for both ARN and toxoplasma chorioretinitis until PCR study of the vitreous and AC returned positive for HSV-2 and negative for toxoplasmosis. Management consisted of a dual therapy regimen of both oral and intravitreal antiviral agents as well as oral corticosteroids. The patient's clinical course was complicated by rhegmatogenous retinal detachment within 2 weeks after symptom onset, requiring pars plana vitrectomy with silicone oil and intraoperative intraocular incubation with foscarnet. We review emerging evidence for pigmented chorioretinal scars in ARN specifically caused by HSV-2, as well as diagnostic and treatment dilemmas in the management of ARN and ARN detachments.
Keywords: Acute retinal necrosis, Herpes simplex virus 2, Pigmented chorioretinal scar, Retinal detachment, Toxoplasma uveitis
Introduction
Acute retinal necrosis (ARN) is defined by the following characteristics: one or more foci of retinal necrosis in the peripheral retina, rapid progression in absence of antiviral therapy, circumferential spread, occlusive vasculopathy with arterial involvement, and inflammatory reaction in the vitreous and anterior chamber (AC). Varicella-zoster virus is thought to be the most common cause of classic ARN, followed by herpes simplex virus (HSV), and only rarely other viral causes [1]. HSV-2 ARN is relatively less common than HSV-1 or VZV ARN, and pathogenesis is thought to be uniquely due to reactivation of virus acquired from perinatal contact with maternal genital lesions [1, 2]. Previous studies have identified HSV-2 as a cause of ARN in younger patients with the mean age at diagnosis of 24 years compared to mean age at diagnosis of 44 years for HSV-1 [3].
ARN has severe visual consequences, with 48% of affected eyes demonstrating a visual acuity of worse than 20/200 6 months after onset of symptoms [1]. Retinal detachment (RD), the most common complication of ARN, occurs in about 75% of patients; on average, detachments occur 2 months after symptom onset [3, 4]. In this report, we present a case of AC reaction, vitritis, mild disc edema, multifocal retinitis, and occlusive vasculitis with a pigmented chorioretinal scar, creating a diagnostic dilemma between ARN and toxoplasmosis chorioretinitis. PCR analysis of the vitreous chamber and AC identified HSV-2 as the causative pathogen.
Case Report
A 53-year-old male who emigrated from Senegal approximately 20 years ago, with a history of phthisical right eye of unclear etiology, presented with 4 days of painless blurry vision and floaters in the left eye. The patient was otherwise healthy with no history of any systemic infection or disease. Review of systems was negative for flashes of light, headache, fever, or other systemic symptoms.
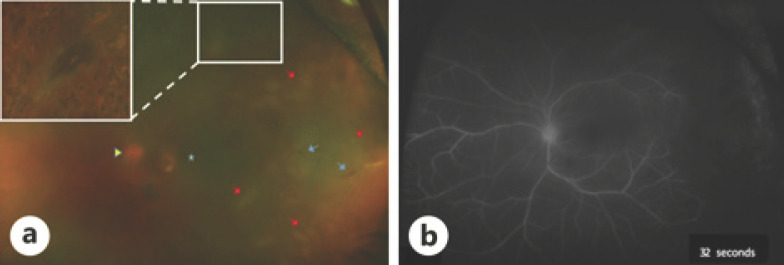
On examination, visual acuity was no light perception in the right eye and 20/60 in the left eye. Intraocular pressures were 2 mm Hg in the right eye and 13 mm Hg in the left eye. Slit lamp examination of the right eye was consistent with a quiet, painless, phthisical eye. Exam of the left eye revealed diffuse conjunctival injection with granulomatous keratic precipitates across the inferior 50% of the cornea and 2+ AC cell. Posterior exam revealed 0.5+ anterior vitreous cell, 3+ posterior vitreous haze, mild disc edema, and multifocal patches of retinal whitening superior and temporal to the arcades, associated with vessel sheathing and rare hemorrhages. A superior pigmented chorioretinal scar with overlying contracted vitreous was noted in the periphery, with no focal overlying vitritis nor adjacent areas of retinal whitening (shown in Fig. 1a). Fluorescein angiography revealed severe occlusive vasculitis (shown in Fig. 1b).
Fig. 1.
a Color fundus photograph demonstrating a pigmented chorioretinal scar (outlined by white box) superior to the arcade with overlying contracted vitreous (Note: enhanced inset view of pigmented chorioretinal scar is postsurgical as demonstrated by surrounding laser photocoagulation scars since clear preoperative views were limited by vitritis). Annotations include optic disc (yellow arrow), macula (yellow star), occlusive vasculitis (blue arrows), and multifocal retinal whitening (red arrows). b Fluorescein angiography demonstrating severe occlusive vasculitis in the temporal periphery.
Given the patient's emigration from Senegal, unknown immune status, and pigmented chorioretinal scar, the differential diagnosis included toxoplasma or tuberculosis uveitis and ARN. He underwent a vitreous chamber and AC tap, followed by intravitreal clindamycin and foscarnet injections. He was started systemically on trimethoprim 60 mg/sulfamethoxazole 800 mg twice daily and valacyclovir 2 g three times daily. HIV, syphilis, and tuberculosis testing were negative. PCR of vitreous and AC fluid was positive for HSV-2 and negative for HSV-1, VZV, CMV, and toxoplasma.
Due to the positive PCR results for HSV-2, trimethoprim-sulfamethoxazole was stopped, and the patient was continued on valacyclovir. He received intravitreal foscarnet injections every 3 days for a period of 10 days. Due to worsening vitritis and stable to improving retinal whitening, he was started on 30 mg of oral prednisone daily 8 days after his initial foscarnet injection. The day after his fourth foscarnet injection (14 days after symptom onset), the patient presented with a large retinal tear and focal RD in the area of temporal necrosis (shown in Fig. 2a, b). The patient underwent perfluorooctane-assisted 25-gauge pars plana vitrectomy with temporal retinectomy, extensive 360° endolaser, and intraocular tamponade with silicone oil. Prior to fluid-air exchange and silicone oil injection, a standard 2.4 mg/0.1 mL dose of foscarnet was injected into the balanced salt solution-filled vitreous cavity and incubated for 5 min before washout. Since foscarnet does not need activation, brief incubation is thought to be sufficient for viral inactivation [5]. He was continued on oral valacyclovir and prednisone postoperatively, with an attached retina and best-corrected visual acuity of 20/80+1 five weeks after surgery.
Fig. 2.
a Color fundus photograph on the day of the patient's fourth foscarnet injection (white arrow indicates pigmented scar). b Color fundus photograph 12 h later demonstrating large tear and focal detachment of the retina in the area of temporal necrosis (blue arrow) with superior pigmented lesion (white arrow).
Discussion/Conclusion
A pigmented chorioretinal scar in the setting of posterior or panuveitis typically heralds toxoplasma as the inciting agent [6]. Our patient, however, also demonstrated classic signs of ARN, including multifocal retinal whitening and occlusive vasculitis [1]. A pigmented chorioretinal scar has rarely been reported in HSV-2 ARN, possibly as a consequence of asymptomatic perinatal retinal infection [3]. Unlike toxoplasma retinitis, where encystment of the organism typically causes reactivation at the site of the old pigmented chorioretinal scar, HSV-2 reactivation involves retrograde travel into the retina from neuronal ganglia, resulting in retinitis well away from the pigmented chorioretinal scar [6, 7]. In our patient, the temporal location of HSV-2 reactivation away from the superior pigmented scar was, in retrospect, a clinical clue favoring the diagnosis of HSV-2 over toxoplasma, despite the patient being significantly older than the average age of onset for HSV-2 ARN [3].
Severe toxoplasma chorioretinitis can be mistaken for ARN, even absent a pigmented chorioretinal scar. Moshfeghi et al. [8] reported 22 patients with widespread chorioretinitis from toxoplasmosis, including some with retinal vasculitis. Half the patients were initially treated as ARN prior to definitive diagnosis. The advent of PCR-based diagnostics has greatly improved our ability to distinguish between toxoplasma and viral causes to necrotizing retinitis. The sensitivity of viral PCR from the aqueous or vitreous in ARN is high, ranging from approximately 80–100% across several studies, with the largest case series reporting a 92% sensitivity [1]. In contrast, the sensitivity of aqueous PCR in ocular toxoplasmosis is approximately 53% [9]. Thus, when faced with a diagnostic dilemma between ARN and toxoplasmosis chorioretinitis, a negative PCR for ARN organisms can be very helpful in pointing toward toxoplasma as the causative organism. At present, there is scant data to suggest that diagnostic yields between vitreous and AC taps significantly differ [1]. In our case, both the AC and vitreous humors were positive for HSV-2.
The mainstay of ARN treatment is early induction of oral, intravenous, and/or intravitreal antiviral therapy, with intravitreal injections every 72–96 h until quiescence is achieved [1]. Repeated intravitreal injections in combination with systemic antiviral therapy may lead to better visual outcomes and fewer rhegmatogenous RDs than systemic antiviral therapy alone [10]. Intravitreal foscarnet has additional benefits over systemic valacyclovir or acyclovir. First, foscarnet does not require enzymatic activation and rapidly inactivates the virus after short exposure times [10]. Second, foscarnet covers CMV in cases where the cause of retinitis is ambiguous [11]. Finally, foscarnet works against acyclovir-resistant strains of HSV and VZV [5].
The use of corticosteroids in treatment of ARN remains a controversial topic. Retrospective observational case series on management of ARN with corticosteroids have shown conflicting evidence with regard to improvement in final visual acuity. Although reduction in the inflammatory response with use of corticosteroids may reduce severity of pathology, the time of introduction of corticosteroids is crucial since temporary immunosuppression itself may lead to ARN activation [12]. We started oral steroids 8 days after initial foscarnet injection due to worsening vitritis, with some resulting in mild improvement in the view to the fundus. The patient's retinal tear and associated detachment developed 2 days after start of systemic corticosteroids.
Loss of visual acuity in ARN is commonly due to rhegmatogenous RD, occurring in about 75% of patients on average within 2 months of symptom onset [3, 4]. In our patient, RD occurred in the area of dense temporal retinal necrosis and within 12 h after a foscarnet injection, which was 14 days after symptom onset. Our patient underwent pars plana vitrectomy, retinectomy, endolaser, and silicone oil fill, with intraoperative injection of foscarnet while the posterior segment was filled with BSS. Continued intravitreal foscarnet therapy after silicone oil fill for RD presents risks. The drug presumably disperses only to the aqueous phase in the posterior segment in a silicone oil-filled eye, potentially elevating concentrations well above the intended final vitreous concentration. A case report in which 2 patients received half-dose foscarnet injections over a period of 6–8 weeks after silicone oil fill demonstrated retinitis regression without clear evidence of overt toxicity [11]. In our case, we closely monitored our patient with oral valacyclovir only after vitrectomy with silicone oil fill. He demonstrated no further progression of his retinitis over a 5-week postoperative period.
We present a case of HSV-2 ARN with a pigmented chorioretinal scar in which toxoplasma chorioretinitis was strongly considered during initial exam. The clinical presentation of HSV-2 ARN may partly mimic toxoplasma chorioretinitis due to presence of a pigmented chorioretinal scar. Apart from the pigmented chorioretinal scar, other unusual features of this case include the older age at presentation and the patient's rapid progression to RD.
Statement of Ethics
Written informed consent for patient information and images to be published was provided by the patient.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
J.M.L.M. and C.D.C. are supported in part by the Heed Foundation. The Heed Foundation supports the fellowship salary of both J.M.L.M. and C.D.C.
Author Contributions
C.G.B., R.C.R., N.S.K., and J.M.L.M. were responsible for the conception and design of this project. N.S.K. and J.M.L.M. were responsible for data collection, data analysis, and interpretation. N.S.K. and J.M.L.M. were responsible for drafting of the manuscript. C.G.B., R.C.R., C.D.C., and R.A.H. were responsible for critical revision of the article. Final approval of the version to be submitted for publication was performed by N.S.K., J.M.L.M., R.A.H., C.D.C., R.C.R., and C.G.B.
References
- 1.Schoenberger SD, Kim SJ, Thorne JE, Mruthyunjaya P, Yeh S, Bakri SJ, et al. Diagnosis and Treatment of Acute Retinal Necrosis: A Report by the American Academy of Ophthalmology. Ophthalmology. 2017 Mar;124((3)):382–92. doi: 10.1016/j.ophtha.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Tran TH, Stanescu D, Caspers-Velu L, Rozenberg F, Liesnard C, Gaudric A, et al. Clinical characteristics of acute HSV-2 retinal necrosis. Am J Ophthalmol. 2004 May;137((5)):872–9. doi: 10.1016/j.ajo.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 3.Van Gelder RN, Willig JL, Holland GN, Kaplan HJ. Herpes simplex virus type 2 as a cause of acute retinal necrosis syndrome in young patients. Ophthalmology. 2001 May;108((5)):869–76. doi: 10.1016/s0161-6420(01)00556-5. [DOI] [PubMed] [Google Scholar]
- 4.Lau CH, Missotten T, Salzmann J, Lightman SL. Acute retinal necrosis features, management, and outcomes. Ophthalmology. 2007 Apr;114((4)):756–62. doi: 10.1016/j.ophtha.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 5.Xu K, Chin EK, Mahajan VB, Almeida DR. Intravitreal Foscarnet With Concurrent Silicone Oil Tamponade for Rhegmatogenous Retinal Detachment Secondary to Viral Retinitis. Retina. 2016 Nov;36((11)):2236–8. doi: 10.1097/IAE.0000000000001174. [DOI] [PubMed] [Google Scholar]
- 6.Park YH, Nam HW. Clinical features and treatment of ocular toxoplasmosis. Korean J Parasitol. 2013 Aug;51((4)):393–9. doi: 10.3347/kjp.2013.51.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grose C. Acute retinal necrosis caused by herpes simplex virus type 2 in children: reactivation of an undiagnosed latent neonatal herpes infection. Semin Pediatr Neurol. 2012 Sep;19((3)):115–8. doi: 10.1016/j.spen.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moshfeghi DM, Dodds EM, Couto CA, Santos CI, Nicholson DH, Lowder CY, et al. Diagnostic approaches to severe, atypical toxoplasmosis mimicking acute retinal necrosis. Ophthalmology. 2004 Apr;111((4)):716–25. doi: 10.1016/j.ophtha.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Bou G, Figueroa MS, Martí-Belda P, Navas E, Guerrero A. Value of PCR for detection of Toxoplasma gondii in aqueous humor and blood samples from immunocompetent patients with ocular toxoplasmosis. J Clin Microbiol. 1999 Nov;37((11)):3465–8. doi: 10.1128/jcm.37.11.3465-3468.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flaxel CJ, Yeh S, Lauer AK. Combination systemic and intravitreal antiviral therapy in the management of acute retinal necrosis syndrome (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2013 Sep;111:133–44. [PMC free article] [PubMed] [Google Scholar]
- 11.Meshi A, Friehmann A, Sella S, Gepstein R, Armarnik S, Assia EI, et al. Intravitreal Administration of Antiviral Agents in Silicone Oil-Filled Human Eyes. Ophthalmol Retina. 2017 Jul-Aug;1((4)):288–93. doi: 10.1016/j.oret.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Shantha JG, Weissman HM, Debiec MR, Albini TA, Yeh S. Advances in the management of acute retinal necrosis. Int Ophthalmol Clin. 2015;55((3)):1–13. doi: 10.1097/IIO.0000000000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]