ABSTRACT
Fertility and gamete reserves are maintained by asymmetric divisions of the germline stem cells to produce new stem cells or daughters that differentiate as gametes. Before entering meiosis, differentiating germ cells (GCs) of sexual animals typically undergo cystogenesis. This evolutionarily conserved process involves synchronous and incomplete mitotic divisions of a GC daughter (cystoblast) to generate sister cells connected by intercellular bridges that facilitate the exchange of materials to support rapid expansion of the gamete progenitor population. Here, we investigated cystogenesis in zebrafish and found that early GCs are connected by ring canals, and show that Deleted in azoospermia-like (Dazl), a conserved vertebrate RNA-binding protein (Rbp), is a regulator of this process. Analysis of dazl mutants revealed the essential role of Dazl in regulating incomplete cytokinesis, germline cyst formation and germline stem cell specification before the meiotic transition. Accordingly, dazl mutant GCs form defective ring canals, and ultimately remain as individual cells that fail to differentiate as meiocytes. In addition to promoting cystoblast divisions and meiotic entry, dazl is required for germline stem cell establishment and fertility.
KEY WORDS: Dazl, Germline cyst, Fertility, Incomplete cytokinesis, Germline stem cells, Ring canals
Summary: Zebrafish dazl is required for incomplete cytokinesis to generate germline cysts during cystogenesis, for differentiation of PGCs into germline stem cells and meiotic cells, and for fertility.
INTRODUCTION
In many organisms, the germline is among the first cell types to be set aside (Ginsburg, 1994; Illmensee and Mahowald, 1976; Illmensee et al., 1976; Wolf et al., 1983). Early germ cells, called primordial germ cells (PGCs), are specified by maternal factors or by inductive signals (Farrell et al., 2018; Lawson et al., 1999; Lawson and Hage, 1994). Once specified, PGCs ignore somatic differentiation programs and migrate to the site in which the gonad forms (Braat et al., 1999; Gross-Thebing et al., 2017; Extavour and Akam, 2003; Marlow, 2015; Nieuwkoop and Sutasurya, 1979; Strome and Updike, 2015). There, the PGCs proliferate, enter meiosis and differentiate to produce the gametes: sperm in males and oocytes in females. Although the earliest stages of PGC development in zebrafish have been studied (Barton et al., 2016; Marlow, 2015; Paksa and Raz, 2015; Raz, 2003), the cellular and molecular mechanisms mediating the transition from PGC to germline stem cell (GSC) specification are less well understood.
Germline stem cells
Nanos, a conserved marker of GSCs in zebrafish, medaka and mice (Nakamura et al., 2010; Sada et al., 2009; Aoki et al., 2009; Beer and Draper, 2013; Cao et al., 2019), is required for the maintenance of GSCs in vertebrates and invertebrates (Forbes and Lehmann, 1998; Sada et al., 2009). The mechanisms that establish the GSCs are unknown, but blocking Wnt diminishes nanos2 and impedes ovary regeneration (Cao et al., 2019). In mouse testis and zebrafish germ cells (GCs), nanos2 is required for the maintenance of GSCs (Sada et al., 2009; Cao et al., 2019). In zebrafish, the related nanos3 contributes to the maintenance of GSCs and may act redundantly to nanos2 in females but not males (Beer and Draper, 2013; Draper et al., 2007). Although zebrafish nanos2 is expressed in a subset of premeiotic gonocytes, neither it nor nanos3 are required for GSC specification (Beer and Draper, 2013; Cao et al., 2019). Instead, Nanos family members in zebrafish may prevent premature differentiation of GSCs, in part by translational repression of meiosis factors like their counterparts in flies (Wang and Lin, 2004) and mice (Barrios et al., 2010; Suzuki et al., 2010). Thus, the cellular and molecular mechanisms that establish zebrafish GSCs have yet to be discovered.
Germline cyst formation
A common and evolutionarily conserved feature of GCs is the asymmetric division of GSCs to produce a stem cell and a premeiotic daughter. These differentiating divisions have been classified as type I or type II divisions (Saito et al., 2007; Saito and Tanaka, 2009). Cells resulting from type-I divisions directly differentiate as meiotic cells and are observed in juvenile and adult teleosts (Marlow and Mullins, 2008; Saito et al., 2007). Type II divisions generate cystoblast cells that divide mitotically with incomplete cytokinesis to generate interconnected sisters in Drosophila (Cox and Spradling, 2003), Xenopus (Kloc et al., 2004) and medaka (Saito et al., 2007). The interconnections generated from incomplete cytokinesis are known as ring canals, or ring channels (Brown and King, 1964; de Cuevas et al., 1997; Fawcett et al., 1959; Greenbaum et al., 2011; Haglund et al., 2011; Koch and King, 1966, 1969; Koch et al., 1967; Lei and Spradling, 2016; Lin and Spradling, 1993; Mahowald, 1971; Marlow and Mullins, 2008; Pepling and Spradling, 1998; Robinson and Cooley, 1996; Seidel et al., 2018; Spradling, 1993; Wolke et al., 2007). In Drosophila, ring canal formation involves the regulation of actin to maintain midbody structures (Tilney et al., 1996). During normal cell division, sister cells are separated by the cytokinetic furrow which severs the midbody, a transient connection between cells. For incomplete cytokinesis, the midbody actomyosin meshwork is stabilized to block abscission and maintain the contractile ring such that ring canals form between connected cells (Greenbaum et al., 2011; Haglund et al., 2011; Hime et al., 1996; Robinson and Cooley, 1996). In mice, the inactive serine-threonine kinases TEX14 and CEP55 regulate intercellular bridge stability in part by blocking abscission factors (Greenbaum et al., 2011, 2006; Kim et al., 2015; Morita et al., 2007). Intercellular bridge and germline cyst formation is a conserved feature of germ cell biology and is crucial for fertility (e.g. Greenbaum et al., 2006). Intercellular bridges of GCs could fulfill several functions, including facilitating intercellular communication to yield synchronous cells (Lei and Spradling, 2013; Pepling and Spradling, 1998), coordinating crucial stages, such as meiotic entry (Stanley et al., 1972), maintaining gamete equivalence and potentially acting to detect and remove abnormal cells (Braun et al., 1989; LeGrand, 2001).
Dazl and fertility
The RNA-binding protein (Rbp) Deleted in azoospermia-like (Dazl) is a member of the Deleted in azoospermia (Daz) family, which is composed of Daz, Daz-like (Dazl) and BOULE (Fu et al., 2015). Daz family members are germline specific and contribute to various aspects of GC development in invertebrates and vertebrates (Fu et al., 2015). Although dazl is required for GC development, loss of daz family members disrupts distinct aspects of GC development in different species (Alphey et al., 1992; Courtot et al., 1992; Eberhart et al., 1996; Fukuda et al., 2018; Gill et al., 2011; Iyer et al., 2016; Karashima et al., 2000; Maines and Wasserman, 1999; Ruggiu et al., 1997; Saunders et al., 2003; Schrans-Stassen et al., 2001). Depletion of Xenopus dazl disrupts PGC migration and causes PGC deficiency (Houston and King, 2000). In mammals, Dazl also regulates meiotic RNAs (Haston et al., 2009; Kim et al., 2012; Li et al., 2019; Medrano et al., 2012; Zagore et al., 2018) and commitment to germline fate (Nicholls et al., 2019). These studies identify diverse and opposing activities for Dazl, including regulation of RNA stability, acting both as translational activator and repressor of its target RNAs in different contexts (Chen et al., 2014; Li et al., 2019; Mikedis et al., 2020; Yang et al., 2020; Zagore et al., 2018). In zebrafish PGCs, Dazl overexpression antagonizes miR-430 and promotes polyadenylation of maternally provided germline RNAs (Maegawa et al., 2002; Takeda et al., 2009); however, the role of Dazl in the zebrafish germline remains unknown.
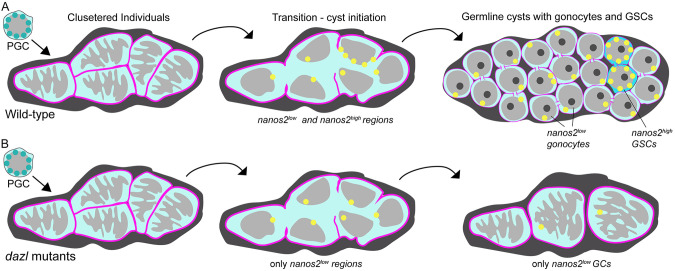
We examined the earliest stages of gonadogenesis and provide evidence that Dazl is required for germline cyst formation and is crucial for GC amplification establishment of GSCs and fertility. We describe zebrafish cystogenesis: the process by which PGCs transition from spatially separated individual cells to closely positioned GC clusters that undergo complex cytoplasmic and nuclear morphological changes to form germline cysts. Additionally, we show that GC numbers increase concomitantly with cyst formation. Analyzing F-actin distribution revealed that premeiotic cyst cells are interconnected by intercellular actin rings that resemble ring canals based on confocal and ultrastructural analyses, and contain aggregated or branched actin-rich structures reminiscent of spectrosomes and fusomes (Cooley and Theurkauf, 1994; Deng and Lin, 1997; Lighthouse et al., 2008; Lin et al., 1994; Robinson and Cooley, 1997; Tilney et al., 1996; Xue and Cooley, 1993; Yue and Spradling, 1992). Analysis of zinc finger nuclease (ZFN) and CRISPR-induced dazl mutant alleles revealed that zygotic dazl is essential for incomplete cytokinesis and germline cyst formation. In contrast to wild type, dazl mutant GCs form defective ring structures, and ultimately remain as individual cells that fail to differentiate as GSCs or meiocytes. Our findings support a novel requirement for dazl in GCs for cystogenesis and GSC establishment, linking cyst formation to PGC specification and fertility in zebrafish.
RESULTS
Germline cyst formation
After arriving at the gonad, PGCs proliferate to form a bipotential gonad (Leu and Draper, 2010; Tong et al., 2010; Tzung et al., 2015; Wang et al., 2007). Sex determination is influenced by the timing of meiotic entry and oocyte abundance, such that more GCs promote female fate (Ye et al., 2019). Conversely, low numbers of GCs result in male development (Dai et al., 2015; Orban et al., 2009; Tzung et al., 2015). Yet, despite these correlations, little is known about GSC specification in zebrafish. Type II divisions of GSCs are thought to be amplifying divisions and have been observed in juvenile and adult teleosts (medaka and in zebrafish) (Beer and Draper, 2013; Marlow and Mullins, 2008; Saito et al., 2007). In zebrafish, GCs proliferate between 5 and 14 days (Leerberg et al., 2017; Leu and Draper, 2010; Tong et al., 2010; Tzung et al., 2015; Wang et al., 2007); however, how cysts form is unknown.
To investigate cystogenesis, we labeled wild-type GCs with anti-Vasa antibody between 7 and 14 days (Fig. S1). At 7-10 days, Vasa+ cells were detected in wild type as individual GCs or clustered groups of individuals (Fig. S1A,B). At this stage, GC nuclei were condensed DNA, as revealed by DAPI, with no visible nucleolus, and the nuclear cytoplasm interface was highly folded with a high nucleus to cytoplasm ratio (Fig. S1A). Following clustering, GCs underwent a morphological transition; individual GCs became difficult to discern within a Vasa+ mass (Fig. S1C). Previous studies indicate that GC proliferation occurs during this stage (Leerberg et al., 2017); thus, these morphological changes may represent mitotic divisions (Tong et al., 2010). The simultaneous obscuring of individual cell boundaries within clusters suggests the phenomena is synchronous (Fig. S1D). As this phenomenon likely corresponds to the amplification of GC numbers preceding germline cyst emergence, hereafter, we refer to this process as cystogenesis. Subsequently, GCs within forming cysts adopted a compact round nucleus with a prominent nucleolus, and perinuclear Vasa aggregates (n=7 gonads) (Fig. S1E). The nuclei of somatic gonadal cells elongated as the cysts grew (Fig. S1F). β-Catenin immunostaining revealed incomplete boundaries between cyst cells (Fig. S1G); but it was unclear whether cyst cells were interconnected by cytoplasmic bridges like those observed in juvenile ovaries (Marlow and Mullins, 2008).
To examine cyst architecture, cyst size and to investigate whether cysts were interconnected by cytoplasmic bridges, we labeled GCs with Vasa antibody and F-actin with phalloidin to visualize cortical actin and the cytoskeleton. At 10 days, cysts of various stages were observed in wild-type gonads (Fig. 1A,B-B″; Fig. S2A-F″), including GCs in two-cell (Fig. 1A,C-C″; Fig. S2A-F″) and four-cell (Fig. 1A,D-D″) configurations. Cyst cells were connected by prominent actin ring structures (Fig. 1E-E″; Fig. S3). These structures appeared before the reported onset of meiotic marker expression in zebrafish (Beer and Draper, 2013; Rodriguez-Mari et al., 2013; Tzung et al., 2015); therefore, we conclude that these are premeiotic GC cysts.
Fig. 1.
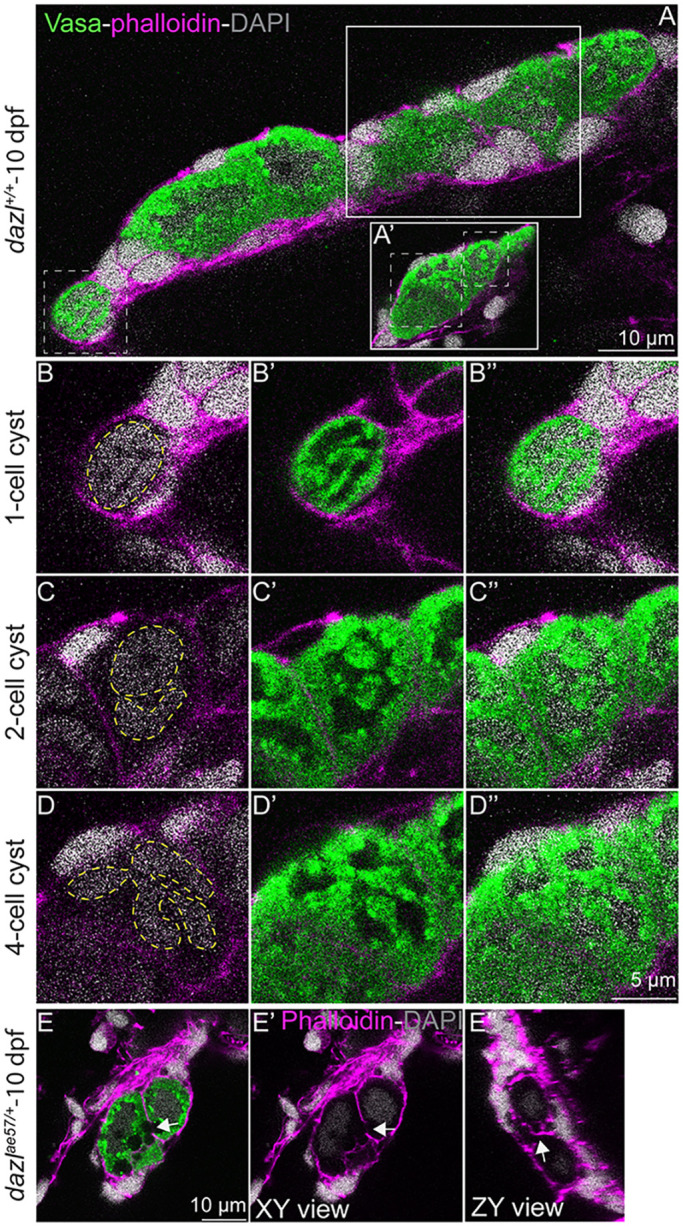
Cyst and actin ring formation in zebrafish. (A,A′) Single confocal plane of a 10-day larval gonad with DAPI-labeled nuclei (gray), GCs marked with Vasa (green), and F-actin with phalloidin (pink). Cystogenesis stages are boxed (white dotted lines). Solid white boxes delineate the focal plane showing the two- or four-cell stage cysts. (B-B″) Images showing a one-cell stage cyst with compact DNA (B) and a high cytoplasm/nucleus ratio (B′). Yellow dashed line indicates the nucleus. (C-C″) Division produces a two-cell stage cyst with two nuclei (yellow dashed line) surrounded by perinuclear Vasa. (D-D″) A four-cell cyst with four nuclei (yellow dashed line). (E-E″) Intercellular bridges (white arrow) in a 10-day larval gonad. (E′) XY view without Vasa (green). White arrow indicates the intercellular bridge. (E″) ZY view of the actin ring (white arrow).
dazl mutants
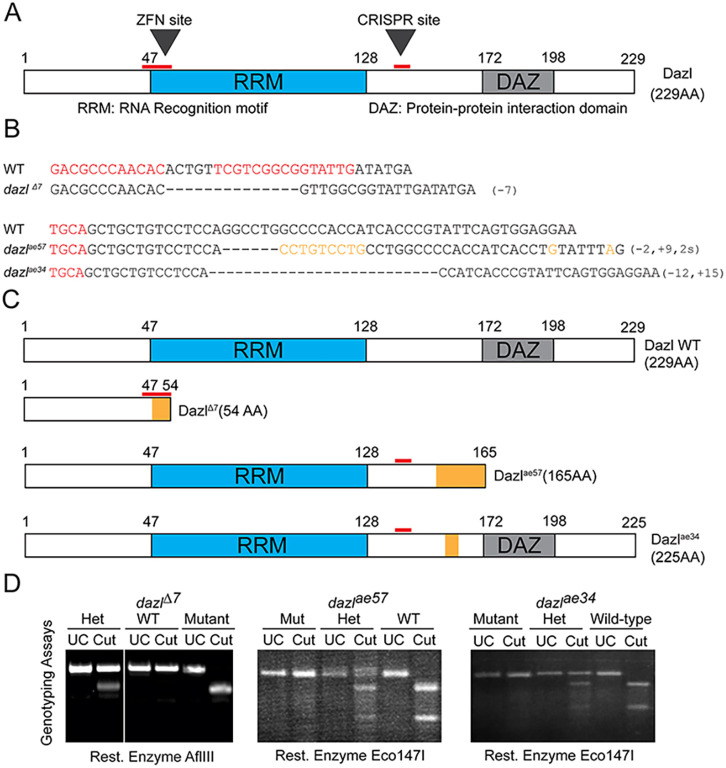
Although Dazl has not been previously implicated in cyst formation, Dazl was a compelling candidate regulator of this conserved process, as in mouse and human fetal ovaries it interacts with Tex14 RNA, a regulator of ring canal formation (Greenbaum et al., 2006; Reynolds et al., 2005; Rosario et al., 2017; Zagore et al., 2018). In zebrafish, dazl is expressed throughout GC development (Hashimoto et al., 2004; Howley and Ho, 2000; Kosaka et al., 2007; Maegawa et al., 1999). To test the hypothesis that dazl regulates germline cyst formation, we generated three mutant alleles disrupting zebrafish dazl using ZFNs (Foley et al., 2009a) and CRISPR/Cas9 mutagenesis (Gagnon et al., 2014). We recovered dazlΔ7 using ZFNs targeting the first exon of dazl (Fig. 2A). Sequencing of the genomic and mutant cDNA revealed a frameshift mutation that caused a premature stop codon within dazl, truncating all functional domains (Fig. 2B,C). Using CRISPR/Cas9 methods targeting exon 6, we recovered two additional alleles: dazlae57and dazlae34. Sequencing of genomic DNA (gDNA) and mutant cDNA revealed two distinct insertion-deletion mutations (Fig. 2B,C; Fig. S4). The dazlae57 allele harbors a 2 bp deletion, a 9 bp insertion and 2 bp substitution that result in a premature stop codon predicted to truncate Dazl and eliminate the Daz motif (Fig. 2B,C). The dazlae34 allele, a 15 bp insertion with a 12 bp deletion, results in a five amino acid in-frame deletion generating a truncated protein of 225 amino acids with intact RNA recognition motif (RRM) and Daz motifs. Genotyping assays were developed for each dazl mutant allele (Fig. 2D; Fig. S5).
Fig. 2.
dazl mutagenesis and fertility phenotypes. (A) Dazl protein diagram illustrating the RRM and DAZ domains with their respective amino acid regions. ZFN and CRISPR sites are indicated by red lines. (B) dazl alleles generated by ZFN (dazlΔ7) and CRISPR (dazlae57and dazlae34). Partial ZFNs and Cas9 binding sites are highlighted in red. Dashed lines represent deletions and orange denotes substitutions. (C) Diagram of alleles generated. The ZFN allele (top) causes a 7 bp deletion and leads to a premature stop codon eliminating all functional domains. dazlae57 (second from top) creates a 9 bp insertion and 2 bp substitution leading to a premature stop codon after the RRM domain. The third allele, induced a 12 bp deletion and 15 bp insertion causing an in-frame deletion between the RRM and DAZ domains. (D) Representative PCR/derived cleaved amplified polymorphic sequences genotyping assay for each allele. Heterozygotes harbor the wild-type allele (upper band) and the mutant allele (lower band) in the case of dazlΔ7. dazlae57 or dazlae34 heterozygotes harbor wild-type (lower band) and mutant alleles (upper band).
Dazl protein is first detected in PGCs just before cystogenesis
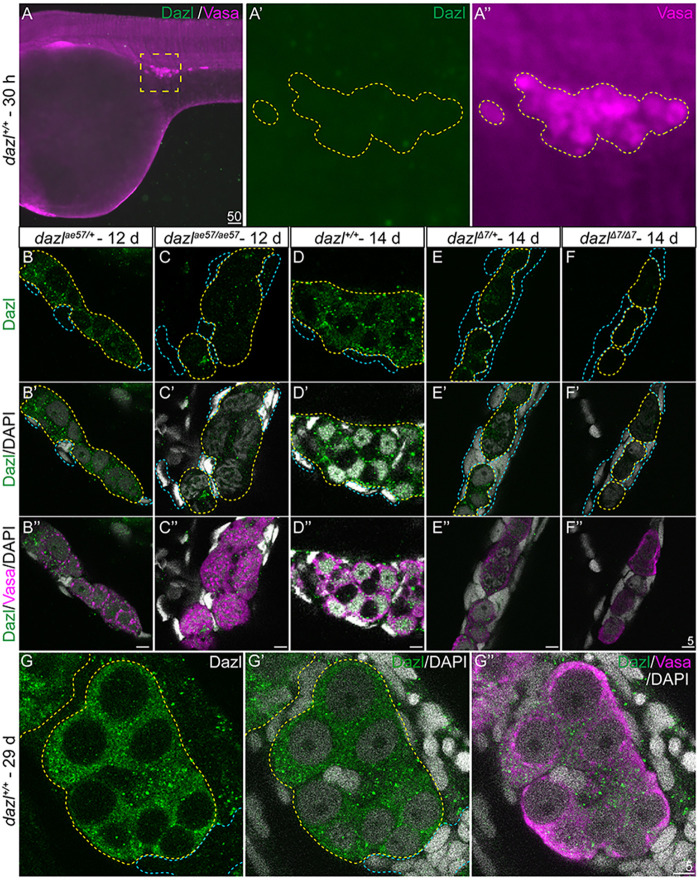
Although dazl transcripts are maternally provided in zebrafish (Kosaka et al., 2007), the distribution of Dazl protein was not known. To examine Dazl protein, we generated C and N-terminal anti-Dazl antibodies (Yenzym) and found that although endogenous Dazl was not detectable in PGCs at 30 hours post fertilization (hpf) (Fig. 3A), it was first detectable just before cyst formation. In 12-14 day wild-type gonads, Dazl protein was detected as puncta in Vasa+ GCs, but not somatic gonad cells, which lack Vasa (Fig. 3B,D; Fig. S6), indicating that Dazl is germline specific. Dazl protein appeared reduced in dazlae57 mutants (Fig. 3C) and was not detectable in dazlΔ7 (Fig. 3E,F). At 29 days, in wild type, Dazl protein was diffusely localized in the cytoplasm of the GCs (Fig. 3G). Thus, the N-terminal antibody is specific for Dazl and detects the truncated dazlae57 protein, revealing that zygotic Dazl is produced before cystogenesis. Consistent with lack of detectable Dazl protein in PGCs at 30 hpf, we observed no deficits in PGC specification, PGC number, germ granule formation or GC migration in dazlae57/ae57, dazlΔ7/Δ7 or dazlae57/Δ7 mutants (Fig. S7). Therefore, if dazl is required for GC specification, PGC granule formation or GC viability, maternal dazl must fulfill these functions.
Fig. 3.
Dazl protein in the germline. (A-A″) Dazl is not detectable in PGCs marked with Vasa at 30 hpf. (A′,A″) Enlargement of the yellow dotted box in A with Dazl (A′, green) and Vasa (A″, magenta). (B-F″) Single confocal plane of representative 12-day dazlae57/+ and dazlae57/ae57 gonads (B-C″), and dazl+/+, dazlΔ7/+ or dazlΔ7/Δ7 14-day gonads (D-F″) immunostained with Dazl (green), Vasa (magenta) and DAPI (gray) as nuclear marker. Yellow dotted lines delineate GCs and blue lines outline somatic cells. (B-C″) Localization of Dazl in Vasa+ GC of dazlae57/+ (B-B″, n=1) and dazlae57/ae57 (C-C″, n=1). There was less abundant Dazl in dazlae57/ae57 gonads. (D-D″) Localization of Dazl in Vasa+ GC of dazl+/+ (n=1). Image is 63× without 2.5× zoom to show full gonad at resolution 512×512. (E-E″) Localization of Dazl in Vasa+ GC of dazlΔ7/+ (n=1). (F-F″) No detecatable Dazl in Vasa+ GC of dazlΔ7/Δ7 (n=2). Yellow dotted line delineates the gonad. (G-G″) Twenty-nine-day gonads labeled with Dazl (green) and Vasa (magenta). DAPI (gray) marks nuclei. Dazl protein was diffusely localized in the cytoplasm of the GCs.
dazl regulates cystogenesis
To determine whether dazl was required for cystogenesis, we examined Vasa and actin, labeled with phalloidin, in dazl mutants. Between 7 and 10 days, Vasa persisted in dazl mutants, indicating that zygotic Dazl is not required for Vasa expression (Fig. 4; Figs S8, S9). As in wild type (Fig. 4A; Figs S8-S11), Vasa+ cells (GCs) of dazl mutants were single and clustered individuals (Fig. 4B; Figs S8-S11), adjacent to somatic gonadal cells (Fig. 4; Fig. S8). Like wild type at 10 days (Fig. 4C; Figs S8, S10), dazl mutant cells transitioned to a cystogenic state and underwent synchronous nuclear/cytoplasmic morphological changes (Fig. 4D; Figs S8, S11). Some dazl mutant GCs showed evidence of amplification, including a compact nucleus (DAPI), but Vasa was diffusely cytoplasmic, and Vasa perinuclear aggregates were less apparent at this time (Figs S8, S10, S11). During the transition-amplification step, somatic gonadal cells enclosed GCs in both wild type and mutants. These results indicate that initiation of the transition and early amplification phases does not require dazl.
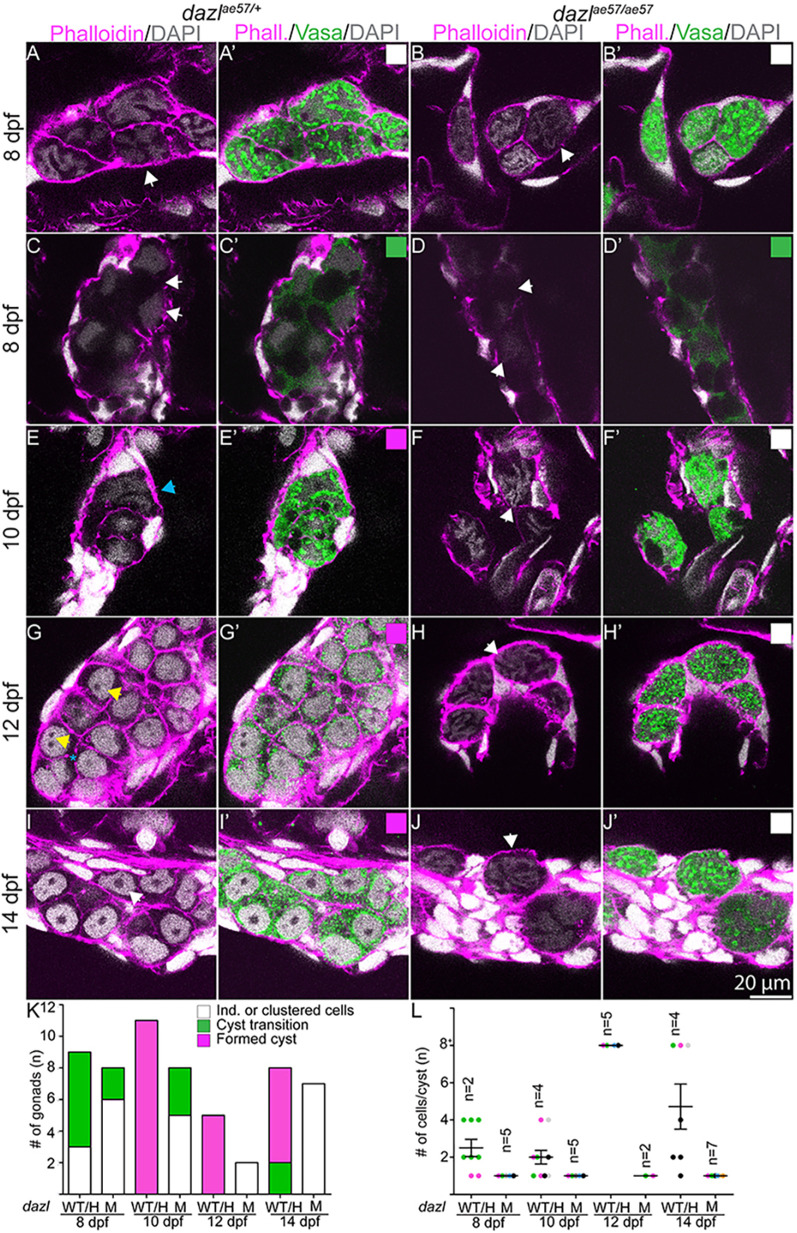
Fig. 4.
Zebrafish cystogenesis. Larval gonads labeled with Vasa (green), DAPI and F-actin (phalloidin). (A,A′,C,C′,E,E′,G,G′,I,I′) Single confocal plane of dazlae57/+ gonad showing cystogenesis between 8-14 days. (A,A′,C,C′,E,E′) Clustered Vasa+ individuals (white arrowheads) in the first step of cystogenesis with compaction and compartmentalization into irregular nuclear Vasa+ and Vasa− domains (C,C′,D,D′) at 8-10 days. Although initial compaction and compartmentalization occurs in mutants, wild-type GCs form cysts with multiple nuclei evident (E,E′, aqua arrowhead), whereas the pretransition morphology is observed in dazl mutants (F,F′). (G,G′) Amplification of GC numbers and round shaped nuclei with one to two nucleoli (yellow arrowheads) at 12 days (n=3). The shared tricellular junction is marked by a blue asterisk. (I,I′) Cyst cells with premeiotic nuclei and prominent nucleolus (white arrowhead). dazlae57/+ [8 days, n=3 (A,A′); 8 days, n=6 (C,C′); 10 days, n=11; 12 days, n=5; 14 days, n=6]. (B,B′,D,D′,F,F′,H,H′,J,J′) age-matched dazlae57/ae57 gonads. All dazlae57/ae57 germ cells end up as individuals with convoluted DNA (white arrows) lacking cyst organization. dazlae57/ae57 [8 days, n=6 (B,B′); 8 days, n=2 (D,D′); 10 days, n=5; 12 days, n=2; 14 days, n=7). (K) Quantification of categories between 8-14 days in dazlae57/+ and dazlae57/ae57 gonads. Categories match the corresponding box at the top right. (L) Quantification of cells/cyst at 8-14 days in dazlae57/+ and dazlae57/ae57 gonads. Cells in transition-amplification stage were not quantified because cell boundaries were not reliably distinguishable. Error bars represent s.d.
Next, we examined dazl mutants at 12 days, when cysts emerge in wild type. In contrast to the interconnected Vasa+ cyst cells in heterozygotes (Fig. 4E), no organized cysts formed in dazl mutants; instead, dazl mutant cells resembled the earlier individual GC morphology (Fig. 4F; Fig. S8). The cytoplasm of the mutant cells appeared convoluted, and perinuclear enrichment of Vasa granules was not evident (Fig. S8). At this stage, cortical actin accumulated at cell membranes, making the membrane appear thicker. In addition, at 8 days and 10 days, dazl mutant GCs became larger than wild type. In contrast, although wild-type gonads were full of cysts at 14 days (Fig. 4G,I; Figs S8, S10), dazl mutant GCs remained as individuals, indicating failed or severely delayed cystogenesis (Fig. 4H,J-L; Figs S8, S10, S11). Because wild-type cells appeared smaller after cystogenesis, we measured cell size before and after the cystogenesis transition. Using three-dimensional imaging software, we determined cell area and volume at 8-14 days. dazlae57/+ GCs decreased significantly in area and volume, indicating the divisions are reductive (Fig. S12). In contrast, dazl mutant cell area and volume did not decrease, suggesting failed division (Fig. S12). Because somatic gonadal cells were intact, and because dazl expression is GC specific (Fig. 3) (Hashimoto et al., 2004; Maegawa et al., 1999), Dazl likely acts within the GCs to promote cystogenesis.
The unusual nuclear morphologies observed during the transition from individual PGC to cyst prompted examination of the nuclear envelope by immunostaining dazlΔ7/+ or dazlΔ7/Δ7 gonads at 8 days and dazlae57/+, dazlΔ7/+ or dazlΔ7/ae57 at 10 days with the nuclear envelope marker LaminB1 (Knaut et al., 2000; Strasser et al., 2008), DAPI to mark DNA, and Vasa to label GCs (Fig. 5). In dazlΔ7/+ at 8 days, GC nuclei had a raisin-like folded appearance (Fig. 5A-A″). No differences were detected in dazlΔ7/Δ7 mutants at this stage (Fig. 5B-B″). At 10 days, the germ cell nuclei of dazlae57/+ became smooth in appearance (Fig. 5C-C″) as they initiated amplification. After this stage, postmitotic GC nuclei had smooth nuclear membranes and large nucleoli (Fig. 5E-E″). Although dazlae57/Δ7 mutants initiated amplification (Fig. 5D-D″, Fig. 4D; Figs S8, S10), mutant GCs did not maintain a smooth nuclear morphology at 10 days or thereafter, and instead of forming cysts were individual cells with folded raisin-like nuclear membranes resembling wild-type GCs at 10 days (Fig. 5F-F″).
Fig. 5.
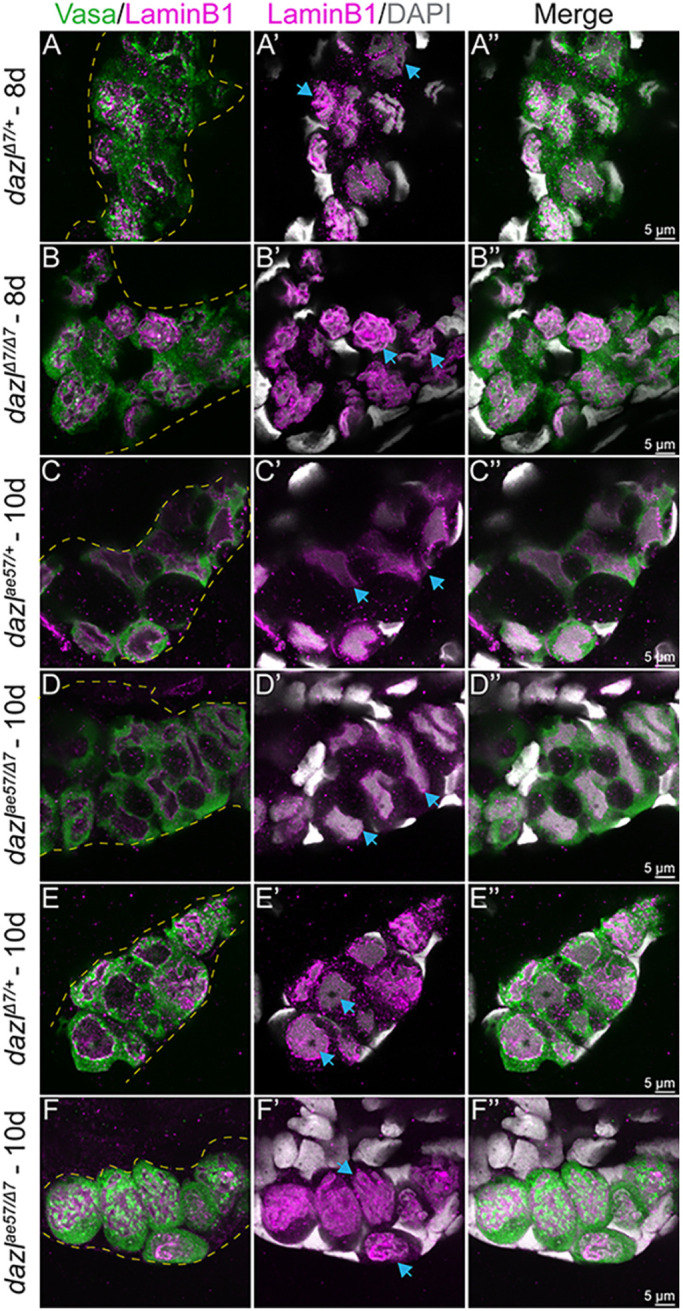
Nuclear morphology during the PGC to cyst transition. Single confocal plane of representative dazlΔ7/+ or dazlΔ7/Δ7 day 8 gonads and dazlae57/+, dazlΔ7/+ or dazlΔ7/ae57 at 10 days labeled with Vasa (green), LaminB1 (magenta; nuclear envelope,) and DAPI (gray; DNA). (A-A″) Vasa+ GC of dazlΔ7/+ (n=2) at day 8, with a folded raisin-like nuclear envelope. (B-B″) Vasa+ GC of dazlΔ7/Δ7 (n=3) at day 8, with an unfolded smooth nuclear envelope of Vasa+ GC of dazlae57/+ (C-C″, n=2) at 10 days. (D-D″) The change in nuclear appearance corresponds to amplification during cyst formation and is intact in dazlae57/Δ7. (E-E″) After the transition at 10 days, the nuclei of Vasa+ GC of dazlΔ7/+ remain smooth (n=2). The postmitotic GC nuclei also have a smooth nuclear membrane and a large nucleolus. (F-F″) Vasa+ GC of dazlae57/Δ7 (n=5) at 10 days. Mutant GCs are individuals with a folded nuclear membrane rather than a smooth morphology at 10 days. Yellow dotted line delineates the gonad. Blue arrows indicate each note for the corresponding panel.
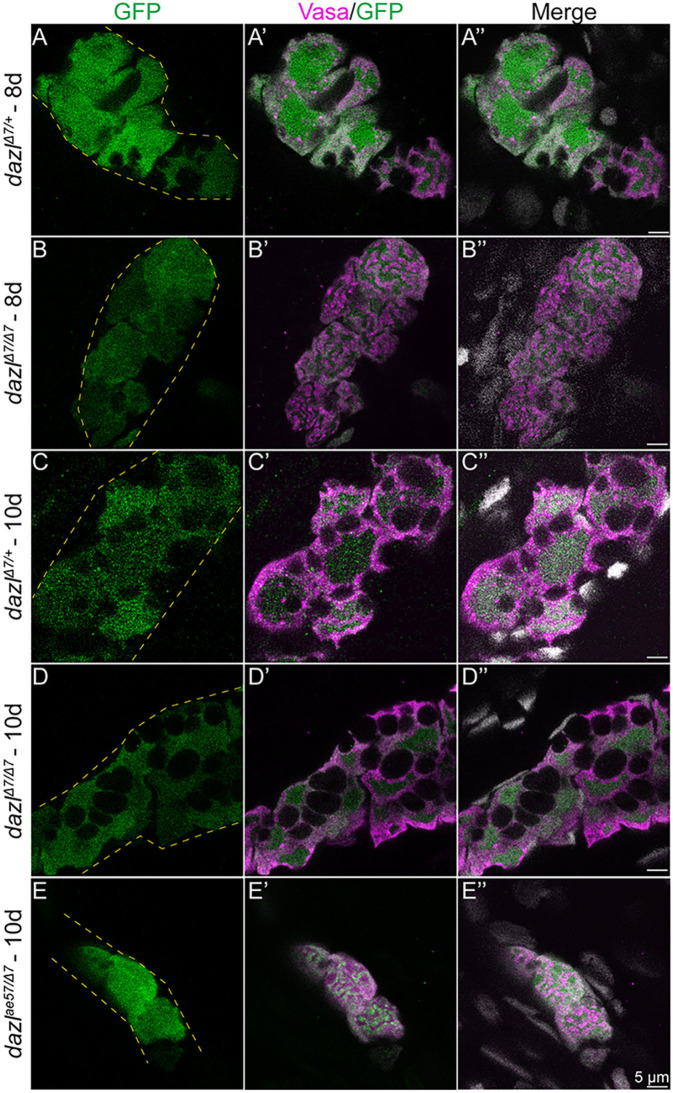
Formation of nuclear indentations has been reported in other cell types and is associated with cell state or cell cycle progression in cultured cells (Aureille et al., 2019), and transcriptional modulation during mouse oogenesis (Almonacid et al., 2019). Whether the change in nuclear morphology from the smooth appearance of early PGCs (Knaut et al., 2000; Strasser et al., 2008) to the raisin-like morphology that precedes the amplification step reflects activation of transcriptional programs necessary for the PGC to GSC transition is unknown. To determine whether germline-specific zygotic programs are activated without Dazl, we examined ziwi, a zygotically expressed GC marker, using the established ziwi:GFP reporter (Leu and Draper, 2010). At 8 days, zygotic activation of ziwi is detected in the germline, marked with Vasa, of dazlΔ7/+ (Fig. 6A) and dazlΔ7/Δ7 mutants (Fig. 6B) undergoing amplification. At 10 days, the reporter persists in dazlΔ7/+ (Fig. 6C) dazlΔ7/Δ7 (Fig. 6D) and dazlΔ7/ae57 mutants (Fig. 6E-E″). Based on the activation of the ziwi reporter and persistent expression of Vasa in dazl mutant GCs, we conclude that at least some zygotic transcriptional programs are activated without Dazl.
Fig. 6.
Transcription is activated in dazl mutant gonads. (A-E″) Single confocal plane of representative dazlΔ7/+ and dazlΔ7/Δ7 at day 8, and dazlΔ7/+, dazlΔ7/Δ7 and dazlae57/Δ7 gonads at day 10. Fish are transgenic for [ziwi:GFP], expressed zygotically in the germ cells. Gonads were immunostained with GFP (green, germ cells), Vasa (magenta) as a GC-specific cytoplasmic marker, and DAPI (gray; nuclei). (A-A″) GFP expression in Vasa+ GC of day 8 dazlΔ7/+ (n=2, B-B″) and dazlΔ7/Δ7 (n=2) gonads, and day 10 dazlΔ7/+ (n=2, C-C″), dazlΔ7/Δ7 (n=4, D-D″) and dazlae57/Δ7 (n=2, E-E″) gonads. Yellow dashed lines delineate the gonad.
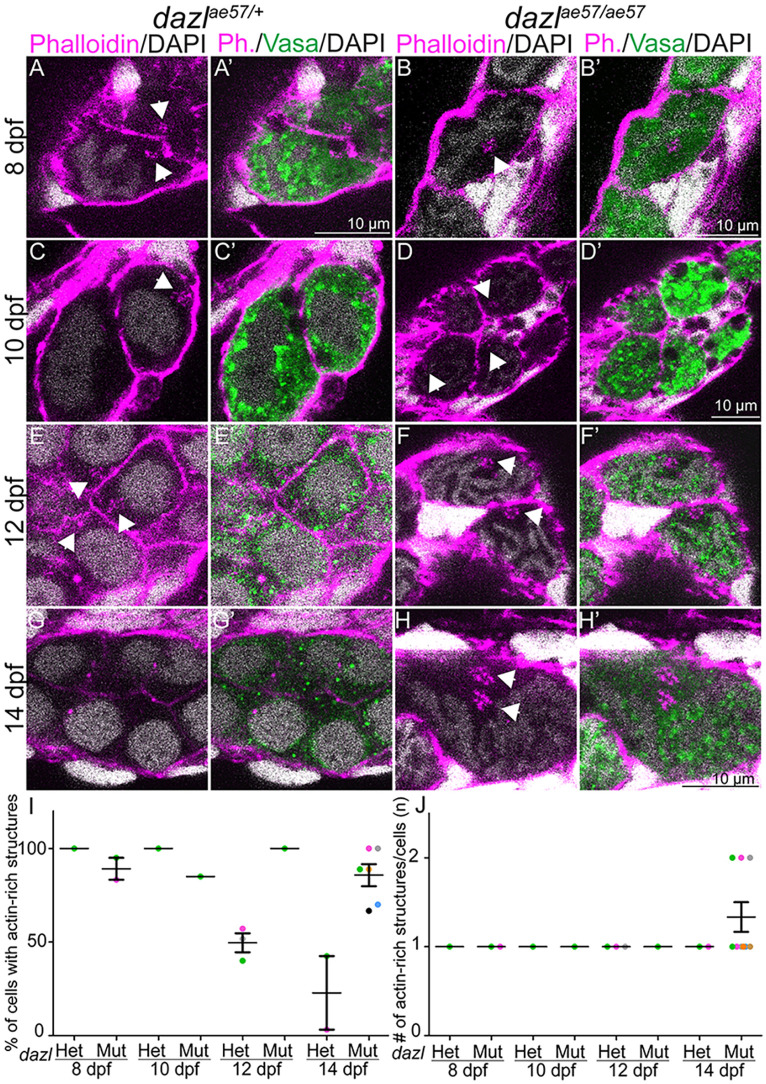
Failure to generate cysts in dazl mutants could be due to abnormal cyst architecture or cytokinesis, such as failure to arrest the cytokinetic furrow, particularly given that Dazl interacts with cytokinesis and ring canal factors (Reynolds et al., 2005; Rosario et al., 2019, 2017; Zagore et al., 2018). Actin is a prominent marker of intercellular connections and subcellular structures of GC cysts in other species, including the spectrosome of GSCs, and the branched fusome that connects germline cyst cells (de Cuevas et al., 1997; Djagaeva et al., 2005; Hime et al., 1996; Kloc et al., 2004; Lin and Spradling, 1995; Snapp et al., 2004; Spradling et al., 1997) and the ring channels of Caenorhabditis elegans (Seidel et al., 2018). Close inspection of actin (labeled with phalloidin) in wild-type gonads at 8 days and 10 days revealed an actin-rich density within GCs (Fig. 7A,A′,C,C′). These actin aggregates were also present in dazl mutant GCs (Fig. 7B,B′,D,D′). By 12 days, when cysts are abundant in wild-type gonads, actin was present in branched structures (Fig. 7E,E′). In contrast, at this stage in dazl mutants, the actin-rich aggregates persisted in Vasa+ cells (Fig. 7F,F′). By 14 days, these actin structures were no longer detected in wild-type cysts (Fig. 7G,G′,I). However, the actin densities persisted with up to two actin aggregates in dazl mutant GCs (n=3/6 individuals), as opposed to the single aggregate observed earlier in dazl mutants (compare Fig. 7H,H′ and Fig. 7B,B′,J), possibly reflecting failed division or cell fusion.
Fig. 7.
dazl mutants retain actin-rich structures. Single confocal plane of dazlae57/+ or dazlae57/ae57 8-14-day gonad labeled with Vasa and fluorescently conjugated phalloidin, which marks actin-rich structures and DAPI. (A,A′,C,C′, E,E′,G,G′) The actin-rich structures (white arrowheads) are present transiently in dazlae57/+ 8-12-day gonads. Number of gonads analyzed: 8 days, n=1 (A,A′); 10 days, n=1 (C,C′); 12 days, n=3 (E,E′); 14 days, n=2 (G,G′). (B,B′,D,D′,F,F′,H,H′) Actin-rich structures (white arrowheads) are present in 8-14-day dazl mutant GCs. (H,H′) At 14 days, actin-rich doublets are observed. Number of gonads analyzed: 8 days, n=1 (B,B′); 10 days, n=2 (D,D′); 12 days, n=2 (F,F′); 14 days, n=6 (H,H′). (I) Quantification of cells containing an actin-rich structure within each gonad. (J) Quantification of actin aggregates per cell. Error bars represent s.d.
The main structures connecting cells within cysts are ring canals or ring channels, thought to be the products of arrested cytokinesis (Haglund et al., 2011; Hime et al., 1996; Robinson and Cooley, 1996; Seidel et al., 2018). Although full ring canal structures were not apparent within a single plane of a z-stack, projections of ten slices spanning the structures combined with supplemental three-dimensional projections revealed circular actin-rich structures between cells (Fig. 8; Movies 1-6).
Fig. 8.
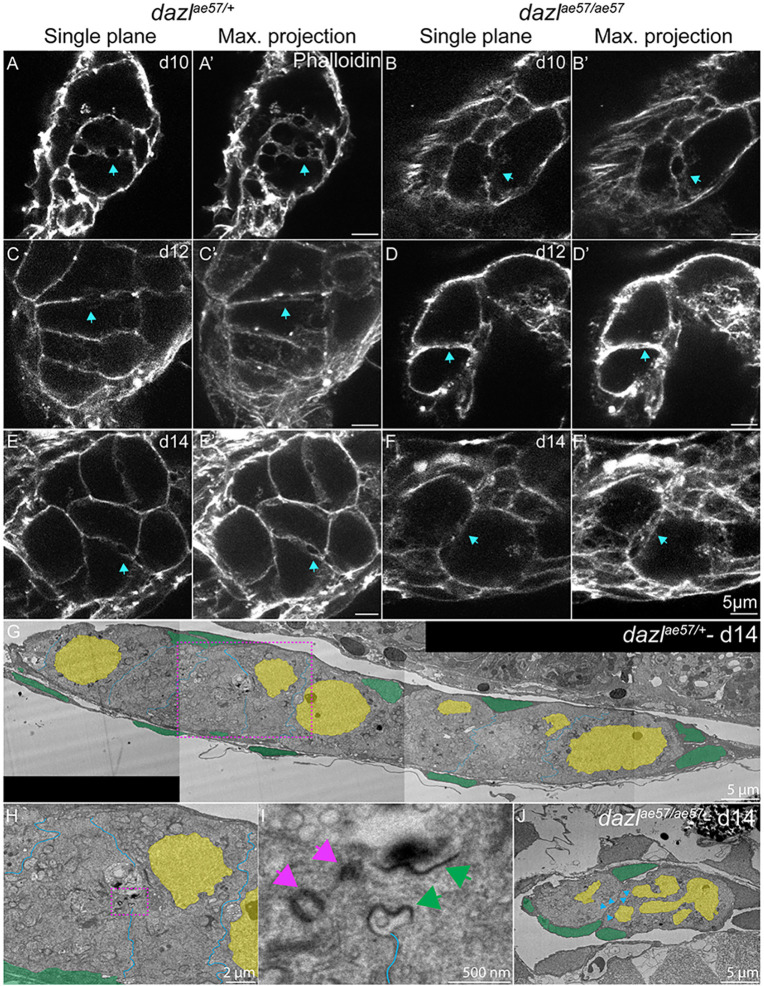
Actin rings are not maintained in dazl mutants. Single confocal plane of representative dazlae57/+ or dazlae57/ae57 10-14-day gonads labeled with fluorescently conjugated phalloidin (actin) depicting the actin ring. Each stage is represented with a sagittal view (XY) or maximum projection (ten planes of a z-stack). (A,A′,C,C′,E,E′) Actin rings are indicated by a blue arrow in dazlae57/+ 8-14-day gonads. Number of gonads analyzed: 10 days, n=3 (A,A′); 12 days, n=3 (C,C′); 14 days, n=3 (E,E′). (B,B′,D, D′,F,F′) Actin rings at 10 days in dazlae57/ae57 cells. At 12- and 14-days, actin rings are not maintained (blue arrows). Gonads analyzed: 10 days, n=4 (B,B′); 12 days, n=2 (D,D′); 14 days, n=3 (F,F′). (G) TEM image of day 14 wild-type gonad stitched from tiled images (magnification 700×). (H) Magnified image of (G, pink dashed line box) showing a ring canal connecting sister GCs within a cyst. (I) Higher magnification of H (pink dashed line box). (J) TEM image of 14-day dazl mutant gonad stitched from tiled images (magnification 700×). No cysts or ring canals were detected. Pink arrows indicate the centrioles and green arrows indicate the ring canal. Blue arrowheads indicate the germ cell membranes.
At 10 days, actin rings were present between wild-type and dazl mutant cyst cells (Fig. 8A-A″, Movie 1; Fig. 8B-B″, Movie 2), consistent with normal onset of cystogenesis without dazl. In wild-type cysts, conspicuous actin rings remained at 12-14 days (Fig. 8C-C″,E-E″; Movies 3, 5). In contrast, the actin rings appeared compromised, possibly collapsed, in dazl−/−, with membranes closely abutting one another as cells individualized (Fig. 8D-D″,F-F″; Movies 4, 6). Altogether, we conclude that dazl is essential for incomplete cellularization and germline cyst formation.
To further examine the architecture of the actin rings, we examined gonad ultrastructure using transmission electron microscopy (TEM). Consistent with the confocal data, at 14 days electron dense intracellular bridges were detected between wild-type GCs (Fig. 8G-I; Fig. S13). In contrast, no ring canals or cysts were detected in the mutant GCs, which were all individual cells (Fig. 8J). Based on the confocal and ultrastructural analysis, we conclude that GC progenitors are connected by intercellular ring canals, resembling those previously observed in zebrafish ovaries (Marlow and Mullins, 2008), and that Dazl is required for cyst structure and GC amplification.
Type II/cystogenic divisions are required for fertility
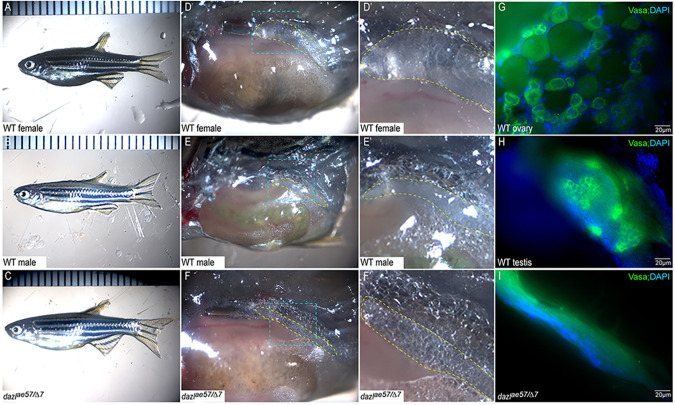
As discussed, two modes of GSC divisions, type I/direct differentiating and type II/cystogenic, have been described (Marlow and Mullins, 2008; Nakamura et al., 2010; Saito et al., 2007). In mouse, ring canals are dispensable for fertility in females but are required in males (Greenbaum et al., 2006). Conversely, defects in ring canal formation in Drosophila disrupt the fertility of both sexes (Hime et al., 1996; Yue and Spradling, 1992). Likewise, type II divisions fail in dazl mutants. To determine whether cystogenic divisions are required for fertility in zebrafish, dazl mutants were raised to adulthood. As expected, based on the germline specific expression of dazl (Kosaka et al., 2007; Maegawa et al., 1999), dazl−/− had no overt morphological defects; however, dazlΔ7/Δ7 (n=11) and dazlae57/ae57 (n=6) adults were exclusively sterile males, whereas dazlae34/ae34 adults were fertile (n=23 males; n=6 females).
To confirm that infertility of dazl mutants was specific to the dazl mutation, and to assess mutant allele strengths, we performed complementation tests. Although heterozygosity for each allele caused no phenotypes or fertility deficits (Fig. 9A,B,D′,D′,E,E′; n=9), dazlae57/Δ7 mutants were exclusively sterile males (Fig. 9C,F,F′; n=2). Examination of Vasa-labeled GCs (Leerberg et al., 2017) and DNA (DAPI) at 2 months of age revealed oocytes (Fig. 9G) or normal testis morphology (Fig. 9H) in wild-type siblings, and lack of Vasa+ GCs in dazlae57/Δ7 mutants (Fig. 9I; n=2). Like wild type, dazlae57/ae34 mutants were fertile males or females (n=5 females, n=10 males). Based on these observations, we conclude that dazl-mediated cystogenesis/type II division is essential for GSC establishment and fertility in zebrafish. Moreover, dazlae57 and dazlΔ7 are strong loss-of-function alleles, but dazlae34 retains all functional domains and sufficient activity to support normal germline development.
Fig. 9.
Zygotic dazl mutants are sterile males. (A-C) Overall morphology of representative adult wild-type female (A), male (B) and dazlΔ7/ae57 compound heterozygote (C). Males and females are distinguished based on secondary sex traits (color and tubercles on male fins). (D-F) Dissected trunks with the gonad region, indicated with yellow dashed lines, of wild-type female (D), male (E) and dazlΔ7/ae57 compound heterozygote (F). (D′-F′) Higher magnification views of blue outlined region in D-F. (D′,E′) Oocytes in females (D′) and sperm/testicular structures of males (E′). No identifiable gametes or GCs were detected in compound heterozygotes (F′). (G-I) Gonads labeled with Vasa reveal the oocytes (G), spermatocytes (H) and a gonad lacking GCs (I).
Checkpoint inactivation cannot suppress germ cell loss in dazl mutants
Loss of germline can occur by cell death, failure to maintain GC fate and differentiation, or an absence of supporting somatic cell types (Gross-Thebing et al., 2017; Kossack and Draper, 2019; Leerberg et al., 2017; Rodriguez-Mari et al., 2010; Slanchev et al., 2005; Uchida et al., 2002; Wang et al., 2007). During normal development, apoptosis of GCs is associated with male-specific differentiation (Pradhan et al., 2012; Rodriguez-Mari et al., 2010; Siegfried and Nusslein-Volhard, 2008; Slanchev et al., 2005; Tzung et al., 2015; Uchida et al., 2002; Wang et al., 2007). Mutation of the tumor suppressor tp53 can block cell death and oocyte loss in some zebrafish mutants (Miao et al., 2017; Rodriguez-Mari et al., 2010; Shive et al., 2010). Similarly, in mouse, infertility phenotypes associated with germline cell death are suppressed by chek2 mutation, which acts via Tp53 and Tp63 (Bolcun-Filas et al., 2014). Zebrafish have a single chek2 gene on chromosome 5. The zebrafish chek2sa20350 mutant allele was recovered in the Sanger screen (Kettleborough et al., 2013). By sequencing gDNA and cDNA from mutants, we confirmed that chk2sa20350 is a nonsense allele harboring a C to A mutation that creates a premature stop codon (Q-stop), yielding a truncated Chek2 protein lacking the kinase domain (Fig. S14). As in Drosophila (Abdu et al., 2002) or mouse (Bolcun-Filas et al., 2014), mutation of zebrafish chek2 caused no overt phenotypes and did not interfere with fertility (n=9 females and n=13 males). Therefore, chek2, on its own, is dispensable for germline development and fertility in zebrafish.
To determine whether eliminating tp53 or chk2 could suppress germline loss in dazl mutants, we generated double mutants lacking dazl and tp53 or chk2. Analysis of dissected 20 dpf, 40 dpf and adult (>6 months) gonads revealed ovary or testis in tp53 or chk2 mutants that were heterozygous for dazlae57 (n=9). In contrast, all dazlae57/57 whether tp53 heterozygous (n=4) or tp53−/− (n=5) lacked GCs and were sterile males (Fig. S15). Similarly, loss of chk2 did not prevent germline loss, as adult dazl;chk2 mutants were sterile males (n=3) like dazl single mutants (n=4), whereas dazl heterozygous siblings were either fertile males or females (n=11) (Fig. S16). These results indicate that dazl is required for cyst development and germline maintenance independently of chk2 and Tp53.
Dazl promotes germline stem cell fate
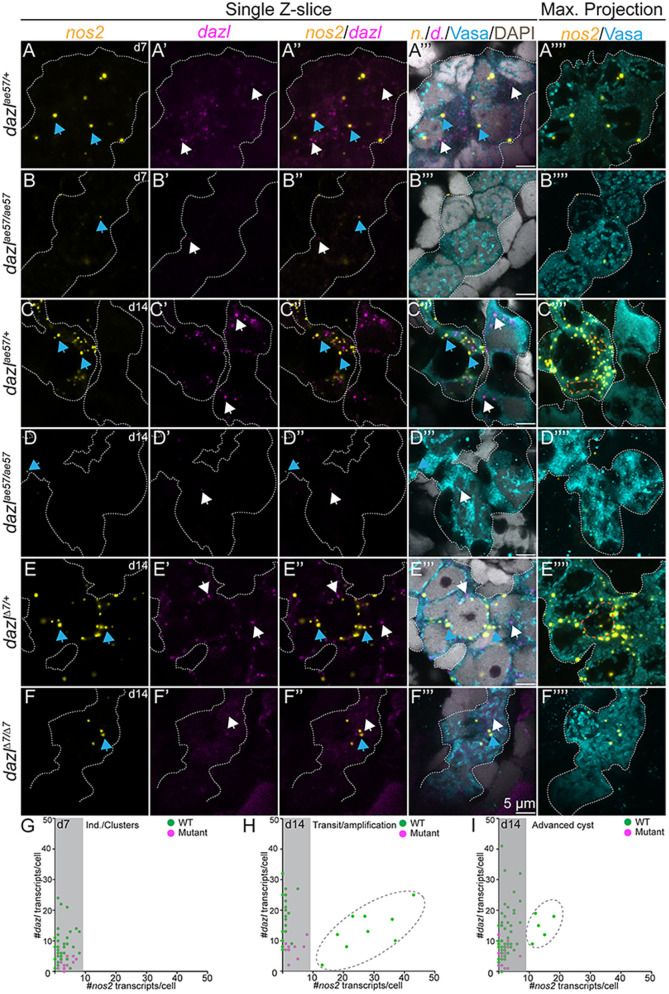
Although migration of PGCs has been extensively studied (reviewed by Barton et al., 2016; Marlow, 2015; Paksa and Raz, 2015), the cellular and molecular mechanisms by which PGCs transition to GSCs, in zebrafish, remain unknown. The only established marker of zebrafish GSCs is nanos2 (Beer and Draper, 2013; Cao et al., 2019), but neither it nor nanos3 are required to specify GSCs in zebrafish (Beer and Draper, 2013; Cao et al., 2019). To determine whether germline loss in dazl mutants was a result of impaired GSC specification or maintenance, we used RNAscope to analyze nanos2 expression in wild type and dazl mutants at 7-14 days, earlier than previously analyzed. In addition, we examined dazl mRNA expression, which also had not been analyzed during this period, using Vasa to mark all GCs. At 7 days and 14 days, dazl transcripts were detected as cytoplasmic puncta in Vasa+ wild-type GCs (Fig. 10A,C,E,G), and were less abundant in dazlae57/ae57 (Fig. 10B,D,E,F). Beginning at 7 days, nanos2 transcripts were detectable as 1-3 foci per cell in wild-type (Fig. 10A,G) and dazlae57/ae57 (Fig. 10B,G) GCs. By 14 days, there were two populations of nanos2-expressing cells in wild-type nanos2 low cells and nanos2 high cells (Fig. 10C,F,H,I), likely reflecting the PGCs and GSCs, respectively. In contrast, only nanos2 low cells were present in dazlae57/ae57 mutants at this stage (Fig. 10D,F,H,I). Therefore, we conclude that all GCs initiate expression of nanos2, but only a few become the nanos2 high GSCs. Because induction of nanos2 is intact, but high nanos2 is not achieved in dazl mutants, we conclude that dazl is required for GSC specification, either by directly promoting stabilization of nanos2 transcripts or by promoting the PGC to GSC transition required for their specification.
Fig. 10.
Absence of germline stem cells in dazl mutants. (A-F⁗) Single confocal plane of representative dazl+/+ or dazlae57/ae57 gonads at 7 days (individuals/clustered cells) and 14 days (transition/amplification), and dazl+/+ or dazlΔ7Δ7(advanced cyst) at 14 days labeled with nanos2 (yellow) or dazl (magenta) RNA probes, Vasa (cyan) and DAPI (gray). Sagittal view (XY) (left side) or maximum projection (right side) of 5-10 planes of a z-stack. (A-A⁗) Early nanos2 (blue arrows) and dazl (white arrows) foci in individuals/clustered cells in dazl+/+ at 7 days. (B-B⁗) Expression of nanos2 and dazl in individuals/clustered cells in dazlae57/ae57 at 7 days. (C-C⁗) Presence of abundant nanos2 foci during transition/amplification (orange dotted line surrounding the nuclear envelope). All GCs express dazl RNA. (D-D⁗) Scattered nanos2 and dazl foci in dazlae57/ae57 mutant cells that failed to progress beyond transition/amplification at day 14. (E-E⁗) Germline cysts in dazl+/+ with two populations: nanos2+ low-expressing cells (cells without an orange dashed line) and nanos2+ high-expressing cells (cells with an orange dotted line). (F-F⁗) nanos2 (blue arrows) foci in dazlΔ7Δ7 cells. (G) Quantification of nanos2 foci/cell compared with dazl foci/cell at 7 days (clustered stage). (H) Quantification of nanos2 foci/cell compared with dazl foci/cell at 14 days (transition/amplification stage). Dark gray dashed line encircles the nanos2+ high population. (I) Quantification of nanos2 foci/cell compared with dazl foci/cell at 14 days (advanced cyst stage). Dark gray dashed line encircles the nanos2+ high population. Gonads analyzed: 7 days, dazl+/+, n=5 (A-A⁗); 7 days, dazlae57/ae57, n=2 (B-B⁗); 14 days, dazl+/+ n=3 (C-C⁗); 14 days, dazlae57/ae57 n=2 (D-D⁗); 14 days, dazl+/+, n=4 (E-E⁗); 14 days, dazlΔ7Δ7, n=2 (F-F⁗).
DISCUSSION
Our study examines the earliest stages of gonadogenesis and provides evidence that the conserved Rbp Dazl is required for germline cyst formation and plays crucial roles in GC amplification and establishment of GSCs to promote fertility. We provide genetic evidence that Dazl is required to form germline cysts interconnected by actin rings that resemble ring canals, based on confocal and ultrastructural analysis. Specifically, dazl mutant GCs are able to initiate cyst formation, but ring canals are not maintained. It is unclear whether failed cysts return to the individual state or are quickly eliminated, such that only pre-cystogenic cells remain (Fig. 11). In either case, mutant cells ultimately remain as individuals that fail to differentiate as meiocytes (Fig. 11). Consistent with our findings, regulators of incomplete cytokinesis were among the Dazl targets identified in immunoaffinity screens (Kim et al., 2015; Reynolds et al., 2005; Rosario et al., 2019, 2017; Zagore et al., 2018). In addition to promoting type II cystoblast divisions, we show dazl is required for GSC establishment. Finally, we show that Dazl and type II cystogenic divisions are essential for fertility, as GCs are lost from dazl mutants by a mechanism independent of checkpoint regulators.
Fig. 11.
Schematics depicting the PGC to germline cysts with germline stem cell transition. (A) In wild-type gonads, individual PGCs cluster near one another and undergo dramatic nuclear rearrangements, possibly reflecting the onset of differentiation into germline stem or progenitor cells. During the transition step, the cell boundaries (pink) are not apparent as nanos2 (yellow) becomes detectable as discrete puncta with low and high regions of expression. Incomplete cytokinesis generates progenitors with round nuclei connected by intracellular bridges. Most cells express low levels of nanos2, whereas a few express high levels of nanos2, the likely GSCs. (B) In dazl mutants the first steps are intact. Mutant germ cells enter the transition state and express low levels of nanos2, but not high levels. As gonad development ensues, only individual GCs are detected in mutants and no GSCs are specified. It is unclear whether GCs revert to the pre-transition state or whether they are lost; however, blocking cell death does not prevent the germline loss or sterility of dazl mutants. Thus, Dazl is essential for germline cyst formation and GSC specification, either directly or indirectly.
Conserved dazl functions in gametogenesis and the PGC to GSC transition
In cultured cells, dazl promotes meiotic entry (Chen et al., 2014; Haston et al., 2009; Jung et al., 2017; Yu et al., 2009). Similarly, in mouse, dazl is required for ‘licensing’, or acquisition, of meiotic competence and to generate gametes; dazl mutant GCs remain PGC-like and fail to develop as male or female (Gill et al., 2011; Hu et al., 2015). In medaka, dazl also regulates gametogenesis and GC development (Li et al., 2016; Xu et al., 2007), but is not required for sexually dimorphic gonad development (Nishimura et al., 2018). We show that zygotic dazl is required for gametogenesis in zebrafish. However, in contrast to mouse dazl mutant gonads, which fail to sexually differentiate, and medaka dazl mutants, which develop as either sex (Nishimura et al., 2018), zebrafish dazl mutants develop exclusively as males. In medaka, recovery of male or female dazl mutants with only early PGCs indicated that PGC-like cells could support development of both sexes (Nishimura et al., 2018). Although PGCs form in zebrafish dazl mutants, no females were recovered. Thus, zebrafish PGCs devoid of dazl cannot support cystogenesis, establishment of GSCs or fertility. Similarly, work in mouse and pig indicates that commitment to germline fate only occurs after the PGCs reach the gonad and requires dazl (Nicholls et al., 2019).
A role for dazl in germline cyst formation
In many organisms, proliferation of GCs involves synchronous incomplete divisions that form germline cysts. During cyst formation, GSCs form cystoblasts by mitotic divisions (Leu and Draper, 2010; Pepling and Spradling, 1998). We characterized cystogenesis in juvenile zebrafish gonads and demonstrate a requirement for Dazl in germline cyst formation. This process begins with the congregation of individual migratory GCs, which then undergo synchronous division to generate cysts. Notably, as in zebrafish dazl mutants, dazl−/− GCs of mouse (Chen et al., 2014; Gill et al., 2011; Hu et al., 2015; Lin et al., 2008) and medaka (Nishimura et al., 2018) remain as individual cells, which were described as PGC-like (Chen et al., 2014; Gill et al., 2011; Nishimura et al., 2018). We found that PGCs lacking dazl initiate cystogenesis, including changes in nuclear morphology and gene expression, but fail to maintain intercellular bridges.
Nuclear indentations have been associated with cell state, behavioral changes or transcriptional modulation (Hulspas et al., 1994; Seirin-Lee et al., 2019; Aureille et al., 2019; Almonacid et al., 2019). Whether the transformation of nuclear morphology from smooth in PGCs (Knaut et al., 2000; Strasser et al., 2008) to the raisin-like morphology that precedes amplification represents the activation of transcriptional programs necessary for PGC differentiation remains to be determined. Initiation of this step independently of Dazl, combined with de novo expression from the ziwi promoter and persistent Vasa expression, indicate that zygotic transcriptional programs are initiated; however, this transition subsequently fails in mutants.
Although GCs without Dazl initiated cystogenesis, ring canal integrity was compromised, possibly due to a failure to mature bridges or arrest cytokinesis. Although Dazl was not previously implicated in cyst formation, it interacts with Tex14 RNA, a known ring canal regulator formation (Greenbaum et al., 2006; Reynolds et al., 2005; Rosario et al., 2017; Zagore et al., 2018). Thus, Dazl likely promotes ring canal formation or maintenance via Tex14 or a related protein. In wild type, actin aggregates progress to branched structures that ultimately resolve in late cysts. It is unclear why these structures seem to disappear after cysts form. Labeling and lineage tracing will be required to determine whether cyst breakdown occurs, as in mice, or if instead cysts are stable and there is selection, like in Drosophila (de Cuevas et al., 1997; Lei and Spradling, 2013; Pepling and Spradling, 1998). In contrast, actin remains in aggregates, which are subsequently duplicated in dazl mutants. These duplicated structures potentially indicate cell fusion or, more likely, failed cytokinesis because although wild-type GCs become smaller, cell size does not change in dazl mutant GCs.
Interestingly, during cystogenesis, the characteristic perinuclear Vasa granules of PGCs (Knaut et al., 2000) are transiently lost and are later re-established in premeiotic cystocytes. Significantly, zygotic Dazl is not required for Vasa translation, but Dazl protein or successful cyst formation is required to re-establish perinuclear Vasa aggregates. In zebrafish, all premeiotic GCs express Vasa, but a subset also express nanos2; those expressing both are the presumed GSCs (Beer and Draper, 2013; Cao et al., 2019; Draper, 2017). Because all GCs seem to enter a cyst state in wild type from which Vasa+ premeiotic cells and a few nanos2+ GSCs emerge, and because GCs of both populations are lost in dazl mutants, it is tempting to speculate that the germline cyst not only serves to amplify premeiotic GC numbers but also plays a role in GSC specification. Work in mouse and pig similarly implicates dazl in commitment to germline fates (Nicholls et al., 2019), suggesting that whether PGCs are specified by maternal inheritance or are induced later, dazl plays an evolutionarily conserved role in commitment or specification of the germline after PGCs reach the gonad anlage.
A Dazl-mediated PGC to GSC transition
The PGCs in zebrafish are specified by inheritance of maternal factors and are maintained by maternal and zygotic programs. As the precursors for the GSC, PGCs are essential for germline fate. Nanos is a conserved marker of GSCs in zebrafish, medaka and mice (Nakamura et al., 2010; Sada et al., 2009; Aoki et al., 2009; Beer and Draper, 2013; Cao et al., 2019), and is required for the maintenance of GSCs in vertebrates and invertebrates (Forbes and Lehmann, 1998; Sada et al., 2009; Cao et al., 2019). In zebrafish, the related nanos3 contributes to GSC maintenance (Beer and Draper, 2013; Draper, 2017). Nanos family members in zebrafish are thought to prevent premature differentiation of stem cells in part by translational repression of meiotic factors like their counterparts in flies (Wang and Lin, 2004) and mice (Barrios et al., 2010; Suzuki et al., 2010). The mechanisms that establish GSCs in zebrafish remain unknown. Here, we show that all GCs are poised to develop as GSCs, based on induction of nanos2 in GCs, which had undergone a change in nuclear morphology. Despite initial expression, few germ cells achieve high nanos2 expression. Induction of nanos2 occurs independent of dazl, but restriction and acquisition of high nanos2 expression requires dazl. Therefore, Dazl is required to promote GSC fate. Future analysis is required to determine whether Dazl directly regulates nanos2 stability or promotes GSC fate by another mechanism, potentially via allocation of GSC versus cystoblast fate during the cystogenic divisions.
MATERIALS AND METHODS
Fish strains
dazlae57 and dazlae34 mutant fish strains were generated using CRISPR/Cas9 mutagenesis as previously described (Gagnon et al., 2014). dazlΔ7 was generated using ZFNs (Ekker, 2008; Foley et al., 2009b) as detailed below. Complementation tests were performed by intercrossing carriers of each dazl mutant allele. To generate double mutants, dazlae57 was crossed with tp53M214K or the chk2sa20350 allele, generated in The Sanger Institute's Zebrafish Mutation Project (Kettleborough et al., 2013) and obtained through the Zebrafish International Resource Center (ZIRC). Genomic DNA and cDNA from chk2sa20350 mutant tissues were sequenced to verify the genomic mutation and determine the transcript produced from the mutant allele (primers in Table S1). To visualize the GCs, mutations were crossed into the ziwi:GFP GC reporter line (Leu and Draper, 2010). All procedures and experimental protocols were performed in accordance with National Institutes of Health guidelines and were approved by the Einstein (protocol 20140502) and Icahn School of Medicine at Mount Sinai Institutional (ISMMS) Animal Care and Use Committees (IACUC, 2017-0114).
Dazl genotyping
Genomic DNA was obtained either from dissected adult trunk, fin clip or whole larvae. Samples were lysed in an alkaline lysis buffer [(25 mM NaOH and 0.2 mM EDTA (pH 12)], heated at 95°C for 20 min, then cooled to 4°C before the addition of neutralizing buffer [(20 mM Tris-HCl and 0.1 mM EDTA (pH 8.1)] (Truett et al., 2000). gDNA from dazlae57 or dazlae34 was PCR-amplified for 40 cycles with an annealing temperature of 59°C, followed by an Eco147I restriction enzyme digestion for 1 h (fast digest, Eco147I, Thermo Scientific). Undigested and digested products were resolved in a 3% MetaPhor 1:1 (Lonza)/agarose gel (Invitrogen) gel. Eco147I cuts the wild-type fragment. A PCR amplified fragment annealed at 57°C flanking the dazlΔ7 region was digested with AflIII enzyme and then resolved on a 3% MetaPhor/agarose gel. AflIII cuts the mutant fragment.
tp53M214K was identified as described previously (Berghmans et al., 2005). High resolution melt analysis assays were also developed for dazlae57, dazlae34, tp53M214K, chk2sa20350 and the dazldelta7 (described below) alleles (primers listed Table S1).
Mutagenesis
dazlae34 and dazlae57 alleles were generated by CRISPR/Cas9 mutagenesis as previously described (Gagnon et al., 2014). dazl single-guide RNAs (sgRNA) targeting exon 6 were designed using the CHOPCHOP web tool (Montague et al., 2014) (Table S1). Following annealing of the gene-specific target and the constant oligonucleotides, the fragment was filled using T4 DNA polymerase. The resulting fragment was transcribed and purified to yield sgRNA using the MEGAscript SP6 kit (Life Technologies, Ambion) (Jinek et al., 2012). The sgRNA [1 nl of 12.5 ng/µl of sgRNA and 1 nl of Cas9 protein (300 ng/µl)] was co-injected with phenol red (Sigma-Aldrich) at the one-cell stage. At 24 hpf, uninjected and injected embryos (n=8 each) were assayed by PCR amplification (primers listed in Table S1), followed by T7 endonuclease digest (Hwang et al., 2013), and those with new banding patterns were sequenced to confirm mutagenesis. Injected embryos were raised to adulthood, and individuals carrying mutations were identified by genotyping their progeny, as described above. Smaller bands compared with the wild-type allele, indicative of de novo mutations, were extracted from the gel, cloned into a PCR4 TOPO vector (Sigma-Aldrich) and sequenced in both directions to determine the mutated sequence. Fish harboring dazlae34 or dazlae57 mutations were outcrossed to AB fish. All mutations were verified by sequencing both gDNA and cDNA from mutant animals. Total RNA was extracted from pooled embryos from heterozygote intercrosses or AB strain wild type (n=20-30) using TRIzol (Life Technologies, 15596). cDNA was prepared using a SuperScript III/IV Reverse Transcription Kit (Life Technologies, 18080-051). RT-PCR was performed to amplify the dazl coding region using Easy-A High Fidelity Taq polymerase (Agilent, 600400) (primers listed in Table S1). The PCR fragments were TOPO cloned into pCR8/GW/TOPO (Invitrogen, K250020) and sequenced (Macrogen). Sequences were analyzed using Sequencher or MacVector software.
The dazlΔ7 allele, a 7 bp deletion resulting in a frame shift and subsequent premature stop codon at amino acid 54, was generated using ZFNs (Foley et al., 2009a). The dazl genomic region was PCR amplified and sequenced. ZFNs targeting the region in exon 2 just upstream of the RRM were purchased from Sigma-Aldrich and injected (500 pg) at the one-cell-stage into wild-type embryos. The dazl ZFN set (spacer is underlined) was as follows: 5′-GACGCCCAACACACTGTTCGTCGGCGGTATTG-3′.
Genomic lesions induced by ZFNs were subsequently identified by PCR amplification and restriction fragment length polymorphism analysis to identify founders carrying mutations in the germline. Recovered alleles were confirmed by sequencing. PCR primers to identify lesions were as follows: 5′-GTTCAGTTACCCGTGTGCCTGATAT-3′ and 3′-ATTAAACATTATAGTCCAATTAAACTAATCTGCATCA-5′.
Dissections
Fish were anesthetized with a lethal dose of tricaine (MS-22) (400 mg/l) and dissected. Fish were positioned laterally, and anteroposterior body length was measured before dissection. Images were acquired using an Olympus SZ61 dissecting microscope equipped with a high-resolution digital camera (model S97809) and Picture Frame 2.0 software.
Dazl antibodies
The YenZym custom antibody service was used to design, produce and raise chicken polyclonal antibodies against the following Dazl epitope: (residues 16-35) CYSQDIQKHRQGFPSSLKLSN–amide (YZ6741 and YZ6742).
Immunostaining
For whole-mount immunofluorescence-stained embryos or ovaries, tissues were fixed in 3.7% paraformaldehyde overnight at 4°C. The next day, samples were washed in PBS then dehydrated in MeOH and stored at −20°C. Chicken anti-Vasa antibody (Blokhina et al., 2019) was used at a 1:5000 dilution or rabbit anti-Vasa (SAB2702444, MilliporeSigma, 1:500), anti-β-catenin (C7202, Sigma-Aldrich, 1:500), anti-LaminB1 (Ab16048, abcam, 1:100), Dazl (YZ6741, 1:500). Alexa Fluor 488, Alexa Fluor 568, CY3 and C5 (Molecular Probes) secondary antibodies were diluted 1:500. For F-actin labeling, samples were fixed overnight at 4°C in 3.7% paraformaldehyde in actin stabilization buffer (Becker and Hart, 1999) and were then permeabilized in 2% Triton X-100. Following the primary antibody solution, Alexa Fluor 568 phalloidin was added. Samples were mounted in Vectashield with DAPI and images were acquired using a Zeiss Axio Observer inverted microscope equipped with ApotomeII and a charged-coupled device (CCD) camera, a Zeiss Zoom dissecting scope equipped with ApotomeII, or a Leica SP5 DMI or SP8 DMI at the Microscopy CoRE at the Icahn School of Medicine Mount Sinai, NY, USA. Image processing was performed using Zenpro (Zeiss), Leica Application Suite, ImageJ/FIJI, Adobe Photoshop, Adobe Illustrator and Imaris (Oxford Instruments). Confocal images were acquired using a Leica SP5 DMI with a 40× or 63× objective. The acquisition setting was set between sample and experiments to XY resolution 1024×1024 (or 512×512 as indicated), zoom 2.5x, pinhole was adjusted to 1.1 µm of the z thickness and increments between images in stacks were 0.2 µm. Laser power and gain were set for each antibody or fluorescent compound to 2-10% below saturation condition.
Fluorescent in situ hybridization
Fluorescent in situ hybridization (RNAscope) was performed using the Multiplex Fluorescent Reagent Kit v2 (ACD Bio, 323100), according to the whole-mount RNAscope protocol described previously (Campbell et al., 2015). Animal trunks were fixed overnight in 4% paraformaldehyde at 4°C, washed with PBT (0.1% Tween), dehydrated in MeOH and placed at −20°C overnight. RNAscope Blank C1 probe, Dr-nanos2-C2 probe (ACD Bio, 843461-C2) and Dr-dazl-C3 probe (ACD Bio, 469261-C3) were used. The signal was amplified using Opal 570 (Akoya Biosciences, FP1488001KT) for Dr-nanos2-C2 probe and Opal 690 (FP1497001KT) for Dr-dazl-C3 probe, respectively. Fluorescent in situ hybridization was followed by immunostaining for Vasa as described above and DNA was labeled by DAPI.
Electron microscopy
Fish were euthanized in tricaine and trunks were immediately fixed in Karnovsky's solution (2% glutaraldehyde and 2% paraformaldehyde in 0.1 M sodium cacodylate) for at least 24 h. The samples were then washed with cacodylate buffer, osmicated with 1% osmium tetroxide for 1 h, and en bloc stained with 2% uranyl acetate for 1 h. After a quick rinse in water and seven 10-min incremental ethanol dehydration steps (25%-100%), samples were infiltrated with a mixture of propylene oxide and an epoxy resin (Epon, Electron Microscopy Sciences). Samples were polymerized in pure resin in a vacuum oven at 60°C for 72 h. Semi-thin sections (2 µm) were cut until the gonadal zone was reached. Five consecutive ultrathin sections were cut at 90 nm using a diamond knife (Diatome) on an ultramicrotome (Leica EM UC7) and mounted onto a formvar-supported slot grid (Electron Microscopy Sciences). Serial sections were imaged using a Hitachi H7500 TEM at 75 kV and 2048X2048 pixel, and 16 bit images were taken using a CCD camera (AMT Imaging).
Germ cell, cyst and early oocyte quantification
Vasa protein was used as a marker to identify and count individual GCs. Z-series stacks of gonads at each stage were obtained using a Zeiss Axio Observer inverted microscope equipped with ApotomeII and a CCD camera, a Zeiss Zoom dissecting scope equipped with ApotomeII, Leica SP5 DMI or Leica SP8 DMI at the Microscopy CoRE at the ISMMS. Cells were manually counted by analyzing each slice within each z-stack for Vasa positivity and nuclear morphology (DAPI), defining each category during the cystogenesis process and the number of cells per cyst. Quantification of actin-rich structures was performed by counting each actin-rich structure through the z-stack. Cell area and volume was measured after a manual segmentation of the cell in each plane through the z-stack. Quantification of nanos2 or dazl foci was performed manually by counting each corresponding dot in each Vasa+ cell in the gonad for the specified stages. In the case of the transition/amplification stage, only the foci surrounding the nucleus were counted because cell boundaries between cells were not well defined. Image analysis for all experiments above were performed using ZEN pro (Zeiss), Leica Application Suite, ImageJ/FIJI or Imaris (Oxford instruments).
Statistical analysis
Statistical differences were assessed using GraphPad Prism software and paired two-tailed Student's t-test. Significant differences are indicated in figures by asterisks (*P<0.01; **P<0.05; ***P<0.001; n.s., not significant).
Supplementary Material
Acknowledgements
We thank members of the Marlow lab for helpful discussions, our animal care staff for fish care (Einstein and Center for Comparative Medicine at ISMMS) and the Microscopy CoRE at the Icahn School of Mount Sinai. We thank Dr Derek Stemple and the Zebrafish Mutation Project for providing chk2sa20350 mutants and generating this valuable community resource, and the Zebrafish Information Network and ZIRC for curating and making this resource available to the community.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: S.B., M.C., T.U.B., J.B., E.R., F.L.M.; Methodology: S.B., T.U.B., J.B., E.R., F.L.M.; Validation: S.B., F.L.M.; Formal analysis: S.B., M.C., T.U.B., J.B., F.L.M.; Investigation: S.B., M.C., T.U.B., J.B., F.L.M.; Resources: E.R., F.L.M.; Data curation: S.B., M.C., T.U.B., J.B., F.L.M.; Writing - original draft: S.B., F.L.M.; Writing - review & editing: S.B., M.C., T.U.B., J.B., E.R., F.L.M.; Visualization: S.B., F.L.M.; Supervision: E.R., F.L.M.; Project administration: S.B., F.L.M.; Funding acquisition: E.R., F.L.M.
Funding
Work in the Marlow lab is supported by the National Institutes of Health (R01-GM089979 to F.L.M.). M.C. was supported by an REI fellowship. Work in the Raz lab is supported by the Deutsche Forschungsgemeinschaft (Clinical Research Unit 326, Male germ cells). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at https://dev.biologists.org/lookup/doi/10.1242/dev.187773.supplemental
Peer review history
The peer review history is available online at https://dev.biologists.org/lookup/doi/10.1242/dev.187773.reviewer-comments.pdf
References
- Abdu, U., Brodsky, M. and Schüpbach, T. (2002). Activation of a meiotic checkpoint during Drosophila oogenesis regulates the translation of Gurken through Chk2/Mnk. Curr. Biol. 12, 1645-1651. 10.1016/S0960-9822(02)01165-X [DOI] [PubMed] [Google Scholar]
- Almonacid, M., Al Jord, A., El-Hayek, S., Othmani, A., Coulpier, F., Lemoine, S., Miyamoto, K., Grosse, R., Klein, C., Piolot, T.et al. (2019). Active fluctuations of the nuclear envelope shape the transcriptional dynamics in oocytes. Dev. Cell 51, 145-157.e10. 10.1016/j.devcel.2019.09.010 [DOI] [PubMed] [Google Scholar]
- Alphey, L., Jimenez, J., White-Cooper, H., Dawson, I., Nurse, P. and Glover, D. M. (1992). Twine, a cdc25 homolog that functions in the male and female germline of Drosophila. Cell 69, 977-988. 10.1016/0092-8674(92)90616-K [DOI] [PubMed] [Google Scholar]
- Aoki, Y., Nakamura, S., Ishikawa, Y. and Tanaka, M. (2009). Expression and syntenic analysis of four nanos genes in Melaka. Zoolog. Sci.26, 112-118. 10.2108/zsj.26.112 [DOI]
- Aureille, J., Buffière-Ribot, V., Harvey, B. E., Boyault, C., Pernet, L., Andersen, T., Bacola, G., Balland, M., Fraboulet, S., Van Landeghem, L.et al. , (2019). Nuclear envelope deformation controls cell cycle progression in response to mechanical force. EMBO Rep. 20, e48084. 10.15252/embr.201948084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios, F., Filipino, D., Pellegrini, M., Paronetto, M. P., Di Siena, S., Geremia, R., Rossi, P., De Felici, M., Jeannine, E.A. and Dolci, S. (2010). Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J. Cell Sci.123, 871-880. 10.1242/jcs.057968 [DOI] [PubMed]
- Barton, L. J., LeBlanc, M. G. and Lehmann, R. (2016). Finding their way: themes in germ cell migration. Curr. Opin. Cell Biol. 42, 128-137. 10.1016/j.ceb.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, K. A. and Hart, N. H. (1999). Reorganization of filamentous actin and myosin-II in zebrafish eggs correlates temporally and spatially with cortical granule exocytosis. J. Cell Sci. 112, 97-110. [DOI] [PubMed] [Google Scholar]
- Beer, R. L. and Draper, B. W. (2013). nanos3 maintains germline stem cells and expression of the conserved germline stem cell gene nanos2 in the zebrafish ovary. Dev. Biol. 374, 308-318. 10.1016/j.ydbio.2012.12.003 [DOI] [PubMed] [Google Scholar]
- Berghmans, S., Murphey, R. D., Wienholds, E., Neuberg, D., Kutok, J. L., Fletcher, C. D., Morris, J. P., Liu, T. X., Schulte-Merker, S., Kanki, J. P.et al. , (2005). tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc. Natl. Acad. Sci. USA 102, 407-412. 10.1073/pnas.0406252102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokhina, Y. P., Nguyen, A. D., Draper, B. W. and Burgess, S. M. (2019). The telomere bouquet is a hub where meiotic double-strand breaks, synapsis, and stable homolog juxtaposition are coordinated in the zebrafish, Danio rerio. PLoS Genet. 15, e1007730. 10.1371/journal.pgen.1007730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolcun-Filas, E., Rinaldi, V. D., White, M. E. and Schimenti, J. C. (2014). Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science 343, 533-536. 10.1126/science.1247671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braat, A. K., Speksnijder, J. E. and Zivkovic, D. (1999). Germ line development in fishes. Int. J. Dev. Biol. 43, 745-760. [PubMed] [Google Scholar]
- Braun, R. E., Behringer, R. R., Peschon, J. J., Brinster, R. L. and Palmiter, R. D. (1989). Genetically haploid spermatids are phenotypically diploid. Nature 337, 373-376. 10.1038/337373a0 [DOI] [PubMed] [Google Scholar]
- Brown, E. H. and King, R. C. (1964). Studies on the events resulting in the formation of an egg chamber in Drosophila melanogaster. Growth 28, 41-81. [PubMed] [Google Scholar]
- Campbell, P. D., Heim, A. E., Smith, M. Z. and Marlow, F. L. (2015). Kinesin I interacts with Bucky ball to form germ cells and is required to pattern the zebrafish body axis. Development142, 2996-3008. 10.1242/dev.124586 [DOI]
- Cao, Z., Mao, X. and Luo, L. (2019). Germline stem cells drive ovary regeneration in Zebrafish. Cell Rep. 26, 1709-1717.e1703. 10.1016/j.celrep.2019.01.061 [DOI] [PubMed] [Google Scholar]
- Chen, H. H., Welling, M., Bloch, D. B., Munoz, J., Mientjes, E., Chen, X., Tramp, C., Wu, J., Yabuuchi, A., Chou, Y. F.et al. , (2014). DAZL limits pluripotency, differentiation, and apoptosis in developing primordial germ cells. Stem Cell Rep. 3, 892-904. 10.1016/j.stemcr.2014.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley, L. and Theurkauf, W. E. (1994). Cytoskeletal functions during Drosophila oogenesis. Science 266, 590-596. 10.1126/science.7939713 [DOI] [PubMed] [Google Scholar]
- Courtot, C., Fankhauser, C., Simanis, V. and Lehner, C. F. (1992). The Drosophila cdc25 homolog twine is required for meiosis. Development 116, 405-416. [DOI] [PubMed] [Google Scholar]
- Cox, R. T. and Spradling, A. C. (2003). A Balbiani body and the fusome mediate mitochondrial inheritance during Drosophila oogenesis. Development 130, 1579-1590. 10.1242/dev.00365 [DOI] [PubMed] [Google Scholar]
- Dai, X., Jin, X., Chen, X., He, J. and Yin, Z. (2015). Sufficient numbers of early germ cells are essential for female sex development in zebrafish. PLoS ONE 10, e0117824. 10.1371/journal.pone.0117824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cuevas, M., Lilly, M. A. and Spradling, A. C. (1997). Germline cyst formation in Drosophila. Annu. Rev. Genet. 31, 405-428. 10.1146/annurev.genet.31.1.405 [DOI] [PubMed] [Google Scholar]
- Deng, W. and Lin, H. (1997). Spectrosomes and fusomes anchor mitotic spindles during asymmetric germ cell divisions and facilitate the formation of a polarized microtubule array for oocyte specification in Drosophila. Dev. Biol. 189, 79-94. 10.1006/dbio.1997.8669 [DOI] [PubMed] [Google Scholar]
- Djagaeva, I., Doronkin, S. and Beckendorf, S. K. (2005). Src64 is involved in fusome development and karyosome formation during Drosophila oogenesis. Dev. Biol. 284, 143-156. 10.1016/j.ydbio.2005.05.012 [DOI] [PubMed] [Google Scholar]
- Draper, B. W. (2017). Identification of germ-line stem cells in Zebrafish. Methods Mol. Biol. 1463, 103-113. 10.1007/978-1-4939-4017-2_8 [DOI] [PubMed] [Google Scholar]
- Draper, B. W., McCallum, C. M. and Moens, C. B. (2007). nanos1 is required to maintain oocyte production in adult zebrafish. Dev. Biol.305, 589-598. 10.1016/j.ydbio.2007.03.007 [DOI]
- Eberhart, C. G., Maines, J. Z. and Wasserman, S. A. (1996). Meiotic cell cycle requirement for a fly homologue of human Deleted in Azoospermia. Nature 381, 783-785. 10.1038/381783a0 [DOI] [PubMed] [Google Scholar]
- Ekker, S. C. (2008). Zinc finger-based knockout punches for zebrafish genes. Zebrafish 5, 121-123. 10.1089/zeb.2008.9988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Extavour, C. G. and Akam, M. (2003). Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development 130, 5869-5884. 10.1242/dev.00804 [DOI] [PubMed] [Google Scholar]
- Farrell, J. A., Wang, Y., Riesenfeld, S. J., Shekhar, K., Regev, A. and Schier, A. F. (2018). Single-cell reconstruction of developmental trajectories during zebrafish embryogenesis. Science 360, eaar3131. 10.1126/science.aar3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett, D. W., Ito, S. and Slautterback, D. (1959). The occurrence of intercellular bridges in groups of cells exhibiting synchronous differentiation. J. Biophys. Biochem. Cytol. 5, 453-460. 10.1083/jcb.5.3.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley, J. E., Maeder, M. L., Pearlberg, J., Joung, J. K., Peterson, R. T. and Yeh, J. R. (2009a). Targeted mutagenesis in zebrafish using customized zinc-finger nucleases. Nat. Protoc. 4, 1855-1867. 10.1038/nprot.2009.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley, J. E., Yeh, J. R., Maeder, M. L., Reyon, D., Sander, J. D., Peterson, R. T. and Joung, J. K. (2009b). Rapid mutation of endogenous zebrafish genes using zinc finger nucleases made by Oligomerized Pool ENgineering (OPEN). PloS one 4, e4348. 10.1371/journal.pone.0004348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes, A. and Lehman, R. (1998). Nanos and Pumilio have critical roles in the development and function of Drosophila germ line stem cells. Development125, 679-690. https://dev.biologists.org/content/125/4/679.long [DOI] [PubMed]
- Fu, X. F., Cheng, S. F., Wang, L. Q., Yin, S., De Felici, M. and Shen, W. (2015). DAZ Family proteins, key players for germ cell development. Int. J. Biol. Sci. 11, 1226-1235. 10.7150/ijbs.11536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, K., Masuda, A., Naka, T., Suzuki, A., Kato, Y. and Saga, Y. (2018). Requirement of the 3'-UTR-dependent suppression of Dazl in oocytes for pre-implantation mouse development. PLoS Genet. 14, e1007436. 10.1371/journal.pgen.1007436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon, J. A., Valen, E., Thyme, S. B., Huang, P., Ahkmetova, L., Pauli, A., Montague, T. G., Zimmerman, S., Richter, C. and Schier, A. F. (2014). Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PLoS ONE 9, e98186. 10.1371/journal.pone.0098186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, M. E., Hu, Y.-C., Lin, Y. and Page, D. C. (2011). Licensing of gametogenesis, dependent on RNA binding protein DAZL, as a gateway to sexual differentiation of fetal germ cells. Proc. Natl. Acad. Sci. USA 108, 7443-7448. 10.1073/pnas.1104501108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg, M. (1994). Primordial germ cell formation in birds. Ciba Found Symp. 182, 52-61; discussion 61–57. 10.1002/9780470514573.ch4 [DOI] [PubMed] [Google Scholar]
- Greenbaum, M. P., Yan, W., Wu, M.-H., Lin, Y.-N., Agno, J. E., Sharma, M., Braun, R. E., Rajkovic, A. and Matzuk, M. M. (2006). TEX14 is essential for intercellular bridges and fertility in male mice. Proc. Natl. Acad. Sci. USA 103, 4982-4987. 10.1073/pnas.0505123103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum, M. P., Iwamori, T., Buchold, G. M. and Matzuk, M. M. (2011). Germ cell intercellular bridges. Cold Spring Harb. Perspect Biol. 3, a005850. 10.1101/cshperspect.a005850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Thebing, T., Yigit, S., Pfeiffer, J., Reichman-Fried, M., Bandemer, J., Ruckert, C., Rathmer, C., Goudarzi, M., Stehling, M., Tarbashevich, K.et al. , (2017). The vertebrate protein dead end maintains primordial germ cell fate by inhibiting somatic differentiation. Dev. Cell 43, 704-715.e705. 10.1016/j.devcel.2017.11.019 [DOI] [PubMed] [Google Scholar]
- Haglund, K., Nezis, I. P. and Stenmark, H. (2011). Structure and functions of stable intercellular bridges formed by incomplete cytokinesis during development. Commun. Integr. Biol. 4, 1-9. 10.4161/cib.13550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto, Y., Maegawa, S., Nagai, T., Yamaha, E., Suzuki, H., Yasuda, K. and Inoue, K. (2004). Localized maternal factors are required for zebrafish germ cell formation. Dev. Biol. 268, 152-161. 10.1016/j.ydbio.2003.12.013 [DOI] [PubMed] [Google Scholar]
- Haston, K. M., Tung, J. Y. and Reijo Pera, R. A. (2009). Dazl functions in maintenance of pluripotency and genetic and epigenetic programs of differentiation in mouse primordial germ cells in vivo and in vitro. PLoS ONE 4, e5654. 10.1371/journal.pone.0005654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hime, G. R., Brill, J. A. and Fuller, M. T. (1996). Assembly of ring canals in the male germ line from structural components of the contractile ring. J. Cell Sci. 109, 2779-2788. [DOI] [PubMed] [Google Scholar]
- Houston, D. W. and King, M. L. (2000). A critical role for Xdazl, a germ plasm-localized RNA, in the differentiation of primordial germ cells in Xenopus. Development 127, 447-456. [DOI] [PubMed] [Google Scholar]
- Howley, C. and Ho, R. K. (2000). mRNA localization patterns in zebrafish oocytes. Mech. Dev. 92, 305-309. 10.1016/S0925-4773(00)00247-1 [DOI] [PubMed] [Google Scholar]
- Hu, Y. C., Nicholls, P. K., Soh, Y. Q., Daniele, J. R., Junker, J. P., van Oudenaarden, A. and Page, D. C. (2015). Licensing of primordial germ cells for gametogenesis depends on genital ridge signaling. PLoS Genet. 11, e1005019. 10.1371/journal.pgen.1005019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulspas, R., Houtsmuller, A. B., Bauman, J. G. and Nanninga, N. (1994). The centrosome moves out of a nuclear indentation in human lymphocytes upon activation. Exp. Cell Res. 215, 28-32. 10.1006/excr.1994.1310 [DOI] [PubMed] [Google Scholar]
- Hwang, W. Y., Fu, Y., Reyon, D., Maeder, M. L., Kaini, P., Sander, J. D., Joung, J. K., Peterson, R. T. and Yeh, J. R. (2013). Heritable and precise zebrafish genome editing using a CRISPR-Cas system. PLoS ONE 8, e68708. 10.1371/journal.pone.0068708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illmensee, K. and Mahowald, A. P. (1976). The autonomous function of germ plasm in a somatic region of the Drosophila egg. Exp. Cell Res. 97, 127-140. 10.1016/0014-4827(76)90662-5 [DOI] [PubMed] [Google Scholar]
- Illmensee, K., Mahowald, A. P. and Loomis, M. R. (1976). The ontogeny of germ plasm during oogenesis in Drosophila. Dev. Biol. 49, 40-65. 10.1016/0012-1606(76)90257-8 [DOI] [PubMed] [Google Scholar]
- Iyer, H., Issigonis, M., Sharma, P. P., Extavour, C. G. and Newmark, P. A. (2016). A premeiotic function for boule in the planarian Schmidtea mediterranea. Proc. Natl. Acad. Sci. USA 113, E3509-E3518. 10.1073/pnas.1521341113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J. A. and Charpentier, E. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816-821. 10.1126/science.1225829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, D., Xiong, J., Ye, M., Qin, X., Li, L., Cheng, S., Luo, M., Peng, J., Dong, J., Tang, F.et al. , (2017). In vitro differentiation of human embryonic stem cells into ovarian follicle-like cells. Nat. Commun. 8, 15680. 10.1038/ncomms15680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima, T., Sugimoto, A. and Yamamoto, M. (2000). Caenorhabditis elegans homologue of the human azoospermia factor DAZ is required for oogenesis but not for spermatogenesis. Development 127, 1069-1079. [DOI] [PubMed] [Google Scholar]
- Kettleborough, R. N., Busch-Nentwich, E. M., Harvey, S. A., Dooley, C. M., de Bruijn, E., van Eeden, F., Sealy, I., White, R. J., Herd, C., Nijman, I. J.et al. , (2013). A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 496, 494-497. 10.1038/nature11992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, B., Cooke, H. J. and Rhee, K. (2012). DAZL is essential for stress granule formation implicated in germ cell survival upon heat stress. Development 139, 568-578. 10.1242/dev.075846 [DOI] [PubMed] [Google Scholar]
- Kim, H. J., Yoon, J., Matsuura, A., Na, J. H., Lee, W. K., Kim, H., Choi, J. W., Park, J. E., Park, S. J., Kim, K. T.et al. , (2015). Structural and biochemical insights into the role of testis-expressed gene 14 (TEX14) in forming the stable intercellular bridges of germ cells. Proc. Natl. Acad. Sci. USA 112, 12372-12377. 10.1073/pnas.1418606112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloc, M., Bilinski, S., Dougherty, M. T., Brey, E. M. and Etkin, L. D. (2004). Formation, architecture and polarity of female germline cyst in Xenopus. Dev. Biol. 266, 43-61. 10.1016/j.ydbio.2003.10.002 [DOI] [PubMed] [Google Scholar]
- Knaut, H., Pelegri, F., Bohmann, K., Schwarz, H. and Nusslein-Volhard, C. (2000). Zebrafish vasa RNA but not its protein is a component of the germ plasm and segregates asymmetrically before germline specification. J. Cell Biol. 149, 875-888. 10.1083/jcb.149.4.875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, E. A. and King, R. C. (1966). The origin and early differentiation of the egg chamber of Drosophila melanogaster. J. Morphol. 119, 283-303. 10.1002/jmor.1051190303 [DOI] [PubMed] [Google Scholar]
- Koch, E. A. and King, R. C. (1969). Further studies on the ring canal system of the ovarian cystocytes of Drosophila melanogaster. Z Zellforsch Mikrosk Anat 102, 129-152. 10.1007/BF00336421 [DOI] [PubMed] [Google Scholar]
- Koch, E. A., Smith, P. A. and King, R. C. (1967). The division and differentiation of Drosophila cystocytes. J. Morphol. 121, 55-70. 10.1002/jmor.1051210106 [DOI] [PubMed] [Google Scholar]
- Kosaka, K., Kawakami, K., Sakamoto, H. and Inoue, K. (2007). Spatiotemporal localization of germ plasm RNAs during zebrafish oogenesis. Mech. Dev. 124, 279-289. 10.1016/j.mod.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Kossack, M. E. and Draper, B. W. (2019). Genetic regulation of sex determination and maintenance in zebrafish (Danio rerio). Curr. Top. Dev. Biol. 134, 119-149. 10.1016/bs.ctdb.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, K. A., Dunn, N. R., Roelen, B. A., Zeinstra, L. M., Davis, A. M., Wright, C. V., Korving, J. P. and Hogan, B. L. (1999). Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 13, 424-436. 10.1101/gad.13.4.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, K. A. and Hage, W. J. (1994). Clonal analysis of the origin of primordial germ cells in the mouse. Ciba Foundation Symposium 182, 68-84; discussion 84–91. 10.1002/9780470514573.ch5 [DOI] [PubMed] [Google Scholar]
- Leerberg, D. M., Sano, K. and Draper, B. W. (2017). Fibroblast growth factor signaling is required for early somatic gonad development in zebrafish. PLoS Genet. 13, e1006993. 10.1371/journal.pgen.1006993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGrand, E. K. (2001). Genetic conflict and apoptosis. Perspect. Biol. Med. 44, 509-521. 10.1353/pbm.2001.0066 [DOI] [PubMed] [Google Scholar]
- Lei, L. and Spradling, A. C. (2013). Mouse primordial germ cells produce cysts that partially fragment prior to meiosis. Development 140, 2075-2081. 10.1242/dev.093864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, L. and Spradling, A. C. (2016). Mouse oocytes differentiate through organelle enrichment from sister cyst germ cells. Science 352, 95-99. 10.1126/science.aad2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu, D. H. and Draper, B. W. (2010). The ziwi promoter drives germline-specific gene expression in zebrafish. Dev. Dyn. 239, 2714-2721. 10.1002/dvdy.22404 [DOI] [PubMed] [Google Scholar]
- Li, H., Liang, Z., Yang, J., Wang, D., Wang, H., Zhu, M., Geng, B. and Xu, E. Y. (2019). DAZL is a master translational regulator of murine spermatogenesis. Natl. Sci. Rev. 6, 455-468. 10.1093/nsr/nwy163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M. Y., Zhu, F., Li, Z. D., Hong, N. and Hong, Y. H. (2016). Dazl is a critical player for primordial germ cell formation in medaka. Sci Rep-Uk 6, 411-421. 10.1038/srep28317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthouse, D. V., Buszczak, M. and Spradling, A. C. (2008). New components of the Drosophila fusome suggest it plays novel roles in signaling and transport. Dev. Biol. 317, 59-71. 10.1016/j.ydbio.2008.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, H. and Spradling, A. C. (1993). Germline stem cell division and egg chamber development in transplanted Drosophila germaria. Dev. Biol. 159, 140-152. 10.1006/dbio.1993.1228 [DOI] [PubMed] [Google Scholar]
- Lin, H. and Spradling, A. C. (1995). Fusome asymmetry and oocyte determination in Drosophila. Dev. Genet. 16, 6-12. 10.1002/dvg.1020160104 [DOI] [PubMed] [Google Scholar]
- Lin, H., Yue, L. and Spradling, A. C. (1994). The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development 120, 947-956. [DOI] [PubMed] [Google Scholar]
- Lin, Y., Gill, M. E., Koubova, J. and Page, D. C. (2008). Germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science 322, 1685-1687. 10.1126/science.1166340 [DOI] [PubMed] [Google Scholar]
- Maegawa, S., Yasuda, K. and Inoue, K. (1999). Maternal mRNA localization of zebrafish DAZ-like gene. Mech. Dev. 81, 223-226. 10.1016/S0925-4773(98)00242-1 [DOI] [PubMed] [Google Scholar]
- Maegawa, S., Yamashita, M., Yasuda, K. and Inoue, K. (2002). Zebrafish DAZ-like protein controls translation via the sequence ‘GUUC’. Genes Cells 7, 971-984. 10.1046/j.1365-2443.2002.00576.x [DOI] [PubMed] [Google Scholar]
- Mahowald, A. P. (1971). The formation of ring canals by cell furrows in Drosophila. Z. Zellforsch Mikrosk Anat. 118, 162-167. 10.1007/BF00341561 [DOI] [PubMed] [Google Scholar]
- Maines, J. Z. and Wasserman, S. A. (1999). Post-transcriptional regulation of the meiotic Cdc25 protein Twine by the Dazl orthologue Boule. Nat. Cell Biol. 1, 171-174. 10.1038/11091 [DOI] [PubMed] [Google Scholar]
- Marlow, F. (2015). Primordial germ cell specification and migration. F1000Res 4, F1000 Faculty Rev-1462. 10.12688/f1000research.6995.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow, F. L. and Mullins, M. C. (2008). Bucky ball functions in Balbiani body assembly and animal-vegetal polarity in the oocyte and follicle cell layer in zebrafish. Dev. Biol. 321, 40-50. 10.1016/j.ydbio.2008.05.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano, J. V., Ramathal, C., Nguyen, H. N., Simon, C. and Reijo Pera, R. A. (2012). Divergent RNA-binding proteins, DAZL and VASA, induce meiotic progression in human germ cells derived in vitro. Stem Cells 30, 441-451. 10.1002/stem.1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao, L., Yuan, Y., Cheng, F., Fang, J., Zhou, F., Ma, W., Jiang, Y., Huang, X., Wang, Y., Shan, L.et al. , (2017). Translation repression by maternal RNA binding protein Zar1 is essential for early oogenesis in zebrafish. Development 144, 128-138. 10.1242/dev.144642 [DOI] [PubMed] [Google Scholar]
- Mikedis, M. M., Fan, Y., Nicholls, P. K., Endo, T., Jackson, E. K., Cobb, S. A., de Rooij, D. G. and Page, D. C. (2020). DAZL mediates a broad translational program regulating expansion and differentiation of spermatogonial progenitors. eLife 9, e56523. 10.7554/eLife.56523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague, T. G., Cruz, J. M., Gagnon, J. A., Church, G. M. and Valen, E. (2014). CHOPCHOP: a CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 42, W401-W407. 10.1093/nar/gku410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita, E., Sandrin, V., Chung, H. Y., Morham, S. G., Gygi, S. P., Rodesch, C. K. and Sundquist, W. I. (2007). Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 26, 4215-4227. 10.1038/sj.emboj.7601850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, S., Kobayashi, K., Nishimura, T., Higashijima, S. and Tanaka, M. (2010). Identification of germline stem cells in the ovary of the teleost medaka. Science 328, 1561-1563. 10.1126/science.1185473 [DOI] [PubMed] [Google Scholar]
- Nicholls, P. K., Schorle, H., Naqvi, S., Hu, Y. C., Fan, Y., Carmell, M. A., Dobrinski, I., Watson, A. L., Carlson, D. F., Fahrenkrug, S. C.et al. (2019). Mammalian germ cells are determined after PGC colonization of the nascent gonad. Proc. Natl. Acad. Sci. USA 116, 25677-25687. 10.1073/pnas.1910733116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop, P. D. and Sutasurya, L. A. (1979). Primordial Germ Cells in the Chordates: Embryogenesis and Phylogenesis. Cambridge Eng; New York: Cambridge University Press. [Google Scholar]
- Nishimura, T., Yamada, K., Fujimori, C., Kikuchi, M., Kawasaki, T., Siegfried, K. R., Sakai, N. and Tanaka, M. (2018). Germ cells in the teleost fish medaka have an inherent feminizing effect. PLoS Genet. 14, e1007259. 10.1371/journal.pgen.1007259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban, L., Sreenivasan, R. and Olsson, P. E. (2009). Long and winding roads: testis differentiation in zebrafish. Mol. Cell. Endocrinol. 312, 35-41. 10.1016/j.mce.2009.04.014 [DOI] [PubMed] [Google Scholar]
- Paksa, A. and Raz, E. (2015). Zebrafish germ cells: motility and guided migration. Curr. Opin. Cell Biol. 36, 80-85. 10.1016/j.ceb.2015.07.007 [DOI] [PubMed] [Google Scholar]
- Pepling, M. E. and Spradling, A. C. (1998). Female mouse germ cells form synchronously dividing cysts. Development 125, 3323-3328. [DOI] [PubMed] [Google Scholar]
- Pradhan, A., Khalaf, H., Ochsner, S. A., Sreenivasan, R., Koskinen, J., Karlsson, M., Karlsson, J., McKenna, N. J., Orban, L. and Olsson, P. E. (2012). Activation of NF-kappaB protein prevents the transition from juvenile ovary to testis and promotes ovarian development in zebrafish. J. Biol. Chem. 287, 37926-37938. 10.1074/jbc.M112.386284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz, E. (2003). Primordial germ-cell development: the zebrafish perspective. Nat. Rev. Genet. 4, 690-700. 10.1038/nrg1154 [DOI] [PubMed] [Google Scholar]
- Reynolds, N., Collier, B., Maratou, K., Bingham, V., Speed, R. M., Taggart, M., Semple, C. A., Gray, N. K. and Cooke, H. J. (2005). Dazl binds in vivo to specific transcripts and can regulate the pre-meiotic translation of Mvh in germ cells. Hum. Mol. Genet. 14, 3899-3909. 10.1093/hmg/ddi414 [DOI] [PubMed] [Google Scholar]
- Robinson, D. N. and Cooley, L. (1996). Stable intercellular bridges in development: the cytoskeleton lining the tunnel. Trends Cell Biol. 6, 474-479. 10.1016/0962-8924(96)84945-2 [DOI] [PubMed] [Google Scholar]
- Robinson, D. N. and Cooley, L. (1997). Genetic analysis of the actin cytoskeleton in the Drosophila ovary. Annu. Rev. Cell Dev. Biol. 13, 147-170. 10.1146/annurev.cellbio.13.1.147 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mari, A., Canestro, C., Bremiller, R. A., Nguyen-Johnson, A., Asakawa, K., Kawakami, K. and Postlethwait, J. H. (2010). Sex reversal in zebrafish fancl mutants is caused by Tp53-mediated germ cell apoptosis. PLoS Genet. 6, e1001034. 10.1371/journal.pgen.1001034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Mari, A., Canestro, C., BreMiller, R. A., Catchen, J. M., Yan, Y. L. and Postlethwait, J. H. (2013). Retinoic acid metabolic genes, meiosis, and gonadal sex differentiation in zebrafish. PLoS ONE 8, e73951. 10.1371/journal.pone.0073951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario, R., Smith, R. W., Adams, I. R. and Anderson, R. A. (2017). RNA immunoprecipitation identifies novel targets of DAZL in human foetal ovary. Mol. Hum. Reprod. 23, 177-186. 10.1093/molehr/gax004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario, R., Crichton, J. H., Stewart, H. L., Childs, A. J., Adams, I. R. and Anderson, R. A. (2019). Dazl determines primordial follicle formation through the translational regulation of Tex14. FASEB J. 33, 14221-14233. 10.1096/fj.201901247R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiu, M., Speed, R., Taggart, M., McKay, S. J., Kilanowski, F., Saunders, P., Dorin, J. and Cooke, H. J. (1997). The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature 389, 73-77. 10.1038/37987 [DOI] [PubMed] [Google Scholar]
- Sada, A., Suzuki, A., Suzuki, H. and Saga, Y. (2009). The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science325, 1394-1398. 10.1126/science.1172645 [DOI]
- Saito, D. and Tanaka, M. (2009). Comparative aspects of gonadal sex differentiation in medaka: a conserved role of developing oocytes in sexual canalization. Sex Dev. 3, 99-107. 10.1159/000223075 [DOI] [PubMed] [Google Scholar]
- Saito, D., Morinaga, C., Aoki, Y., Nakamura, S., Mitani, H., Furutani-Seiki, M., Kondoh, H. and Tanaka, M. (2007). Proliferation of germ cells during gonadal sex differentiation in medaka: Insights from germ cell-depleted mutant zenzai. Dev. Biol. 310, 280-290. 10.1016/j.ydbio.2007.07.039 [DOI] [PubMed] [Google Scholar]
- Saunders, P. T., Turner, J. M., Ruggiu, M., Taggart, M., Burgoyne, P. S., Elliott, D. and Cooke, H. J. (2003). Absence of mDazl produces a final block on germ cell development at meiosis. Reproduction 126, 589-597. 10.1530/rep.0.1260589 [DOI] [PubMed] [Google Scholar]
- Schrans-Stassen, B. H., Saunders, P. T., Cooke, H. J. and de Rooij, D. G. (2001). Nature of the spermatogenic arrest in Dazl -/- mice. Biol. Reprod. 65, 771-776. 10.1095/biolreprod65.3.771 [DOI] [PubMed] [Google Scholar]
- Seidel, H. S., Smith, T. A., Evans, J. K., Stamper, J. Q., Mast, T. G. and Kimble, J. (2018). C. elegans germ cells divide and differentiate in a folded tissue. Dev. Biol. 442, 173-187. 10.1016/j.ydbio.2018.07.013 [DOI] [PubMed] [Google Scholar]
- Seirin-Lee, S., Osakada, F., Takeda, J., Tashiro, S., Kobayashi, R., Yamamoto, T. and Ochiai, H. (2019). Role of dynamic nuclear deformation on genomic architecture reorganization. PLoS Comput. Biol. 15, e1007289. 10.1371/journal.pcbi.1007289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shive, H. R., West, R. R., Embree, L. J., Azuma, M., Sood, R., Liu, P. and Hickstein, D. D. (2010). brca2 in zebrafish ovarian development, spermatogenesis, and tumorigenesis. Proc. Natl. Acad. Sci. U.S.A. 107, 19350-19355. 10.1073/pnas.1011630107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried, K. R. and Nusslein-Volhard, C. (2008). Germ line control of female sex determination in zebrafish. Dev. Biol. 324, 277-287. 10.1016/j.ydbio.2008.09.025 [DOI] [PubMed] [Google Scholar]
- Slanchev, K., Stebler, J., de la Cueva-Mendez, G. and Raz, E. (2005). Development without germ cells: the role of the germ line in zebrafish sex differentiation. Proc. Natl. Acad. Sci. U.S.A. 102, 4074-4079. 10.1073/pnas.0407475102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapp, E. L., Reinhart, G. A., Bogert, B. A., Lippincott-Schwartz, J. and Hegde, R. S. (2004). The organization of engaged and quiescent translocons in the endoplasmic reticulum of mammalian cells. J. Cell Biol. 164, 997-1007. 10.1083/jcb.200312079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling, A. C. (1993). Germline cysts: communes that work. Cell 72, 649-651. 10.1016/0092-8674(93)90393-5 [DOI] [PubMed] [Google Scholar]
- Spradling, A. C., de Cuevas, M., Drummond-Barbosa, D., Keyes, L., Lilly, M., Pepling, M. and Xie, T. (1997). The Drosophila germarium: stem cells, germ line cysts, and oocytes. Cold Spring Harb. Symp. Quant. Biol. 62, 25-34. 10.1101/SQB.1997.062.01.006 [DOI] [PubMed] [Google Scholar]
- Stanley, H. P., Bowman, J. T., Romrell, L. J., Reed, S. C. and Wilkinson, R. F. (1972). Fine structure of normal spermatid differentiation in Drosophila melanogaster. J Ultrastruct Res 41, 433-466. 10.1016/S0022-5320(72)90049-4 [DOI] [PubMed] [Google Scholar]
- Strasser, M. J., Mackenzie, N. C., Dumstrei, K., Nakkrasae, L. I., Stebler, J. and Raz, E. (2008). Control over the morphology and segregation of Zebrafish germ cell granules during embryonic development. BMC Dev. Biol. 8, 58. 10.1186/1471-213X-8-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome, S. and Updike, D. (2015). Specifying and protecting germ cell fate. Nat. Rev. Mol. Cell Biol. 16, 406-416. 10.1038/nrm4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, H., Saba, R., Sada, A. and Saga, Y. (2010). The Nanos3-3'UTR is required for germ cell specific NANOS3 expression in mouse embryos. PLoS ONE5, e9300. 10.1371/journal.pone.0009300 [DOI] [PMC free article] [PubMed]
- Takeda, Y., Mishima, Y., Fujiwara, T., Sakamoto, H. and Inoue, K. (2009). DAZL relieves miRNA-mediated repression of germline mRNAs by controlling poly(A) tail length in zebrafish. PloS one 4, e7513. 10.1371/journal.pone.0007513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney, L. G., Tilney, M. S. and Guild, G. M. (1996). Formation of actin filament bundles in the ring canals of developing Drosophila follicles. J. Cell Biol. 133, 61-74. 10.1083/jcb.133.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, S. K., Hsu, H. J. and Chung, B. C. (2010). Zebrafish monosex population reveals female dominance in sex determination and earliest events of gonad differentiation. Dev. Biol. 344, 849-856. 10.1016/j.ydbio.2010.05.515 [DOI] [PubMed] [Google Scholar]
- Truett, G. E., Heeger, P., Mynatt, R. L., Truett, A. A., Walker, J. A. and Warman, M. L. (2000). Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). BioTechniques 29, 54. 10.2144/00291bm09 [DOI] [PubMed] [Google Scholar]
- Tzung, K. W., Goto, R., Saju, J. M., Sreenivasan, R., Saito, T., Arai, K., Yamaha, E., Hossain, M. S., Calvert, M. E. and Orban, L. (2015). Early depletion of primordial germ cells in Zebrafish promotes testis formation. Stem Cell Reports 5, 156. 10.1016/j.stemcr.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida, D., Yamashita, M., Kitano, T. and Iguchi, T. (2002). Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile Zebrafish. J. Exp. Biol. 205, 711-718. [DOI] [PubMed] [Google Scholar]
- Wang, Z. and Lin, H. (2004). Nanos maintains germ line stem cell self-renewal by preventing differentiation. Science303, 2016-2019. 10.1126/science.1093983 [DOI]
- Wang, X. G., Bartfai, R., Sleptsova-Freidrich, I. and Orban, L. (2007). The timing and extent of ‘juvenile ovary’ phase are highly variable during zebrafish testis differentiation. J. Fish Biol. 70, 33-44. 10.1111/j.1095-8649.2007.01363.x [DOI] [Google Scholar]
- Wolf, N., Priess, J. and Hirsh, D. (1983). Segregation of germline granules in early embryos of Caenorhabditis elegans: an electron microscopic analysis. J. Embryol. Exp. Morphol. 73, 297-306. [PubMed] [Google Scholar]
- Wolke, U., Jezuit, E. A. and Priess, J. R. (2007). Actin-dependent cytoplasmic streaming in C. elegans oogenesis. Development 134, 2227-2236. 10.1242/dev.004952 [DOI] [PubMed] [Google Scholar]
- Xu, H. Y., Li, M. Y., Gui, J. F. and Hong, Y. H. (2007). Cloning and expression of medaka dazl during ernbryogenesis and gametogenesis. Gene Expr. Patterns 7, 332-338. 10.1016/j.modgep.2006.08.001 [DOI] [PubMed] [Google Scholar]
- Xue, F. and Cooley, L. (1993). kelch encodes a component of intercellular bridges in Drosophila egg chambers. Cell 72, 681-693. 10.1016/0092-8674(93)90397-9 [DOI] [PubMed] [Google Scholar]
- Yang, C. R., Rajkovic, G., Daldello, E. M., Luong, X. G., Chen, J. and Conti, M. (2020). The RNA-binding protein DAZL functions as repressor and activator of mRNA translation during oocyte maturation. Nat. Commun. 11, 1399. 10.1038/s41467-020-15209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, D., Zhu, L., Zhang, Q., Xiong, F., Wang, H., Wang, X., He, M., Zhu, Z. and Sun, Y. (2019). Abundance of early embryonic primordial germ cells promotes Zebrafish female differentiation as revealed by lifetime labeling of germline. Mar. Biotechnol (NY) 21, 217-228. 10.1007/s10126-019-09874-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Z., Ji, P., Cao, J. P., Zhu, S., Li, Y., Zheng, L., Chen, X. J. and Feng, L. X. (2009). Dazl promotes germ cell differentiation from embryonic stem cells. J Mol Cell Biol 1, 93-103. 10.1093/jmcb/mjp026 [DOI] [PubMed] [Google Scholar]
- Yue, L. and Spradling, A. C. (1992). Hu-li tai shao, a gene required for ring canal formation during Drosophila oogenesis, encodes a homolog of adducin. Genes Dev. 6, 2443-2454. 10.1101/gad.6.12b.2443 [DOI] [PubMed] [Google Scholar]
- Zagore, L. L., Sweet, T. J., Hannigan, M. M., Weyn-Vanhentenryck, S. M., Jobava, R., Hatzoglou, M., Zhang, C. and Licatalosi, D. D. (2018). DAZL Regulates germ cell survival through a network of PolyA-proximal mRNA interactions. Cell reports 25, 1225-1240.e1226. 10.1016/j.celrep.2018.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.