Abstract
Acute generalized exanthematous pustulosis (AGEP) is a rare drug-related adverse skin reaction caused mainly by antibiotics. Erlotinib is an epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) used to treat lung cancer. A 69-year-old woman with primary lung cancer (adenocarcinoma, cT3N1M1b, stage IVB) developed erythema and multiple skin pustules on her abdomen and both thighs after 7 weeks of erlotinib treatment. She also had fever and general fatigue. Histological examination of a skin biopsy specimen showed intraepidermal pustules with neutrophil and eosinophil infiltration. She was diagnosed with erlotinib-induced AGEP. AGEP resolved by erlotinib discontinuation and systemic corticosteroid treatment. The lung cancer progressed when erlotinib was discontinued, so afatinib, a second-generation EGFR-TKI, was administrated without any skin adverse effects. Afatinib successfully decreased the lung cancer, and maintained the disease stable for 1 year. Although acneiform rash was the most common skin adverse event caused by EGFR, AGEP rarely occurred. The present case also demonstrated that it is possible to switch agents, from erlotinib to afatinib, even though they have the same pharmacological effects. Although AGEP is a rare drug-related skin disorder, physicians should be aware that erlotinib may induce AGEP.
Keywords: Acute generalized exanthematous pustulosis, Lung cancer, Erlotinib, Skin disorder
Introduction
Acute generalized exanthematous pustulosis (AGEP) is a drug-related adverse reaction that results in skin eruptions; it is most frequently caused by antibiotics, including penicillins, macrolides, and quinolones [1, 2]. AGEP is similar to drug-induced hypersensitivity syndrome or Stevens-Johnson syndrome [3]. It is characterized by the rapid development of non-follicular and sterile pustules, and the time period from drug exposure to onset is typically within 48 h [4].
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) are the first-line treatment for patients with lung cancer who have an activating EGFR mutation [5]. Although acne-like eruptions are a common skin side effect of EGFR-TKIs, there are few reports of EGFR-TKI-induced AGEP [6, 7]. We report herein a rare case of AGEP caused by erlotinib in a patient with lung cancer.
Case Presentation
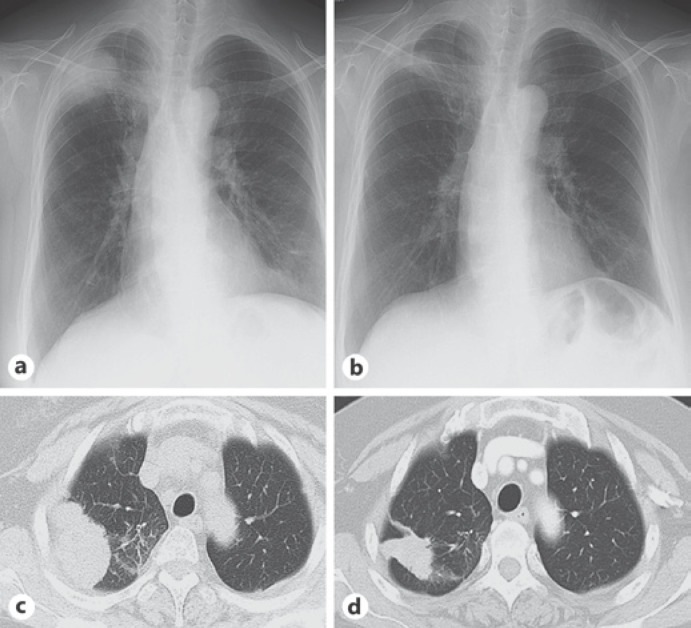
A 69-year-old Japanese woman visited our hospital with a right upper lung mass shadow on chest radiography (Fig. 1a) and computed tomography (CT; Fig. 1c). She was diagnosed as having primary lung cancer (adenocarcinoma, cT3N1M1b, stage IVB). An activating EGFR mutation, L858R, was detected in her lung cancer, and she was administrated with 150 mg erlotinib as the first-line treatment. After 7 weeks of treatment with erlotinib, she developed erythema and small pustules on her abdomen and both thighs, and had fever and general fatigue. She had not taken any drugs other than erlotinib.
Fig. 1.
Findings of chest radiography and computed tomography (CT). a Chest radiography before treatment with erlotinib revealing a mass shadow in the right upper lung field. b Chest CT before erlotinib treatment revealing a lung mass shadow in the right upper lobe. The mass shadow has lobulated margins. c Chest radiography 7 weeks after treatment with erlotinib. d Chest CT 7 weeks after treatment with erlotinib.
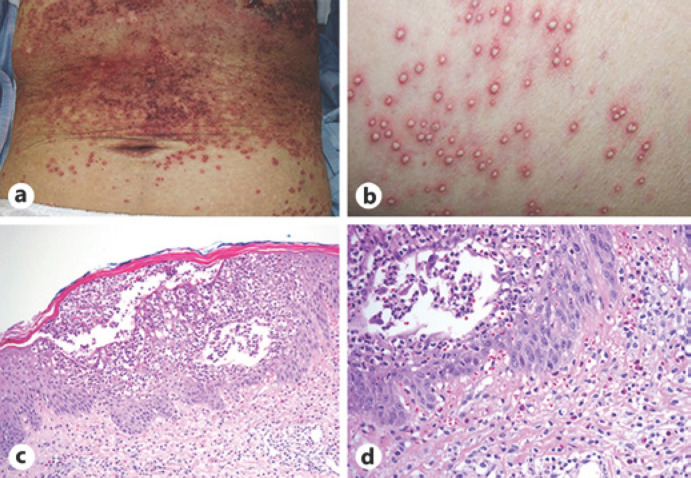
On admission, her body temperature was 38.6°C. Multiple pustules and purpura were found on the skin of her abdomen, lower back, hips, and thighs (Fig. 2a, b). Results of other physical examinations, including assessments of consciousness, chest auscultation, and joints, were normal. Her laboratory findings on admission revealed the following: white blood cell count, 8.6 × 103/µL with 67.5% neutrophils and 2.5% eosinophils; hemoglobin, 16.0 g/dL; platelet count, 18.9 × 104/µL; total protein, 6.4 g/dL; albumin, 2.7 g/dL; aspartate aminotransferase, 26 U/L; alanine aminotransferase, 106 U/L, lactate dehydrogenase, 201 U/L; and C-reactive protein, 21.49 mg/dL. The results of other biochemical tests and urine tests were within the normal ranges, including direct bilirubin, indirect bilirubin, the coagulation profile, and immunoglobulin E. Chest radiography showed a mass shadow in the right upper lung field, which decreased remarkably in size after erlotinib treatment (Fig. 1c). Chest CT also showed a reduction in the size of the lung mass in the right upper lobe (Fig. 1d). Histological examination of a skin biopsy specimen showed intraepidermal pustules with neutrophil and eosinophil infiltration (Fig. 2c, d). There were no findings suggestive of bacterial or fungal infection. According to these results, the patient was diagnosed as having AGEP due to erlotinib. Erlotinib was discontinued, and the patient received systemic corticosteroid treatment with 1.0 mg/kg/day prednisolone because she had a high fever and systemic inflammation. Her AGEP skin lesions, fever, and elevated C-reactive protein level gradually improved with the prednisolone treatment, and the dose of prednisolone was subsequently tapered over 3 weeks and discontinued.
Fig. 2.
Skin features and pathological findings at skin biopsy. a Macroscopic findings of the abdominal skin. Diffuse edematous erythema and scattered small pustules are observed. b Close-up findings of the skin. c, d Microscopic findings of a skin lesion showing intraepidermal pustules with inflammatory cell infiltration. Hematoxylin and eosin stain, ×40 (c), ×100 (d).
A month after she discontinued erlotinib, chest radiography revealed enlargement of the lung mass shadow. Since the lung cancer had relapsed, she was administrated with afatinib, a second-generation EGFR-TKI, with her consent. The lung cancer was well-controlled by afatinib for 1 year.
Discussion
We report a rare case of a lung cancer patient who developed erlotinib-induced AGEP; the case was subsequently successfully treated with afatinib. Only one other case of erlotinib-induced AGEP has been reported to date [6].
AGEP is mostly found as an adverse reaction to several antibiotics, including aminopenicillins, quinolones, hydroxychloroquine, ketoconazole, and fluconazole [3]. The period from drug administration to AGEP onset ranged from 24 h to more than 1 month. AGEP is characterized by multiple non-follicular pustules on an erythematous base that are mainly distributed in the trunk and intertriginous regions. The reported pathophysiology of AGEP includes vesicles composed mainly of CD4 T cells and keratinocytes at the initial stage; the cells then release increased amounts of interleukin 8, leading to the chemotaxis of neutrophils into the vesicles, which causes the formation of sterile pustules [8, 9]. A previous study showed that 10 of 58 (17%) patients with AGEP had organ involvement, including 7 patients with abnormal hepatic function test results, 6 patients with renal insufficiency, and 2 patients who developed acute respiratory distress [10]. Fever (>38.0°C) and leukocytosis with an elevated neutrophil count (>7.5 × 103/mL) were frequently observed in AGEP patients [4]. The present case also had subacute fever onset, liver dysfunction, and systemic inflammation without renal dysfunction or respiratory failure.
The most significant treatment for AGEP is the removal of the causative drug. In prolonged cases, topical corticosteroids are appropriate for the treatment of pruritus and inflammation, and topical corticosteroids are correlated with a decreased duration of hospitalization [11]. In cases with systemic symptoms, systemic corticosteroids have been reported to be effective [12]. The present case was treated with a systemic corticosteroid, and after her skin lesions improved, the corticosteroid was tapered over 3 weeks and discontinued.
In the present case, the lung cancer progressed for 1 month after the discontinuation of erlotinib. Thereafter, she was treated with afatinib as the second-line treatment. The reasons were that the initial treatment with erlotinib was highly effective, and the patient did not consent to cytotoxic chemotherapy. Afatinib successfully decreased both the primary and metastatic lesions, and maintained the disease stable for 1 year. The present case demonstrated that it is possible to switch agents, from erlotinib to afatinib, even though they have the same pharmacological effects.
Erlotinib has been reported to cause a high incidence of the following adverse skin effects: acneiform rash (63%), dry skin (7.7%), pruritus (3.8%), and paronychia (6.0%) [13]. Erlotinib-related acneiform rash typically manifests as red papules and/or pustules on the face, chest, abdomen, or thighs [14]. Although the frequency of liver dysfunction due to erlotinib is low, gefitinib, a first-generation EGFR-TKI, often causes the elevation of transaminase levels [15]. Therefore, physicians should pay attention for AGEP when administering erlotinib in cases complicated by liver dysfunction or a systemic inflammatory reaction with multiple non-follicular pustules.
Conclusion
In conclusion, although AGEP is a rare drug-related skin disorder, physicians should be aware that it can occur in patients with lung cancer treated with erlotinib.
Statement of Ethics
The patient has given written informed consent to publish this case report (including publication of images).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was obtained for this report.
Author Contributions
All authors were involved in the acquisition of data. N.K. and K.T. drafted the article. G.K., M.K., H.T., C.N., T.N., K.I., K.S. and N.S.-A. revised the manuscript critically for important intellectual content. All authors approved the final manuscript.
Acknowledgements
Not applicable.
References
- 1.Roujeau JC, Bioulac-Sage P, Bourseau C, Guillaume JC, Bernard P, Lok C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991;127((9)):1333–8. [PubMed] [Google Scholar]
- 2.Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP)–a clinical reaction pattern. J Cutan Pathol. 2001;28((3)):113–9. doi: 10.1034/j.1600-0560.2001.028003113.x. [DOI] [PubMed] [Google Scholar]
- 3.Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): A review and update. J Am Acad Dermatol. 2015;73((5)):843–8. doi: 10.1016/j.jaad.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JN, Naldi L, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) Br J Dermatol. 2007;157((5)):989–96. doi: 10.1111/j.1365-2133.2007.08156.x. [DOI] [PubMed] [Google Scholar]
- 5.Greenhalgh J, Dwan K, Boland A, Bates V, Vecchio F, Dundar Y, et al. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst Rev. 2016;((5)):CD010383. doi: 10.1002/14651858.CD010383.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Liquete E, Ali S, Kammo R, Ali M, Alali F, Challa H, et al. Acute Generalized Exanthematous Pustulosis Induced by Erlotinib (Tarceva) with Superimposed Staphylococcus aureus Skin Infection in a Pancreatic Cancer Patient: A Case Report. Case Rep Oncol. 2012;5((2)):253–9. doi: 10.1159/000338806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shih HC, Hsiao YP, Wu MF, Yang JH. Gefitinib-induced acute generalized exanthematous pustulosis in two patients with advanced non-small-cell lung cancer. Br J Dermatol. 2006;155((5)):1101–2. doi: 10.1111/j.1365-2133.2006.07511.x. [DOI] [PubMed] [Google Scholar]
- 8.Britschgi M, Steiner UC, Schmid S, Depta JP, Senti G, Bircher A, et al. T-cell involvement in drug-induced acute generalized exanthematous pustulosis. J Clin Invest. 2001;107((11)):1433–41. doi: 10.1172/JCI12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaerli P, Britschgi M, Keller M, Steiner UC, Steinmann LS, Moser B, et al. Characterization of human T cells that regulate neutrophilic skin inflammation. J Immunol. 2004;173((3)):2151–8. doi: 10.4049/jimmunol.173.3.2151. [DOI] [PubMed] [Google Scholar]
- 10.Hotz C, Valeyrie-Allanore L, Haddad C, Bouvresse S, Ortonne N, Duong TA, et al. Systemic involvement of acute generalized exanthematous pustulosis: a retrospective study on 58 patients. Br J Dermatol. 2013;169((6)):1223–32. doi: 10.1111/bjd.12502. [DOI] [PubMed] [Google Scholar]
- 11.Ingen-Housz-Oro S, Hotz C, Valeyrie-Allanore L, Sbidian E, Hemery F, Chosidow O, et al. Acute generalized exanthematous pustulosis: a retrospective audit of practice between 1994 and 2011 at a single centre. Br J Dermatol. 2015;172((5)):1455–7. doi: 10.1111/bjd.13540. [DOI] [PubMed] [Google Scholar]
- 12.Choi MJ, Kim HS, Park HJ, Park CJ, Lee JD, Lee JY, et al. Clinicopathologic manifestations of 36 korean patients with acute generalized exanthematous pustulosis: a case series and review of the literature. Ann Dermatol. 2010;22((2)):163–9. doi: 10.5021/ad.2010.22.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagawa K, Kudoh S, Ohe Y, Johkoh T, Ando M, Yamazaki N, et al. Postmarketing surveillance study of erlotinib in Japanese patients with non-small-cell lung cancer (NSCLC): an interim analysis of 3488 patients (POLARSTAR) J Thorac Oncol. 2012;7((8)):1296–303. doi: 10.1097/JTO.0b013e3182598abb. [DOI] [PubMed] [Google Scholar]
- 14.Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol. 2013;69((3)):463–72. doi: 10.1016/j.jaad.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 15.Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2015;88((1)):74–9. doi: 10.1016/j.lungcan.2015.01.026. [DOI] [PubMed] [Google Scholar]