Abstract
BACKGROUND
Cyclooxygenase (COX) inhibitors including non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used to reduce pain, fever, and inflammation but have been associated with complications in community acquired pneumonia and other respiratory tract infections (RTIs). Conclusive data are not available about potential beneficial or adverse effects of COX inhibitors on COVID-19 patients.
METHODS
We conducted a retrospective, multi-center observational study by leveraging the harmonized, high-granularity electronic health record data of the National COVID Cohort Collaborative (N3C). Potential associations of eight COX inhibitors with COVID-19 severity were assessed using ordinal logistic regression (OLR) on treatment with the medication in question after matching by treatment propensity as predicted by age, race, ethnicity, gender, smoking status, comorbidities, and BMI. Cox proportional hazards analysis was used to estimate the correlation of medication use with morbidity for eight subcohorts defined by common indications for COX inhibitors.
RESULTS
OLR revealed statistically significant associations between use of any of five COX inhibitors and increased severity of COVID-19. For instance, the odds ratio of aspirin use in the osteoarthritis cohort (n=2266 patients) was 3.25 (95% CI 2.76 – 3.83). Aspirin and acetaminophen were associated with increased mortality.
CONCLUSIONS
The association between use of COX inhibitors and COVID-19 severity was consistent across five COX inhibitors and multiple indication subcohorts. Our results align with earlier reports associating NSAID use with complications in RTI patients. Further research is needed to characterize the precise risk of individual COX inhibitors in COVID-19 patients.
As of 3 April 2021, severe acute respiratory syndrome associated with coronavirus-2 (SARS-CoV-2) has infected more than 128 million people and caused more than 2.8 million deaths worldwide.1 SARS-CoV-2 is the cause of the coronavirus disease of 2019 (COVID-19), a condition characterized by pneumonia, hyperinflammation, hypoxemic respiratory failure, a prothrombotic state, cardiac dysfunction, substantial mortality, and persistent morbidity in some survivors.2
Cyclooxygenase (COX) inhibitors represent a large and heterogeneous class of medications defined by their ability to inhibit COX, an enzyme that catalyzes the conversion of arachidonic acid to prostaglandins. The two COX isoforms differ in their distribution and physiologic roles. COX-1 is ubiquitously and constitutively expressed and produces prostaglandins implicated in homeostatic functions such as maintenance of gastrointestinal (GI) mucosal integrity. Many of the adverse effects of conventional non-steroidal anti-inflammatory drugs (NSAIDs) (including GI bleeding, peptic ulceration, hemorrhagic cerebrovascular accident, renal impairment, wheezing, and rash) are thought to be primarily related to inhibition of COX-1. In contrast, COX-2 is induced by cytokines and produces prostaglandins that mediate pain and inflammation.3 Due to their widespread use, NSAIDs are common causes of serious adverse events that frequently necessitate hospitalization.4
NSAIDs have numerous potentially deleterious effects on immune function and may also mask warning signs of severe infection such as fever and pain during the course of community-acquired pneumonia. NSAID exposure in the early stage of community-acquired pneumonia has been associated with a delayed diagnosis and more severe clinical course,5,6 but the quality of available research has been called into doubt and recent studies have failed to reproduce the proposed association.7,8 Some NSAIDs have been associated with abnormalities of immune function.9,10 Here, we investigate seven NSAIDs and acetaminophen, which has COX-2 inhibitory and other pharmacological activities.11 For conciseness, we will refer to the group of NSAIDs and acetaminophen as COX inhibitors.
Existing evidence regarding the effect on COVID-19 of exposure to COX inhibitors is inconclusive. Indomethacin has a direct antiviral effect against the SARS-CoV virus,12 and a clinical trial to assess the effects of adding naproxen to the treatment of critically ill patients hospitalized for COVID-19 infection is underway (ClinicalTrials.gov identifier: NCT04325633). On the other hand, an apparent increase in the severity of clinical symptoms was noted in French COVID-19 patients taking ibuprofen.7 In the early months of the pandemic, a study was conducted with data from OpenSAFELY, a secure health analytics platform that covers 40% of all patients in England. The study ran from 1 March 2020 to 14 June 2020, looking at two (partially overlapping) cohorts: 2,463,707 patients in the general population with a history of NSAID use, and 1,708,781 patients with a diagnosis of rheumatoid arthritis or osteoarthritis. In both cohorts, the treatment group was defined as all patients who were prescribed NSAIDs within 4 months of the study’s start date; all others were considered controls. The primary outcome was COVID-19 related death: no significant increase in mortality was shown in the treatment group of either cohort.13 Other small cohort studies ranging in size from 293 to 1222 patients failed to show increased risks with use of various NSAIDs.14–17 An observational study on 98 patients who had received aspirin within 24 hours of admission or 7 days before admission compared to 314 patients who had not received aspirin showed significant associations of aspirin use with decreased severity and mortality of COVID-19.18
Given the common use of COX inhibitors and the inconclusiveness of these prior studies, additional insight on potential risks of COX inhibitor use by COVID-19 patients is needed. Our research leverages data from the National COVID Cohort Collaborative (N3C), a centralized, harmonized, high-granularity electronic health record (EHR) repository with the largest, most representative U.S. cohort of COVID-19 cases and controls to date. We assessed associations of eight COX inhibitors with clinical severity and mortality of COVID-19 patients, demonstrating significant associations of five COX-inhibitors with increased COVID-19 severity and in two cases, mortality.
Methods
STUDY POPULATION
All patient data were accessed through the National COVID Cohort Collaborative (N3C) (covid.cd2h.org). N3C aggregates and harmonizes EHR data across 38 clinical organizations in the United States, including the Clinical and Translational Science Awards (CTSA) Program hubs. For this analysis, data were derived from the 24 centers that provided data for all predictors used in the regression analysis described below. Fourteen centers did not provide data on Body Mass Index (BMI) and were not included in this study. N3C harmonizes data across four clinical data models and provides a unified analytical platform in which data are encoded using the Observational Medical Outcomes Partnership (OMOP)19 version 5.3.1 and provides shared phenotype definitions such as those for positive COVID-19 laboratory tests and COVID-19 clinical severity categories.20,21
ELIGIBILITY CRITERIA
Criteria for the current study were determined as follows. The COVID-19 positive cohort (Supplemental Table S1) was defined as those patients with any encounter after January 1, 2020 and positive SARS-CoV-2 laboratory test (polymerase chain reaction or antigen). For this study, data from up to February 2, 2021 were included. For each medication, we defined subcohorts based on an indication for use of one of eight COX inhibitors (acetaminophen, aspirin, celecoxib, diclofenac, ibuprofen, ketorolac, meloxicam, naproxen) that were neither sex-specific nor limited to pediatric age groups on the basis of information in the DrugCentral resource.22 Subcohorts of COVID-19 patients who had a history of that indication prior to contracting COVID-19 were identified, as determined by a condition era23 that began before the date of COVID-19 diagnosis. A treatment group within the subcohort was defined as patients whose drug era23 for the medication in question began on or before the date of COVID-19 diagnosis and continued for at least one day. All other patients from the subcohort were used in propensity matching to define the control group. A secondary analysis was performed on all patients receiving the COX inhibitor regardless of indication.
Only patients with complete records (no missing values for any covariate used in propensity matching or logistic regression) were included for further analysis. We studied the drug-indication combinations for which there were at least 50 treated patients. OMOP concept ID codes for all drugs and drug indications used in this analysis are listed in Supplemental Tables S2 and S3.
OUTCOMES
The primary outcome of interest was the COVID-19 clinical severity category. Clinical severity was classified into five categories using the Clinical Progression Scale (CPS) established by the World Health Organization (WHO) for COVID-19 clinical research24: “mild” (outpatient, WHO severity 1–3); “mild ED” (outpatient with ED visit, WHO severity 3); “moderate” (hospitalized without invasive ventilation, WHO severity 4–6); “severe” (hospitalized with invasive ventilation or ECMO, WHO severity 7–9); and “mortality/hospice” (hospital mortality or discharge to hospice, WHO Severity 10).21 For the purposes of the ordinal logistic regression (OLR) analysis described below, patients were assigned to severity groups according to the maximum clinical severity during their index encounter,21 which was defined as the medical encounter during which a positive COVID-19 test was documented for the first time. Secondary outcomes were all-cause mortality recorded at any time following the COVID-19 diagnosis as well as analyses of the entire groups of COVID-19 patients treated with the medications of interest without restriction to indications.
STUDY DESIGN
For each of the eight COX Inhibitors, we constructed a codeset containing concept IDs representing all formulations of the medications (see Supplemental Table S3) using ATLAS (http://atlas-covid19.ohdsi.org/), the graphical user interface for the OMOP common data model.25
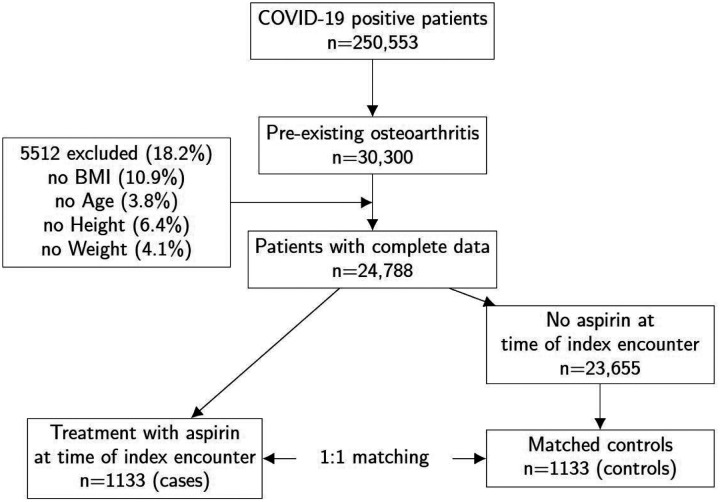
We explored the potential association between treatment with a pharmaceutical agent and severity of COVID-19. Two strategies were chosen to minimize the potential effects of confounding. To control for confounding due to covariate imbalance, we compared outcomes in a propensity-matched group of patients who had an indication associated with the COX inhibitor of interest. This resulted in subchorts of relatively homogeneous patient clusters. Propensity score matching was performed to correct residual covariate imbalance within these subcohorts. Using the DrugCentral resource22, we developed the following list of representative indications: osteoarthritis, rheumatoid arthritis, angina pectoris, migraine, myocardial infarction, pain, headache, and fever (OMOP concept id codes in Supplemental Table S2).19 Figure 1 summarizes the procedure used to define the aspirin-osteoarthritis subcohort; other subcohorts were defined analogously.
Figure 1.
Definition of subcohorts by drug indication. In this example, the osteoarthritis subcohort is used to define case/control groups for treatment with aspirin. Other subcohorts were defined analogously.
Propensity matching
We performed propensity matching using the “nearest” method implemented in the R MatchIt package. Each patient from the treated group was matched to the patient in the untreated group with the closest propensity score. The propensity formula included age, race, ethnicity, gender, smoking status, Charlson Comorbidity Index, and BMI.
Ordinal logistic regression (OLR)
To investigate the association of treatment and other covariates with COVID-19 severity, we performed OLR using the polr function of the MASS R package. The dependent variable was COVID-19 severity, an ordered factor with levels “mild”, “mild ED”, “moderate”, “severe”, and “mortality/hospice”.21 We assessed the relationship between COVID-19 severity and treatment with each medication under consideration as a part of multiple OLR with age, race, ethnicity, gender, smoking status, Charlson Comorbidity Index, and BMI as additional predictors. For treatment with the medication we recorded the t value, the corresponding p value, and the odds ratio along with its 95% confidence interval.
Cox proportional hazards modeling
A single index encounter was defined for each laboratory-confirmed positive patient as described previously.21 Survival time was defined with respect to this encounter. For each patient, the latest visit and date of death (if observed) was recorded to measure right censoring (the last date for which death outcome can be ruled out) and survival, respectively. The survival R package was used to produce Kaplan-Meier survival curves, compute Cox proportional hazards regression using the same predictors as with OLR, and visualize Schoenfeld residuals.
RESULTS
We evaluated 250,533 patients with COVID-19 in a retrospective cohort study. Mean age was 41.6 years, and 115,828 patients (46.2%) were men. The Charlson Comorbidity Index was used to account for comorbidities,26 and the Clinical Progression Scale (CPS) established by the World Health Organization (WHO) for COVID-19 clinical research was used to stratify patients according to severity.24 A total of 6138 deaths were recorded in the COVID-19 cohort (1.9%). Supplemental Table S1 summarizes the demographics of the cohort.
We evaluated eight COX inhibitors. For each medication, we chose one or more representative indications with at least 50 cases, collected all individuals diagnosed with COVID-19 who additionally had been diagnosed with the representative drug indication, and then divided this cohort into individuals treated with the medication (cases) and those who were not (controls). We reasoned that this would define more balanced case and control groups than if we compared all COVID-19 patients who were receiving the medication with those who were not, since medication use is a proxy for the distribution of comorbidities, some of which may correlate strongly with COVID-19 outcome. To further reduce the effect of confounders, we performed propensity matching27 to match drug-treated and untreated patients according to age, race, ethnicity, gender, smoking status, Charlson Comorbidity Index, and BMI. Table 1 shows a representative cohort before and after propensity matching.
Table 1.
Aspirin-treated osteoarthritis subcohort. SMD: standardized mean difference.
| Before propensity matching | After propensity matching | ||||
|---|---|---|---|---|---|
| Aspirin | Control | SMD | Control | SMD | |
| n | 1133 | 23655 | - | 1133 | - |
| Age | 67.5 ± 12.0 | 61.4 ± 14.1 | 0.465 | 67.9 ± 11.9 | 0.035 |
| BMI | 32.1 ± 8.4 | 32.4 ± 8.2 | 0.037 | 32.2 ± 8.4 | 0.016 |
| Charlson | 2.55 ± 2.71 | 1.87 ± 2.47 | 0.264 | 2.53 ± 2.85 | 0.007 |
| Race | |||||
| - African American | 307 (27.2%) | 5069 (21.4%) | 271 (24.0%) | ||
| - Asian | 21 (1.9%) | 360 (1.5%) | 19 (1.7%) | ||
| - Pacific Islander | 1 (0.1%) | 23 (0.1%) | 0 | ||
| - White | 715 (63.3%) | 15900 (67.2%) | 752 (66.6%) | ||
| - Other | 10 (0.9%) | 289 (1.2%) | 14 (1.2%) | ||
| - Unknown | 75 (6.6%) | 2018 (8.5%) | 73 (6.5%) | ||
| Ethnicity | |||||
| - Hispanic or Latino | 81 (7.2%) | 1897 (8.0%) | 84 (7.4%) | ||
| - Not Hispanic or Latino | 1018 (90.2%) | 21145 (89.4%) | 1013 (89.7%) | ||
| - Unknown | 30 (2.7%) | 617 (2.6%) | 32 (2.8%) | ||
| Sex | |||||
| - Female | 591 (52.3%) | 14348 (60.6%) | 599 (53.1%) | ||
| - Male | 538 (47.7%) | 9310 (39.4%) | 530 (46.9%) | ||
| - Other | 0 (0.0%) | 1 (0.0%) | 0 | ||
| Smoking status | |||||
| - Current or Former | 142 (12.6%) | 2746 (11.6%) | 135 (12.0%) | ||
| - Non smoker | 987 (87.4%) | 20913 (88.4%) | 994 (88.0%) | ||
| Severity Type | |||||
| - Mild | 245 (21.7%) | 13806 (58.4%) | 576 (51.0%) | ||
| - Mild ED | 73 (6.5%) | 2463 (10.4%) | 109 (9.7%) | ||
| - Moderate | 621 (55.0%) | 5879 (24.8%) | 337 (29.8%) | ||
| - Severe | 66 (5.8%) | 523 (2.2%) | 23 (2.0%) | ||
| - Mortality/hospice | 124 (11.0%) | 988 (4.2%) | 84 (7.4%) | ||
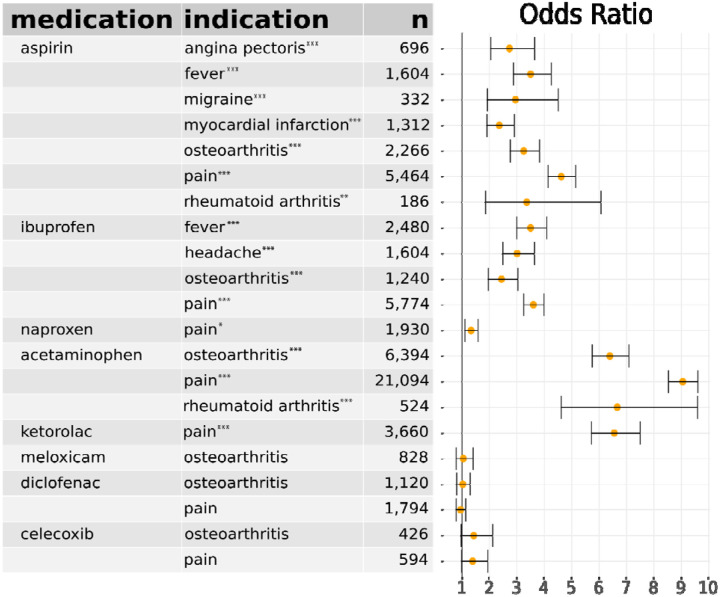
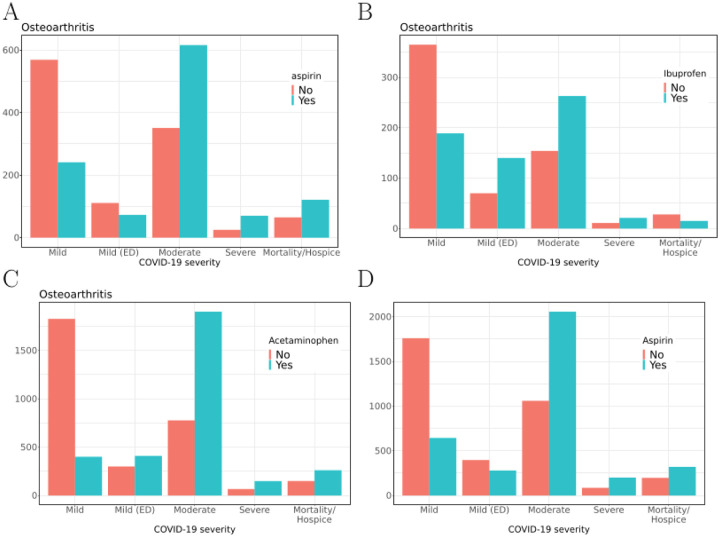
OLR was performed to assess the relationship between use of the candidate medication and other covariates (age, race, ethnicity, gender, smoking status, Charlson comorbidity, and BMI) and COVID-19 severity (“mild”, “mild ED”, “moderate”, “severe”, “mortality/hospice”). Sixteen of the 21 cohort comparisons indicated a significant association of COX inhibitor use with increased COVID-19 severity (Table 2). Figure 2 shows the distribution of severity classes in osteoarthritis cohorts for aspirin, ibuprofen, and acetaminophen, as well as the distribution of severity classes in the entire COVID-19+ cohort for aspirin. In agreement with previous studies28,29, we also consistently observed a significant association of age, male sex, and Charlson comorbidity with COVID-19 severity (Supplemental Table S1 – S21). OLR relies on an assumption of proportional odds, which we verified is tenable for these data (Supplemental Figure S22). We calculated the E-value for the observed values of the odds ratio to assess the sensitivity of our findings to uncorrected confounders.30 The observed odds ratio of 3.3 for the association of aspirin with increased COVID-19 severity in the aspirin-osteoarthritis subcohort could not be explained away by an unmeasured confounder that was associated with both the treatment and the outcome by a odds ratio of less than 3.0-fold each above and beyond the confounders included in the regression. E-values for other subcohorts are shown in Supplemental Table S4.
Table 2. Association of COX inhibitors with COVID-19 severity.
For each combination of medication and disease indication, subcohorts were constructed using propensity matching. Ordinal logistic regression was used to assess association with COVID-19 severity. Odds ratios, s, 95% confidence intervals, and sample sizes (treated plus control) are shown.
p < 0.001,
p < 0.01,
p < 0.05 after Bonferroni correction for 21 tests. Details for each subcohort are provided in Supplemental Tables S5–S25 and Supplemental Figures S1–S21.
Figure 2.
COVID-19 severity in a subcohort of osteoarthritis patients taking vs. not taking A) aspirin, B) ibuprofen or C) acetaminophen; and D) entire COVID-19 cohort taking vs. not taking aspirin. Severity of COVID-19 by group is shown on the x-axis, and number of patients is shown on the y-axis.
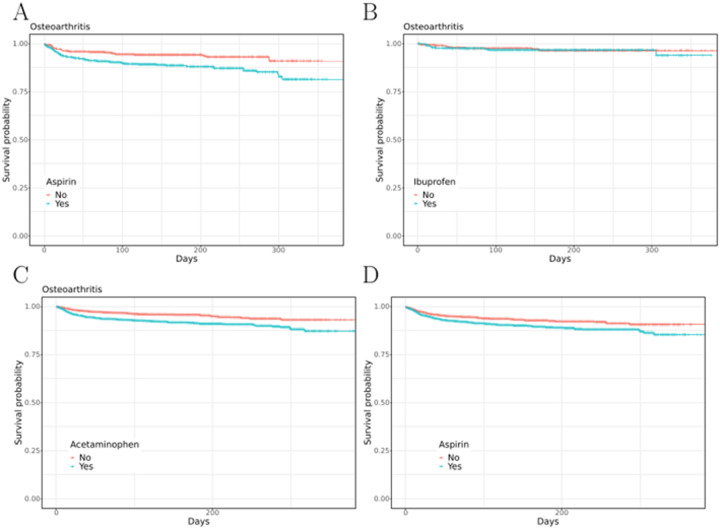
To assess the effect of COX inhibitors on mortality, survival was measured in cohorts using the Cox proportional hazards model (Figure 3). The proportional hazards assumption was assessed by visualizing Schoenfeld residuals (Supplemental Fig. S23). We observed that aspirin and acetaminophen but not the other six tested COX inhibitors were associated with increased mortality. Supplemental Tables S21–S60 and Supplemental Figures S1–S25 show detailed results. Comparable results were obtained by analyzing the entire cohort of COVID-19 per medication (Supplemental Figures S23–S31 and Tables S47–S62).
Figure 3.
Kaplan-Meier survival curves for a subcohort of osteoarthritis patients taking vs. not taking A) aspirin, B) ibuprofen or C) acetaminophen and D) entire COVID-19+ cohort taking vs. not taking aspirin.
Discussion
Our findings show an association of COX inhibitors with increased COVID-19 severity across five of the eight tested agents (aspirin, ibuprofen, ketorolac, naproxen, and acetaminophen). Diclofenac, meloxicam, and celecoxib did not show significant associations with severity in our study. Interestingly, these three agents display selectivity for COX-2.31
COX-2 selective inhibitors were developed with the goal of avoiding the adverse GI effects associated with non-selective NSAIDs, but were found to be associated with a higher risk of cardiovascular adverse events including stroke and myocardial infarction.32,33 Increased cardiovascular risk was subsequently shown to be associated also with non-selective NSAIDs in multiple studies.34
NSAIDs have multiple effects on the immune system, including inhibition of neutrophil adherence, decreased neutrophil degranulation and oxidant production, inhibition of neutrophil elastase activity and induction of neutrophil apoptosis, and inhibition of antibody production.10,35 Several studies have documented an association between NSAID use and risk of severe pulmonary complications in the setting of community acquired pneumonia and acute viral infection.5 Other studies have failed to find an association of NSAIDs with a worse prognosis among patients admitted with influenza.8 Conceivably, the increased risk could be related to effects of NSAIDs on the immune system or to a delay in treatment related to the masking of symptoms of infection by an NSAID.36 We did not observe an association of NSAID use with neutrophil counts in COVID-19 patients (data not shown). Insufficient data was available to assess other potential associations with immune cell function. Acetaminophen, which has a mechanism of action different from that of NSAIDs, has previously been associated with decreased mortality in critically ill patients.37 These considerations, together with isolated reports of exacerbation of the clinical course of COVID-19 following ibuprofen exposure, have led to recommendations against the use of ibuprofen for managing symptoms of COVID-19.38
Strengths and limitations of this study
Observational studies such as retrospective EHR cohort analysis are subject to confounding. In the case of our study, the decision of whether to treat a patient with a COX inhibitor could in principle be correlated with the outcome of interest (COVID-19 severity). We applied two strategies to mitigate confounding: (i) analysis of subcohorts according to indication, and (ii) propensity matching. However, in observational studies a risk of residual confounding persists because the efficacy of propensity matching is limited to known and measured factors. Exposure to the drugs of interest, most of which are available without a prescription, was likely to be captured incompletely in the EHR data used in the analysis. Thus, it is possible that there was unrecorded use of COX inhibitors in the untreated group.
Our study uses a five-level ordinal measure of COVID-19 outcome that may allow increased sensitivity compared with some previous efforts that use only COVID-19 mortality to measure outcome.
Conclusions
The many NSAIDs vary with respect to their ability to inhibit each isoenzyme (COX-1 and COX-2). Aspirin shows the lowest degree of COX-2 selectivity, while celecoxib is one of the most COX-selective NSAIDs.39 In addition, celecoxib possess activities unrelated to COX inhibition.40 Therefore, it appears plausible that the adverse effect profile of COX inhibitors in individuals with COVID-19 may differ from medication to medication. The results of our observational study demonstrated a significant association of the use of five of eight different COX inhibitors with increased clinical severity and mortality. We did not find evidence for an association of increased COVID-19 severity with diclofenac, meloxicam and celecoxib, which are selective for COX-2,31 raising the possibility of agent-specific risk profiles for individual COX inhibitors. Additionally, we demonstrate a significant association between COVID-19 severity and acetaminophen use, which does not provide support for recent recommendations for acetaminophen for symptom relief in COVID-19. While our findings suggest the importance for clinicians to carefully consider which COX inhibitor to prescribe, additional research will be required to confirm our results in other settings and to address the question of whether different COX inhibitors have different adverse effect profiles in treating COVID-19.
IRB
The N3C data transfer to NCATS is performed under a Johns Hopkins University Reliance Protocol #IRB00249128 or individual site agreements with NIH. The N3C Data Enclave is managed under the authority of the NIH; information can be found at https://ncats.nih.gov/n3c/resources.
Supplementary Material
Acknowledgements
The analyses described in this publication were conducted with data or tools accessed through the NCATS N3C Data Enclave (covid.cd2h.org/enclave) and supported by NCATS U24 TR002306. This research was possible because of the patients whose information is included within the data from participating organizations: https://ncats.nih.gov/n3c/resources/data-contribution/data-transfer-agreement-signatories and scientists who have contributed to the ongoing development of this community resource (doi.org/10.5281/zenodo.3979622). Authorship was determined using ICMJE recommendations.
We are grateful to the following data partners with released data, made possible by supporting grants:
Stony Brook University — None (Voluntary) • University of Oklahoma Health Sciences Center — U54GM104938: Oklahoma Clinical and Translational Science Institute (OCTSI) • West Virginia University — U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI) • University of Mississippi Medical Center — U54GM115428: Mississippi Center for Clinical and Translational Research (CCTR) • University of Nebraska Medical Center — U54GM115458: Great Plains IDeA-Clinical & Translational Research • Maine Medical Center — U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • Wake Forest University Health Sciences — UL1TR001420: Wake Forest Clinical and Translational Science Institute • Northwestern University at Chicago — UL1TR001422: Northwestern University Clinical and Translational Science Institute (NUCATS) • University of Cincinnati — UL1TR001425: Center for Clinical and Translational Science and Training • The University of Texas Medical Branch at Galveston — UL1TR001439: The Institute for Translational Sciences • Medical University of South Carolina — UL1TR001450: South Carolina Clinical & Translational Research Institute (SCTR) • University of Massachusetts Medical School Worcester — UL1TR001453: The UMass Center for Clinical and Translational Science (UMCCTS) • University of Southern California — UL1TR001855: The Southern California Clinical and Translational Science Institute (SC CTSI) • Columbia University Irving Medical Center — UL1TR001873: Irving Institute for Clinical and Translational Research • George Washington Children’s Research Institute — UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • University of Kentucky — UL1TR001998: Appalachian Translational Research Network (ATRN) • University of Rochester — UL1TR002001: UR Clinical & Translational Science Institute • University of Illinois at Chicago — UL1TR002003: UIC Center for Clinical and Translational Science • Penn State Health Milton S. Hershey Medical Center — UL1TR002014: Penn State Clinical and Translational Science Institute • The University of Michigan at Ann Arbor — UL1TR002240: Michigan Institute for Clinical and Health Research • Vanderbilt University Medical Center — UL1TR002243: Vanderbilt Institute for Clinical and Translational Research • University of Washington — UL1TR002319: Institute of Translational Health Sciences • Washington University in St. Louis — UL1TR002345: Institute of Clinical and Translational Sciences • Oregon Health & Science University — UL1TR002369: Oregon Clinical and Translational Research Institute • University of Wisconsin-Madison — UL1TR002373: Wisconsin Network For Health Research • Rush University Medical Center — UL1TR002389: The Institute for Translational Medicine (ITM) • The University of Chicago — UL1TR002389: The Institute for Translational Medicine (ITM) • University of North Carolina at Chapel Hill — UL1TR002489: North Carolina Translational and Clinical Science Institute • University of Minnesota — UL1TR002494: Clinical and Translational Science Institute • Children’s Hospital Colorado — UL1TR002535: Colorado Clinical and Translational Sciences Institute • The University of Iowa — UL1TR002537: Institute for Clinical and Translational Science • The University of Utah — UL1TR002538: Uhealth Center for Clinical and Translational Science • Tufts Medical Center — UL1TR002544: Tufts Clinical and Translational Science Institute • Duke University — UL1TR002553: Duke Clinical and Translational Science Institute • Virginia Commonwealth University — UL1TR002649: C. Kenneth and Dianne Wright Center for Clinical and Translational Research • The Ohio State University — UL1TR002733: Center for Clinical and Translational Science • The University of Miami Leonard M. Miller School of Medicine — UL1TR002736: University of Miami Clinical and Translational Science Institute • University of Virginia — UL1TR003015: iTHRIVL Integrated Translational health Research Institute of Virginia • Carilion Clinic — UL1TR003015: iTHRIVL Integrated Translational health Research Institute of Virginia • University of Alabama at Birmingham — UL1TR003096: Center for Clinical and Translational Science • Johns Hopkins University — UL1TR003098: Johns Hopkins Institute for Clinical and Translational Research • University of Arkansas for Medical Sciences — UL1TR003107: Consortium of Rural States (CORES) • Nemours — U54GM104941: Delaware CTR ACCEL Program • University of Colorado Denver, Anschutz Medical Campus — UL1TR002535: Colorado Clinical and Translational Sciences Institute • Mayo Clinic Rochester — UL1TR002377: Mayo Clinic Center for Clinical and Translational Science (CCaTS) • Loyola University Medical Center — UL1TR002389: The Institute for Translational Medicine (ITM) • Advocate Health Care Network — UL1TR002389: The Institute for Translational Medicine (ITM)
We are grateful to the following data partners whose data release is pending: The Rockefeller University — UL1TR001866: Center for Clinical and Translational Science • The Scripps Research Institute — UL1TR002550: Scripps Research Translational Institute • University of Texas Health Science Center at San Antonio — UL1TR002645: Institute for Integration of Medicine and Science • The University of Texas Health Science Center at Houston — UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • NorthShore University HealthSystem — UL1TR002389: The Institute for Translational Medicine (ITM) • Yale New Haven Hospital — UL1TR001863: Yale Center for Clinical Investigation • Emory University — UL1TR002378: Georgia Clinical and Translational Science Alliance • Weill Medical College of Cornell University — UL1TR002384: Weill Cornell Medicine Clinical and Translational Science Center • Montefiore Medical Center — UL1TR002556: Institute for Clinical and Translational Research at Einstein and Montefiore • Medical College of Wisconsin — UL1TR001436: Clinical and Translational Science Institute of Southeast Wisconsin • University of New Mexico Health Sciences Center — UL1TR001449: University of New Mexico Clinical and Translational Science Center • George Washington University — UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • Stanford University — UL1TR003142: Spectrum: The Stanford Center for Clinical and Translational Research and Education • Regenstrief Institute — UL1TR002529: Indiana Clinical and Translational Science Institute • Cincinnati Children’s Hospital Medical Center — UL1TR001425: Center for Clinical and Translational Science and Training • Boston University Medical Campus — UL1TR001430: Boston University Clinical and Translational Science Institute • University Medical Center New Orleans — U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • The State University of New York at Buffalo — UL1TR001412: Clinical and Translational Science Institute • Aurora Health Care — UL1TR002373: Wisconsin Network For Health Research • Brown University — U54GM115677: Advance Clinical Translational Research (Advance-CTR) • Rutgers, The State University of New Jersey — UL1TR003017: New Jersey Alliance for Clinical and Translational Science • Loyola University Chicago — UL1TR002389: The Institute for Translational Medicine (ITM) • New York University Grossman School of Medicine — UL1TR001445: Langone Health’s Clinical and Translational Science Institute • Children’s Hospital of Philadelphia — UL1TR001878: Institute for Translational Medicine and Therapeutics • University of Kansas Medical Center — UL1TR002366: Frontiers: University of Kansas Clinical and Translational Science Institute • Massachusetts General Brigham — UL1TR002541: Harvard Catalyst • OCHIN — INV-018455: Bill and Melinda Gates Foundation grant to Sage Bionetworks • Icahn School of Medicine at Mount Sinai — UL1TR001433: ConduITS Institute for Translational Sciences • Ochsner Medical Center — U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • HonorHealth — None (Voluntary) • University of California, Irvine — UL1TR001414: The UC Irvine Institute for Clinical and Translational Science (ICTS) • University of California, San Diego — UL1TR001442: Altman Clinical and Translational Research Institute • University of California, Davis — UL1TR001860: UCDavis Health Clinical and Translational Science Center • University of California, San Francisco — UL1TR001872: UCSF Clinical and Translational Science Institute • University of California, Los Angeles — UL1TR001881: UCLA Clinical Translational Science Institute
Justin T. Reese supported by Director, Office of Science, Office of Basic Energy Sciences of the U.S. Department of Energy Contract No. DE-AC02-05CH11231; Nomi L. Harris supported by Director, Office of Science, Office of Basic Energy Sciences of the U.S. Department of Energy Contract No. DE-AC02-05CH11231; Rachel Deer supported by UTMB CTSA, 2P30AG024832-16 (PI: Volpi); Christopher G. Chute supported by U24 TR002306; Heidi Spratt supported by NIH UL1TR001439 ; Christopher J. Mungall supported by Director, Office of Science, Office of Basic Energy Sciences of the U.S. Department of Energy Contract No. DE-AC02-05CH11231; Peter N. Robinson supported by Donald A. Roux Family Fund at the Jackson Laboratory
Conflicts of Interest
Katie Rebecca Bradwell: employee of Palantir Technologies; Melissa A. Haendel: co-founder Pryzm Health; Julie A. McMurry: Cofounder, Pryzm Health; Jasvinder Singh: JAS has received consultant fees from Crealta/Horizon, Medisys, Fidia, PK Med, Two labs Inc, Adept Field Solutions, Clinical Care options, Clearview healthcare partners, Putnam associates, Focus forward, Navigant consulting, Spherix, MedIQ, Jupiter Life Science, UBM LLC, Trio Health, Medscape, WebMD, and Practice Point communications; and the National Institutes of Health and the American College of Rheumatology. JAS owns stock options in TPT Global Tech, Vaxart pharmaceuticals and Charlotte’s Web Holdings, Inc. JAS previously owned stock options in Amarin, Viking and Moderna pharmaceuticals. JAS is on the speaker’s bureau of Simply Speaking. JAS is a member of the executive of Outcomes Measures in Rheumatology (OMERACT), an organization that develops outcome measures in rheumatology and receives arms-length funding from 12 companies.
References
- 1.COVID-19 Map - Johns Hopkins Coronavirus Resource Center [Internet]. [cited 2021 Mar 16];Available from: https://coronavirus.jhu.edu/map.html [Google Scholar]
- 2.Ortiz-Prado E, Simbaña-Rivera K, Gómez-Barreno L, et al. Clinical, molecular, and epidemiological characterization of the SARS-CoV-2 virus and the Coronavirus Disease 2019 (COVID-19), a comprehensive literature review. Diagn Microbiol Infect Dis 2020;98(1):115094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 2000;284(10):1247–55. [DOI] [PubMed] [Google Scholar]
- 4.Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 2004;329(7456):15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Micallef J, Soeiro T, Jonville-Béra A-P, French Society of Pharmacology, Therapeutics (SFPT). Non-steroidal anti-inflammatory drugs, pharmacology, and COVID-19 infection. Therapie 2020;75(4):355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voiriot G, Dury S, Parrot A, Mayaud C, Fartoukh M. Nonsteroidal antiinflammatory drugs may affect the presentation and course of community-acquired pneumonia. Chest 2011;139(2):387–94. [DOI] [PubMed] [Google Scholar]
- 7.Vaja R, Chan JSK, Ferreira P, et al. The COVID-19 ibuprofen controversy: A systematic review of NSAIDs in adult acute lower respiratory tract infections. Br J Clin Pharmacol 2021;87(3):776–84. [DOI] [PubMed] [Google Scholar]
- 8.Lund LC, Reilev M, Hallas J, et al. Association of Nonsteroidal Anti-inflammatory Drug Use and Adverse Outcomes Among Patients Hospitalized With Influenza. JAMA Netw Open 2020;3(7):e2013880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham NM, Burrell CJ, Douglas RM, Debelle P, Davies L. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus-infected volunteers. J Infect Dis 1990;162(6):1277–82. [DOI] [PubMed] [Google Scholar]
- 10.Bancos S, Bernard MP, Topham DJ, Phipps RP. Ibuprofen and other widely used non-steroidal anti-inflammatory drugs inhibit antibody production in human cells. Cell Immunol 2009;258(1):18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J 2008;22(2):383–90. [DOI] [PubMed] [Google Scholar]
- 12.Amici C, Di Caro A, Ciucci A, et al. Indomethacin has a potent antiviral activity against SARS coronavirus. Antivir Ther 2006;11(8):1021–30. [PubMed] [Google Scholar]
- 13.Wong AY, MacKenna B, Morton CE, et al. Use of non-steroidal anti-inflammatory drugs and risk of death from COVID-19: an OpenSAFELY cohort analysis based on two cohorts. Ann Rheum Dis [Internet] 2021;Available from: 10.1136/annrheumdis-2020-219517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi MH, Ahn H, Ryu HS, et al. Clinical Characteristics and Disease Progression in Early-Stage COVID-19 Patients in South Korea. J Clin Med Res [Internet] 2020;9(6). Available from: 10.3390/jcm9061959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2020;79(7):859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rinott E, Kozer E, Shapira Y, Bar-Haim A, Youngster I. Ibuprofen use and clinical outcomes in COVID-19 patients. Clin Microbiol Infect 2020;26(9):1259.e5–1259.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruce E, Barlow-Pay F, Short R, et al. Prior Routine Use of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Important Outcomes in Hospitalised Patients with COVID-19. J Clin Med Res [Internet] 2020;9(8). Available from: 10.3390/jcm9082586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow JH, Khanna AK, Kethireddy S, et al. Aspirin Use is Associated with Decreased Mechanical Ventilation, ICU Admission, and In-Hospital Mortality in Hospitalized Patients with COVID-19. Anesth Analg [Internet] 2020;Available from: 10.1213/ANE.0000000000005292 [DOI] [PubMed] [Google Scholar]
- 19.Voss EA, Makadia R, Matcho A, et al. Feasibility and utility of applications of the common data model to multiple, disparate observational health databases. J Am Med Inform Assoc 2015;22(3):553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haendel MA, Chute CG, Bennett TD, et al. The National COVID Cohort Collaborative (N3C): Rationale, design, infrastructure, and deployment. J Am Med Inform Assoc 2021;28(3):427–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett TD, Moffitt RA, Hajagos JG, et al. The National COVID Cohort Collaborative: Clinical Characterization and Early Severity Prediction. medRxiv [Internet] 2021;Available from: 10.1101/2021.01.12.21249511 [DOI] [Google Scholar]
- 22.Ursu O, Holmes J, Knockel J, et al. DrugCentral: online drug compendium. Nucleic Acids Res 2017;45(D1):D932–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hripcsak G, Duke JD, Shah NH, et al. Observational Health Data Sciences and Informatics (OHDSI): Opportunities for Observational Researchers. Stud Health Technol Inform 2015;216:574–8. [PMC free article] [PubMed] [Google Scholar]
- 24.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020;20(8):e192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maier C, Kapsner LA, Mate S, Prokosch H-U, Kraus S. Patient Cohort Identification on Time Series Data Using the OMOP Common Data Model. Appl Clin Inform 2021;12(1):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin SR, Wong Y-N, Uzzo RG, Beck JR, Egleston BL. Why Summary Comorbidity Measures Such As the Charlson Comorbidity Index and Elixhauser Score Work. Med Care 2015;53(9):e65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z. Propensity score method: a non-parametric technique to reduce model dependence. Ann Transl Med 2017;5(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss P, Murdoch DR. Clinical course and mortality risk of severe COVID-19 [Internet]. The Lancet. 2020;395(10229):1014–5. Available from: 10.1016/s0140-6736(20)30633-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendren NS, de Lemos JA, Ayers C, et al. Association of Body Mass Index and Age With Morbidity and Mortality in Patients Hospitalized With COVID-19: Results From the American Heart Association COVID-19 Cardiovascular Disease Registry. Circulation 2021;143(2):135–44. [DOI] [PubMed] [Google Scholar]
- 30.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med 2017;167(4):268–74. [DOI] [PubMed] [Google Scholar]
- 31.FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med 2001;345(6):433–42. [DOI] [PubMed] [Google Scholar]
- 32.Cannon CP, Cannon PJ. Physiology. COX-2 inhibitors and cardiovascular risk. Science 2012;336(6087):1386–7. [DOI] [PubMed] [Google Scholar]
- 33.Yu Y, Ricciotti E, Scalia R, et al. Vascular COX-2 modulates blood pressure and thrombosis in mice. Sci Transl Med 2012;4(132):132ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest 2006;116(1):4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology 2010;49(9):1618–31. [DOI] [PubMed] [Google Scholar]
- 36.Torjesen I. Ibuprofen can mask symptoms of infection and might worsen outcomes, says European drugs agency. BMJ 2020;369:m1614. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki S, Eastwood GM, Bailey M, et al. Paracetamol therapy and outcome of critically ill patients: a multicenter retrospective observational study. Crit Care 2015;19:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Day M. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ 2020;368:m1086. [DOI] [PubMed] [Google Scholar]
- 39.Voiriot G, Philippot Q, Elabbadi A, Elbim C, Chalumeau M, Fartoukh M. Risks Related to the Use of Non-Steroidal Anti-Inflammatory Drugs in Community-Acquired Pneumonia in Adult and Pediatric Patients. J Clin Med Res [Internet] 2019;8(6). Available from: 10.3390/jcm8060786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schönthal AH. Direct non-cyclooxygenase-2 targets of celecoxib and their potential relevance for cancer therapy. Br J Cancer 2007;97(11):1465–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.