Abstract
Introduction
Tranexamic acid (TXA) is the standard medication to prevent or treat hyperfibrinolysis. However, prolonged inhibition of lysis (so-called “fibrinolytic shutdown”) correlates with increased mortality. A new viscoelastometric test enables bedside quantification of the antifibrinolytic activity of TXA using tissue plasminogen activator (TPA).
Materials and Methods
Twenty-five cardiac surgery patients were included in this prospective observational study. In vivo, the viscoelastometric TPA test was used to determine lysis time (LT) and maximum lysis (ML) over 96 h after TXA bolus. Additionally, plasma concentrations of TXA and plasminogen activator inhibitor 1 (PAI-1) were measured. Moreover, dose effect curves from the blood of healthy volunteers were performed in vitro. Data are presented as median (25–75th percentile).
Results
In vivo TXA plasma concentration correlated with LT (r = 0.55; p < 0.0001) and ML (r = 0.62; p < 0.0001) at all time points. Lysis was inhibited up to 96 h (LT<sub>TPA-test</sub>: baseline: 398 s [229–421 s] vs. at 96 h: 886 s [626–2,175 s]; p = 0.0013). After 24 h, some patients (n = 8) had normalized lysis, but others (n = 17) had strong lysis inhibition (ML <30%; p < 0.001). The high- and low-lysis groups differed regarding kidney function (cystatin C: 1.64 [1.42–2.02] vs. 1.28 [1.01–1.52] mg/L; p = 0.002) in a post hoc analysis. Of note, TXA plasma concentration after 24 h was significantly higher in patients with impaired renal function (9.70 [2.89–13.45] vs.1.41 [1.30–2.34] µg/mL; p < 0.0001). In vitro, TXA concentrations of 10 µg/mL effectively inhibited fibrinolysis in all blood samples.
Conclusions
Determination of antifibrinolytic activity using the TPA test is feasible, and individual fibrinolytic capacity, e.g., in critically ill patients, can potentially be measured. This is of interest since TXA-induced lysis inhibition varies depending on kidney function.
Keywords: Tranexamic acid, Fibrinolysis shutdown, Hyperfibrinolysis, Cardiac surgery, Viscoelastometry, Thromboelastometry
Introduction
Intravenous administration of tranexamic acid (TXA) is highly recommended for treatment and prophylaxis of hyperfibrinolysis [1, 2], not least due to the fact that alternatives like aprotinin and ε-aminocaproic acid are not widely available or have very restricted indications [3, 4]. TXA administration to trauma patients and/or those undergoing major surgery reduces the need for red blood cell transfusions [4, 5, 6, 7, 8, 9, 10, 11]. It is known that hyperfibrinolysis is associated with increased mortality in severely injured patients [12, 13]. On the other hand, an extensive inhibition of fibrinolysis, so-called fibrinolytic shutdown, occurs frequently in trauma patients and is correlated with increased mortality, organ failure, and thrombotic complications [14, 15, 16, 17]. Nevertheless, administration of TXA remains the standard therapy for various conditions.
Rotational thromboelastometry (ROTEM) is established as routine bedside monitoring, and is sensitive in detecting hyperfibrinolysis and also inhibiting it by adding TXA or aprotinin. In contrast, thromboelastometric assays are not commonly used to measure fibrinolysis inhibition in clinical practice [18]. A few groups have used modified thrombelastography (TEG) and ROTEM by adding recombinant tissue plasminogen activator (TPA) to evaluate fibrinolytic capacity, e.g., in patients with fibrinolytic shutdown [17, 18, 19, 20]. However, these techniques are difficult to execute and are not available for bedside monitoring. Alternatively, direct measurement of TXA plasma concentration with standardized laboratory tests is possible but is only available in specialized institutions and is not suitable for emergency situations. We evaluated a newly available standardized TPA-based viscoelastometric assay, which induces lysis, thus enabling the monitoring of fibrinolysis inhibition, not only with TXA but also aprotinin.
We performed a prospective, observational study on patients undergoing cardiothoracic surgery, and evaluated the duration of the antifibrinolytic effect of TXA. We hypothesized that the TPA test correlates with TXA plasma concentration and can thus be used to evaluate the individual fibrinolytic capacity.
Material and Methods
Viscoelastometry
Viscoelastometry was performed using the commercially available point-of-care device ClotPro (enicor GmbH, Munich, Germany) and its consumables by following manufacturer's instructions [21]. ClotPro is a new system that uses a modified viscoelastometric technique, similar to the original technique introduced by Hartert [22]. Briefly, clot formation is measured using a cup and a pin, with the cup being rotated using an elastic element and the pin stationary during the measurement. When the blood clots, the rotation of the cup is compromised, which is continuously detected by the analyzer and transformed into a viscoelastometry amplitude. ClotPro has 6 independent test channels with up to 8 different assays, depending on the reagent added. Of note, all tests are heparin-insensitive due to the addition of polybrene. All reagents are provided in a ready-to-use dry format loaded in a syringe, and they are released into the blood by pipetting the blood up the syringe. Blood sample volume for each assay is 340 µL. In our study, we used the TPA test, where coagulation is initiated by recalcification and recombinant tissue factor, and TPA (650 ng/mL; concentration of the blood sample) is added to induce fibrinolysis within the blood sample.
All samples were analyzed under standardized conditions, with the temperature set to 37°C and the testing time to 4,500 s. Tests were performed on 3 ClotPro analyzers in a randomized order within 2 h after blood collection. Quality controls were routinely performed as recommended by the manufacturer. Furthermore, round-robin tests for intermachine and interday variability were performed once a week.
Parameters provided by the system are clotting time (CT; time from initiation of the clotting process to a 2-mm clot amplitude), clot formation time (CFT; CT until a clot amplitude of 20 mm is reached), A5 (clot amplitude 5 min after CT), A10 (clot amplitude 10 min after CT), maximum clot firmness (MCF; the maximum amplitude of the clot), maximum lysis (ML; the degree of lysis in relation to MCF during measurement), lysis time (LT; the time from CT until 50% lysis), and lysis onset time (LOT; the time from CT until 15% lysis). When no LT was recorded (because no lysis of 50% of MCF was recorded) during the measurement, the LT was set as 4,500 s.
Normal values for the TPA test (LT and ML) were determined in 60 healthy volunteers (online suppl. Table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000511230). Exclusion criteria were coagulation disorders and the intake of anticoagulants or platelet inhibitors within 30 days prior to study inclusion. Venous blood samples (S-Monovette Sarstedt, Nürnbrecht, Germany) were withdrawn once for viscoelastometric analysis and processed within 2 h.
TXA Plasma Concentration
TXA plasma concentration was assessed by ultra-high-performance liquid chromatography/mass spectrometry (UHPLC-MS/MS) as described by Barreiros et al. [23].
Plasminogen Activator Inhibitor and Tissue Plasminogen Activator
To further specify antifibrinolytic activity, antigen concentrations of plasminogen activator inhibitor 1 (PAI-1) and active PAI-1 as well as tissue plasminogen activator (tPA) were measured using Technozym ELISA (Technoclone GmbH, Vienna, Austria; PAI-1 antigen ELISA, normal range 7–43 ng/mL; PAI-1 Actibind ELISA, normal range 1–7 IU/mL; tPA antigen, normal range 2–8 ng/mL).
Standard Laboratory Tests
Standard laboratory tests including international normalized ratio (INR; Thromborel S), thrombin time (TT; Berichrom Thrombinreagenz), and activated partial thromboplastin time (aPTT; Actin FSL), all from Siemens Healthcare GmbH, Erlangen, Germany, were performed. Fibrinogen plasma concentration was measured by the Clauss method (optical measurement, Multifibren U) and tests were performed using an BCS XP analyzer (both from Siemens Healthcare).
Renal function was assessed using both serum creatinine (creatinine OSR6178; AU 5,800/AU 680 [Beckman Coulter]) and cystatin C [24], using a turbidimetric immunoassay (Biomed Diagnostics GmbH, Germany) with a stand-alone analyzer (Cobas 8000c702, Roche, Germany). All standard laboratory tests were performed by the LMU Munich Institute for Laboratory Medicine, according to institutional standards.
In vivo Study
Twenty-five patients undergoing elective cardiothoracic surgery on cardiopulmonary bypass (CPB) were included. Modified viscoelastometry was analyzed at different time points (see below). We included all adult patients undergoing cardiothoracic surgery on CPB. We excluded all patients with coagulation disorders, convulsive disorders, intake of TXA prior to surgery within the last 2 weeks, or haematopoietic disorders.
All patients obtained standardized instrumentation and anesthesia according to institutional standards and guidelines. In detail, patients were monitored including central venous and arterial line insertion. General anesthesia was initiated using sufentanil 0.6–1 µg/kg body weight (BW), propofol 2 mg/kg BW, and rocuronium 0.6 mg/kg BW, and then maintained with propofol 5 mg/kg BW/h and sufentanil 1 µg/kg BW/h. A bolus of 2,500 mg TXA (Carinopharm GmbH, Elze, Germany) was given after induction of anesthesia. Afterwards, 400 IE/kg BW unfractionated heparin was administered before initiation of cardiopulmonary bypass as well as a second dose of 2,500 mg TXA when extracorporeal circulation (priming) was initiated (after time point T2) according to institutional standard operating procedures (SOP). The priming volume of the extracorporeal circuit consisted of Jonosteril (Fresenius Kabi Deutschland GmbH, Bad Homburg, Germany) and an additional dosage of 10.000 I.E. heparin (ratiopharm GmbH, Ulm, Germany) for all patients.
Blood samples were taken immediately before TXA administration (baseline) as well as 0.5, 6, 24, 48, 72, and 96 h after TXA application. At every time point abovementioned coagulation tests were performed within 2 h and plasma was immediately stored at −80°C.
In vitro Study
Furthermore, we simulated the effect of TXA dosing on TPA-modified viscoelastometry in vitro, using blood samples from 6 different healthy individuals. Blood samples were spiked with 6 different TXA concentrations (0, 1, 2, 3, 5, and 10 µg/mL) and viscoelastometric variables were analyzed to determine and specify the amount of TXA-induced fibrinolysis inhibition.
Statistics
Data are presented as median (25–75th percentile) unless indicated otherwise. Statistical analysis was performed using GraphPad Prism 8 (La Jolla, USA) and SPSS v25 (IBM Corp., USA). Statistical differences between conditions/time points were analyzed using one-way-ANOVA and correction for multiple testing using Holm-Sidak's multiple comparison test. Correlations between viscoelastometric variables and cTXA were done by Spearman's correlation. Statistical differences between subgroups were analyzed using Mann-Whitney U test. Alpha error was adjusted for multiple testing (p = 0.05). An a priori sample size calculation was performed, using G * Power 3.1 (http://www.gpower.hhu.de/; Düsseldorf, Germany), and revealed 24 patients (f = 0.25; power = 0.8, ANOVA repeated measures).
Results
In vivo Study
All patients obtained elective cardiac surgery on cardiopulmonary bypass. Patients' characteristics, operations, and preoperative medications are displayed in Tables 1 and 2. Viscoelastometric reference values from healthy individuals are given in online supplementary Table 1. No patient received TXA postoperatively at the intensive care unit. Median intraoperative blood loss was 600 mL (400–900). Viscoelastometric variables at baseline differ from reference values (LT 298 s [339–421 s] vs. 210 s [186–262] s; p < 0.0001; ML 97% [96–97%] vs. 95% [94–96%]; p < 0.0001).
Table 1.
Characteristics of 25 patients
| Female/male | 8/17 (32/68) |
| Age, years | 61 (56–74) |
| Height, m | 1.75 (l.65–1.82) |
| Body weight, kg | 85 (66–93) |
| Body mass index | 26.6 (22.5–30.1) |
| ASA status | |
| I | 0 (0) |
| II | 0 (0) |
| III | 7 (28) |
| IV | 18 (72) |
| Surgery | |
| CABG | 5 (20) |
| CABG + valve surgery | 3 (12) |
| Aortic surgery | 6 (24) |
| Valve surgery | 11 (44) |
| CPB time, min | 144 (112–195) |
| Duration of surgery, min | 256 (211–320) |
| RBC, mL | 0 (0–500) |
| FFP, mL | 0 (0–750) |
| PC, mL | 300 (0–600) |
| Hemoglobin, g/dL | 12.8 (11.5–13.9) |
| INR | 1.0 (1.0–1.0) |
| Platelets, 109/L | 226 (178–253) |
| Fibrinogen, mg/dL | 340 (261–368) |
| aPTT, s | 26 (25–28) |
| GFR, mL/min | 94 (77–102) |
| Serum creatinine, mg/dL | 1.0 (0.8–1.2) |
| Serum cystatin C, mg/L | 1.58 (1.33–1.80) |
Values express n (%) or median (25–75th percentile). ASA, American Society of Anesthesiologists; CABG, coronary artery bypass surgery; CPB, cardiopulmonary bypass; RBC, red blood cell concentrate; FFP, fresh frozen plasma; PC, platelet concentrate; INR, international normalized ratio; aPTT, activated partial thromboplastin time; GFR, glomerular filtration rate.
Table 2.
Patients' preoperative medication (n = 25)
| Betablockers | 11 (46) |
| ACE inhibitors | 6 (25) |
| Sartanes | 7 (29) |
| Calcium channel blockers | 4 (17) |
| Diuretics | 11 (46) |
| DOAC | 2 (8) |
| Phenprocumon | 0 |
| ASA | 13 (54) |
| Clopidogrel | 2 (8) |
| Enoxaparine | 2 (8) |
| Antidiabetics | 2 (8) |
| Statins | 10 (42) |
| Antidepressants | 2 (8) |
| Pantoprazole | 5 (21) |
| Benzodiazepines | 2 (8) |
| Levothyroxine | 4 (17) |
| Digoxin | 1 (4) |
| Allopurinol | 2 (8) |
Values express n (%). ACE, angiotensin-converting enzyme; ASA, acetylsalicylic acid; DOAC, direct oral anticoagulant.
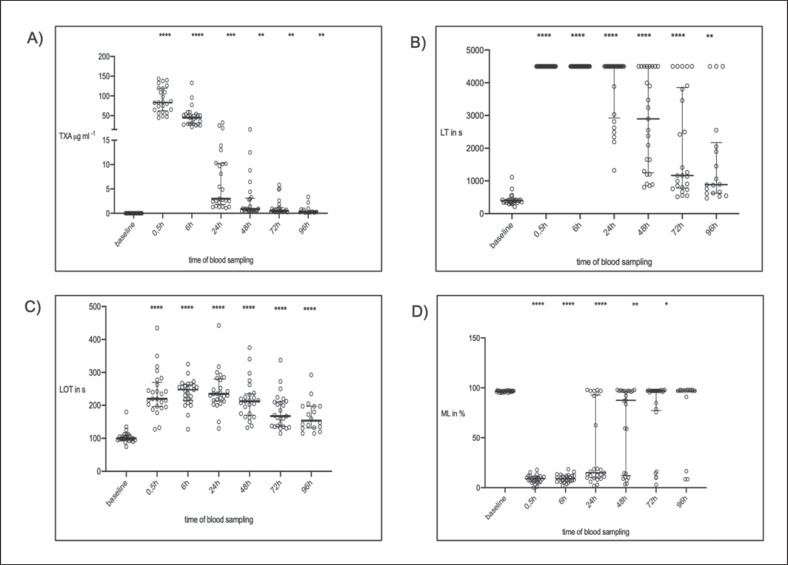
Following TXA administration, plasma concentration increased compared to baseline (0 µg/mL) at all time points with a peak concentration 0.5 h after application (83.3 [62.3–120] µg/mL; p < 0.0001). TXA plasma concentration remained detectable for as long as 96 h after application (0.29 [0.19–0.75] µg/mL; p = 0.005; Fig. 1A).
Fig. 1.
In vivo thromboelastometric variables (median + IQR; n = 25) over time. A Tranexamic acid (TXA) plasma concentration. B Lysis time (LTTPA-test) = time span between CT and 50% lysis. C Lysis onset time (LOTTPA-test) = time span from clotting time to 15% lysis. D Maximum lysis (MLTPA-test) = difference between MCF and lowest amplitude in % of MCF. * p < 0.05 vs. baseline (before TXA); ** p < 0.01 vs. baseline; *** p < 0.001 vs. baseline; **** p < 0.0001 vs. baseline.
There was a good correlation between TXA plasma concentration and LTTPA-test (r = 0.55; p < 0.0001). Effective lysis inhibition ex vivo could be seen in all samples 30 min after the application of TXA, expressed by a prolonged LTTPA-test compared to baseline (baseline: 398 s [229–421 s] vs. at 30 min: 4,500 s [4,500–4,500 s]; p < 0.001). At the following time points, LTTPA-test decreased (Fig. 1B). Even 96 h after application, a prolonged LTTPA-test was seen (baseline: 398 s [339–421 s] vs. at 96 h: 886 s [626–2,175 s]; p < 0.0013).
There was a moderate correlation between LOTTPA-test and cTXA (r = 0.3507; p < 0.0001). LOTTPA-test increased 0.5 h after administration of TXA compared to baseline (baseline: 100 s [95–110 s] vs. at 30 min: 220 s [196–270 s]; p < 0.0001; Fig. 1C) and was still prolonged after 96 h (baseline: 100 s [95–110 s] vs. at 96 h: 154 s [132–198 s]; p < 0.0001; Fig. 1C).
MLTPA-test correlated well with TXA plasma concentration (r = −0.62; p < 0.0001). MLTPA-test decreased from baseline to 0.5 h after TXA application (baseline: 97% [96–97%] vs. at 0.5 h: 9% [6–11%]; p < 0.0001), and remained decreased until 72 h after TXA application (Fig. 1D). It returned to baseline value 96 h after application (97% [96–98%]; p = 0.072; Fig. 1D).
The duration of lysis inhibition differed between patients; 24 h after TXA administration, some patients (n = 8) already had normal lysis compared to baseline (MLTPA-test >30%), but others (n = 17) had compromised lysis up to 96 h later (MLTPA-test <30%; Fig. 1D). For this aspect, we performed a post hoc analysis.
There was an association of the recovery of MLTPA-test 24 h after TXA application with endogenous antifibrinolytic activity and renal function: active PAI-1 plasma concentrations were almost 3 times greater in patients showing low lysis (MLTPA-test <30%: 62.62 [12.6–100.0] ng/mL vs. patients with higher lysis MLTPA-test >30%: 21.5 [9.88–53.07] ng/mL; p = 0.189). However, this difference was not significant. Additionally, the tissue plasminogen activator plasma concentration differed between both groups (MLTPA-test <30%: 3.8 [3.115–5.64] ng/mL vs. MLTPA-test >30%: 2.015 [1.975–3.513] ng/mL; p = 0.023). When stratifying patients by renal function, it turned out that patients with MLTPA-test <30% had higher serum creatinine values at baseline than patients with an MLTPA-test >30% (MLTPA-test <30%: 1 [1.0–1.3] mg/dL vs. MLTPA-test >30%: 0.8 [0.7–0.9] mg/dL; p = 0.023). The second renal marker at baseline, cystatin C, was also significantly different between groups (MLTPA-test <30%: 1.64 [1.42–2.02] mg/L vs. MLTPA-test >30%: 1.28 [1.01–1.52] mg/L; p = 0.002). According to the differences in renal function, TXA plasma concentration was significantly higher in the group with MLTPA-test <30% than the other group (MLTPA-test <30%: 9.70 [2.89–13.45] µg/mL vs. MLTPA-test >30%: 1.41 [1.30–2.34] µg/mL; p < 0.0001). All results in terms of these both groups are also displayed in online supplementary Table 2.
Of interest, there was only a moderate association between the lysis variables of active PAI-1 (LTTPA-test: r = 0.143; p = 0.066; LOTTPA-test: r = 0.158; p = 0.042; MLTPA-test: r = −0.139; p = 0.073).
In vitro Study
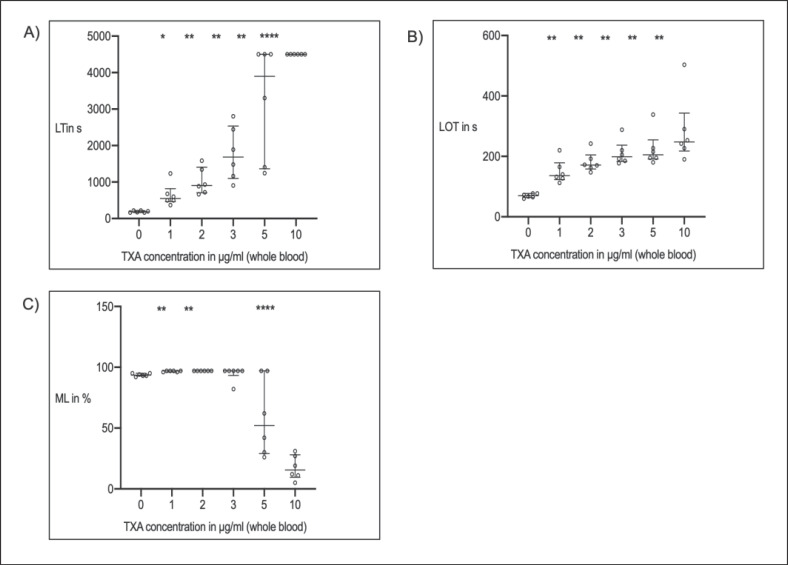
Following spiking of TXA to whole-blood samples of 6 healthy volunteers, the LTTPA-test and LOTTPA-test increased with escalating doses of TXA compared to baseline (Fig. 2A, B), and MLTPA-test decreased (Fig. 2C). There was a strong correlation between spiked dosages and LTTPA-test (r = 0.894, p < 0.0001), LOTTPA-test (r = 0.705; p < 0.0001), and MLTPA-test (r = −0.879; p < 0.0001), respectively. With TXA concentrations of 10 µg/mL MLTPA-test was highly inhibited (MLTPA-test <30%) and LTTPA-test exceeded the maximum run time (4,500 s) in all blood samples. Volunteer characteristics are displayed in Table 3.
Fig. 2.
In vitro, dose-effect curves of thromboelastometric variables (median + IQR; n = 25) and increasing whole-blood concentrations of tranexamic acid (TXA). A Lysis time (LTTPA-test) = time span between CT and 50% lysis. B Lysis onset time (LOTTPA-test) = time span from clotting time to 15% lysis. C Maximum lysis (MLTPA-test) = difference between MCF and lowest amplitude in % of MCF. * p < 0.05 vs. baseline (before TXA); ** p < 0.01 vs. baseline; *** p < 0.001 vs. baseline; **** p < 0.0001 vs. baseline.
Table 3.
Characteristics of 6 healthy volunteers
| Female/male | 3/3 (50/50) |
| Age, years | 49 (36–53) |
| Body mass index | 23.6 (20.3–26.6) |
| Height, m | 1.73 (l.66–1.83) |
| Mass, kg | 73 (57–83) |
| ASA status | |
| I | 6 (100) |
| II | 0 |
| III | 0 |
| IV | 0 |
| Hemoglobin, g/dL | 13.8 (13.1–15.8) |
| INR | 0.9 (0.9–1.0) |
| Platelets, 109/L | 248 (203–362) |
| Fibrinogen, mg/dL | 315 (266–398) |
| aPTT, s | 25 (25–27) |
| GFR, mL/min | 99 (86–105) |
| Serum creatinine, mg/dL | 0.8 (0.7–1.0) |
Values express n (%) or median (25–75th percentile). ASA, American Society of Anesthesiologists; INR, international normalized ratio; aPTT, activated partial thromboplastin time; GFR, glomerular filtration rate.
Discussion
Time effects of TXA on fibrinolysis using a bedside available TPA-triggered viscoelastometric assay (TPA test) were analyzed in cardiac surgery patients. TXA plasma concentrations correlated significantly with the lysis variables of the TPA test.
TXA-induced lysis inhibition varied greatly in cardiac surgery patients and was detectable up to 96 h. Variations in lysis inhibition were seen according to differences in endogenous antifibrinolytic activity and renal function.
Furthermore, in vitro, we observed a dose-dependent increase of LOTTPA-test and LTTPA-test and a dose-dependent decrease of MLTPA-test after the administration of TXA, with effective inhibition of fibrinolysis at a concentration of 10 µg/mL.
Optimal dosing of TXA is still under debate and has been the subject of several studies [4, 25, 26]. Higher doses of TXA have been shown to have greater efficacy in reducing postoperative bleeding than lower doses, but they may lead to dose-dependent complications such as seizures [27, 28]. Therefore, several trials involving trauma and obstetric patients recommended an initial dose of 1 g, which became the recommendation in the current guidelines on bleeding related to trauma and surgery [1, 2, 29]. Of note, this recommendation is regardless of BW. TXA administration is standard in orthopedic patients, i.e., those undergoing total hip arthroplasty [30], although these patients usually receive doses of 5–10 mg/kg BW which is far lower than the doses administered during cardiac surgery. Furthermore, uncertainty exists as to whether oral or intravenous administration is more favorable. In this regard, we performed a study analyzing whether there are differences between these regarding peak plasma concentrations and drug half-life. In cardiac surgery, higher TXA doses of up to 30–50 mg/kg are common, resulting in dosages up to 5 g [27]. Although the guidelines recommend the abovementioned dose, effective minimal TXA plasma concentrations and potentially reducing this dose in cases of renal insufficiency are still under discussion [31, 32]. The in vitro and in vivo results of our study, using a viscoelastometric approach, suggest that TXA plasma concentrations of 10 µg/mL are able to inhibit fibrinolysis completely; this was supported by the significant correlations of TXA plasma concentrations and TPA test lysis variables. These results are in agreement with recent recommendations of 10–15 µg/mL for the inhibition of fibrinolysis [32].
Another aspect of this trial concerning individual dosing is that the duration of fibrinolysis inhibition following the administration of a single dose of TXA seemed to be considerably longer than previously reported, as TXA was detectable in the plasma as long as 96 h after administration [33, 34]. A possible explanation could be both the relatively high dose of TXA administered in our study and the impaired renal function in some patients. This could be harmful if fibrinogen, as an acute-phase protein, is additionally upregulated postoperatively in a situation where fibrinolytic capacity is needed, e.g., during disseminated intravascular coagulation. In this context, BW-dependent dosing may be preferred over the guideline recommendations of 1 g TXA. This could also be of interest as renal impairment is an independent risk factor for TXA-associated seizures, as recently shown in 6,200 CABG patients [35]. These results provide a rationale for using lower TXA doses. Thromboelastometric monitoring could be a tool for monitoring individual fibrinolytic capacity in these cases. Several authors have investigated individual fibrinolytic capacity by the addition of rTPA to the standard TEG and ROTEM [18, 19, 20]. However, individual spiking is more complex to handle and is often not available at the bedside. In contrast, the system we used here is much easier to handle and the results therefore more reproducible and comparable.
Our data present a broad variation in lysis inhibition following TXA administration: while some patients showed an almost normal lytic response 24 h after application of TXA, other individuals had pronounced inhibition of TPA-induced clot lysis as late as 96 h after TXA administration. This phenotype with prolonged lysis inhibition might be considered “fibrinolytic shutdown.” These interindividual differences in lytic response were reproducible (double determinations of the TPA test showed similar phenotypes of fibrinolysis or clot stability). In order to understand the observed variability, we stratified patients according to their lytic response 24 h after TXA application; those showing a normalized lytic response to TPA or ongoing lysis inhibition were compared with respect to markers of renal function and endogenous antifibrinolytic capacity. Patients with ongoing lysis inhibition at 24 h had higher plasma concentrations of cystatin C and creatinine. Absolute differences in serum creatinine were small (1 vs. 0.8 mg/dL). The differences for the more sensitive marker cystatin C were higher (1.64 vs. 1.28 mg/L), which underlines the sensitivity of this parameter in mild renal dysfunction [24]. Most important, patients with prolonged lysis inhibition had impaired renal function, but also significantly higher TXA plasma concentrations (9.70 [2.89–13.45] µg/mL), which were comparable to the minimally effective spiked TXA concentrations in the whole-blood samples of healthy volunteers (10 µg/mL). This fact can be attributed to the almost complete renal elimination of TXA [31, 36].
In addition, patients with limited fibrinolysis at 24 h differed with regard to the levels of tPA and active PAI-1, an endogenous antifibrinolytic protein. The latter points to a synergism between TXA and PAI-1, resulting in a fibrinolytic shutdown associated with adverse outcomes in some publications (although other authors found no association between fibrinolytic shutdown and mortality in polytraumatized patients) [16, 17, 37]. PAI-1 is a major regulator of the balance between clot lysis and formation, and acts by reversibly binding tPA in its active center [38, 39]. PAI-1 concentration can increase drastically during diseases and after major surgery, which may explain the fibrinolysis inhibition observed in some samples with detectable but very low TXA concentrations. The higher levels of active PAI-1 in some of our patients could be explained by a better capacity of endothelial cells to release PAI-1 and a different capacity of hepatocytes to produce PAI-1 as well as by individual variability. Nevertheless, even if patients with inhibited fibrinolysis at 24 h after TXA and individuals with normalized lysis differed regarding active PAI-1 and tPA plasma concentrations, both values were within the normal range. The value of these results should therefore not be overestimated.
Key findings of our study were the prolonged lysis inhibition as well as prolonged measurable TXA plasma concentrations combined with changes in endogenous antifibrinolytic substances. One might emphasize that it is a combination of these factors that leads to the prolongation of lysis variables. Of note, we only observed a good correlation between LT and cTXA (r = 0.55) but not between LT and endogenous antifibrinolytic substances (active PAI; r = 0.143). The association between lysis variables and active PAI was weak, which may be explained by the stronger effect of TXA on fibrinolysis compared to PAI-1. There may be additional endogenous mechanisms contributing to altered induced clot lysis (TPA test) which were not analyzed in this study.
Our study has some limitations. First, our investigation was not designed to detect clinical outcome variables like bleeding, seizures, or thromboembolic complications. It was conducted to show the feasibility and reliability of a bedside-available new viscoelastometric assay. Our results are therefore still not conclusive regarding patient outcomes. Second, the in vitro evaluations might only partly reflect the in vivo situation. However, dose-effect curves help to verify the functionality of the test and supported recent recommendations concerning effective TXA plasma concentrations. Third, patients in the in vivo part were heterogeneous according to their medication, preexisting conditions, and surgical procedure. Last, TXA dosing was at the upper limit of the dose recommendation (50 mg/kg BW) [31, 40]. However, several trials reported even higher dosages of TXA with target plasma concentrations >114 µg/mL to increase thrombin formation, which might be another mechanism of TXA action [41]. Our findings do not reflect the situation in patients receiving lower TXA doses and should therefore be considered with caution.
Conclusions
The measured TXA plasma concentrations correlated with the results of a new viscoelastometric assay representing fibrinolytic capacity. The functional method applied in our study is reproducible and seems well-suited to the bedside monitoring of TXA effects in clinical routine. Our study revealed a marked interindividual variability of TXA effects using the new functional TPA test. The differences observed were related to renal function. Inhibition of fibrinolysis by high-dose TXA lasted for several days in a proportion of individuals. This could be of interest, e.g., for critically ill patients with a risk of fibrinolytic shutdown or patients who need an urgent reoperation and may need a second dose of TXA. Our data suggest that differentiated and individualized dose adjustments of TXA are advisable, even in patients with mild renal impairment. However, these data should be evaluated in further randomized trials with clinical outcome measurements.
Statement of Ethics
The study was approved by the LMU Munich Ethics Committee (No. 17-525-4; August 8, 2018), registered by the German clinical trials database (ID: DRKS00015269; August 17, 2018), and performed at the University Hospital Munich in accordance with the Declaration of Helsinki. Viscoelastometric reference values were determined using blood samples of healthy volunteers at the University Hospital Augsburg after approval of the institutional review board (No. 2018-13). Written consent was obtained from healthy volunteers and patients prior to study inclusion.
Conflict of Interest Statement
The authors declare there are no competing interests.
Funding Sources
The study was funded by institutional resources as well as a FÖFOLE research grant by the medical faculty of the LMU Munich to P.G. Reagents and thromboelastometric machines were provided by enicor GmbH, Munich.
Author Contributions
S.T.S., T.K., and P.G.: study design. S.T.S., T.K., P.G., S.P., and P.S.: literature search. S.R.S., P.G., and T.S.: data extraction. C.H., S.P., and P.S.: assessment of bias. P.G., T.K., S.T.S., and S.R.S.: data analysis. T.K., P.G., S.T.S., and T.S.: drafting of the manuscript. P.G., S.R.S., and P.S.: patient/volunteer recruitment. S.R.S., P.A.S., and P.G.: blood sample analysis of patients and volunteers. A.G.: reference values. All authors: revision of the manuscript.
Supplementary Material
Supplementary data
Supplementary data
Acknowledgments
We thank Marcela Alves Segundo (Department of Chemistry of the Faculty of Pharmacy, University of Porto) for the measurement of TXA plasma concentrations.
References
- 1.Spahn DR, Bouillon B, Cerny V, Duranteau J, Filipescu D, Hunt BJ, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care. 2019 Mar;23((1)):98. doi: 10.1186/s13054-019-2347-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kozek-Langenecker SA, Ahmed AB, Afshari A, Albaladejo P, Aldecoa C, Barauskas G, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: First update 2016. Eur J Anaesthesiol. 2017 Jun;34((6)):332–95. doi: 10.1097/EJA.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 3.European Society of Anaesthesiology task force reports on place of aprotinin in clinical anaesthesia. Aprotinin: is it time to reconsider? Eur J Anaesthesiol. 2015 Sep;32((9)):591–5. doi: 10.1097/EJA.0000000000000284. [DOI] [PubMed] [Google Scholar]
- 4.Levy JH, Koster A, Quinones QJ, Milling TJ, Key NS. Antifibrinolytic Therapy and Perioperative Considerations. Anesthesiology. 2018 Mar;128((3)):657–70. doi: 10.1097/ALN.0000000000001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishijima DK, Kuppermann N, Roberts I, VanBuren JM, Tancredi DJ. The Effect of Tranexamic Acid on Functional Outcomes: An Exploratory Analysis of the CRASH-2 Randomized Controlled Trial. Ann Emerg Med. 2019 Jan;74((1)):79–87. doi: 10.1016/j.annemergmed.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Henry DA, Carless PA, Moxey AJ, O'Connell D, Stokes BJ, Fergusson DA, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011 Mar;((3)):CD001886. doi: 10.1002/14651858.CD001886.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, Society of Thoracic Surgeons Blood Conservation Guideline Task Force et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011 Mar;91((3)):944–82. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 8.Myles PS, Smith JA, Painter T. Tranexamic Acid in Patients Undergoing Coronary-Artery Surgery. N Engl J Med. 2017 May;376((19)):1893. doi: 10.1056/NEJMc1703369. [DOI] [PubMed] [Google Scholar]
- 9.Polanco-García M, Capielo AM, Miret X, Chamero A, Sainz J, Revilla E, et al. Effectiveness of a patient blood management protocol on reduction of allogeneic red blood cell transfusions in orthopedic surgery. Med Clin (Barc) 2019 Feb;152((3)):90–7. doi: 10.1016/j.medcli.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Hooda B, Chouhan RS, Rath GP, Bithal PK, Suri A, Lamsal R. Effect of tranexamic acid on intraoperative blood loss and transfusion requirements in patients undergoing excision of intracranial meningioma. J Clin Neurosci. 2017 Jul;41:132–8. doi: 10.1016/j.jocn.2017.02.053. [DOI] [PubMed] [Google Scholar]
- 11.Houston BL, Uminski K, Mutter T, Rimmer E, Houston DS, Menard CE, et al. Efficacy and Safety of Tranexamic Acid in Major Non-cardiac Surgeries at High Risk of Transfusion: A Systematic Review and Meta-Analysis. Transfus Med Rev. 2019 Oct;34((1)):51–62. doi: 10.1016/j.tmrv.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Cotton BA, Harvin JA, Kostousouv V, Minei KM, Radwan ZA, Schöchl H, et al. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012 Aug;73((2)):365–70. doi: 10.1097/TA.0b013e31825c1234. [DOI] [PubMed] [Google Scholar]
- 13.Ives C, Inaba K, Branco BC, Okoye O, Schochl H, Talving P, et al. Hyperfibrinolysis elicited via thromboelastography predicts mortality in trauma. J Am Coll Surg. 2012 Oct;215((4)):496–502. doi: 10.1016/j.jamcollsurg.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Moore HB, Moore EE, Liras IN, Gonzalez E, Harvin JA, Holcomb JB, et al. Acute Fibrinolysis Shutdown after Injury Occurs Frequently and Increases Mortality: A Multicenter Evaluation of 2,540 Severely Injured Patients. J Am Coll Surg. 2016 Apr;222((4)):347–55. doi: 10.1016/j.jamcollsurg.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meizoso JP, Dudaryk R, Mulder MB, Ray JJ, Karcutskie CA, Eidelson SA, et al. Increased risk of fibrinolysis shutdown among severely injured trauma patients receiving tranexamic acid. J Trauma Acute Care Surg. 2018 Mar;84((3)):426–32. doi: 10.1097/TA.0000000000001792. [DOI] [PubMed] [Google Scholar]
- 16.Nicolau-Raducu R, Beduschi T, Vianna R, Diez C, Sleem M, Singh BP, et al. Fibrinolysis Shutdown Is Associated With Thrombotic and Hemorrhagic Complications and Poorer Outcomes After Liver Transplantation. Liver Transpl. 2019 Mar;25((3)):380–7. doi: 10.1002/lt.25394. [DOI] [PubMed] [Google Scholar]
- 17.Moore HB, Moore EE, Huebner BR, Dzieciatkowska M, Stettler GR, Nunns GR, et al. Fibrinolysis shutdown is associated with a fivefold increase in mortality in trauma patients lacking hypersensitivity to tissue plasminogen activator. J Trauma Acute Care Surg. 2017 Dec;83((6)):1014–22. doi: 10.1097/TA.0000000000001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuiper GJ, Kleinegris MC, van Oerle R, Spronk HM, Lancé MD, Ten Cate H, et al. Validation of a modified thromboelastometry approach to detect changes in fibrinolytic activity. Thromb J. 2016 Jan;14((1)):1. doi: 10.1186/s12959-016-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kupesiz A, Rajpurkar M, Warrier I, Hollon W, Tosun O, Lusher J, et al. Tissue plasminogen activator induced fibrinolysis: standardization of method using thromboelastography. Blood Coagul Fibrinolysis. 2010 Jun;21((4)):320–4. doi: 10.1097/MBC.0b013e32833464e9. [DOI] [PubMed] [Google Scholar]
- 20.Dirkmann D, Radü-Berlemann J, Görlinger K, Peters J. Recombinant tissue-type plasminogen activator–evoked hyperfibrinolysis is enhanced by acidosis and inhibited by hypothermia but still can be blocked by tranexamic acid. J Trauma Acute Care Surg. 2013;74((2)):482–8. doi: 10.1097/TA.0b013e318280dec1. [DOI] [PubMed] [Google Scholar]
- 21.Calatzis A, Wittwer M, Leyser H, Hipp Q, Spannagl M. ClotPro − a new generation viscoelastic whole blood coagulation analyzer. Hämostaseologie 2018. Schattauer; 2018. p. p. P013. [Google Scholar]
- 22.Hartert H. Die Thrombelastographie. Z Gesamte Exp Med. 1951;117((2)):189–203. [PubMed] [Google Scholar]
- 23.Barreiros L, Amoreira JL, Machado S, Fernandes SR, Silva EM, Sá P, et al. Determination of tranexamic acid in human plasma by UHPLC coupled with tandem mass spectrometry targeting sub-microgram per milliliter levels. Microchem J. 2019;144:144–50. [Google Scholar]
- 24.Shlipak MG, Matsushita K, Arnlov J, Inker LA, Katz R, Polkinghorne KR, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369((10)):932–43. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl. 1980;14(Suppl 14):41–7. [PMC free article] [PubMed] [Google Scholar]
- 26.Sigaut S, Tremey B, Ouattara A, Couturier R, Taberlet C, Grassin-Delyle S, et al. Comparison of two doses of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. Anesthesiology. 2014 Mar;120((3)):590–600. doi: 10.1097/ALN.0b013e3182a443e8. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Bai Y, Chen M, Zhou Y, Yu X, Zhou H, et al. The safety and efficiency of intravenous administration of tranexamic acid in coronary artery bypass grafting (CABG): a meta-analysis of 28 randomized controlled trials. BMC Anesthesiol. 2019 Jun;19((1)):104. doi: 10.1186/s12871-019-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manji RA, Grocott HP, Leake J, Ariano RE, Manji JS, Menkis AH, et al. Seizures following cardiac surgery: the impact of tranexamic acid and other risk factors. Can J Anaesth. 2012 Jan;59((1)):6–13. doi: 10.1007/s12630-011-9618-z. [DOI] [PubMed] [Google Scholar]
- 29.Collis RE, Collins PW. Haemostatic management of obstetric haemorrhage. Anaesthesia. 2015 Jan;70((Suppl 1)):78–86. doi: 10.1111/anae.12913. e27–8. [DOI] [PubMed] [Google Scholar]
- 30.Xiao C, Zhang S, Long N, Yu W, Jiang Y. Is intravenous tranexamic acid effective and safe during hip fracture surgery? An updated meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. 2019 Jul;139((7)):893–902. doi: 10.1007/s00402-019-03118-6. [DOI] [PubMed] [Google Scholar]
- 31.Jerath A, Yang QJ, Pang KS, Looby N, Reyes-Garces N, Vasiljevic T, et al. Tranexamic Acid Dosing for Cardiac Surgical Patients With Chronic Renal Dysfunction: A New Dosing Regimen. Anesth Analg. 2018 Dec;127((6)):1323–32. doi: 10.1213/ANE.0000000000002724. [DOI] [PubMed] [Google Scholar]
- 32.Picetti R, Shakur-Still H, Medcalf RL, Standing JF, Roberts I. What concentration of tranexamic acid is needed to inhibit fibrinolysis? A systematic review of pharmacodynamics studies. Blood Coagul Fibrinolysis. 2019 Jan;30((1)):1–10. doi: 10.1097/MBC.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eriksson O, Kjellman H, Pilbrant A, Schannong M. Pharmacokinetics of tranexamic acid after intravenous administration to normal volunteers. Eur J Clin Pharmacol. 1974 Aug;7((5)):375–80. doi: 10.1007/BF00558210. [DOI] [PubMed] [Google Scholar]
- 34.Ausen K, Pleym H, Liu J, Hegstad S, Nordgård HB, Pavlovic I, et al. Serum Concentrations and Pharmacokinetics of Tranexamic Acid after Two Means of Topical Administration in Massive Weight Loss Skin-Reducing Surgery. Plast Reconstr Surg. 2019 Jun;143((6)):1169e–78e. doi: 10.1097/PRS.0000000000005620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hulde N, Zittermann A, Deutsch MA, von Dossow V, Gummert JF, Koster A. Tranexamic acid and convulsive seizures after off-pump coronary artery bypass surgery: the role of renal insufficiency. Interact Cardiovasc Thorac Surg. 2019 Dec;29((6)):852–4. doi: 10.1093/icvts/ivz188. [DOI] [PubMed] [Google Scholar]
- 36.Kawashima E, Yuasa M, Maehira M, Soga M, Aoi R, Takahashi K, et al. Masui. 2017 Mar;66((3)):306–8. [PubMed] [Google Scholar]
- 37.Gomez-Builes JC, Acuna SA, Nascimento B, Madotto F, Rizoli SB. Harmful or Physiologic: Diagnosing Fibrinolysis Shutdown in a Trauma Cohort With Rotational Thromboelastometry. Anesth Analg. 2018 Oct;127((4)):840–9. doi: 10.1213/ANE.0000000000003341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griemert EV, Schwarzmaier SM, Hummel R, Gölz C, Yang D, Neuhaus W, et al. Plasminogen activator inhibitor-1 augments damage by impairing fibrinolysis after traumatic brain injury. Ann Neurol. 2019 May;85((5)):667–80. doi: 10.1002/ana.25458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawrence DA, Olson ST, Muhammad S, Day DE, Kvassman JO, Ginsburg D, et al. Partitioning of serpin-proteinase reactions between stable inhibition and substrate cleavage is regulated by the rate of serpin reactive center loop insertion into beta-sheet A. J Biol Chem. 2000 Feb;275((8)):5839–44. doi: 10.1074/jbc.275.8.5839. [DOI] [PubMed] [Google Scholar]
- 40.Menkis AH, Martin J, Cheng DC, Fitzgerald DC, Freedman JJ, Gao C, et al. Drug, devices, technologies, and techniques for blood management in minimally invasive and conventional cardiothoracic surgery: a consensus statement from the International Society for Minimally Invasive Cardiothoracic Surgery (ISMICS) 2011. Innovations. 2012 Jul;7((4)):229–41. doi: 10.1097/IMI.0b013e3182747699. [DOI] [PubMed] [Google Scholar]
- 41.Guo J, Gao X, Ma Y, Lv H, Hu W, Zhang S, et al. Different dose regimes and administration methods of tranexamic acid in cardiac surgery: a meta-analysis of randomized trials. BMC Anesthesiol. 2019 Jul;19((1)):129. doi: 10.1186/s12871-019-0772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data