Hospital pharmacists: making the difference by improving medication safety
Patient safety and quality of care are a priority for hospital pharmacists all across Europe. The European Association of Hospital Pharmacists (EAHP) has manifested the importance of patient safety for hospital pharmacy practice in a dedicated section of its European Statements of Hospital Pharmacy.1 As stewards of patients’ medication safety, hospital pharmacists are the key stakeholders ensuring the safe, effective and rational use of medicines by upholding the ‘rights’ of patients. This includes improving the safety of using medications, especially high-risk medication and look-alike and sound-alike (LASA) medications, through their close surveillance as well as advising on the most appropriate use of medicines. While some prescribing, dispensing and administration may not cause harm, it may not be cost-effective or beneficial for the patient. The abovementioned phrase 'appropriate use' encompasses a multitude of situations.
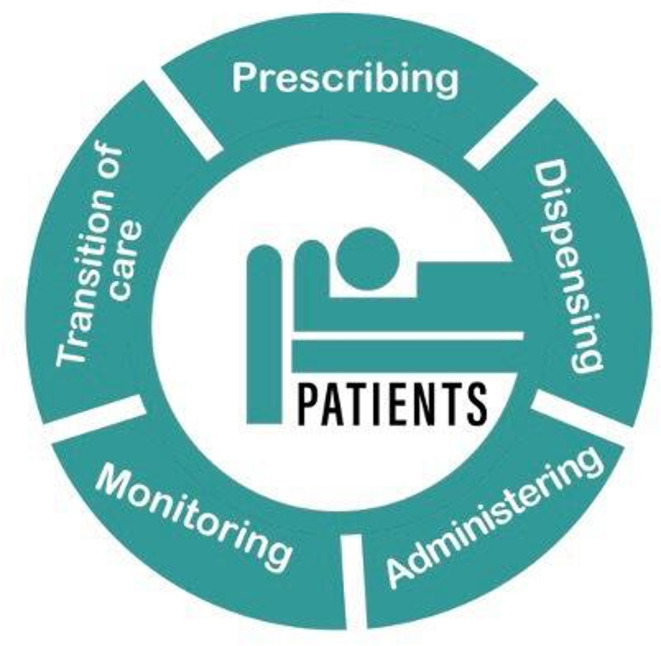
Patient safety, which lies at the heart of all representations made by EAHP and its members, covers many aspects. EAHP’s position on patient safety specifically focuses on medication safety. In particular, medication errors, which occur when a medicine has been inappropriately prescribed, prepared, dispensed or administered to a patient, are a key concern for hospital pharmacists. Throughout the medication use process specific measures must be taken by healthcare professionals (figure 1). For hospital pharmacists these include improving medication safety by reducing available harm through using risk assessment procedures.
Figure 1.
The medication use process from a patient perspective.
Even before a patient is treated for a specific disease, hospital pharmacists ensure the safety of the patient throughout the care pathway. These include actions linked to the hospital formulary, such as selecting narrow-spectrum antibiotics to prevent resistance, and implementation of the decision of the Drug and Therapeutics Committee, the proper selection and procurement as well as the application of appropriate quality assurance strategies for the medication use processes to detect errors and identify priorities for improvement. Also, awareness raising about prophylactic measures is an important factor that will be included in the quality assurance strategies for the medication use processes.
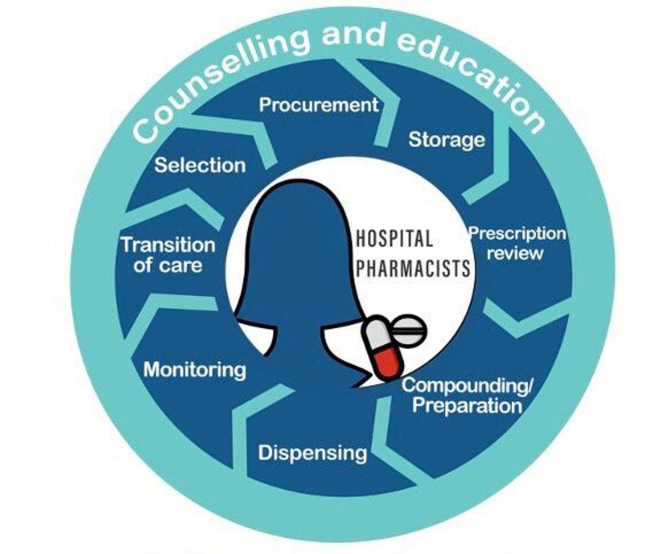
During the selection and procurement processes, the hospital pharmacist reviews the needs of the healthcare system and takes into account important safety aspects linked to, for example, LASA medications, standard concentrations, different pharmaceutical forms or active substances and safety alert monitoring (figure 2). Storage fulfilling all safety standards is carried out to separate similar medicines from each other so that errors during dispensing may be avoided. Transcribing errors are among the most common errors that can compromise patient safety. These are caused by illegible handwriting, error-prone abbreviations, including inappropriate defaults in electronic health records, and the lack of familiarity with drug names, doses and frequencies. The use of IT systems, such as computerised physician order entry systems or the Kardex information system for nurses, can reduce the risk of transcription errors. Additionally, the prescription review carried out by the hospital pharmacist prior to the medicine preparation helps to avoid safety problems caused by incorrect medications, doses, patients and routes. Other safeguards which are taken before administration include the preparation/compounding of medicines under the correct conditions and the supervision of reconstitution activities. Also, dispensing with pharmacy oversight ensures that the item dispensed matches the item ordered for the patient.
Figure 2.
The role of the hospital pharmacist in the medication use process.
Before administration, it is important that all involved parties including the patient and the practitioner are informed of the correct procedures to prevent problems and medication errors. This process can be facilitated by different tools, such as visual guidance, written instructions, checklists and barcoding.
Other areas of avoidable harm can be overcome by improving an individual’s knowledge about their condition and treatment, including preventive medicines such as vaccines, increasing medication concordance, reducing the incidence of side effects and adverse events, and carrying out medication reconciliation. To further foster patient safety in European hospitals different steps, including more far-reaching interprofessional collaboration, are needed to reduce medication errors and healthcare-associated infections (HAIs), heighten the uptake of closed-loop medication administration, increase medication concordance by patients and improve medication reconciliation. To achieve these aims:
EAHP recommends a wider application of different risk management tools, including but not limited to single-unit dose barcoding, risk management and quality control committees and computerised order entry systems in hospitals to lower medication errors for the benefits of patients.
EAHP advocates for the uptake of closed-loop medication management to facilitate a faster and more accurate administration of medicines in hospitals.
EAHP recommends the implementation of clinical pharmacy services that can guarantee the medication appropriateness, reconciliation and the personalisation of therapies to further enhance the safety and the quality of pharmaceutical care in Europe.
EAHP calls on national governments and health system providers to guarantee an adequate number of hospital pharmacists and the full utilisation of pharmacy services to improve concordance.
EAHP requests the strengthening of interprofessional collaboration and communication among healthcare personnel in all healthcare settings.
EAHP recommends the universal application of infection prevention and control measures by the European Centre for Disease Prevention and Control (ECDC) and the World Health Organization (WHO) among healthcare professionals.
Medication error reduction: increasing patient safety through the use of risk management tools
Unsafe medication practices and errors are the leading cause of avoidable harm in healthcare settings around the world.2 Data from European hospitals consistently demonstrate that medical errors and healthcare-related adverse events occur in 8% to 12% of hospitalisations and 18% of European Union citizens claim to have experienced a serious medical error in a hospital.3 A significant portion of these errors leads to longer hospital stays, needless readmissions and higher costs.4–7 These errors are preventable through comprehensive and systematic approaches to patient safety throughout the medication use process. Mandatory action is necessary to prevent and mitigate the harm that patients can suffer due to a medication error. Such action includes, for example, the conduct of active and proactive risk assessments by all healthcare professionals involved in the care and at the transition of care.8 Also, risk management and quality control committees, reliable IT systems, the use of computerised physician order entry systems or Kardex information system for nurses as well as educated healthcare professionals can contribute immensely.9
Special attention should be paid to high-risk medication as well as LASA products. These should be separated clearly to avoid confusion. Different tools exist to lower the risks to patient safety when selecting these medicines. Barcoding medicinal products to the single-unit dose and bedside scanning of medications have for example proven to be an efficient way to diminish medication errors and adverse drug events.10 In Dutch hospitals alone the use of single-unit dose barcoding would save 47 patient lives each year.11 While in Denmark, a 57% decrease in medication administration errors on a haematological ward was observed after the introduction of single-unit dose barcoding. This measure resulted in a cost-effectiveness ratio of €2.01 per avoided administration error.4 Similar financial findings were reported by a study for Dutch hospitals which concluded that the countrywide usage of the/a single-unit dose barcoding could lower healthcare spending by €21 million per year.11 EAHP recommends a wider application of different risk management tools, including but not limited to single-unit dose barcoding, risk management and quality control committees and computerised order entry systems in hospitals to lower medication errors for the benefits of patients. The application of these tools should, however, not neglect staffing considerations since only an adequately staffed hospital pharmacy can ensure that the different risk management tools are correctly applied. Another important factor for medication error reduction is the promotion of a positive safety culture in hospitals following a transparent and non-punitive approach. The education and training of healthcare professionals regarding medication safety should be fostered.
Closed-loop medication management: facilitating the uptake of closed-loop medication management
To further enhance patient safety, closed-loop medicines administration should be used. ‘Closed-loop medicines administration’ is defined as a process that integrates automated and intelligent systems to completely close the inpatient medication management and administration loop. As shown by a study conducted in the UK, closed-loop medication management reduced medication administration and prescription error rates and freed up pharmacists' time so they could concentrate on other care aspects.12 The German Association of Hospital Pharmacists declared the adoption of closed-loop medication management as its goal for 2021, which should go hand in hand with the regular use of ward pharmacists for better medication management.13 In view of these benefits, EAHP advocates for the uptake of closed-loop medication management to facilitate a faster and more accurate administration of medicines in hospitals.
Transition of care: expanding patient safety by providing medication reconciliation at care transitions
Medication reconciliation is the formal process by which healthcare professionals interact with patients to ensure accurate and complete medication information transfer at all stages of care.14 This intervention is particularly important at the admission and the discharge of the patient. Hospital pharmacists, as the health system’s experts in medication management, dosing, adverse drug-related reactions and cost-effectiveness, have a critical role to play during these interactions.15 16 The acknowledgement of hospital pharmacists’ drug expertise and the investment in medication reconciliation and optimisation roles in all healthcare settings, including nursing homes, should be seen as a key component of the European response to the increasing prevalence of polypharmacy. EAHP recommends the implementation of clinical pharmacy services that can guarantee the medication appropriateness, reconciliation and the personalisation of therapies to further enhance the safety and the quality of pharmaceutical care in Europe. The suggestions contained within the Council of Europe Resolution on the implementation of pharmaceutical care for the benefit of patients and health services should be used.17
Concordance: improving patient safety by increasing medication concordance
Medication concordance is a key factor in ameliorating patient safety. The concept of concordance was recognised in the UK in the 2000s and stretches from prescribing communication to patient support in medicine taking. It is wider than adherence, which mainly covers compliance with the recommendations of a healthcare professional.18
Pharmacists interact frequently with patients and are thus a valuable source of information on adverse effects, contraindications and interactions of combinations of different medications. Their involvement in medication reviews in the hospital setting is very effective in preventing adverse drug reactions, which are oftentimes feared by patients and may result in non-adherence.19 Studies have shown that older patients in particular benefit the most from these interactions.20 These should be carried out in accordance with patient-centred care through a therapeutic alliance between the healthcare professional and the patient in which both interact as equals.21 Consequently, EAHP calls on national governments and health system managers to guarantee an adequate number of hospital pharmacists and the full utilisation of pharmacy services to improve concordance.
Interprofessional collaboration: advancing patient safety through interprofessional collaboration and decreasing HAIs
Interprofessional collaboration is an important means for facilitating communication between health professionals in clinical practice. Involving different professions in multidisciplinary team discussions is not only beneficial for the exchange among professionals but contributes immensely to patient safety. Exchanges between physicians and hospital pharmacists at the stage of ordering and prescribing medicines can effectively solve and prevent clinically significant drug-related problems leading to reduced length of hospital stay, fewer readmissions and fewer disease events.22 The close collaboration between nurses and pharmacists has been proven to improve the safety of medication management on hospital wards.23 Incorporating hospital pharmacists into multidisciplinary teams on hospital wards is a cost-effective way to advance patient safety by increasing treatment safety and reducing the use of non-necessary drugs.24 Consequently, EAHP requests strengthened interprofessional collaboration and communication among healthcare personnel in all healthcare settings.
Healthcare-associated infections: reducing HAIs through infection prevention and control measures
HAIs are a patient safety issue that is affecting all types of healthcare organisations. Hand hygiene, as the single most important prophylactic measure, needs to be further promoted.25 EAHP recommends the universal application of infection prevention and control measures by the ECDC and WHO among healthcare professionals.
Footnotes
Collaborators: Delegates at the 50th EAHP General Assembly.
Contributors: This position paper was approved and adopted by the delegates at the 50th EAHP General Assembly in October 2020.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Commissioned; internally peer reviewed.
References
- 1. The European Statements of Hospital Pharmacy. Eur J Hosp Pharm 2014;21:256–8. 10.1136/ejhpharm-2014-000526 [DOI] [Google Scholar]
- 2. World Health Organization (WHO) . 10 facts about patient safety. Available: https://www.who.int/features/factfiles/patient_safety/en/ [Accessed 3 Oct 2020].
- 3. World Health Organization (WHO) Regional Office for Europe . Patient safety - data and statistics. Available: http://www.euro.who.int/en/health-topics/Health-systems/patient-safety/data-and-statistics [Accessed 3 Oct 2020].
- 4. Risør BW, Lisby M, Sørensen J. Cost-effectiveness analysis of an automated medication system implemented in a Danish hospital setting. Value Health 2017;20:886–93. 10.1016/j.jval.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 5. Cavell GF, Mandaliya D. Magnitude of error: a review of wrong dose medication incidents reported to a UK Hospital voluntary incident reporting system. Eur J Hosp Pharm:doi: 10.1136/ejhpharm-2019-001987. [Epub ahead of print: 21 Aug 2019] 10.1136/ejhpharm-2019-001987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karnon J, Campbell F, Czoski-Murray C. Model-based cost-effectiveness analysis of interventions aimed at preventing medication error at hospital admission (medicines reconciliation). J Eval Clin Pract 2009;15:299–306. 10.1111/j.1365-2753.2008.01000.x [DOI] [PubMed] [Google Scholar]
- 7. Kantelhardt P, Süle A, Saar M, et al. PS-001 Identification of risk factors frequently associated with medication errors – Pan-European Project for Patient Safety (PEPPAS). Eur J Hosp Pharm 2016;23:A214.3–5. 10.1136/ejhpharm-2016-000875.486 [DOI] [Google Scholar]
- 8. Billstein-Leber M, Carrillo CJD, Cassano AT, et al. ASHP guidelines on preventing medication errors in hospitals. Am J Health Syst Pharm 2018;75:1493–517. 10.2146/ajhp170811 [DOI] [PubMed] [Google Scholar]
- 9. Nuckols TK, Smith-Spangler C, Morton SC, et al. The effectiveness of computerized order entry at reducing preventable adverse drug events and medication errors in hospital settings: a systematic review and meta-analysis. Syst Rev 2014;3:56. 10.1186/2046-4053-3-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Truitt E, Thompson R, Blazey-Martin D, et al. Effect of the implementation of barcode technology and an electronic medication administration record on adverse drug events. Hosp Pharm 2016;51:474–83. 10.1310/hpj5106-474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Capgemini Consulting . Barcodering op de primaire verpakking van geneesmiddelen in ziekenhuizen – Een kosten-baten analyse. Available: https://www.eahp.eu/sites/default/files/barcodering-op-de-primaire-verpakking-van-geneesmiddelen-in-ziekenhuizen.pdf [Accessed 3 Oct 2020].
- 12. Franklin BD, O'Grady K, Donyai P, et al. The impact of a closed-loop electronic prescribing and administration system on prescribing errors, administration errors and staff time: a before-and-after study. Qual Saf Health Care 2007;16:279–84. 10.1136/qshc.2006.019497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dörje F, Haber M, Baehr M. Closed loop medication management – ein Muss für die Klinik 4.0. Available: https://www.adka.de/index.php?eID=dumpFile&t=f&f=2721&token=e20eac748cf3cfbe83e422b7ef4a0df81e1ff4c2 [Accessed 3 Oct 2020].
- 14. World Health Organization (WHO) . High 5S standard operating protocol assuring medication accuracy at transitions in care. Available: https://www.who.int/patientsafety/implementation/solutions/high5s/h5s-sop.pdf?ua=1 [Accessed 3 Oct 2020].
- 15. González-García L, Belda-Rustarazo S, García-Lirola MA, et al. Implementation of medicines reconciliation at hospital admission†. Eur J Hosp Pharm 2012;19:134.1–134. 10.1136/ejhpharm-2012-000074.130 [DOI] [Google Scholar]
- 16. Son H, Kim J, Kim C, et al. Pharmacist-led interdisciplinary medication reconciliation using comprehensive medication review in gynaecological oncology patients: a prospective study. Eur J Hosp Pharm 2018;25:21–5. 10.1136/ejhpharm-2016-000937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Council of Europe . Resolution CM/Res(2020)3 on the implementation of pharmaceutical care for the benefit of patients and health services.
- 18. Horn R, Weinman J, Barber N, et al. Concordance, adherence and compliance in medicine taking, Report for the National Co-ordinating Centre for NHS Service Delivery and Organisation R & D (NCCSDO). Available: http://www.netscc.ac.uk/hsdr/files/project/SDO_FR_08-1412-076_V01.pdf [Accessed 3 Oct 2020].
- 19. Hughes CM. Medication non-adherence in the elderly. Drugs Aging 2004;21:793–811. 10.2165/00002512-200421120-00004 [DOI] [PubMed] [Google Scholar]
- 20. Kearney A, Halleran C, Walsh E, et al. Medication reviews by a clinical pharmacist at an Irish university teaching hospital. Pharmacy 2017;5:60. 10.3390/pharmacy5040060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bell JS, Airaksinen MS, Lyles A, et al. Concordance is not synonymous with compliance or adherence. Br J Clin Pharmacol 2007;64:710–1. 10.1111/j.1365-2125.2007.02971_1.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Viktil KK, Blix HS. The impact of clinical pharmacists on drug-related problems and clinical outcomes. Basic Clin Pharmacol Toxicol 2008;102:275–80. 10.1111/j.1742-7843.2007.00206.x [DOI] [PubMed] [Google Scholar]
- 23. Karim-Letournel C, Cormier E, Disnard L. La collaboration infirmier-pharmacien pour La Prévention des erreurs médicamenteuses. La Revue de l'Infirmière 2019;68:42–4. 10.1016/j.revinf.2019.02.013 [DOI] [PubMed] [Google Scholar]
- 24. Arenas Villafranca JJ, Maria Eugenia B, Miriam N, et al. CP-124 Impact of hospital pharmacist integration on patient safety in a general surgery service and the related direct financial savings. Eur J Hosp Pharm 2015;22:A49.3–50. 10.1136/ejhpharm-2015-000639.118 [DOI] [Google Scholar]
- 25. World Health Organization (WHO) . Evidence of hand hygiene to reduce transmission and infections by multidrug resistant organisms in healthcare settings. Available: http://www.who.int/gpsc/5may/MDRO_literature-review.pdf [Accessed 3 Oct 2020].