Abstract
Granulomatosis with polyangiitis (GPA) is a granulomatous-necrotic systemic vasculitis with a lesion of predominantly the upper and lower respiratory tracts at the onset of the disease (vasculitis, accompanied by granulomatous inflammation), and subsequently renal (glomerulonephritis). In addition, GPA may manifest as inflammation of small arteries and veins. Despite many years of study of this disease, the etiology of GPA remains unknown. The present case is about a 47-year-old female, who had been suffering from necrotizing scleritis, corneal ulcer, and secondary glaucoma in both eyes for 3 months, and she was treated with anti-inflammatory and antimicrobial therapy that showed no effect; the patient's general condition became worse. In the second week of treatment, multiple abscess ruptures exposed the sclera. Sampling of the affected conjunctival tissue and positive HLA B8 haplotype and ANCA (PR3-ANCA) testings make it clear that GPA was the main reason of necrotizing scleritis with inflammation. The targeted treatment of the underlying disease allows to stabilize an inflammation of corneal and scleral lesions.
Keywords: Necrotizing scleritis, Corneal ulcer, Wegener's granulomatosis, Granulomatosis with polyangiitis
Introduction
Granulomatosis with polyangiitis (GPA; formerly Wegener's granulomatosis) is a systemic necrotizing, granulomatous vasculitis with primarily affected upper and lower respiratory tracts and kidneys. H. Klinger in 1936 and F. Wegener in 1939 identified the disease as an independent syndrome characterized by the classic triad of necrotizing granulomatous vasculitis of the upper and lower respiratory tract, focal segmental glomerulonephritis, and necrotizing vasculitis of small arteries and veins [1, 2]. Eye manifestations are common and occur in 28–58 percent of cases of generalized GPA and in 8 percent of patients lead to irreversible damage to the eyes, blindness and, in complicated cases, to enucleation [3, 4]. Irreversible changes in the respiratory, cardiovascular systems, and kidneys, in particular, chronic renal failure, malignant neoplasms, and intercurrent infections in some cases are fatal; with adequate treatment of GPA, 5 years of survival is over 65 percent [5, 6, 7].
Many different types of ocular involvement have been described, including conjunctivitis, episcleritis, necrotizing scleritis, corneoscleral ulceration, uveitis, optic neuritis, retinal artery occlusion, and orbital involvement with proptosis from pseudotumor of the orbit or orbital cellulitis [3]. The aim of this case report was to describe a case of GPA with severe ophthalmic manifestations in both eyes.
Case Report
Patient, a 47-year-old female, was referred to Kazakh Eye Research Institute in April 2017 with a diagnosis of necrotizing scleritis, corneal ulcer, and secondary glaucoma in both eyes. She was presented with pain, conjunctival irritation, diminished vision in both eyes, and headache. The first time she noticed the symptoms 3 months ago. She was unsuccessfully treated by topical, subconjunctival antibiotics, and topical antiseptic Miramistin (benzyl-dimethyl [3-(myristoylamino) propyl] ammonium chloride monohydrate) eye drops in a local hospital. There was no previous history of any autoimmune systemic disease.
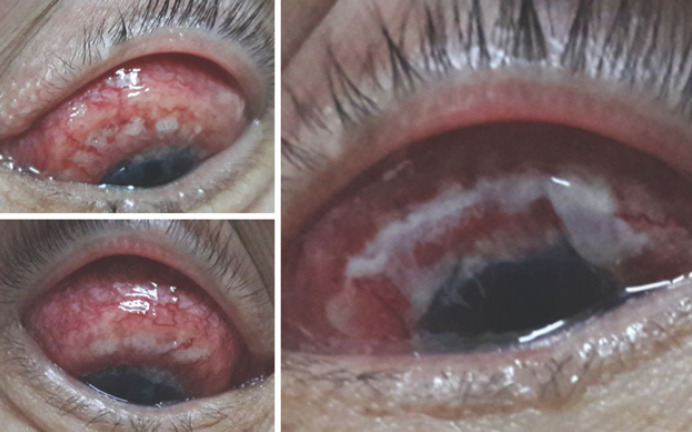
At presentation, her best-corrected visual acuity was 0.2 on the right and 0.3 on the left eye. Both eyelids were hyperemic, swollen, the palpebral fissure was narrowed, mixed conjunctival injection, subconjunctival hemorrhages, chemosis, creeping into the limbal zone, white-yellow infiltrates, a deep crescent-shaped cornea defect in the limbal area, covered with mucopurulent discharge. The central and paracentral corneal zones were transparent, and the epithelium was rough and dull (shown in Fig. 1). The patient started on systemic dehydration therapy (mannitol 75 g per day), topical antifungal therapy (fluconazole 0.2% 10 times a day), and topical steroids (dexamethasone 3 times a day) in both eyes.
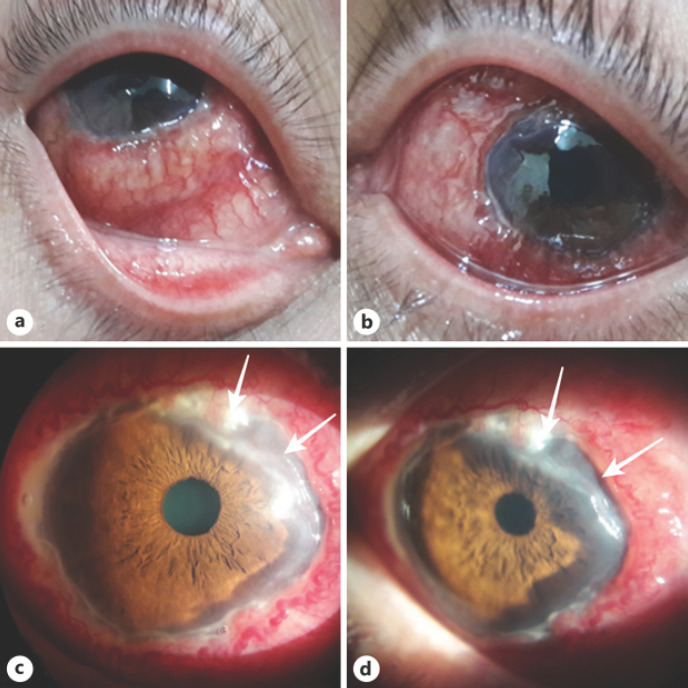
Fig. 1.
The condition of the right and left eyes at admission: subconjunctival hemorrhage, chemosis, dense, in the form of a “roller” infiltration of the conjunctiva, partially covering the limb zone (a, b); zone of corneal ulceration (white arrow) (c, d).
On the 10th day of treatment appeared − sore throat, fever up to 37.2°C, a runny nose, nasal breathing difficulties, a therapist diagnosed the acute respiratory viral infections, prescribed symptomatic treatment. In the following days, the general condition rapidly worsened: bilateral ear congestion, “stunned” feeling, an increase in headache appeared. Keratotopography (OCULUS Pentacam®) of the patient's both eyes showed gross changes in the axial, tangential, and profile maps, which was associated with a deep defect of the corneal stroma in the paralimbal zone, on the contrary, in the adjacent corneal paraoptic zone, there was a sharp elevation of the lunate shape due to perifocal edema and cicatricial process (shown in Fig. 2).
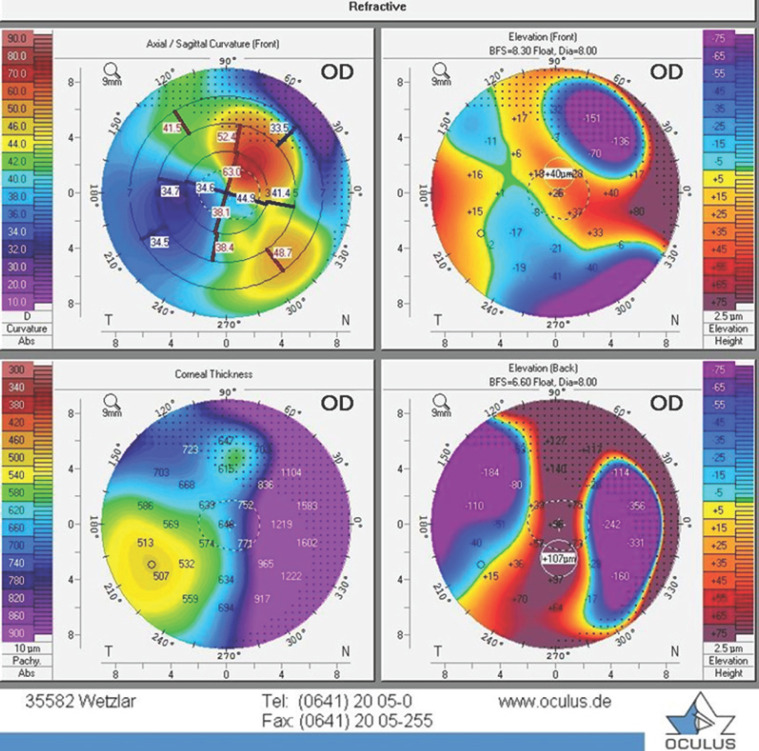
Fig. 2.
Right eye. Keratotopography (OCULUS Pentacam®) showed a deep defect of the corneal stroma in the paralimbal zone, on the contrary, in the adjacent corneal paraoptic zone, there was a sharp elevation of the lunate shape due to perifocal edema and cicatricial process.
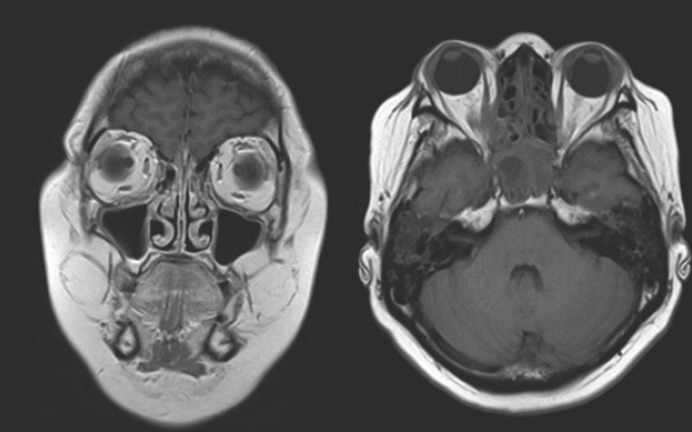
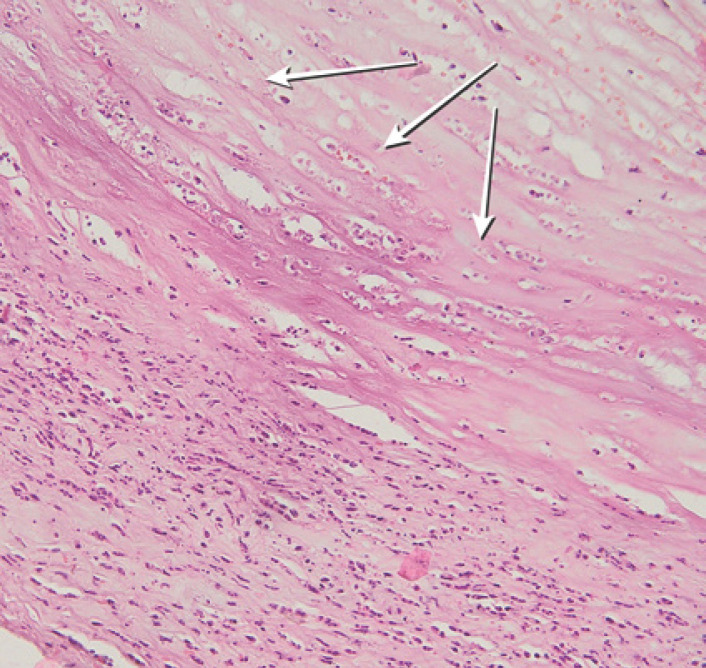
Optical coherent tomography (Heidelberg Spectralis®) of the anterior segment showed a zone of sharp thinning of the stroma in the paralimbal zone, the structure of the stroma was loose, adjacent to the zone of the ulcer defect was accompanied by a zone of perifocal edema (shown in Fig. 3, 4). MRI demonstrated right-sided sinusitis, frontal sinusitis, and residual effects of postponed sphenoiditis and edema of eye muscles on the left eye (shown in Fig. 5). In addition to rapid deterioration of the general condition, an abscess formed in the zones of tense conjunctiva; a day later, it began to spontaneously open, exposing the sclera, covered with abundant purulent discharge (shown in Fig. 6). Biopsy demonstrated granulomatous inflammation with infiltrates and necrosis (shown in Fig. 7).
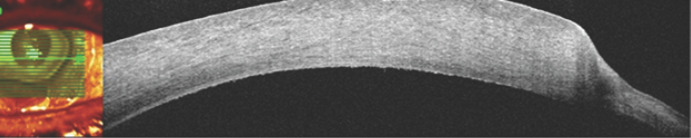
Fig. 3.
Right eye. Optical coherence tomography (Heidelberg Spectralis®) a zone of sharp thinning of the stroma in the paralimbal zone, the structure of the stroma was loose, adjacent to the zone of the ulcer defect was accompanied by a zone of perifocal edema. Similar changes were observed at all levels of scanning along the limbal zone.
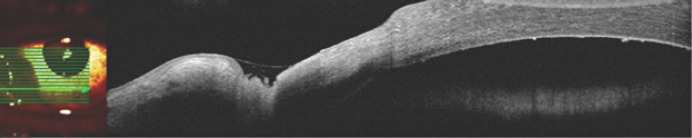
Fig. 4.
Left eye. In the paralimbal zone with the established zone of destruction of the deep layers of the stroma, covered with a tear film. Peripheral damage was noted as “stepped,” which was biomicroscopically corresponding to the active edge of an ulcerative defect. In contrast to the heterogeneous structure of the endothelium, corresponding to single precipitates.
Fig. 5.
MRI demonstrated right-sided sinusitis, frontal sinusitis, and residual signs of sphenoiditis and edema of eye muscles on the left eye.
Fig. 6.
Abscessing and spontaneous opening of the abscess with the presence of mucopurulent discharge.
Fig. 7.
Hematoxylin and eosin stain. 20× of biopsy showing a patchy infiltrative pattern. Chronic granulomatous inflammatory infiltrate and focal areas of necrosis (white arrows).
Bacteriological seeding showed Staphylococcus epidermidis, Streptococcus agalactiae with sensitivity to almost all antibiotics. The edges of the ulcer margin showed necrotizing granulomatous vasculitis.
In laboratory tests, the erythrocytes sedimentation rate was from 28 to 35 mm/h. The patient was tested for HLA which showed B8 haplotype which is frequently associated with ANCA-associated vasculitis, particularly with GPA [8].
To provide a differential diagnosis of necrotizing scleritis with associated diseases like GPA, rheumatoid arthritis, systemic lupus erythematosus, the patient was consulted by rheumatologist; PR3-ANCA testing was positive. GPA was diagnosed. Therapy was begun with cytostatic and prednisolone, and the ulcer improved as her systemic disease became quiescent. But the defect of the cornea and limbal conjunctiva was still existed. Due to the high risk of perforation, on the third week of treatment blepharorrhaphy was performed on both eyes in order to prevent the progression of corneoscleromalacia. The patient died in September 2017 of an acute myocardial infarction which could be associated with GPA [9, 10].
Discussion/Conclusion
In our case, corneoscleral ulcer was the first sign of GPA. It progressed despite antibiotic and antiseptic drops used; administered systemic corticosteroid therapy showed no effect. Once GPA was diagnosed and cytostatic therapy started the corneoscleral ulcer was improved and control of systemic disease was achieved.
In general, treatment of keratitis and scleritis in GPA is largely aimed at treating the main disorder. Topical corticosteroids are considered ineffective and may cause thinning of the cornea and its perforation [11, 12]. In the cases of a high risk of perforation in peripheral ulcerative keratitis, local measures such as blepharorrhaphy or graft may be necessary [11]. Patients presenting with scleritis often need to be investigated for underlying systemic disease. General steroids should be considered if conventional therapy is ineffective or in cases of concomitant corneal ulcer. In most cases of necrotizing scleritis and keratitis, a systemic steroid and cytostatic therapy are necessary [13, 14]. The case is consistent with findings of other studies; we would like to emphasize that GPA may involve various ocular tissues, most commonly sclera, and systemic immunosuppressive medications are frequently necessary for successful treatment.
Statement of Ethics
This case complies with the principles of the Declaration of Helsinki. The patient's relatives have given written informed consent for publication of this case report.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors declare no financial disclosures.
Author Contributions
Makpal Assainova and Botagoz Issergepova: coordinators of the patient's examinations, treatment procedures, and follow-ups. Lukpan Orazbekov and Kairat Ruslanuly: provide analysis and interpretation of the data; preparation, review, or approval of the manuscript.
References
- 1.Tarabishy AB, Schulte M, Papaliodis GN, Hoffman GS. Wegener's granulomatosis: clinical manifestations, differential diagnosis, and management of ocular and systemic disease. Surv Ophthalmol. 2010 Sep-Oct;55((5)):429–44. doi: 10.1016/j.survophthal.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Allen SD, Harvey CJ. Imaging of Wegener's granulomatosis. Br J Radiol. 2007 Sep;80((957)):757–65. doi: 10.1259/bjr/34705892. [DOI] [PubMed] [Google Scholar]
- 3.Karampatakis V, Konidaris V, Michailidou M, Gerofotis A, Daniilidis M. Peripheral corneal ulceration associated with rheumatoid arthritis. Am J Case Rep. 2013 Aug;14:318–21. doi: 10.12659/AJCR.883998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubal AA, Perez VL. Ocular manifestations of ANCA-associated vasculitis. Rheum Dis Clin North Am. 2010 Aug;36((3)):573–86. doi: 10.1016/j.rdc.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Khan AR, Chapman PT, Stamp LK, Wells JE, O'Donnell JL. Wegener's granulomatosis: treatment and survival characteristics in a high-prevalence southern hemisphere region. Intern Med J. 2012 Apr;42((4)):e23–6. doi: 10.1111/j.1445-5994.2011.02700.x. [DOI] [PubMed] [Google Scholar]
- 6.Leavitt RY, Fauci AS, Bloch DA, Michel BA, Hunder GG, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis. Arthritis Rheum. 1990 Aug;33((8)):1101–7. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- 7.Montagnac R, Nyandwi J, Loiselet G, Pradel J, Schillinger F. Manifestations ophtalmologiques de la granulomatose de Wegener. Revue de la littérature à propos d'une observation. Nephrol Ther. 2009 Dec;5((7)):603–13. doi: 10.1016/j.nephro.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Patricia M., Stassen, Jan W., Cohen-Tervaert, Simon P., Lems M., Bouke G., Hepkema, Cees G., Kallenberg M., Coen A. Stegeman, HLA-DR4, DR13(6) and the ancestral haplotype A1B8DR3 are associated with ANCA-associated vasculitis and Wegener's granulomatosis/ Rheumatology. 2009;48((6)):622–625. doi: 10.1093/rheumatology/kep057. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 9.McGeoch L, Carette S, Cuthbertson D, Hoffman GS, Khalidi N, Koening CL, Vasculitis Clinical Research Consortium et al. Cardiac Involvement in Granulomatosis with Polyangiitis. J Rheumatol. 2015 Jul;42((7)):1209–12. doi: 10.3899/jrheum.141513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suppiah R, Judge A, Batra R, Flossmann O, Harper L, Höglund P, et al. A model to predict cardiovascular events in patients with newly diagnosed Wegener's granulomatosis and microscopic polyangiitis. Arthritis Care Res (Hoboken) 2011 Apr;63((4)):588–96. doi: 10.1002/acr.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tauber J, Sainz de la Maza M, Hoang-Xuan T, Foster CS. An analysis of therapeutic decision making regarding immunosuppressive chemotherapy for peripheral ulcerative keratitis. Cornea. 1990 Jan;9((1)):66–73. [PubMed] [Google Scholar]
- 12.Shiuey Y, Foster CS. Peripheral ulcerative keratitis and collagen vascular disease. Int Ophthalmol Clin. 1998;38((1)):21–32. doi: 10.1097/00004397-199803810-00004. [DOI] [PubMed] [Google Scholar]
- 13.Jabs DA, Mudun A, Dunn JP, Marsh MJ. Episcleritis and scleritis: clinical features and treatment results. Am J Ophthalmol. 2000 Oct;130((4)):469–76. doi: 10.1016/s0002-9394(00)00710-8. [DOI] [PubMed] [Google Scholar]
- 14.McCluskey PJ, Watson PG, Lightman S, Haybittle J, Restori M, Branley M. Posterior scleritis: clinical features, systemic associations, and outcome in a large series of patients. Ophthalmology. 1999 Dec;106((12)):2380–6. doi: 10.1016/S0161-6420(99)90543-2. [DOI] [PubMed] [Google Scholar]