Abstract
Background:
Targeted testing and treatment (TTT) for latent tuberculosis infection (LTBI) is a recommended strategy to accelerate TB reductions and further tuberculosis elimination in the United States (US). Evidence on cost-effectiveness of TTT for key populations can help advance this goal.
Methods:
We used a model of TB transmission to estimate the numbers of individuals who could be tested by interferon-γ release assay (IGRA) and treated for LTBI with three months of self-administered rifapentine and isoniazid (3HP) under various TTT scenarios. Specifically, we considered rapidly scaling up TTT among people who are non-US-born, diabetic, HIV-positive, homeless or incarcerated in California, Florida, New York, and Texas – states where more than half of US TB cases occur. We projected costs (from the healthcare system perspective, in 2018 dollars), thirty-year reductions in TB incidence, and incremental cost effectiveness (cost per quality-adjusted life year [QALY] gained) for TTT in each modeled population.
Results:
The projected cost effectiveness of TTT differed substantially by state and population, while the health impact (number of TB cases averted) was consistently greatest among the non-US-born. TTT was most cost-effective among persons living with HIV (from $2,828/QALY gained in Florida to $11,265/QALY gained in New York) and least cost-effective among people with diabetes (from $223,041/QALY gained in California to $817,753 /QALY in New York).
Conclusions:
The modeled cost-effectiveness of TTT for LTBI varies across states but was consistently greatest among people living with HIV, moderate among people who are non-US-born, incarcerated, or homeless, and least cost-effective among people living with diabetes.
Keywords: latent tuberculosis, cost effectiveness, United States, targeting TB preventive therapy
Summary:
The cost-effectiveness of TTT for LTBI varies across states but was greatest among people living with HIV, moderate among people who are non-US-born, incarcerated, or homeless, and least cost effective among people living with diabetes.
Introduction
Although substantial progress has been made in reducing the burden of tuberculosis (TB)[1], the United States (US) is far from achieving TB elimination, defined as less than one incident case per million population per year.[2] The Centers for Disease Control and Prevention (CDC) Division of Tuberculosis Elimination’s strategic plan includes accelerating the decline in TB through targeted testing and treatment (TTT) of latent TB infection (LTBI).[3] Reactivation of LTBI accounts for approximately 80% of cases of TB in the US. Previous studies have found that interferon-gamma release assay (IGRA) testing and LTBI treatment after a positive IGRA result can be cost-effective in certain key populations, including people living with HIV and non-US-born individuals.[4, 5]
Since most TTT programs are implemented at the state or county level, decision-makers need to know the cost-effectiveness of TTT in populations within their locale. For US decision-makers considering national-level resource allocation, understanding how TTT cost-effectiveness varies by state and what factors generate this heterogeneity might be useful. We adapted a state-level epidemiological model of TB transmission [6] to project the effectiveness, costs, and cost-effectiveness of hypothetical TTT interventions in the four most populous US states: California, Texas, New York, and Florida. [7]
Methods
Effectiveness model & Interventions.
We used a previously developed individual-based TB modeling framework[8] structured to capture the demographic and epidemiological processes underpinning the TB epidemics in California, Florida, New York, and Texas. We incorporated five key populations, namely people who are (i) non-US-born; (ii) living with diabetes; (iii) HIV-positive; (iv) experiencing recent homelessness; or (v) incarcerated. The model includes TB-uninfected, LTBI, active TB, and recovered states, and calibrates reactivation rates to observed state-specific incidence (by age and calendar time) as a function of individuals’ age, region of birth, and time since infection, as well as calendar time. Full details of model population demography and TB natural history are described in the Appendix and elsewhere.[8, 9] In previous analyses describing the impact of interventions by state [6], we estimated baseline levels of TTT in each state. Here, we expand those analyses and link those estimates of effectiveness to corresponding cost estimates to compare the cost-effectiveness of alternative TTT strategies at the state level. We assumed a one-time TTT intervention, occurring in the first year, in which key populations are tested for LTBI using IGRAs, screened for TB using chest radiograph if IGRA-positive, and treated with 12 weeks of self-administered weekly isoniazid and rifapentine (3HP) if ruled out for TB. We assumed 85% sensitivity of IGRA testing, 85% initiation [10] and 78% completion [11] of LTBI treatment for 3HP among all who test positive, and 93% efficacy among those who complete therapy.[12] For each state, we considered testing 50% of all non-US born residents; 80% of residents living with diabetes; and 100% of residents living with HIV, experiencing recent homelessness, or experiencing incarceration. These populations were considered independently, without excluding overlaps; for instance, residents living with diabetes include both US-born and non-US born individuals.
We assessed the cost effectiveness of TTT from the perspective of the healthcare system in each state. The populations in each state were followed over a 30-year horizon, modeled as open cohorts with immigration, birth, and death. We estimated effectiveness as TB cases (and TB-related deaths) averted and quality-adjusted life years [QALYs] gained over the analytic horizon, by comparing simulations of the model at baseline, which assumes continuation of current trends in TB and TB services. We also estimated the costs of LTBI tests and chest radiographs performed, and persons treated in each population. We estimated net QALYs gained under 20 separate scenarios, including TTT in each of the five risk populations listed above, in each of the four states considered. We included estimates of mild and severe LTBI treatment toxicity (with attendant hospitalization costs and losses in quality of life). Our primary outcome was the incremental cost-effectiveness ratio (ICER), expressed as 2018 US dollars per QALY gained, comparing each TTT strategy to the baseline.
Unit cost estimates.
We used published literature and other publicly available sources (Table 1) to estimate unit costs per person tested for LTBI, per person completing LTBI treatment, and per case of TB disease averted. In estimating these unit costs, we assumed that states followed CDC testing and treatment protocols, including IGRA testing and self-administered 3HP for treatment. We included an initial complete blood count (CBC), liver function test (LFT), and HIV test (except people living with HIV). We used published costs of treatment for TB. [13, 14] Unit cost estimates were inflated to the year 2018 using the US GDP deflator, and costs were indexed to the cost of living in each state.[15] TTT costs and QALY losses resulting from the intervention were assumed to occur in the initial year and therefore not discounted; future costs and QALYs gained from averted cases of TB were discounted at 3% annually.
Table 1.
Selected input parameters
| Group | Parameters | California | Florida | New York | Texas |
|---|---|---|---|---|---|
| Costs | Cost inputs | ||||
| Direct cost of Diagnostic tests | IGRA (Interferon gamma release assay QuantiFERON-Gold)a [28] | $85 | $77 | $84 | $75 |
| Chest radiograph [29] | $22 | $20 | $22 | $20 | |
| Direct cost of latent tuberculosis infection (LTBI) treatment | 3HP (isoniazid & rifapentine, 3 months, self-administered)b [30] | $451 | $404 | $443 | $394 |
| Lab testsc [28] | $41 | $36 | $40 | $36 | |
| Toxicity not requiring hospitalization [30] | $216 | $193 | $212 | $189 | |
| Toxicity requiring hospitalization [31] | $6,926 | $6,208 | $6,802 | $6,053 | |
| Direct cost of (drug susceptible) TB treatment | Inpatient costs [32] | $42,356 | $23,378 | $41,685 | $18,350 |
| Outpatient costs [33] | $3,550 | $3,181 | $3,486 | $3,102 | |
| Cost of living adjustment | Percentage of the national average price for healthcare [15] | 110.84 | 98.55 | 109.40 | 96.91 |
| Effectiveness | Effectiveness inputs | ||||
| Probabilities and discount rate | Probability of toxicity during LTBI treatment (without hospitalization) [11] | 3.2% | |||
| Probability of toxicity during LTBI treatment (with hospitalization) [34] | 0.015% | ||||
| Probability of case fatality of TB disease [17, 30] | 5.8% (9.2% for people living with HIV) | ||||
| Discount rate, per year [16] | 3% | ||||
| Probability of TB hospitalization [35] | 49% | ||||
| QALY estimates d | Estimated quality-adjusted life years (QALY) lost per case of non-fatal TB disease (QALYs gained per TB case averted) | 0.12 | |||
| Estimated QALYs lost per LTBI treatment completed [16] (probability * disutility of toxicity) | 0.002 | ||||
Costs of additional testing among people with abnormal radiographs, such as for Mtb smear/culture, nucleic acid amplification testing, and drug susceptibility were not incorporated because these costs are not typically considered part of LTBI testing, though are part of testing persons to rule out TB disease prior to LTBI treatment.
We estimated 3HP costs as $369 per patient from the healthcare system perspective excluding directly observed therapy (DOT) costs from Shepardson et al, 2013 [30] and adjusted using the Gross Domestic Product (GDP) deflator to 2018, including cost-of-living adjustment by state. Based on 1 initial clinic visit, 2 monthly follow-up clinic visits, 3 months of medication, and 12 DOT visits
Lab tests include liver function test (hepatic function panel), complete blood count, and human immunodeficiency virus (HIV) testing in the first month (except for people living with HIV after HIV diagnosis). We used the lab tests cost for people living with HIV as $21.84 in California, $19.58 Florida, $21.45 in New York, and $19.09 in Texas and by excluding the HIV testing cost.
Estimated results based on the QALY equation from Sassi et al. [16]
QALY estimates.
The QALY is a measure of disease burden including both health-related quality of life and the quantity of life lived (see Appendix Table E1 note for further details).[16] We used published data to estimate the case-fatality of TB [17] in the US within each key population and the future life expectancy of individuals who die of TB (based on the age at TB disease diagnosis by risk populations/states estimated from the transmission model).[18] We also incorporated disutilities associated with HIV[19] and diabetes[20], for those specific populations.[21] The net number of QALYs gained for each intervention was estimated as the difference between QALYs gained due to averted TB and QALYs lost due to the toxicity of LTBI treatment.
Sensitivity analyses.
We performed one-way sensitivity analyses to describe the association between each input variable in our model and the primary outcome (i.e., ICER), for each key population in each state. Since we calibrated our model’s effectiveness outputs to TB incidence in each state, we evaluated the cost-effectiveness of TTT under simulations with different estimated TTT effectiveness, in terms of number and timing of TB cases and deaths averted, rather than directly varying LTBI prevalence and reactivation rates (as these were calibrated to historical TB incidence within each simulation; see Appendix for further details). To better explore the simultaneous effect of uncertainty ranges across all of our model parameters, we also conducted a probabilistic sensitivity analysis (PSA) in which all model parameter values were randomly sampled over pre-specified distributions (shown in Appendix Table E3). This process was repeated 10,000 times to generate uncertainty estimates around the primary ICER estimate, with 95% uncertainty ranges reported as the 2.5th and 97.5th percentiles of the corresponding distributions.
Results
The numbers of patients screened for LTBI, diagnosed, and completing treatment reflected the relative size of each key population in each state. For example, in California, testing of 50% of non-US-born people – the largest key population studied – involved testing an estimated 5.3 million people, 1.1 million people testing positive for LTBI (based on age specific LTBI prevalence of 24%−43% and 85% IGRA sensitivity), and 660,000 people completing treatment (assuming 66% net initiation and completion). The cost of this intervention was estimated as $944 million (in 2018 dollars), including $470 million for testing and $474 million for treatment. Over the subsequent 29 years, treating this number of people was estimated to avert 9,800 cases of TB, resulting in a net present value of $179 million in treatment costs averted. By comparison, TTT for all HIV-positive individuals in California would involve testing 141,000 people, 18,000 testing positive, and 10,500 completing treatment, at a cost of $19 million ($12 million for testing and $7 million for treatment). This intervention averted an estimated 670 TB cases, resulting in a net present value of $13 million in TB treatment costs averted over the next 30 years. Similar results are shown for other states and populations in Appendix Table E2. When categorizing costs as LTBI testing versus treatment, testing comprised about 47% of costs (versus 53% for treatment) among the non-US-born (out of total intervention cost as $944 million), homeless ($60 million) and incarcerated ($30 million) populations, whereas testing comprised 64% of costs (versus 36% for treatment) among people living with diabetes ($408 million) and HIV ($19 million), reflecting these populations’ slightly lower LTBI prevalence.
As shown in Figure 1, the estimated health impact and cost-effectiveness of TTT differed substantially across states and risk populations. TTT among non-US-born populations resulted in an ICER for the baseline scenario of $81,790 (95% uncertainty interval: $20,000, $139,000) in Texas and $104,098 ($36,000, $498,000) in California, but $144,096 ($36,000, $306,000) in Florida and $174,152 ($40,000, $517,000) in New York. TTT among people living with HIV was the most cost-effective intervention in all states, with ICERs ranging from $2,828 (cost saving, $11,000) in Florida to $11,265 (cost saving, $119,000) in New York. The cost effectiveness of TTT was intermediate in incarcerated persons (but the strategy was dominated in New York) and homeless persons (however, in New York, cost effectiveness in this population was over $511,000 per QALY gained). This is because incidence among homeless and incarcerated individuals is low in New York, partially reflecting baseline and historical TB practices in the state. As a result, the net QALYs gained for incarcerated individuals in New York (QALYs gained from averted TB minus QALYs lost due to toxicity of 3HP) is even negative, thereby causing the cost per QALY gained to be dominated. The ICER for TTT among people living with diabetes was over $223,000 in each state and reached $818,000 per QALY gained in New York because of older age and competing risk of death. Cost-effectiveness of self-administered 3HP was more favorable than for directly observed treatment (Appendix Table E3).
Figure 1.
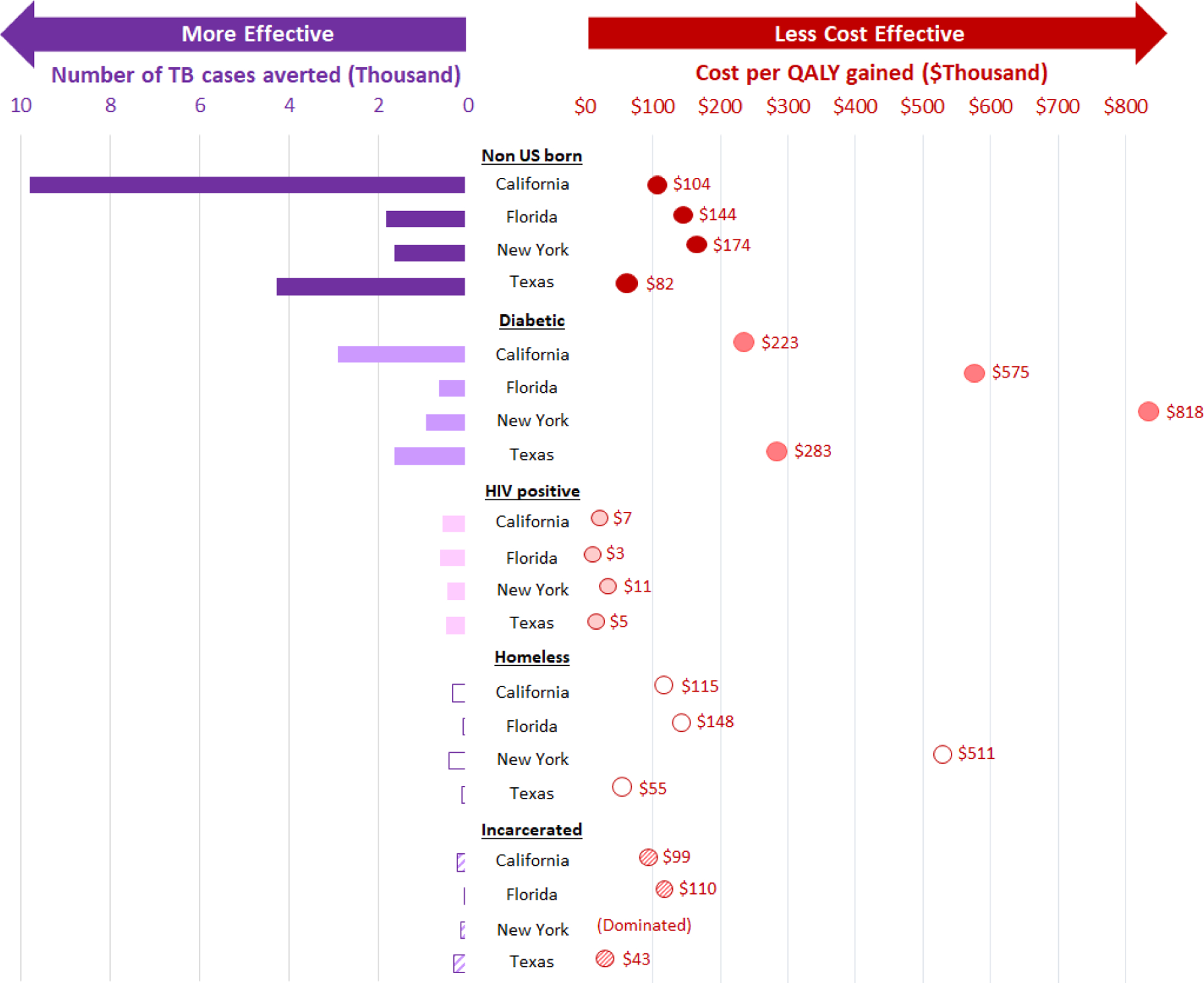
Health Impact and Incremental Cost-effectiveness in Deterministic Analyses
The most important determinants of overall TTT cost-effectiveness included the expected decline in TB incidence, the direct medical cost of IGRA, QALY lost per LTBI treatment (including proportion of patients experiencing toxicity from 3HP). The rank ordering of these variables in one-way sensitivity analyses varied across key populations and states (Figure 2 and Appendix Figure E3). For example, we varied the expected TB incidence after 30 years (without TTT) by −/+50% from the California baseline of 5.3 cases per 100,000 [22] to either 2.7 or 7.9 per 100,000 persons, which resulted in a change in the discounted number of TB cases averted among non-US-born individuals changed from a baseline of 7,380 to 3,507 and 10,522, respectively. This caused the corresponding ICER to vary from a baseline value of $104,098/QALY gained to $343,061/QALY gained and $61,546/QALY gained, respectively. On the other hand, doubling the disutility of 3HP (e.g., doubling levels of toxicity or incorporating a QALY loss for the inconvenience of treatment) would cause the ICER to increase between $82,656 and $140,015 per QALY gained. In probabilistic sensitivity analysis (shown in Appendix Figure E1 and E2), more than 67% of simulation estimates fell below $150,000 per QALY gained for TTT among the non-US-born in all four states, while fewer than 35% of simulation estimates fell below this level for people living with diabetes.
Figure 2.
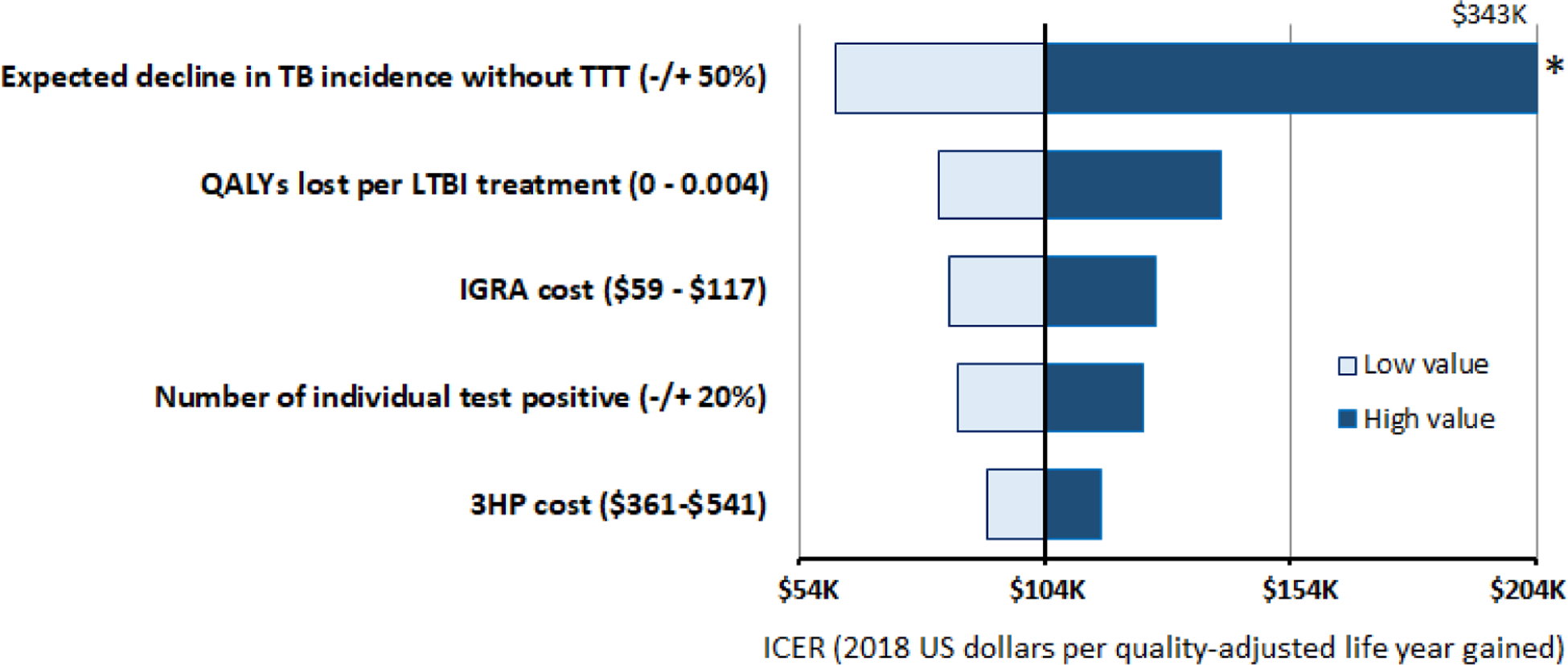
One-Way Sensitivity Analyses in California, Compared with the Baseline Incremental Cost Effectiveness Ratio (ICER)
Discussion
This model-based analysis describes how cost-effectiveness of TTT for LTBI in the United States varies across key populations and the states that we examined. Specifically, cost effectiveness was consistently greatest among people living with HIV; moderate among individuals who are non-US-born, incarcerated or homeless; and lowest for people living with diabetes. The cost effectiveness for TTT of homeless and incarcerated populations was similar as for non-US born persons, but the small size of those populations results in uncertainty in long-term population-level health impact. Cost-effectiveness differed substantially by state, with a tendency for additional TTT above baseline practices to be more cost-effective in Texas (where TB transmission rates may be higher)[6] and less cost-effective in New York (where TB incidence in the populations evaluated was lower).[23] The cost-effectiveness of TTT was most sensitive, in order, to assumptions regarding the state-specific trend in TB incidence, the cost of IGRA and the impact of 3HP toxicity among people treated for LTBI.
Importantly, this analysis suggests that people living with diabetes may experience less benefit from LTBI treatment due to their older age (e.g. mean age of 61 years in California at TB disease diagnosis, consequently with fewer remaining years of life) and lower quality-adjusted life expectancy (i.e., more disability in remaining years of life) – despite their increased risk of reactivation relative to the general population. However, we did not consider in our analysis the overlap of key risk populations, notably of non-US-born people living with diabetes, because of lack of data on the state-level prevalence of diabetes among non-US-born people. By contrast, cost-effectiveness of TTT among people living with HIV was greatest in every scenario tested, reflecting their relatively younger age and the high prevalence of LTBI and risk of progression to TB.[24] For people experiencing recent homelessness or incarceration, our analysis estimated cost-effectiveness somewhere between the highest and lowest levels, but with great uncertainty given their small population sizes and without additional consideration of TTT implementation challenges for these populations.
Decisions about which populations should be tested and treated for LTBI must consider the balance between benefits that are substantial but uncertain and delayed, and harms that are rare, but more immediate.[7, 25] It will be important for policy makers to consider whether the risk-benefit balance of TTT of specific populations (e.g., populations who might experience greater toxicity, such as people with hepatitis C or the very old)[26] might be less favorable than presented in this analysis – or more beneficial than presented here (e.g., populations having higher risks of TB reactivation such as people living with HIV, recent immigrants, and close contacts). Further research is needed that evaluates risk assessment tools to determine efficient ways of identifying candidates for TTT, and whether certain screening and treatment methods are preferable for certain risk groups as well as how often and where LTBI screening should be performed in different risk groups.[7]
Overall, our findings are generally consistent with previous analyses [4, 5] suggesting that cost-effectiveness of TTT (with IGRA and self-administered 3HP) is likely to be greater in non-US-born people and people living with HIV and less among people with diabetes. However, our estimates of the cost per QALY gained through TTT are higher (less favorable) than previous estimates, even after adjustment for inflation. For example, two earlier studies (updated to 2018$) estimated that the cost of TTT among non-US-born populations would fall between $64,000 and $124,000 per QALY gained.[4, 5] These differences are partially explained by the previous studies limiting their analysis to populations (e.g., younger age, recent immigrants) in whom cost-effectiveness is expected to be higher (because of more years of life remaining or higher LTBI prevalence and progression to TB), and also by our model’s use of an open population cohort, in which migration of untreated individuals and death due to other causes result in lower effectiveness estimates over time. Nevertheless, the policy implications – that TTT for people living with HIV and non-US-born individuals is likely to be cost-effective – remain similar.
This study has limitations. There is lack of certainty around many of the most important determinants of cost-effectiveness, including the state-specific LTBI prevalence of each population, long-term reactivation rates, and the disutility associated with taking 3HP.[27] For smaller populations (e.g., HIV, homeless and incarcerated), uncertainty estimates were very wide, reflecting model stochasticity and the relatively small contribution of these populations to TB incidence at the state level. (Appendix Figure E4) Our model analyzes an open cohort over 30 years of the population of each state, rather than the lifetime of the cohort that received the intervention; as such, we may underestimate the cost-effectiveness of TTT but likely better reflect decision-making on the part of individual state TB programs that might plan based on a set number of years. Our costs only include the healthcare system costs of testing and treatment and exclude program implementation costs (identification and recruitment of populations for testing, etc.), patient costs, and other societal costs. We also did not include costs of additional testing among people with abnormal radiographs (e.g., sputum smear and TB culture) because these costs are considered part of an evaluation for persons suspected of having TB disease. While any TTT program may identify active TB cases, this analysis did not incorporate either the costs or the benefits thereof. These simplifications may cause us to underestimate or overestimate the cost-effectiveness of TTT. Finally, we did not consider multidrug-resistant TB, different diagnostic algorithms, or different treatment options, nor did we consider strategies that could enhance cost-effectiveness by additional stratification of risk (e.g., TTT of recent arrivals among non-US-born populations). TTT cost-effectiveness is strongly affected by assumptions about long-term effectiveness, in terms of averted TB cases and deaths over a period of decades in a non-static population. Better empirical estimates to inform these values in different epidemiological contexts are needed. State-level cost-effectiveness will also differ by the conditions and costs of TTT implementation. Accordingly, future work might consider testing and treatment of feasible numbers in each year, which might be a more realistic implementation scenario. Nevertheless, these findings may be useful for prioritization of TTT effort and for resource allocation while awaiting additional data. Research on better ways to target efforts to prevent TB in populations at risk for reactivation and cheaper, but equally effective treatment strategies would help reduce program costs and increase cost-effectiveness.
Conclusions
Testing and treatment of persons with LTBI constitutes an essential component of TB elimination in the United States and globally. This model-based analysis shows that cost-effectiveness of TTT for LTBI varies across key populations and the states. Specifically, cost effectiveness was greatest among people living with HIV, moderate among individuals who were non-US born, homeless, or incarcerated; and least cost effective for the people living with diabetes. While conducting TTT in populations with the greatest cost effectiveness makes efficient use of program dollars, TTT among the non-US-born population results in substantial progress towards TB elimination. These results may help decision-makers prioritize resources at the state level to more effectively advance TB control efforts toward the goal of elimination.
Supplementary Material
Funding:
This work was supported by U.S. Centers for Disease Control and Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Epidemiologic and Economic Modeling Agreement [grant number #5U38PS004646]
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- [1].Centers for Disease Control and Prevention (CDC). Reported Tuberculosis in the United States, 2017. Atlanta, GA: US Department of Health and Human Services, CDC; 2018. [Google Scholar]
- [2].LoBue PA, Mermin JH. Latent tuberculosis infection: the final frontier of tuberculosis elimination in the USA. The Lancet Infectious diseases 2017;17(10):e327–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Centers for Disease Control and Prevention (CDC). Division of Tuberculosis Elimination Strategic Plan 2016–2020. https://www.cdc.gov/tb/about/strategicplan.htm Accessed April 23, 2020.
- [4].Tasillo A, Salomon JA, Trikalinos TA, Horsburgh CR Jr., Marks SM, Linas BP. Cost-effectiveness of Testing and Treatment for Latent Tuberculosis Infection in Residents Born Outside the United States With and Without Medical Comorbidities in a Simulation Model. JAMA Intern Med 2017;177(12):1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Linas BP, Wong AY, Freedberg KA, Horsburgh CR Jr. Priorities for screening and treatment of latent tuberculosis infection in the United States. Am J Respir Crit Care Med 2011;184(5):590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shrestha S, Cherng S, Hill AN, Reynolds S, Flood J, Barry PM, et al. Impact and Effectiveness of State-level Tuberculosis interventions in California, Florida, New York and Texas: A model-based analysis. American journal of epidemiology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bibbins-Domingo K, Grossman DC, Curry SJ, Bauman L, Davidson KW, Epling JW Jr., et al. Screening for Latent Tuberculosis Infection in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2016;316(9):962–9. [DOI] [PubMed] [Google Scholar]
- [8].Shrestha S, Hill AN, Marks SM, Dowdy DW. Comparing Drivers and Dynamics of Tuberculosis in California, Florida, New York, and Texas. Am J Respir Crit Care Med 2017;196(8):1050–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vynnycky E, Fine PE. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect 1997;119(2):183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sandgren A, Vonk Noordegraaf-Schouten M, van Kessel F, Stuurman A, Oordt-Speets A, van der Werf MJ. Initiation and completion rates for latent tuberculosis infection treatment: a systematic review. BMC infectious diseases 2016;16:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Belknap R, Holland D, Feng PJ, Millet JP, Cayla JA, Martinson NA, et al. Self-administered Versus Directly Observed Once-Weekly Isoniazid and Rifapentine Treatment of Latent Tuberculosis Infection: A Randomized Trial. Annals of internal medicine 2017;167(10):689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].International Union Against Tuberculosis Committee on Prophylaxis. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ 1982;60:555–64 [PMC free article] [PubMed] [Google Scholar]
- [13].Castro KG, Marks SM, Chen MP, Hill AN, Becerra JE, Miramontes R, et al. Estimating tuberculosis cases and their economic costs averted in the United States over the past two decades. Int J Tuberc Lung Dis 2016;20(7):926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Oh P, Pascopella L, Barry PM, Flood JM. A systematic synthesis of direct costs to treat and manage tuberculosis disease applied to California, 2015. BMC research notes 2017;10(1):434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Infoplease. Cost of Living Index for Selected U.S. Cities. https://www.infoplease.com/business-finance/us-economy-and-federal-budget/cost-living-index-selected-us-cities1. Accessed April 23, 2020.
- [16].Sassi F. Calculating QALYs, comparing QALY and DALY calculations. Health policy and planning 2006;21(5):402–8. [DOI] [PubMed] [Google Scholar]
- [17].Straetemans M, Glaziou P, Bierrenbach AL, Sismanidis C, van der Werf MJ. Assessing tuberculosis case fatality ratio: a meta-analysis. PloS one 2011;6(6):e20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].United States Social Security Administration. Actuarial Life Table. https://www.ssa.gov/oact/STATS/table4c6.html. Accessed April 23, 2020.
- [19].Salomon JA, Haagsma JA, Davis A, de Noordhout CM, Polinder S, Havelaar AH, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health 2015;3(11):e712–23. [DOI] [PubMed] [Google Scholar]
- [20].Miller TL, McNabb SJ, Hilsenrath P, Pasipanodya J, Weis SE. Personal and societal health quality lost to tuberculosis. PloS one 2009;4(4):e5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang P, Brown MB, Bilik D, Ackermann RT, Li R, Herman WH. Health utility scores for people with type 2 diabetes in U.S. managed care health plans: results from Translating Research Into Action for Diabetes (TRIAD). Diabetes care 2012;35(11):2250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].California Department of Public Health. TB in California: 2018 Snapshot. https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/TBCB-TB-Fact-Sheet-2018.pdf. Accessed April 23, 2020.
- [23].Cherng ST, Shrestha S, Reynolds S, Hill AN, Marks SM, Kelly J, et al. Tuberculosis Incidence Among Populations at High Risk in California, Florida, New York, and Texas, 2011–2015. American journal of public health 2018;108(S4):S311–S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shea KM, Kammerer JS, Winston CA, Navin TR, Horsburgh CR Jr. Estimated rate of reactivation of latent tuberculosis infection in the United States, overall and by population subgroup. American journal of epidemiology 2014;179(2):216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Infect Dis 2017;64(2):111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Riza AL, Pearson F, Ugarte-Gil C, Alisjahbana B, van de Vijver S, Panduru NM, et al. Clinical management of concurrent diabetes and tuberculosis and the implications for patient services. The lancet Diabetes & endocrinology 2014;2(9):740–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Goodell AJ, Shete PB, Vreman R, McCabe D, Porco TC, Barry PM, et al. Outlook for tuberculosis elimination in California: An individual-based stochastic model. PloS one 2019;14(4):e0214532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].United States Department of Health and Human Services Center for Medicare Services. Clinical laboratory fee schedule. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html. Accessed April 23, 2020.
- [29].United States Department of Health and Human Services Center for Medicare Services. Physician fee schedule. https://www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx Accessed April 23, 2020.
- [30].Shepardson D, Marks SM, Chesson H, Kerrigan A, Holland DP, Scott N, et al. Cost-effectiveness of a 12-dose regimen for treating latent tuberculous infection in the United States. Int J Tuberc Lung Dis 2013;17(12):1531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Holland DP, Sanders GD, Hamilton CD, Stout JE. Costs and cost-effectiveness of four treatment regimens for latent tuberculosis infection. Am J Respir Crit Care Med 2009;179(11):1055–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].United States Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality, Rockville, MD. HCUPnet. https://hcupnet.ahrq.gov/ Accessed April 23, 2020. [PubMed] [Google Scholar]
- [33].Marks SM, Flood J, Seaworth B, Hirsch-Moverman Y, Armstrong L, Mase S, et al. Treatment practices, outcomes, and costs of multidrug-resistant and extensively drug-resistant tuberculosis, United States, 2005–2007. Emerg Infect Dis 2014;20(5):812–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sotgiu G, Matteelli A, Getahun H, Girardi E, Sane Schepisi M, Centis R, et al. Monitoring toxicity in individuals receiving treatment for latent tuberculosis infection: a systematic review versus expert opinion. Eur Respir J 2015;45(4):1170–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Taylor Z, Marks SM, Rios Burrows NM, Weis SE, Stricof RL, Miller B. Causes and costs of hospitalization of tuberculosis patients in the United States. Int J Tuberc Lung Dis 2000;4(10):931–9. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


