Abstract
Recent technical advances have led to the discovery of novel functions of extrachromosomal DNA (ecDNA) in multiple cancer types. Studies have revealed that cancer-associated ecDNA shows a unique circular shape and contains oncogenes that are more frequently amplified than that in linear chromatin DNA. Importantly, the ecDNA-mediated amplification of oncogenes was frequently found in most cancers but rare in normal tissues. Multiple reports have shown that ecDNA has a profound impact on oncogene activation, genomic instability, drug sensitivity, tumor heterogeneity and tumor immunology, therefore may offer the potential for cancer diagnosis and therapeutics. Nevertheless, the underlying mechanisms and future applications of ecDNA remain to be determined. In this review, we summarize the basic concepts, biological functions and molecular mechanisms of ecDNA. We also provide novel insights into the fundamental role of ecDNA in cancer.
Keywords: EcDNA, Oncogene amplification, Chromosomal rearrangement, Epigenetic modification, Tumor heterogeneity
Introduction
Extrachromosomal DNA (ecDNA) is a particular type of DNA molecule outside of the chromosome that is usually 1–3 Mb in length [1]. EcDNA does not contain centromeres or telomeres, but it has regulatory regions that control the expression of the encoded genes [2, 3]. Studies have shown that ecDNA accounts for nearly 30% of all DNA particles outside of the chromosome [4, 5]. In addition, there are two major categories of extrachromosomal DNA particles that differ from ecDNA in sequence length: (1) extrachromosomal small circular DNA (eccDNA) and (2) ring- or neochromosome (Table 1) [6–9]. eccDNA is a double-stranded circular molecule less than 1 Mb in length that consists of multiple copies of genome-originated repetitive non-coding DNA sequences and telomeric circles (e.g. small polydispersed circular DNA and microDNA) [10]. In contrast to ecDNA that is rarely seen in normal cells, eccDNA is present in both normal cells and cancer cells, and eccDNA may promote tumorigenesis through the selection of telomere extension and modulation of genomic instability [10, 11]. In ring-chromosomes, the ends of the DNA sequence are fused together to form a ring shape [12]. Neochromosomes contain centromere and telomere sequences, with a typical sequence length of 30–600 Mb [7, 8]. Neochromosomes have been shown to contain high copy numbers of oncogenes and can be created through chromothripsis [9].
Table 1.
Characteristics of ecDNA, eccDNA, neo and ring chromosome
| Size | Single/double strand | Sequence feature | Definition | Origination | Refs. | |
|---|---|---|---|---|---|---|
| ecDNA |
1-3 Mb, visible under microscope |
Double | Oncogene amplification, regulatory regions, no centromeres or telomeres | Extrachromosomal DNA (double minutes) | BFB cycle, translocation-deletion-amplification, episome and chromothripsis | [1, 3] |
| eccDNA | < 1 Mb. Invisible under microscope | Single or double | Small regulatory RNA | Extrachromosomal small circular DNA | Telomere circle, spcDNA, miDNA, episome | [93, 94] |
|
Neo chromosome |
30–600 Mb, visible under microscope | Double | Contains centromere or telomere | Structurally abnormal chromosome | Chromothripsis and BFB cycles with telomere aggregation | [7, 9] |
| Ring chromosome | 1.4–7.3 cms. Visible under microscope | Double | Circular or linear form, contains centromere and telomere | Breaks of telomeric ends, end-to-end fusion of the centric chromosome | End joining of DNA double-strand breaks, telomere_subtelomere junction, or rearrangement | [6] |
Recent findings have revealed the essential roles of ecDNA in cancer [1, 2, 12–14]. ecDNA is widely expressed in multiple types of cancers, including highly aggressive glioblastoma and sarcoma, but not in normal tissues [2, 15]. The presence of ecDNA is also associated with the rapid amplification of oncogenes and elevated intra-tumoral heterogeneity [15]. Moreover, the lack of centromeres in ecDNA leads to the discordant inheritance of ecDNA elements during mitosis, contributing to the hyper-activity of oncogene expression. These features of ecDNA endow cancer cells with the ability of quick adaptation toward the microenvironment, therefore promoting intra-tumoral heterogeneity [1, 16]. The study of ecDNA in cancer is still in its infancy. In this review, we summarize the recent findings of ecDNA regarding the structure, biogenesis, function and therapeutic potentials in cancer.
The biological features of ecDNA
Structure
Early attempts to uncover the structure of ecDNA were limited due to technical obstacles. Recent advances in next-generation sequencing technologies (e.g. whole genome sequencing) and computational analytical approaches have led to the discovery that ecDNA presents in a circular shape and can replicate independently outside of chromosomes [2, 16, 17]. In addition, ecDNA contains not only complete genes, but also regulatory elements such as upstream promoters and enhancers [2, 3]. Importantly, rewiring of adjacent enhancers along with endogenous enhancers was observed in ecDNA [3]. In certain circumstances, ecDNA can incorporate DNA segments from different chromosomes to form chimeric sequences, which may subsequently “invade” and re-integrate into other chromosomes to generate novel DNA sequences [2]. In comparison with linear DNA, ecDNA has a highly accessible chromatin state and significantly higher levels of H3K27ac, a well-established histone marker for super enhancers [2, 3]. These structural characteristics of ecDNA markedly elevate the expression levels of oncogenes in ecDNA and affect chromatin rearrangement to promote intra-tumoral heterogeneity [16]. Verhaak et al. analyzed circular DNA data from 3,212 patients across a variety of cancer types. The association between oncogene amplification and ecDNA structure was observed, however such association could not be applied to the breakpoint; and the distributions of oncogene amplicons were highly nonrandom [15]. These results demonstrate that not only the inherited “genetic code,” but also the topology and three-dimensional chromatin landscape play critical roles in maintaining proper function of the cancer genome [1, 15, 18].
Biogenesis
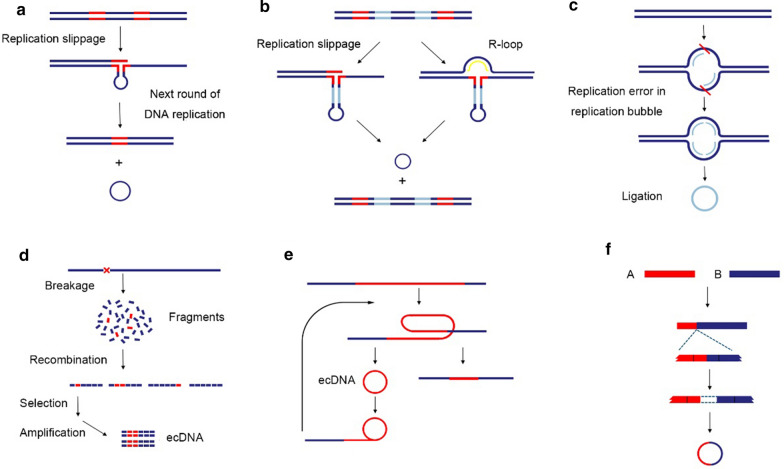
The biological source of ecDNA generation includes endogenous DNA damage (e.g. DNA replication stress), exogenous stress (e.g. carcinogens and pathogens) and aberrations in the DNA damage repair machinery [19]. Both ecDNA and homogeneously staining regions (HSR) of chromosomes can be formed through gene transcription and dramatically increase the complexity and plasticity of the genome [20–22]. Nevertheless, the underlying mechanisms of ecDNA biogenesis are not fully understood. In addition to simple self-ligation after DNA breaks, ecDNA can also be generated in multiple other ways [23, 24], and several models have been proposed, such as the breakage-fusion-bridge cycle [25], translocation-deletion-amplification [26], episome [27] and chromothripsis [28] models. The diverse genome compositions of ecDNA in multiple cancers imply complex multiple-step procedures in its formation, including the generation of DNA fragments from DNA damage (e.g. double-strand breaks), tandem duplication [29], breakage-fusion-bridge cycle [30] and chromothripsis-mediated chromatin rearrangement [31]. Non-homologous end-joining or microhomology-mediated end-joining repair mechanisms facilitate the rewiring of these DNA fragments in a random order, contributing to the generation of ecDNA [15, 32]. Importantly, ecDNA can self-replicate in the absence of tumor suppressors [1]. However, contradictory results have been reported regarding the contribution of DNA replication to ecDNA formation. One report showed that during replication, ecDNA can originate from loop excision and/or ligation of DNA fragments in the replication bubble where DNA replication is paused [32]. In contrast, other studies showed that inhibitors of DNA replication promoted the formation of ecDNA [33]. In addition, the DNA fragments released into peripheral blood by damaged cells in response to oxidative stress further contribute to ecDNA formation [34–36]. Collectively, the formation of ecDNA involves complex mechanisms (Fig. 1).
Fig. 1.
Models of ecDNA formation. a Replication slippage model 1: ecDNA can be generated from replication slippage where DNA polymerase replicates DNA at wrong direction and creates a loop on the template strand. The loop is then excised and ligated into a circle, resulting in microdeletions at chromosomes. b Replication slippage model 2: ecDNA can be derived from replication slippage and recombination without deletion of original locus, leaving no chromosomal micro-gaps as they are filled by R-loop or homogenous recombination. This is more compatible when small regions of DNA containing one or a number of driver genes are selected. c Episome model: The ligation of DNA fragment pairs of inverted repeats in the replication bubble forms a single-strand circle when DNA replication is paused. d DSBs based model: in the process of DNA damage repair, DNA circle can be created by chromosomal rearrangements, which are mediated by the DNA damage repair mechanism non-homologous end-joining or microhomology-mediated end-joining. e Rolling circle model: Intrachromosomal recombination and "circularization" between tandem repeats produce circular molecules and shortened tandem sequences. f Translocation-deletion-amplification model: DNA segments derived from different chromosomes may form chimeric ecDNA sequences, which further invade and re-integrated into other chromosomes to generate novel DNA sequences
Source sequences
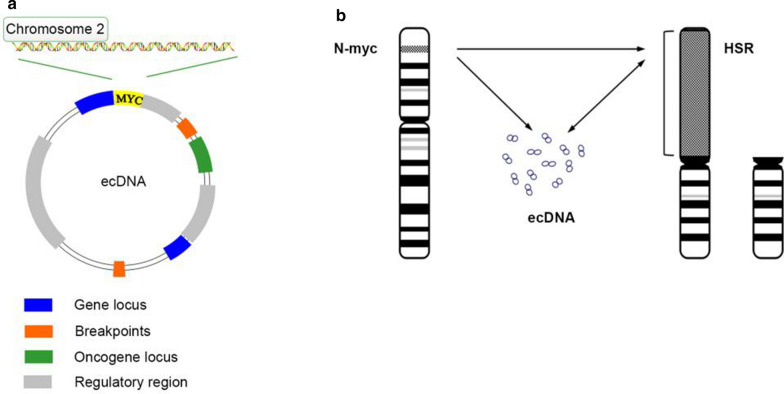
Studies have shown the source sequences of ecDNA originate from multiple genomic sites from various individual chromosomes [23]. Storlazzi et al. demonstrated that ecDNA exhibits a high degree of heterogeneity in the sequence source, even within a single cell [37, 38]. Bioinformatic analyses of ecDNA sequences also indicate that oncogene amplification is unlikely to be the consequence of chromothripsis [23] (Fig. 2).
Fig. 2.
Gene amplification diagram of amplicons on ecDNA or HSR. a EcDNA carries not only complete genes, but also regulatory sequences such as promoters and enhancers. b The N-MYC amplification model. The N-MYC gene can be amplified either at ecDNA or at genome
The oncogenic functions of ecDNA
Recent studies have demonstrated fundamental roles of ecDNA in cancer in modulating cell growth [15, 19, 39], metastasis/invasion [40, 41], autophagy [42, 43], DNA damage repair [34, 35], drug response [40, 44] and clinical outcome [15, 41] (Fig. 3). In addition, ecDNA contributes to intra-tumoral heterogeneity through genetic, epigenetic and microenvironmental factors [1, 2, 13, 18].
Fig. 3.
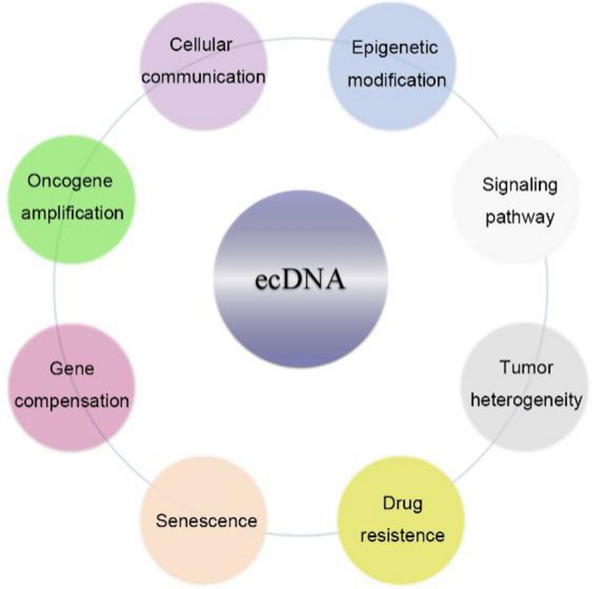
Overview of the oncogenic functions of ecDNA. EcDNA has a profound impact on multiple aspects of cancer phenotypes
Cell growth
The formation of ecDNA correlates with enhanced levels of DNA replication in highly proliferating cancer cells and exhibits survival benefits [2, 41]. In addition, ecDNA enhances the proliferation of cancer cells but suppresses the infiltration of immune cells, thus leading to an aggressive phenotype of elevated number of lymph nodes with micro-metastases in cancer patients [15].
Intra-tumoral heterogeneity
Several studies showed that ecDNA increases the level of intra-tumoral heterogeneity in multiple cancer types [1, 2, 12]. The ecDNA originating from asymmetric chromatin segregation during mitosis and the massive amplification of oncogenes in ecDNA enable cancer cells to readily adapt to the evolving environment. Both primary and recurrent tumors show amplification of ecDNA-encoded genes (e.g. MYC, MYCN, EGFR, PDGFRA and MET), linking ecDNA to the evolvability of cancer cells under the selection pressure of the tumor microenvironment and therapeutic treatment [14]. In addition, as ecDNA is much more abundant in progressive tumors whereas high levels of HSR are more frequently observed in tumors under environmental stress, the balance between ecDNA and HSR of chromosomes is considered essential to determine the evolvability of cancer cells [18, 45].
Autophagy
Several reports showed that ecDNA activates pathogen recognition receptors such as Toll-like receptor (TLR) family proteins, leading to inhibition of apoptosis and promotion of autophagy [42, 44]. In line with these results, some studies showed that ecDNA-containing cell-free DNA may regulate autophagy in a TLR9-dependent manner [42, 44]. Furthermore, studies in colon cancer cells have demonstrated that ecDNA transported in micronuclei or extracellular vesicles (EVs) can facilitate the induction of autophagy thus to promote cancer cell survival in response to chemotherapy [44].
Drug sensitivity
Schimke et al. discovered that methotrexate resistance was attributed to DHFR gene amplification in ecDNA [22]. Meng et al. found that knock-down of DHFR resulted in increased sensitivity to methotrexate in DNA-PKcs-depleted ecDNA-containing cells but not in HSR-containing cells [46]. Glioblastoma cells have high levels of oncogenic EGFRvIII in ecDNA [18, 45]. Turner et al. performed structural analysis of EGFRvIII amplification on glioblastoma cells (GBM39) and found that ecDNA reintegrated into HSR during erlotinib treatment. Importantly, the ecDNA amplicon re-emerged when erlotinib was discontinued [18]. In addition, resistance to tyrosine kinase inhibitors of glioblastoma cells can be strengthened by adjustment of EGFRvIII levels in ecDNA [47]. These findings demonstrate that ecDNA modulates the drug sensitivity of cancer cells.
Metastasis and invasiveness
Recent findings have linked ecDNA to increased cancer metastasis and poor patient outcomes. The overall level of ecDNA is markedly elevated in cancer patients with metastases than in patients without metastases [40, 41]. Mechanistically, ecDNA shuttle between the nucleus and cytoplasm and can be encapsulated in micronuclei or transported in EVs to cross the cell membrane or be exported to the extracellular space by exosomes [19, 48]. Cancer cells may use ecDNA as a messenger to transmit oncogenic information to other cell types in the microenvironment or to satellite tumors. ecDNA-mediated autocrine and paracrine signaling may result in increased invasiveness and chemoresistance and acquisition of the cancer stem cell–like phenotype [48]. In addition, ecDNA positively correlates with poor patient outcome [49]. The overall survival of patients carrying at least one circular amplicon of ecDNA was significantly poorer than that of patients without ecDNA-associated amplicons [15]. A meta-analysis of ecDNA measurement from 1076 patients with metastatic colorectal cancer confirmed a positive correlation between lower basal levels of ecDNA and better patient survival [41]. Furthermore, ecDNA can be used as a non-invasive prognostic tool that predicts the early relapse of thyroid cancer [50] and chemotherapy response in ovarian cancer [51].
Senescence
Senescence is a potent barrier to prevent the malignant transformation of normal cells to cancer cells [52]. EcDNA functions as a reservoir of heterogeneous genetic material that endows cancer cells with rapid adaptation to environmental stress [53]. In yeast, the induction of senescence can be attributed to the accumulation of ecDNA with ribosome genes [54]. Importantly, daughter cells with less ecDNA exhibited a longer lifespan than mother cells with more ecDNA, and the ectopic expression of autonomously replicating sequence of ecDNA can trigger cell cycle arrest, cell death or age-related inflammation [54, 55]. However, the underlying mechanisms of how ecDNA circumvents the barrier of senescence to facilitate malignant transformation remain to be elucidated.
Anti-tumor immunity
The elimination of ecDNA involves the entrapment of ecDNA within micronuclei, disappeared chromosomal γH2AX foci and the stimulation of immune responses [56]. Shimizu et al. found that ecDNA originating from anaphase chromosomes form micronuclei after hydroxyurea treatment [56]. Micronuclei facilitate the generation of aneuploid cells, which exhibit enhanced cell viability [57]. In neuroblastoma, ecDNA-containing micronuclei with amplified MYCN sequences were detected in vivo [58]. Notably, the DNA within micronuclei is prone to be released into the cytosol [59]. Dynamic for extracellular DNA interacts with micronuclei may be important for induction of anti-tumor immune response. Ji et al. found that downregulation of ecDNA-carried genes from colorectal and neuroectodermal tumor cells led to reduction of ecDNA genes by micronuclei expulsion which resulted in a decrease of tumor proliferation and malignancy [60]. As micronuclei are potential biomarkers for inflammation and DNA damage and known to trigger innate immune response including activation of cGAS-STING innate immune signaling [58], the cross-link of ecDNA and anti-tumor immunity is worth further investigation.
The mechanistic actions of ecDNA
Oncogene amplification
Oncogene amplification is one of the driving factors of tumorigenesis and can occur at either the HSR structures on chromosomes or ecDNA [61]. Studies have reported significantly elevated copy numbers of oncogenes encoded in ecDNA (e.g. EGFR, MYC, CDK4, and MDM2) [2]. The amplification of oncogenes in ecDNA markedly increases overall oncogene expression, which can be found in both primary and metastatic tumors regardless of treatment [18]. In addition to elevating oncogene levels by copy number amplification, ecDNA may re-integrate into HSRs of chromosome and/or affect DNA accessibility to further “stabilize” the expression of oncogenes (e.g. EGFR in glioblastoma) [47].
The distinct inheritance pattern of ecDNA differs from the traditional Mendel’s law of inheritance and raises the question of whether and how the location of amplified oncogenes impacts tumorigenesis. In this regard, Lobachev et al. found that the breaking sites of yeast chromosomes determine the consequences of gene amplification [62]. EcDNA is often observed to be produced from oncogene amplification, if the breaking sites locate between the hairpin break and the telomere. In contrast, when the break occurs between the oncogene and telomere, the amplification of oncogenes will generate HSR [62].
Importantly, a positive feedback regulatory loop between the elevated expression of ecDNA-encoded genes and the accumulation of ecDNA has emerged. Hull et al. found that yeast cells obtain high levels of ecDNA containing the copper resistance gene CUP1 under copper exposure, and CUP1 expression may cause further accumulation of CUP1-bearing ecDNA [63]. Moreover, Ji et al. showed that down-regulation of genes in ecDNA may result in the integration of ecDNA into cytoplasmic micronuclei and the subsequent reduction of ecDNA [60]. These results reveal a mechanistic link between the accumulation of ecDNA and oncogene hyper-activity.
Chromosome rearrangements
As one of the major sources of somatic rearrangements, ecDNA exemplifies the mutagenic feature of the cancer genome [64]. Chromosomal rearrangements include translocations and/or insertions, which often result in oncogenes adjoining to transcriptional regulatory elements (e.g. promoters, enhancers) and the formation of fusion genes [65–67].
Rewiring enhancers
Morton et al. found that enhancers of EGFR, including endogenous enhancers as well as rewired enhancers from topological-associated domains, were co-amplified with oncogenes in glioblastoma [3]. These selectively skewed enhancers were also found in multiple cancer types (e.g. medulloblastoma, neuroblastoma and Wilms tumors) [3]. Helmsauer et al. further demonstrated that the majority of genomic rearrangement events involved ecDNA, challenging the current understanding of cancer genome remodeling [64].
Gene fusions
Gene fusions in ecDNA have been widely observed in leukemia and solid tumors, such as multiple myeloma [67], medulloblastoma [66] and gastric cancer [65]. Graux et al. identified a novel mechanism for the activation of tyrosine kinases in which the formation of ecDNA resulted in gene fusion between NUP214 and ABL1 in T-cell acute lymphoblastic leukemia [68]. Additionally, amplification of the BCR-ABL1 fusion gene on ecDNA and the translocation of (9;22) (q34;q11) have been reported in chronic myeloid leukemia during imatinib treatment [69, 70]. Furthermore, L'Abbate et al. identified the PVT1 gene on ecDNA as a hotspot for breakpoints whose amplification and rearrangements positively correlated with drug resistance and poor patient outcome in small cell lung cancer, indicating a crucial role of ecDNA in gene fusions [23].
Epigenetic modifications
Epigenetic modifications, which includes the chemical modification of chromatin, gene compensation, chromatin interaction, and topological reconstruction, alter the accessibility of chromatin and ecDNA and play a key role in a variety of biological processes [2, 71, 72].
Histone modification
Previous studies showed that ecDNA are enriched with active rather than repressive histone markers [2]. Analyses of metaphase glioblastoma cells further demonstrated the high levels of active histone marks (H3K4me1, H3K27ac) on ecDNA, while the levels of repressive markers (H3K9me3, H3K27me3) were low [2].
Gene compensation
EcDNA plays a critical role in the compensation of histone genes. In Saccharomyces cerevisiae, a novel circular ecDNA with HTA2-HTB2 amplification was generated to compensate for the effects of HTA1-HTB1 deletion through the recombination between two Ty1 retrotransposon elements [72]. This finding suggests that loss of histone genes somehow activates a gene compensatory mechanism on ecDNA to maintain the proper expression levels of histone genes that are required for transcriptional activities.
Nucleosome accessibility
Topological studies have shown that ecDNA is packaged into circular chromatin and nucleosome units and lacks the canonical high-order of chromatin structure that is commonly seen in chromosomal DNA [2]. This unique structure of ecDNA leads to enhanced chromatin accessibility to transcriptional machineries to ecDNA-encoded genes [2].
Remote chromatin interaction
The circular chromosome conformation capture technology combining high-throughput sequencing (4C-seq) has been used to assess the chromatin connection on ecDNA [73]. Previous studies have shown that the remote interaction of active chromatin was enhanced via ecDNA, and even ultra-remote chromatin contact could be detected [2].
Signaling pathways regulated by ecDNA
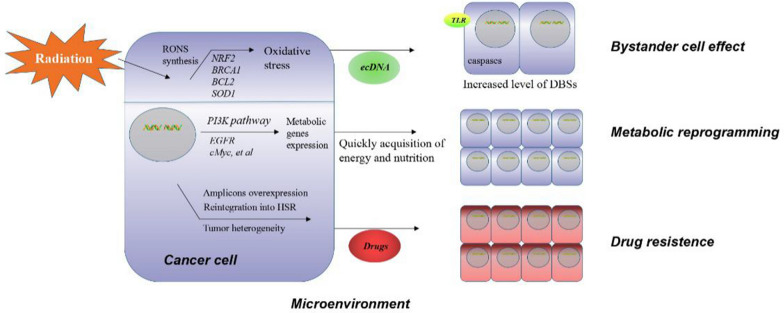
Better understanding of signaling pathways regulated by ecDNA is essential to elucidate the biological functions of ecDNA in cancer. These signals influence oxidative stress, inflammation and the bystander effect (Fig. 4) [34, 35, 74–76].
Fig. 4.
Mechanisms of ecDNA signaling in cancer. In response to oxidative stress, ecDNA induces the activation of inflammation, and to exert the bystander effect. Also, ecDNA regulates the metabolic reprogramming signaling to enhance the acquisition of energy in cancer cells
Oxidative stress signaling and bystander effect
ecDNA signaling contributes to the development of adaptive responses and bystander effect under oxidative stress. Low dose ionizing radiation triggers oxidative stress, DNA modification, apoptosis, ecDNA generation and subsequent changes in bystander cells [35]. The damaged DNA in irradiated cells can be released into the intracellular space and received by bystander cells through caspase 3 and TLR (e.g. TLR9) dependent mechanisms [36, 74, 76]. Consistent with the responses of irradiated cells to oxidative stress, bystander cells also show alterations in nuclei shape, activation of nucleolar organizer regions, promotion of actin polymerization and elevation of double-strand break level (i.e. bystander effect) [76]. Accordingly, the increased level of ecDNA stimulates the rapid synthesis of reactive oxygen and nitrogen species, resulting in secondary oxidative stress and upregulation of anti-oxidant genes (e.g. NRF2, KEAP1, SOD1) [75].
Pro-inflammatory signaling
The presence of ecDNA stimulates the production of pro-inflammatory cytokines that are deleterious to cancer cells [34]. Notably, ecDNA was shown to activate TLR9-MyD88-NF-kB signaling in the plasma of rheumatoid arthritis patients, leading to increases in pro-inflammatory cytokines (e.g. IL-6, TNF-α) [77]. In addition, the high GC content of ecDNA appears to affect the production of pro-inflammatory cytokines. One study reported that the GC-rich elements of ecDNA, but not genomic DNA, activated the production of IL-6 and TNF-α [77].
Metabolic reprogramming signaling
High EGFR expression was shown to drive glycolysis through EGFR signaling, PI3K pathway and c-MYC dysregulation [78]. EGFRvIII signaling is stringently regulated in the metabolic events of glioblastoma. EGFRvIII-dependent metabolic reprogramming includes the synergistic regulation of fatty acid synthesis through Akt-SREBP1-dependent mechanisms [79] and the control of intra-tumoral cholesterol levels through LDLR-dependent signaling [80]. Importantly, MYC was shown to be co-amplified with SQLE, a key metabolic gene that encodes squalene monooxygenase in the sterol biosynthesis pathway [81]. Furthermore, MYC also upregulates PYCRL, a crucial regulator of ornithine to proline conversion, and its isoenzymes to enhance the synthesis of proline [82].
ecDNA as a potential biomarker
An analysis of over 3200 clinical samples revealed that ecDNA was found in at least 14% of human cancers [2]. The frequency of ecDNA is likely to be higher in most aggressive cancer types, including glioblastoma, neuroblastoma, hepatocellular carcinoma, leukemia, lung and ovarian cancer [83]. Recent studies have revealed the potential utility of ecDNA in tumor diagnosis, prognosis and potential treatment of certain cancers on clinic [84, 85] (Table 2). The relationship of oncogenes amplified on ecDNA with drug sensitivity is also summarized below.
Table 2.
The roles and potential applications of ecDNA in cancers
| Cancers | Current advances | The connection between ecDNA and clinical applications | Refs. |
|---|---|---|---|
| I. Serving as potential biomarker to assess clinical outcomes | |||
| Thyroid cancer | Development of a noninvasive diagnostic tool for biopsy | EcDNA is a component in liquid biopsy of thyroid cancer as a new plasma genotyping source | [42] |
| Cervical cancer | Development of a computational diagnosis method | The presence of ecDNA-viral structures is verified in cervical cancer samples | [43] |
| Ovarian cancer | Mouse xenograft model | Ciuculating DNA complements miRNAs and linear DNA for diagnosis | [44] |
| Non-small-cell lung cancer (NSCLC) | Application in the FLAURA phase III trial | Circulating tumor DNA (ctDNA) serves as primary objective to depict genetic tumor profile | [45] |
| Hepatocellular carcinoma (HCC) | Study in biopsy and plasma samples in HCC patients | ecDNA tracks real-time therapeutic responses and could overcome tumor heterogeneity | [98] |
| Cancers | Genes on ecDNA | Functions | Refs. |
|---|---|---|---|
| II. Elimination of oncogenes reside on ecDNA increases drug-sensitivity | |||
| Glioblastoma | MYC, EGFR, PDGFRα, ERBB2, CDK4, MDM2 | Amplification of EGFRvIII results in erlotinib resistance | [48] |
| Colon cancer | DHFR, c-MYC | Down-regulation of DHFR on ecDNA increases MTX sensitivity | [57, 94, 95] |
| Neuroblastoma | MYCN | Elimination of MYCN on ecDNA increases HU sensitivity | [67] |
| Cervical cancer | DHFR | Amplification of DHFR promote MTX resistance | [96] |
| Ovarian cancer | MYCN, EIF5AR, CA125 | Decreased levels of ecDNA-form CA125 after HU treatment | [97] |
| Breast cancer | DHFR, HER2 | Loss of HER2 residing on ecDNA has no effect on trastuzamab therapy | [98, 99] |
| Leukemia | c-MYC | Down-regulation of c-MYC promotes drug sensitivity | [100] |
| Oral squamous cell carcinoma | MDR1 | Loss of MDR1 enhances HU sensitivity | [101] |
| III. Extracellular vesicles carrying ecDNA transfer oncogenes and trigger tumorigenesis | |||
| Ovarian cancer | Studies of EVs from cancer cells remain in the laboratory stage | ecDNA can be encapsulated in EVs. EVs might have applications on clinic for tumor diagnosis, prognosis or potential treatment | [19] |
ecDNA may represent a novel tool for various clinical applications mainly in three aspects. First, ecDNA can be released into the peripheral blood system [86] and may serve as potential prognostic biomarkers of multiple cancers, such as thyroid cancer [50], cervical cancer [87], ovarian cancer [88] and non-small cell lung cancer [89]. As an example, ecDNA has been used in liquid biopsy of thyroid cancer as a new plasma genotyping source [50]. Second, elimination of oncogenes on ecDNA increases drug sensitivity [22, 69, 90, 47], providing a novel adjunctive therapeutic option for chemotherapy. Third, ecDNA-carrying EVs transport oncogenes and trigger tumorigenesis [19]. Thus, detecting and targeting EVs might have potential utility for cancer treatment.
Conclusions and perspectives
Recent findings have revolutionized our understanding of ecDNA in cancer, highlighting the potential of ecDNA as a potential biomarker for personalized therapy. Since ecDNA is usually more stable than linear DNA, ecDNA may potentially be used in liquid biopsy [86]. However, the prognostic and/or diagnostic power of ecDNA remains undetermined. Clinical proof to support the feasibility of ecDNA as a biomarker is still lacking.
Despite the promising findings, several aspects of ecDNA remain to be elucidated. Studies have shown the possible origin and destination of ecDNA [91, 92]; however, the type of stress that initiates the generation of ecDNA and whether and how the ecDNA-encoded genes could be selectively induced under the evolving microenvironment remain unclear. ecDNA may influence bystander cells in response to oxidative stress, but whether the original ecDNA-producing cancer cells affect bystander cells to facilitate tumorigenesis and/or progression is still unknown.
In addition, the functions of ecDNA in multiple biological processes (e.g. cell development, aging, genomic instability, adaptive evolution, drug resistance, tumor development) also need to be further investigated. Elucidation of the underlying mechanisms of ecDNA may further shed light on cancer therapeutics.
Acknowledgements
This work was supported, in whole or in part, by The Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (No. TP2018046 to J.S.), Shanghai Municipal Education Commission-Two Hundred Talents (No. 20191817 to J.S.), and General Program of National Natural Science Foundation of China (No. 81972667 to J.S.).
Abbreviations
- HSR
Homogeneously staining region
- BFB cycle
Breakage-fusion-bridge cycle
- EGFR
Epidermal growth factor receptor
- DHFR
Dihydrofolate reductase
- LDLR
Low density lipoprotein receptors
- SREBP1
Sterol regulatory element binding protein-1
- PYCRL
Pyrroline-5-carboxylatereductase L
- DSB
DNA double strand break
- EV
Extracellular vesicle
Authors' contributions
YHW, RH and GPZ performed the literature review, wrote the review and helped with the revisions. JFS wrote and edited the manuscript. All authors read and approved the final manuscript.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Verhaak RGW, Bafna V, Mischel PS. Extrachromosomal oncogene amplification in tumour pathogenesis and evolution. Nat Rev Cancer. 2019;19(5):283–8. doi: 10.1038/s41568-019-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu S, Turner KM, Nguyen N, Raviram R, Erb M, Santini J, et al. Circular ecDNA promotes accessible chromatin and high oncogene expression. Nature. 2019;575(7784):699–703. doi: 10.1038/s41586-019-1763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morton AR, Dogan-Artun N, Faber ZJ, MacLeod G, Bartels CF, Piazza MS, et al. Functional enhancers shape extrachromosomal oncogene amplifications. Cell. 2019;179(6):1330–41. doi: 10.1016/j.cell.2019.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balaban-Malenbaum G, Gilbert F. Double minute chromosomes and the homogeneously staining regions in chromosomes of a human neuroblastoma cell line. Science. 1977;198(4318):739–41. doi: 10.1126/science.71759. [DOI] [PubMed] [Google Scholar]
- 5.Buoen LC, Brand KG. Double-minute chromosomes in plastic film-induced sarcomas in mice. Sci Nat-Heidelberg. 1968;55(3):135–6. doi: 10.1007/BF00624255. [DOI] [PubMed] [Google Scholar]
- 6.Pristyazhnyuk IE, Menzorov AG. Ring chromosomes: from formation to clinical potential. Protoplasma. 2018;255(2):439–449. doi: 10.1007/s00709-017-1165-1. [DOI] [PubMed] [Google Scholar]
- 7.Garsed DW, Marshall OJ, Corbin VDA, Hsu A, Di Stefano L, Schröder J, et al. The architecture and evolution of cancer neochromosomes. Cancer Cell. 2014;26(5):653–67. doi: 10.1016/j.ccell.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Macchia G, Severgnini M, Purgato S, Tolomeo D, Casciaro H, Cifola I, et al. The hidden genomic and transcriptomic plasticity of giant marker chromosomes in cancer. Genetics. 2018;208(3):951–61. doi: 10.1534/genetics.117.300552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papenfuss AT, Thomas DM. The life history of neochromosomes revealed. Mol Cell Oncol. 2015;2(4):e1000698. doi: 10.1080/23723556.2014.1000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu GH. Protection of the genome and central protein-coding sequences by non-coding DNA against DNA damage from radiation. Mutat Res Rev Mutat Res. 2015;764:108–17. doi: 10.1016/j.mrrev.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Paulsen T, Shibata Y, Kumar P, Dillon L, Dutta A. Small extrachromosomal circular DNAs, microDNA, produce short regulatory RNAs that suppress gene expression independent of canonical promoters. Nucleic Acids Res. 2019;47(9):4586–96. doi: 10.1093/nar/gkz155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koche RP, Rodriguez-Fos E, Helmsauer K, Burkert M, MacArthur IC, Maag J, et al. Extrachromosomal circular DNA drives oncogenic genome remodeling in neuroblastoma. Nat Genet. 2019;12:16. doi: 10.1038/s41588-019-0547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deshpande V, Luebeck J, Nguyen ND, Bakhtiari M, Turner KM, Schwab R, et al. Exploring the landscape of focal amplifications in cancer using AmpliconArchitect. Nat Commun. 2019;10:15. doi: 10.1038/s41467-018-07969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeCarvalho AC, Kim H, Poisson LM, Winn ME, Mueller C, Cherba D, et al. Discordant inheritance of chromosomal and extrachromosomal DNA elements contributes to dynamic disease evolution in glioblastoma. Nat Genet. 2018;50(5):708–17. doi: 10.1038/s41588-018-0105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H, Nguyen N, Turner K, Wu S, Gujar AD, Luebeck J, et al. Extrachromosomal DNA is associated with oncogene amplification and poor outcome across multiple cancers. Nat Genet. 2020;8:17. doi: 10.1038/s41588-020-0678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajkumar U, Turner K, Luebeck J, Deshpande V, Chandraker M, Mischel P, et al. EcSeg: semantic segmentation of metaphase images containing extrachromosomal DNA. iScience. 2019;21:428–35. doi: 10.1016/j.isci.2019.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brettmann EA, Oh IY, de Guzman SC. High-throughput identification of gene regulatory sequences using next-generation sequencing of circular chromosome conformation capture (4C-seq) J Vis Exp. 2018;140:58. doi: 10.3791/58030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner KM, Deshpande V, Beyter D, Koga T, Rusert J, Lee C, et al. Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity. Nature. 2017;543(7643):122–125. doi: 10.1038/nature21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tandon I, Pal R, Pal JK, Sharma NK. Extrachromosomal circular DNAs: an extra piece of evidence to depict tumor heterogeneity. Fut Sci OA. 2019;5(6):O390. doi: 10.2144/fsoa-2019-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohl NE, Kanda N, Schreck RR, Bruns G, Latt SA, Gilbert F, et al. Transposition and amplification of oncogene-related sequences in human neuroblastomas. Cell. 1983;35(2):359–67. doi: 10.1016/0092-8674(83)90169-1. [DOI] [PubMed] [Google Scholar]
- 21.Alitalo K, Schwab M, Lin CC, Varmus HE, Bishop JM. Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc Natl Acad Sci USA. 1983;80(6):1707–11. doi: 10.1073/pnas.80.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haber DA, Schimke RT. Unstable amplification of an altered dihydrofolate reductase gene associated with double-minute chromosomes. Cell. 1981;26(3):355–62. doi: 10.1016/0092-8674(81)90204-X. [DOI] [PubMed] [Google Scholar]
- 23.L'Abbate A, Macchia G, D'Addabbo P, Lonoce A, Tolomeo D, Trombetta D, et al. Genomic organization and evolution of double minutes/homogeneously staining regions with MYC amplification in human cancer. Nucleic Acids Res. 2014;42(14):9131–45. doi: 10.1093/nar/gku590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith G, Taylor-Kashton C, Dushnicky L, Symons S, Wright J, Mai S. c-Myc-induced extrachromosomal elements carry active chromatin. Neoplasia. 2003;5(2):110–20. doi: 10.1016/S1476-5586(03)80002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCLINTOCK B. Chromosome organization and genic expression. Cold Spring Harb Symp Quant Biol. 1951;16:13–47. doi: 10.1101/SQB.1951.016.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Barr FG, Nauta LE, Davis RJ, Schafer BW, Nycum LM, Biegel JA. In vivo amplification of the PAX3-FKHR and PAX7-FKHR fusion genes in alveolar rhabdomyosarcoma. Hum Mol Genet. 1996;5(1):15–21. doi: 10.1093/hmg/5.1.15. [DOI] [PubMed] [Google Scholar]
- 27.Carroll SM, Gaudray P, De Rose ML, Emery JF, Meinkoth JL, Nakkim E, et al. Characterization of an episome produced in hamster cells that amplify a transfected CAD gene at high frequency: functional evidence for a mammalian replication origin. Mol Cell Biol. 1987;7(5):1740–50. doi: 10.1128/MCB.7.5.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephens PJ, Greenman CD, Fu B, Yang F, Bignell GR, Mudie LJ, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144(1):27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menghi F, Barthel FP, Yadav V, Tang M, Ji B, Tang Z, et al. The tandem duplicator phenotype is a prevalent genome-wide cancer configuration driven by distinct gene mutations. Cancer Cell. 2018;34(2):197–210. doi: 10.1016/j.ccell.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gisselsson D, Jin Y, Lindgren D, Persson J, Gisselsson L, Hanks S, et al. Generation of trisomies in cancer cells by multipolar mitosis and incomplete cytokinesis. Proc Natl Acad Sci USA. 2010;107(47):20489–93. doi: 10.1073/pnas.1006829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korbel JO, Campbell PJ. Criteria for inference of chromothripsis in cancer genomes. Cell. 2013;152(6):1226–36. doi: 10.1016/j.cell.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 32.Vogt N, Lefevre SH, Apiou F, Dutrillaux AM, Cor A, Leuraud P, et al. Molecular structure of double-minute chromosomes bearing amplified copies of the epidermal growth factor receptor gene in gliomas. Proc Natl Acad Sci USA. 2004;101(31):11368–73. doi: 10.1073/pnas.0402979101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulsen T, Kumar P, Koseoglu MM, Dutta A. Discoveries of extrachromosomal circles of DNA in normal and tumor cells. TRENDS GENET. 2018;34(4):270–278. doi: 10.1016/j.tig.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ermakov AV, Konkova MS, Kostyuk SV, Izevskaya VL, Baranova A, Veiko NN. Oxidized extracellular DNA as a stress signal in human cells. Oxid Med Cell Longev. 2013;2013:1–12. doi: 10.1155/2013/649747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ermakov AV, Kon'Kova MS, Kostiuk SV, Veiko NN. DNA-signaling pathway mediating development of a radiation-induced bystander effect in human cells. Radiats Biol Radioecol. 2011;51(6):651–9. [PubMed] [Google Scholar]
- 36.Ermakov AV, Konkova MS, Kostyuk SV, Egolina NA, Efremova LV, Veiko NN. Oxidative stress as a significant factor for development of an adaptive response in irradiated and nonirradiated human lymphocytes after inducing the bystander effect by low-dose X-radiation. Mutat Res. 2009;669(1–2):155–161. doi: 10.1016/j.mrfmmm.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Storlazzi CT, Lonoce A, Guastadisegni MC, Trombetta D, D'Addabbo P, Daniele G, et al. Gene amplification as double minutes or homogeneously staining regions in solid tumors: origin and structure. Genome Res. 2010;20(9):1198–206. doi: 10.1101/gr.106252.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Storlazzi CT, Fioretos T, Surace C, Lonoce A, Mastrorilli A, Strombeck B, et al. MYC-containing double minutes in hematologic malignancies: evidence in favor of the episome model and exclusion of MYC as the target gene. Hum Mol Genet. 2006;15(6):933–42. doi: 10.1093/hmg/ddl010. [DOI] [PubMed] [Google Scholar]
- 39.Cohen Z, Bacharach E, Lavi S. Mouse major satellite DNA is prone to eccDNA formation via DNA Ligase IV-dependent pathway. Oncogene. 2006;25(33):4515–24. doi: 10.1038/sj.onc.1209485. [DOI] [PubMed] [Google Scholar]
- 40.Xu K, Ding L, Chang T, Shao Y, Chiang J, Mulder H, et al. Structure and evolution of double minutes in diagnosis and relapse brain tumors. Acta Neuropathol. 2019;137(1):123–137. doi: 10.1007/s00401-018-1912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spindler KLG, Boysen AK, Pallisgård N, Johansen JS, Tabernero J, Sørensen MM, et al. Cell-free DNA in metastatic colorectal cancer: a systematic review and meta-analysis. Oncologist. 2017;22(9):1049–1055. doi: 10.1634/theoncologist.2016-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Műzes G, Kiss AL, Tulassay Z, Sipos F. Cell-free DNA-induced alteration of autophagy response and TLR9-signaling: their relation to amelioration of DSS-colitis. Comp Immunol Microbiol Infect Dis. 2017;52:48–57. doi: 10.1016/j.cimid.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Zhu Z, Liu Y, Wei L, Li B, Mao F, et al. MDM2 inhibition-mediated autophagy contributes to the pro-apoptotic effect of berberine in p53-null leukemic cells. Life Sci. 2020;242:117228. doi: 10.1016/j.lfs.2019.117228. [DOI] [PubMed] [Google Scholar]
- 44.Anunobi R, Boone BA, Cheh N, Tang D, Kang R, Loux T, et al. Extracellular DNA promotes colorectal tumor cell survival after cytotoxic chemotherapy. J Surg Res. 2018;226:181–91. doi: 10.1016/j.jss.2018.02.042. [DOI] [PubMed] [Google Scholar]
- 45.Tuveson D, Clevers H. Cancer modeling meets human organoid technology. Science. 2019;364(6444):952–5. doi: 10.1126/science.aaw6985. [DOI] [PubMed] [Google Scholar]
- 46.Meng X, Qi X, Guo H, Cai M, Li C, Zhu J, et al. Novel role for non-homologous end joining in the formation of double minutes in methotrexate-resistant colon cancer cells. J Med Genet. 2015;52(2):135–144. doi: 10.1136/jmedgenet-2014-102703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nathanson DA, Gini B, Mottahedeh J, Visnyei K, Koga T, Gomez G, et al. Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA. Science. 2014;343(6166):72–6. doi: 10.1126/science.1241328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sadovska L, Santos CB, Kalnina Z, Line A. Biodistribution, uptake and effects caused by cancer-derived extracellular vesicles. J Circ Biomark. 2015;4:2. doi: 10.5772/60522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bailey C, Shoura MJ, Mischel PS, Swanton C. Extrachromosomal DNA - relieving heredity constraints, accelerating tumour evolution. Ann Oncol. 2020;4:7. doi: 10.1016/j.annonc.2020.03.303. [DOI] [PubMed] [Google Scholar]
- 50.Khatami F, Tavangar SM. Liquid biopsy in thyroid cancer: new insight. Int J Hematol Oncol Stem Cell Res. 2018;12(3):235–48. [PMC free article] [PubMed] [Google Scholar]
- 51.Kalavska K, Minarik T, Vlkova B, Manasova D, Kubickova M, Jurik A, et al. Prognostic value of various subtypes of extracellular DNA in ovarian cancer patients. J Ovarian Res. 2018;11(1):85. doi: 10.1186/s13048-018-0459-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calcinotto A, Kohli J, Zagato E, Pellegrini L, Demaria M, Alimonti A. Cellular senescence: aging, cancer, and injury. Physiol Rev. 2019;99(2):1047–78. doi: 10.1152/physrev.00020.2018. [DOI] [PubMed] [Google Scholar]
- 53.Hull RM, Houseley J. The adaptive potential of circular DNA accumulation in ageing cells. Curr Genet. 2020;4:15. doi: 10.1007/s00294-020-01069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sinclair DA, Guarente L. Extrachromosomal rDNA circles–a cause of aging in yeast. Cell. 1997;91(7):1033–42. doi: 10.1016/S0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 55.Storci G, Bacalini MG, Bonifazi F, Garagnani P, De Carolis S, Salvioli S, et al. Ribosomal DNA instability: an evolutionary conserved fuel for inflammaging. Ageing Res Rev. 2020;58:101018. doi: 10.1016/j.arr.2020.101018. [DOI] [PubMed] [Google Scholar]
- 56.Shimizu N, Misaka N, Utani K. Nonselective DNA damage induced by a replication inhibitor results in the selective elimination of extrachromosomal double minutes from human cancer cells. Genes Chromosom Cancer. 2007;46(10):865–874. doi: 10.1002/gcc.20473. [DOI] [PubMed] [Google Scholar]
- 57.Mansilla S, Bataller M, Portugal J. A nuclear budding mechanism in transiently arrested cells generates drug-sensitive and drug-resistant cells. Biochem Pharmacol. 2009;78(2):123–32. doi: 10.1016/j.bcp.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 58.Valent A, Benard J, Clausse B, Barrois M, Valteau-Couanet D, Terrier-Lacombe MJ, et al. In vivo elimination of acentric double minutes containing amplified MYCN from neuroblastoma tumor cells through the formation of micronuclei. Am J Pathol. 2001;158(5):1579–84. doi: 10.1016/S0002-9440(10)64112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwon J, Bakhoum SF. The cytosolic DNA-sensing cGAS–STING pathway in cancer. Cancer Discov. 2020;10(1):26–39. doi: 10.1158/2159-8290.CD-19-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ji W, Bian Z, Yu Y, Yuan C, Liu Y, Yu L, et al. Expulsion of micronuclei containing amplified genes contributes to a decrease in double minute chromosomes from malignant tumor cells. Int J Cancer. 2014;134(6):1279–88. doi: 10.1002/ijc.28467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schimke RT. Gene amplification in cultured animal cells. Cell. 1984;37(3):705–13. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- 62.Lobachev KS, Rattray A, Narayanan V. Hairpin- and cruciform-mediated chromosome breakage: causes and consequences in eukaryotic cells. Front Biosci. 2007;12:4208–20. doi: 10.2741/2381. [DOI] [PubMed] [Google Scholar]
- 63.Hull RM, King M, Pizza G, Krueger F, Vergara X, Houseley J. Transcription-induced formation of extrachromosomal DNA during yeast ageing. Plos Biol. 2019;17(12):e3000471. doi: 10.1371/journal.pbio.3000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koche RP, Rodriguez-Fos E, Helmsauer K, Burkert M, MacArthur IC, Maag J, et al. Extrachromosomal circular DNA drives oncogenic genome remodeling in neuroblastoma. Nat Genet. 2020;52(1):29–34. doi: 10.1038/s41588-019-0547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim HP, Cho GA, Han SW, Shin JY, Jeong EG, Song SH, et al. Novel fusion transcripts in human gastric cancer revealed by transcriptome analysis. Oncogene. 2014;33(47):5434–41. doi: 10.1038/onc.2013.490. [DOI] [PubMed] [Google Scholar]
- 66.Northcott PA, Shih DJ, Peacock J, Garzia L, Morrissy AS, Zichner T, et al. Subgroup-specific structural variation across 1000 medulloblastoma genomes. Nature. 2012;488(7409):49–56. doi: 10.1038/nature11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagoshi H, Taki T, Hanamura I, Nitta M, Otsuki T, Nishida K, et al. Frequent PVT1 rearrangement and novel chimeric genes PVT1-NBEA and PVT1-WWOX occur in multiple myeloma with 8q24 abnormality. Cancer Res. 2012;72(19):4954–62. doi: 10.1158/0008-5472.CAN-12-0213. [DOI] [PubMed] [Google Scholar]
- 68.Graux C, Cools J, Melotte C, Quentmeier H, Ferrando A, Levine R, et al. Fusion of NUP214 to ABL1 on amplified episomes in T-cell acute lymphoblastic leukemia. Nat Genet. 2004;36(10):1084–9. doi: 10.1038/ng1425. [DOI] [PubMed] [Google Scholar]
- 69.Barnes DJ, Palaiologou D, Panousopoulou E, Schultheis B, Yong AS, Wong A, et al. Bcr-Abl expression levels determine the rate of development of resistance to imatinib mesylate in chronic myeloid leukemia. Cancer Res. 2005;65(19):8912–9. doi: 10.1158/0008-5472.CAN-05-0076. [DOI] [PubMed] [Google Scholar]
- 70.Morel F, Bris MJ, Herry A, Calvez GL, Marion V, Abgrall JF, et al. Double minutes containing amplified bcr-abl fusion gene in a case of chronic myeloid leukemia treated by imatinib. Eur J Haematol. 2003;70(4):235–9. doi: 10.1034/j.1600-0609.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 71.Corces MR, Granja JM, Shams S, Louie BH, Seoane JA, Zhou W, et al. The chromatin accessibility landscape of primary human cancers. Science. 2018;362(6413):v1898. doi: 10.1126/science.aav1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Libuda DE, Winston F. Amplification of histone genes by circular chromosome formation in Saccharomyces cerevisiae. Nature. 2006;443(7114):1003–7. doi: 10.1038/nature05205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao Z, Tavoosidana G, Sjölinder M, Göndör A, Mariano P, Wang S, et al. Circular chromosome conformation capture (4C) uncovers extensive networks of epigenetically regulated intra- and interchromosomal interactions. Nat Genet. 2006;38(11):1341–1347. doi: 10.1038/ng1891. [DOI] [PubMed] [Google Scholar]
- 74.Kostyuk SV, Ermakov AV, Alekseeva AY, Smirnova TD, Glebova KV, Efremova LV, et al. Role of extracellular DNA oxidative modification in radiation induced bystander effects in human endotheliocytes. Mutat Res. 2012;729(1–2):52–60. doi: 10.1016/j.mrfmmm.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 75.Loseva P, Kostyuk S, Malinovskaya E, Clement N, Dechesne CA, Dani C, et al. Extracellular DNA oxidation stimulates activation of NRF2 and reduces the production of ROS in human mesenchymal stem cells. Expert Opin Biol Ther. 2012;12:S85–97. doi: 10.1517/14712598.2012.688948. [DOI] [PubMed] [Google Scholar]
- 76.Ermakov AV, Konkova MS, Kostyuk SV, Smirnova TD, Malinovskaya EM, Efremova LV, et al. An extracellular DNA mediated bystander effect produced from low dose irradiated endothelial cells. Mutat Res. 2011;712(1–2):1–10. doi: 10.1016/j.mrfmmm.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 77.Speranskii AI, Kostyuk SV, Kalashnikova EA, Veiko NN. Enrichment of extracellular DNA from the cultivation medium of human peripheral blood mononuclears with genomic CpG rich fragments results in increased cell production of IL-6 and TNF-a via activation of the NF-kB signaling pathway. Biomed Khim. 2016;62(3):331–40. doi: 10.18097/PBMC20166203331. [DOI] [PubMed] [Google Scholar]
- 78.Bi J, Wu S, Zhang W, Mischel PS. Targeting cancer's metabolic co-dependencies: A landscape shaped by genotype and tissue context. Biochimica et Biophysica Acta. 2018;1870(1):76–87. doi: 10.1016/j.bbcan.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo D, Prins RM, Dang J, Kuga D, Iwanami A, Soto H, et al. EGFR signaling through an Akt-SREBP-1-dependent, rapamycin-resistant pathway sensitizes glioblastomas to antilipogenic therapy. Sci Signal. 2009;2(101):a82. doi: 10.1126/scisignal.2000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo D, Reinitz F, Youssef M, Hong C, Nathanson D, Akhavan D, et al. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov. 2011;1(5):442–56. doi: 10.1158/2159-8290.CD-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haider S, McIntyre A, van Stiphout RG, Winchester LM, Wigfield S, Harris AL, et al. Genomic alterations underlie a pan-cancer metabolic shift associated with tumour hypoxia. Genome Biol. 2016;17(1):140. doi: 10.1186/s13059-016-0999-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu W, Hancock CN, Fischer JW, Harman M, Phang JM. Proline biosynthesis augments tumor cell growth and aerobic glycolysis: involvement of pyridine nucleotides. Sci Rep. 2015;5:17206. doi: 10.1038/srep17206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu S, Bafna V, Mischel PS. Extrachromosomal DNA (ecDNA) in cancer pathogenesis. Curr Opin Genet Dev. 2021;66:78–82. doi: 10.1016/j.gde.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 84.Yan Y, Guo G, Huang J, Gao M, Zhu Q, Zeng S, et al. Current understanding of extrachromosomal circular DNA in cancer pathogenesis and therapeutic resistance. J Hematol Oncol. 2020;13:12. doi: 10.1186/s13045-020-00960-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liao Z, Jiang W, Ye L, Li T, Yu X, Liu L. Classification of extrachromosomal circular DNA with a focus on the role of extrachromosomal DNA (ecDNA) in tumor heterogeneity and progression. Biochimica et Biophysica Acta. 2020;1874(1):188392. doi: 10.1016/j.bbcan.2020.188392. [DOI] [PubMed] [Google Scholar]
- 86.Khatami F, Larijani B, Tavangar SM. The presence of tumor extrachomosomal circular DNA (ecDNA) as a component of liquid biopsy in blood. Med Hypotheses. 2018;114:5–7. doi: 10.1016/j.mehy.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 87.Nguyen ND, Deshpande V, Luebeck J, Mischel PS, Bafna V. ViFi: accurate detection of viral integration and mRNA fusion reveals indiscriminate and unregulated transcription in proximal genomic regions in cervical cancer. Nucleic Acids Res. 2018;46(7):3309–25. doi: 10.1093/nar/gky180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumar P, Dillon LW, Shibata Y, Jazaeri AA, Jones DR, Dutta A. Normal and cancerous tissues release extrachromosomal circular DNA (eccDNA) into the circulation. Mol Cancer Res. 2017;15(9):1197–205. doi: 10.1158/1541-7786.MCR-17-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bennouna J, Girard N, Audigier-Valette C, le Thuaut A, Gervais R, Masson P, et al. Phase II study evaluating the mechanisms of resistance on tumor tissue and liquid biopsy in patients with EGFR-mutated non-pretreated advanced lung cancer receiving osimertinib until and beyond radiologic progression: The MELROSE Trial. Clin Lung Cancer. 2020;21(1):e10–4. doi: 10.1016/j.cllc.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 90.Morales C, Ribas M, Aiza G, Peinado MA. Genetic determinants of methotrexate responsiveness and resistance in colon cancer cells. Oncogene. 2005;24(45):6842–7. doi: 10.1038/sj.onc.1208834. [DOI] [PubMed] [Google Scholar]
- 91.Gu X, Yu J, Chai P, Ge S, Fan X. Novel insights into extrachromosomal DNA: redefining the onco-drivers of tumor progression. J Exp Clin Cancer RES. 2020;39:18. doi: 10.1186/s13046-020-1520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wei J, Wu C, Meng H, Li M, Niu W, Zhan Y, et al. The biogenesis and roles of extrachromosomal oncogene involved in carcinogenesis and evolution. Am J Cancer Res. 2020;10(11):3532–50. [PMC free article] [PubMed] [Google Scholar]
- 93.Cai ZX, Chen G, Zeng YY, Dong XQ, Lin MJ, Huang XH, et al. Circulating tumor DNA profiling reveals clonal evolution and real-time disease progression in advanced hepatocellular carcinoma. Int J Cancer. [Journal Article; Research Support, Non-U.S. Gov't]. 2017;141(5):977–85. doi: 10.1002/ijc.30798. [DOI] [PubMed] [Google Scholar]
- 94.Cai M, Zhang H, Hou L, Gao W, Song Y, Cui X, et al. Inhibiting homologous recombination decreases extrachromosomal amplification but has no effect on intrachromosomal amplification in methotrexate-resistant colon cancer cells. Int J Cancer. [Journal Article; Research Support, Non-U.S. Gov't]. 2019;144(5):1037–48. doi: 10.1002/ijc.31781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shimizu N, Itoh N, Utiyama H, Wahl GM. Selective entrapment of extrachromosomally amplified DNA by nuclear budding and micronucleation during S phase. J Cell Biol. [Journal Article; Research Support, Non-U.S. Gov't; Research Support, U.S. Gov't, Non-P.H.S.]. 1998;140(6):1307–20. doi: 10.1083/jcb.140.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ruiz-Herrera A, Smirnova A, Khoriauli L, Nergadze SG, Mondello C, Giulotto E. Gene amplification in human cells knocked down for RAD54. Genome Integr. [Journal Article]. 2011;2(1):5. doi: 10.1186/2041-9414-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang CY, Feng YX, Yu Y, Sun WJ, Bai J, Chen F, et al. The molecular mechanism of resistance to methotrexate in mouse methotrexate-resistant cells by cancer drug resistance and metabolism Super Array. Basic Clin Pharmacol Toxicol. [Journal Article; Research Support, Non-U.S. Gov't]. 2006;99(2):141–5. doi: 10.1111/j.1742-7843.2006.pto_470.x. [DOI] [PubMed] [Google Scholar]
- 98.Hahn P, Nevaldine B, Morgan WF. X-ray induction of methotrexate resistance due to dhfr gene amplification. Somat Cell Mol Genet. [Journal Article; Research Support, U.S. Gov't, Non-P.H.S.; Research Support, U.S. Gov't, P.H.S.]. 1990;16(5):413–23. doi: 10.1007/BF01233191. [DOI] [PubMed] [Google Scholar]
- 99.Vicario R, Peg V, Morancho B, Zacarias-Fluck M, Zhang J, Martinez-Barriocanal A, et al. Patterns of HER2 gene amplification and response to Anti-HER2 therapies. Plos One. [Journal Article; Research Support, Non-U.S. Gov't]. 2015;10(6):e129876. doi: 10.1371/journal.pone.0129876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eckhardt SG, Dai A, Davidson KK, Forseth BJ, Wahl GM, Von Hoff DD. Induction of differentiation in HL60 cells by the reduction of extrachromosomally amplified c-myc. Proc Natl Acad Sci USA. [Journal Article; Research Support, Non-U.S. Gov't; Research Support, U.S. Gov't, P.H.S.]. 1994;91(14):6674–8. doi: 10.1073/pnas.91.14.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Von Hoff DD, Waddelow T, Forseth B, Davidson K, Scott J, Wahl G. Hydroxyurea accelerates loss of extrachromosomally amplified genes from tumor cells. Cancer Res. [Journal Article; Research Support, Non-U.S. Gov't; Research Support, U.S. Gov't, P.H.S.]. 1991;51(23 Pt 1):6273–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.