Abstract
Background
Although acute gastrointestinal injury (AGI) and feeding intolerance (FI) are known independent determinants of worse outcomes and high mortality in intensive care unit (ICU) patients, the incidence of AGI and FI in critically ill COVID-19 patients and their prognostic importance have not been thoroughly studied.
Methods
We reviewed 218 intubated patients at Stony Brook University Hospital and stratified them into three groups based on AGI severity, according to data collected in the first 10 days of ICU course. We used chi-square test to compare categorical variables such as age and sex and two-sample t-test or Mann-Whitney U-tests for continuous variables, including important laboratory values. Cox proportional hazards regression models were utilized to determine whether AGI score was an independent predictor of survival, and multivariable analysis was performed to compare risk factors that were deemed significant in the univariable analysis. We performed Kaplan-Meier survival analysis based on the AGI score and the presence of FI.
Results
The overall incidence of AGI was 95% (45% AGI I/II, 50% AGI III/IV), and FI incidence was 63%. Patients with AGI III/IV were more likely to have prolonged mechanical ventilation (22 days vs 16 days, P-value <0.002) and higher mortality rate (58% vs 28%, P-value <0.001) compared to patients with AGI 0/I/II. This was confirmed with multivariable analysis which showed that AGI score III/IV was an independent predictor of higher mortality (AGI III/IV vs AGI 0/I/II hazard ratio (HR), 2.68; 95% confidence interval (CI), 1.69–4.25; P-value <0.0001). Kaplan-Meier survival analysis showed that both AGI III/IV and FI (P-value <0.001) were associated with worse outcomes. Patients with AGI III/IV had higher daily and mean D-dimer and CRP levels compared to AGI 0/I/II (P-value <0.0001).
Conclusions
The prevalence of AGI and FI among critically ill COVID-19 patients was high. AGI grades III/IV were associated with higher risk for prolonged mechanical ventilation and mortality compared to AGI 0/I/II, while it also correlated with higher D-dimer and C-reactive protein (CRP) levels. FI was independently associated with higher mortality. The development of high-grade AGI and FI during the first days of ICU stay can serve as prognostic tools to predict outcomes in critically ill COVID-19 patients.
Keywords: COVID-19, Critically ill, Acute gastrointestinal injury (AGI), Feeding intolerance (FI), D-dimers, CRP
Introduction
The COVID-19 pandemic, caused by the novel SARS-CoV-2, has led to a global health crisis with 113 million cases and more than 2.5 million deaths globally.1 It has been shown that SARS-CoV-2 affects multiple systems causing widespread physiologic insult and multiorgan failure in critically ill patients, manifesting as acute respiratory distress syndrome, acute kidney and liver injury, heart failure, but also as AGI.2,3 AGI is a term used to broadly describe malfunctioning of the gut affecting digestion and absorption of nutrients that ranges from a temporary partial impairment to intestinal end-organ failure.4 Although it is well known that AGI is closely related to adverse outcomes,5 there is a relative scarcity of data on how it affects COVID-19 patients.
In the most relevant retrospective study on AGI in COVID-19 patients, it was shown that AGI was not only common but also associated with a higher mortality rate.6 FI has also been shown to be an independent prognostic factor of adverse outcomes in critically ill populations,7 and its importance was recently emphasized in ICU COVID-19 patients.8 On the other hand, there is little evidence correlating the known biochemical markers of adverse COVID-19 outcomes, such as CRP and D-dimer,9 with the development of AGI. It has been shown that SARS-CoV-2 infects the target cells through the angiotensin-converting enzyme 2 (ACE-2) receptor, found on the surface of lung alveolar, endothelial, perivascular pericytes, cardiomyocytes, but also enterocytes.10,11 Thus, direct viral injury to GI epithelial cells may contribute to AGI. Alternately, the pathogenicity of SARS-CoV-2 in the gastrointestinal tract could be attributed to the induction of a prothrombotic state and formation of microthrombi.8,12 In our study, we investigated the link between the development of AGI and FI with mortality in COVID-19 intubated patients and its correlation with increased CRP and D-dimer levels as a manifestation of intense inflammatory state and possible intestinal micro-thrombosis, as well as the utility of these findings as prognostic tools.
Methods
Patient Population and Inclusion Criteria
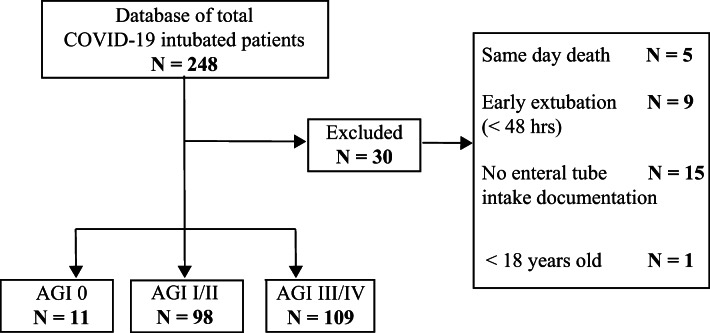
This was a retrospective cohort study of all intubated COVID-19 patients, confirmed with a positive RT-PCR test for SARS-CoV-2, at Stony Brook University Hospital between February 7, 2020, and May 17, 2020. Our inclusion criteria required patients to be 18 years of age or older, intubated and on tube feeds for at least 24 h. Patients were excluded if there was no enteral tube intake documentation on record or if they expired or were extubated in less than 48 h after intubation, since such a brief period of observation did not allow for initiation of tube feeds or the collection of gastrointestinal function parameters. Thirty patients that did not meet the inclusion criteria were excluded from the study, yielding a cohort of 218 patients (Fig. 1).
Fig. 1.
Patient selection algorithm
We collected data on patient demographics (age, gender, body mass index (BMI)), duration of hospital stay, mechanical ventilatory support, and daily values of D-dimer and CRP as well as interleukin-6. We elected to monitor CRP as a surrogate of patients’ inflammatory responses, since high levels have been linked to COVID-19 disease severity,13 but also as a marker of possible bacterial superinfection.14 Due to the susceptibility of critically ill intubated patients to blood stream and urinary tract infections as well as ventilator associated pneumonia, all the patients in our cohort were screened with blood, urine, and sputum cultures at time of intubation and as needed, depending on the clinical course.
D-dimer was assessed daily as part of the complex clinical practice for this patient population. This was used as a prognostic factor based on previous evidence suggesting an association between D-dimer and increased mortality15 but also to guide the choice of anticoagulation regimen. Specifically, in our institution, an aggressive anticoagulation protocol was implemented, which included dose escalation based on D-dimer levels. Patients with D-dimer < 1000 ng/mL received enoxaparin 40 mg daily, those with D-dimer ≥ 1000 ng/mL but < 3000 ng/mL received enoxaparin 40 mg twice a day, and those with D-dimer ≥ 3000 ng/mL received therapeutic anticoagulation with enoxaparin 1 mg/kg twice a day or intravenous heparin drip at a starting rate of 18 units/kg/h to achieve a goal PTT of 60 to 90.16
Sequential organ failure assessment (SOFA) scores were calculated for all patients at the time of intubation, which coincided with the ICU admission and has been used to quantify the degree of organ dysfunction or failure present on admission.17 Specifically, for the cardiovascular component of SOFA, due to the variety and combination of pressors used in the ICU setting, we used the conversion as suggested by Lambden et al,18 to allow for accurate calculations. First-line agents included vasopressin and norepinephrine with addition of phenylephrine and epinephrine as needed. We titrated the doses of vasopressors, following the guidelines of the “Surviving Sepsis Campaign” to maintain a mean arterial pressure (MAP) of 65 mmHg.19 Almost the entirety of our cohort required vasopressor infusion even for a brief time at some point of the hospitalization.
We collected the daily enteral tube intake volume for each patient during the first 10 days of ICU stay. In our institution, the enteral feeds utilized were Nepro, Jevity, Glucerna, Pulmocare, and TwoCal depending on the patients’ clinical characteristics and their nutritional needs. A single ICU nutrition protocol was in place to administer enteral tube feedings over 20 h. The prescribed formula was initiated at 30 mL/h and increased by 30 mL every 4 h as tolerated until the prescribed goal was reached. Gastric residual volume was documented every 4 h. If greater than 200 mL, the prescribed feeding was restarted at half the rate, and volume was rechecked in 4 h. If the residual was still greater than 200 mL, feedings were held for 4 h before restarting at 30 mL/h and increasing by 30 mL every 4 h, until goal reached, provided that the residual volume was not greater than 200 mL at any point. Knowing the caloric density of the different types of feeds, we calculated the daily caloric intake and compared it with the optimal volume and calorie goal as documented in the nutritionists’ notes. Feeding intolerance (FI) was defined if 20 kcal/kg of body weight/day via enteral route could not be reached or if enteral feeding had to be stopped for whatever clinical reason.4
We further gathered information on gastric residual volume, administration of total parenteral nutrition, use of prokinetics, and any relevant imaging, including abdominal x-rays and CT scans. Since our cohort consisted solely of ICU intubated patients, the documentation of self-reported GI symptoms was lacking. All patients were examined by the ICU team at least twice daily, during morning and evening rounds, with documentation of abdominal physical exam in terms of distention, frequency of bowel sounds, and quality of nasogastric tube or rectal tube output when present. To avoid interobserver variability due to the subjective nature and the dynamic evolution of the physical exam, we opted to use as quantifiable markers of gastrointestinal function, the measured daily stool count and volume, and the gastric residual volume. Based on this, we were able to identify whether our patients were having ileus, defined as the absence of bowel movements for three or more consecutive days without mechanical obstruction or diarrhea defined as three or more loose or liquid stools per day.4
Based on collected clinical and imaging data, our patients were categorized into five AGI grades according to the guidelines published by the European Society of Intensive Care Medicine (ESICM). AGI grade 0 was defined as a normal gastrointestinal function (intubated patients without any signs of shock, which is a risk factor of AGI, tolerated enteral tube feeds, had normal gastric residuals, and absence of diarrhea or ileus). AGI grade I was defined as an increased risk of developing gastrointestinal dysfunction or failure (intubated patients with transient signs of shock, with temporary and self-limiting GI dysfunction including ileus or diarrhea, who tolerated enteral feeds and had normal gastric residuals). AGI grade II was defined as gastrointestinal dysfunction (intubated patients with transient signs of shock and feeding intolerance that improved over the first 10 hospital days, normal gastric residuals with or without the presence of diarrhea or ileus). AGI grade III was defined as gastrointestinal failure (intubated patients with persistent signs of shock, feeding intolerance without improvement over the first 10 hospital days, high gastric residuals despite the addition of prokinetic agents, with or without the presence of diarrhea or ileus). AGI grade IV was defined as marked gastrointestinal failure with severe impact on distant organ function (intubated patient with persistent signs of shock, presence of feeding intolerance and diarrhea or ileus and clinically significant upper or lower GI bleeding, imaging-proved mesenteric ischemia, or Ogilvie’s syndrome).4
AGI score was implemented in several studies, and its utility was proven in identifying the severity of GI dysfunction and predicting adverse outcomes.5,20 A prospective study from China showed that differentiating AGI as gastrointestinal dysfunction (AGI grades I/II) or gastrointestinal failure (AGI grades III/IV) appears to be more valid for predicting prognosis than the AGI 4-grade system.21 Similarly, it has been shown that FI can be a reliable prognostic factor when evaluating intestinal dysfunction in critically ill patients.5 It can be an indicator of adverse outcomes even in the absence of intra-abdominal pressure measurements.22 Routine paralytic agents were not part of clinical care for this patient population. Since reliable intra-abdominal pressure measurements require appropriate paralysis, we elected to not include intra-abdominal pressure as a parameter to stratify patients in different AGI grades. Based on the above, we grouped our patients into three AGI categories: no AGI as group 0, AGI grades I and II (gastrointestinal dysfunction), and AGI grades III and IV (gastrointestinal failure).21 We further subdivided our cohort based on whether patients developed FI or not.
This study was a retrospective chart review of a COVID-19 patient database. Stony Brook University Committee on Research in Human Subjects approved the study protocol and supervised all study procedures according to state and federal regulations, with a waiver of informed consent.
Data Analysis
Statistics
Statistical analyses were performed using house-developed coding in MATLAB and SPSS 21.0 software (SPSS Inc., Chicago, IL). All tests’ significance level was set to 0.05, and all reported P values were calculated two-sided. Data were reported as group means, along with the two-tailed Student’s T-statistic for several labs. We utilized chi-square test to compare for any significant difference between two groups for categorical variables (sex, co-infections), two-sample t-test for parametric (age, SOFA, BMI), and Mann-Whitney U-tests for non-parametric continuous variables (D-dimers, CRP, and interleukin-6 levels), which was determined based on normalness and skewness of these distribution. We investigated the evolvement of critical laboratory values such as D-dimer and CRP over days after intubation in correlation with AGI score. We collected and analyzed these laboratory values using MATLAB and time-locked to the intubation date. We assessed the survival of the subgroups using Kaplan-Meier models and a log-rank test. We had more than 5 months of follow-up for each patient without missing a data point in terms of survival. To determine whether the AGI score was an independent predictor of survival, we used Cox proportional hazards regression models. We determined significant factors to be involved in the multivariable Cox regression model based on the univariable analysis. These factors included AGI score, participation in the D-dimer-driven escalation of anticoagulation protocol, gender, age, and SOFA score. BMI greater than thirty-five and being on the steroid were not significant in the Cox model’s univariable analysis. Entry level for multivariable analysis was P-value <0.1. This model provided hazard ratios to estimate which parameters are independent predictors of survival.
Results
A total of 218 critically ill ICU intubated patients with confirmed COVID-19 met the criteria for this study. The patients’ mean age was 59.8 years (SD: 14.24), and most patients were male (69.33%). We categorized the patients into three groups: AGI grade 0 (n = 11), AGI grades I/II (n = 98), and AGI grades III/IV (n = 109) (Fig. 1). The overall incidence of AGI was 95%; 45% of the patients had AGI grades I/II, and 50% had AGI grades III/IV. Our initial analysis showed that the rate of death in those with AGI III/IV was 58.72% compared to 29.59% in those with AGI I/II and 18.18% in those with AGI grade 0 (Table 1). Since there were only a few patients in our cohort with normal gastrointestinal function, we further stratified our population based on the AGI grading into AGI 0/I/II and AGI III/IV and compared for significant variables between the two groups. Those with AGI III/IV had significantly higher mortality (58% vs 28%, P-value <0.001) and duration of mechanical ventilation (22 days vs 16 days, P-value <0.002) compared to AGI 0/I/II (Table 2).
Table 1.
Descriptive analysis, groups AGI 0, AGI I/II, AGI III/IV
| AGI 0 N=11 |
AGI I/II N=98 |
AGI III/IV N=109 |
All patients N=218 |
|
|---|---|---|---|---|
| Age—yr (mean ± SD) | 64.72+13.48 | 60.01+13.9 | 58.8+14.51 | 59.8+14.24 |
| Male (%) | 54.55% | 66.33% | 72.48% | 69.33% |
| BMI (mean ± SD) | 29.31+4.63 | 30.67+6.55 | 30.32+6.68 | 30.39+6.48 |
| SOFA (mean ± SD) | 6+1.54 | 6.21+2.28 | 6.69+2.4 | 6.45+2.3 |
| Days on ventilator | 15.45+10.41 | 16.22+16.25 | 22.3+22.54 | 18.82+19.42 |
| Death (%) | 18.18% | 29.59% | 58.72% | 44.44% |
| Discharged (%) | 81.82% | 70.41% | 41.28% | 55.56% |
| D-dimer (mean ± SE) | 8245+9280 | 5939+6462 | 12276+13941 | 9133+ 762 |
| Admit creatinine (mean ± SE) | 1.17+0.48 | 1.3+1.55 | 1.33+1.22 | 1.33+0.09 |
| Max interleukin-6 (Vivacor) (mean ± SE) | 165.93 | 221.47 | 610.57 | 411+90.75 |
Table 2.
Comparison of variables between groups AGI 0/I/II, AGI III/IV
| AGI 0/I/II N=109 |
AGI III/IV N=109 |
P-value | |
|---|---|---|---|
| Age—yr (mean ± SD) | 60.48+13.8 | 58.8+14.5 | 0.33 |
| Male (%) | 65.14% (71/109) | 72.48% (79/109) | 0.3 |
| BMI (mean ± SD) | 30.53+6.388 | 30.32+6.68 | 0.70 |
| SOFA (mean ± SD) | 6.19+2.21 | 6.69+2.4 | 0.12 |
| Days on ventilator | 16.14 | 22.3 | 0.02 |
| Death (%) | 28.44% (31/109) | 58.72 (64/109) | 0.001 |
| Discharged (%) | 71.56% | 41.28% | 0.001 |
| D-dimer (mean ± SE) | 1467±44.04 | 2603±95.9 | 0.0001 |
| CRP (mean ± SE) | 8.35±0.28 | 10.88±0.24 | 0.0001 |
| Admit creatinine (mean ± SE) | 1.28±0.14 | 1.33±0.11 | 0.43 |
| Max interleukin-6 (Vivacor) (mean ± SE) | 216.3 | 610.5 | 0.09 |
P-values less than 0.05 are shown in bold to highlight the significance
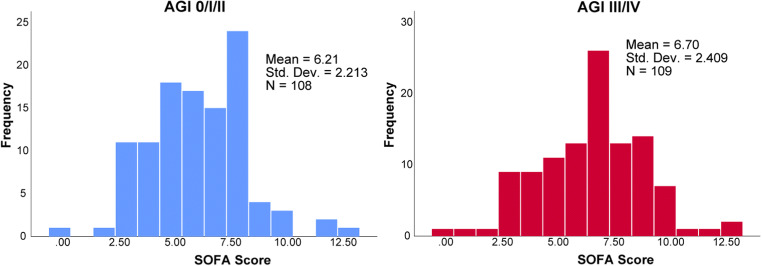
In the univariate survival analysis, AGI grades III/IV (P-value <0.0001), male sex (P-value =0.027), age greater than 70 years old (P-value <0.0001), SOFA score greater than seven (P-value <0.001), and not being on aggressive D-dimer driven anticoagulation protocol (P-value <0.0001) were predictors of mortality. Steroid use (P-value=0.32) and BMI greater than 35 (P-value=0.5) were not predictors of outcome. Multivariate analysis performed on the variables identified in the univariable analysis showed that AGI grades III/IV (hazard ratio (HR), 2.68; 95% confidence interval (CI), 1.69–4.25; P-value <0.0001), male sex (HR, 1.7; 95% CI, 1.02–2.85; P-value =0.04) and age over 70 (HR, 2.8; 95% CI, 1.8-4.36; P-value <0.0001) were predictors of higher mortality, while SOFA score greater than 7 did not reach statistical significance (HR, 1.52; 95% CI, 0.98–2.37; P-value =0.058) (Fig. 2). On the other hand, the implementation of aggressive D-dimer-driven anticoagulation was associated with decreased mortality (HR, 0.433; 95% CI, 0.274–0.683; P-value <0.0001) (Table 3). Kaplan-Meier survival analysis demonstrated that patients with AGI III/IV had significantly lower mortality rates compared to AGI 0/I/II (overall survival 41% vs 71%, P-value <0.001) (Fig. 3).
Fig. 2.
SOFA score at the time of intubation, between patients with AGI 0/I/II and AGI grades III/IV
Table 3.
Multivariable analysis
| Variable | Comparison level | Hazard ratio (95% C.I.) | *P-value |
|---|---|---|---|
| Sex | Male vs female | 1.7 (1.02–2.85) | 0.04 |
| Age | More than 70 vs less than 70 years old | 2.8 (1.8–4.36) | 0.0001 |
| SOFA | More than 7 vs less than 7 | 1.52 (0.98–2.37) | 0.058 |
| AGI grade | Grade III/IV vs grades 0/I/II | 2.68 (1.69–4.25) | 0.0001 |
| Anticoagulation | Aggressive vs routine anticoagulation | 0.433 (0.274–0.683) | 0.0001 |
Fig. 3.
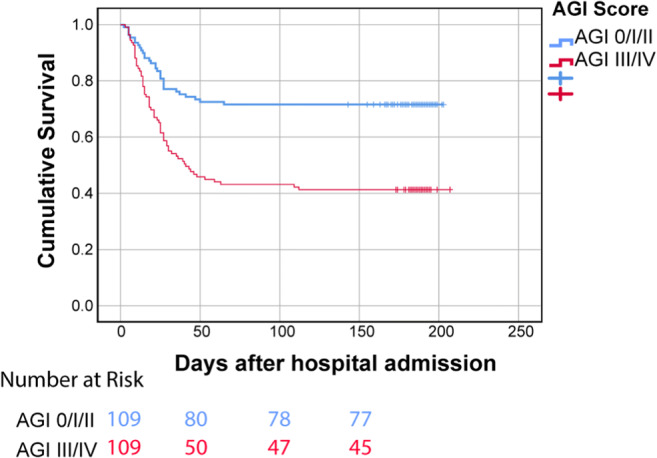
Comparison of cumulative survival rate among patients with AGI 0/I/II and AGI grades III/IV
The incidence of feeding intolerance was 63% (n=138/218) in our cohort. Similarly to the impact of high AGI grade on overall survival, we found that patients with FI had worse cumulative mortality compared to patients with feeding tolerance based on Kaplan-Meier models (44% vs 74%, P-value <0.001) (Fig. 4).
Fig. 4.
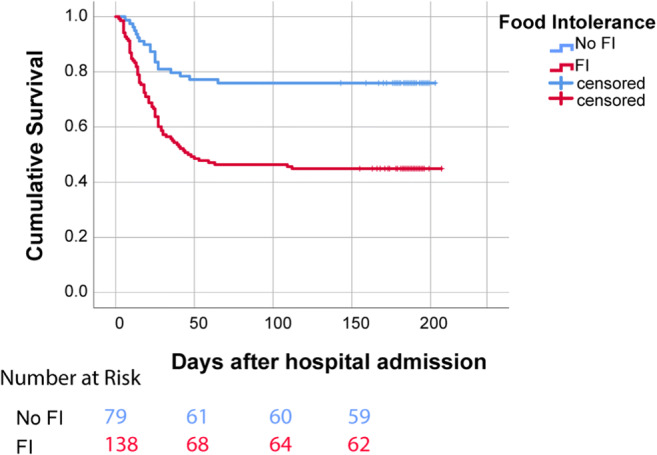
Comparison of cumulative survival rate among patients with FI (n=138) and no FI (n=79)
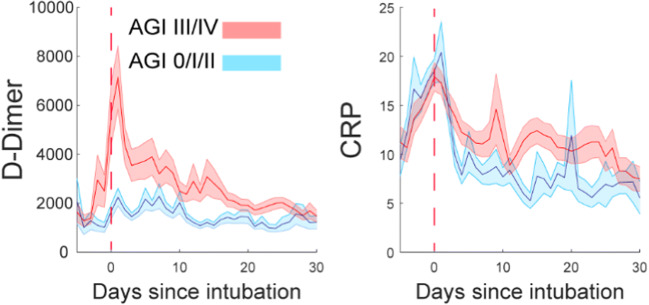
When evaluating the two groups for differences in selected labs that correlate with adverse outcomes, we found that patients with AGI grades III/IV had a significantly greater mean D-dimer and CRP level (P-value <0.0001). Furthermore, the trends of D-dimer and CRP levels in the 30-day post-intubation period were higher in those with AGI III/IV compared to AGI grades 0/I/II. Part of the standard workup in our patient cohort was to measure interleukin-6 (IL-6) levels based on specific clinical criteria indicating cytokine storm. We did not find any significant difference in the maximum IL-6 levels between AGI 0/I/II and AGI III/IV, although the sample was smaller (Table 2, Fig. 5).
Fig. 5.
D-dimer and CRP levels over 30 days post-initial intubation among patients with AGI 0/I/II and AGI grades III/IV
The most common site affected was the urinary tract with the overall incidence being 18% regarding co-infections. The most common pathogens were Enterobacteriaceae (Escherichia coli, Enterobacter, Klebsiella, Citrobacter), Pseudomonas, Enterococcus, and Candida. There was no statistically significant difference in UTI incidence among patients with AGI 0/I/II and AGI III/IV (P-value =0.08). Sputum cultures were routinely checked at the time of intubation and as needed and were negative in our cohort. The overall incidence of bacterial bloodstream infections was 14.5%. The most common pathogens were Escherichia coli, Klebsiella, Pseudomonas, Enterococcus, and Staphylococcus, with microbiology and antimicrobial resistance patterns reflecting our ICU flora before COVID-19. The fungemia incidence was 8.7%, with the most common pathogens being Candida albicans and Candida parapsilosis. Patients with AGI 0/I/II had a statistically significant lower incidence of bacteremia and fungemia than those with AGI III/IV (P-value=0.006) (Table 4).
Table 4.
Patients with AGI 0/I/II had a statistically significant lower incidence of bacteremia and fungemia than those with AGI III/IV (P-value=0.006)
| Species | AGI 0/I/II | AGI III/IV | P-value | |
|---|---|---|---|---|
| UTI | Enterobacteriaceae (Escherichia coli, Klebsiella, Citrobacter) | 3 | 6 | |
| Pseudomonas | 2 | 2 | ||
| Candida | 8 | 12 | ||
| Enterococcus | 2 | 5 | ||
| Total UTI | 15 | 25 | 0.08 | |
| Bacteremia | Enterobacteriaceae (Escherichia coli, Klebsiella) | 3 | 5 | |
| Pseudomonas | 2 | 3 | ||
| Enterococcus | 3 | 9 | ||
| Staphylococcus (MSSA/MRSA) | 2 | 5 | ||
| Fungemia | Candida | 7 | 12 | |
| Total bacteremia and fungemia | 17 | 34 | 0.006 |
Discussion
In our study, we divided the patients into three categories of acute gastrointestinal injury. No AGI was defined as grade 0, gastrointestinal dysfunction was AGI grades I/II, and gastrointestinal failure was AGI grades III/IV. We further evaluated FI as an independent prognostic factor. Because short periods of feeding could not precisely demonstrate the problems associated with enteral nutrition and knowing that the presence of FI in the first week of ICU stay can be predictive of adverse outcomes,5 we investigated the effect of both AGI and FI in the 10-day post-intubation period on patients’ clinical course.
We found that the presence of AGI was widely prevalent in this patient population and that those with AGI grades III and IV required prolonged mechanical ventilation and had a higher mortality rate than AGI grades 0/I/II. Our findings agree with studies prior to the COVID-19 pandemic, which showed that the presence of higher AGI grade in critically ill patients positively correlated with increased mortality and rate of mechanical ventilation.5
In a recent retrospective study, Sun et al. came to similar conclusions. In their cohort, the incidence of AGI was 86.7% among COVID-19 patients. They also concluded that AGI correlated with 28-day mortality and the development of septic shock. At the same time, the duration of mechanical ventilation was an independent risk factor for the development of AGI grade II and above.6 It has been shown that gastrointestinal symptoms in COVID-19 are associated with a higher risk of acute respiratory distress syndrome, resulting in mortality.23 Furthermore, although a systematic review failed to show a statistically significant correlation between gastrointestinal manifestations and disease severity or mortality rates,24 the proportion of severe disease in patients with GI symptoms was higher than that in patients without GI symptoms. In our cohort of critically ill patients, the development of AGI was predictive of worse outcomes, although these symptoms may not have any prognostic value in mild COVID-19.25
In addition to AGI, we independently investigated the prevalence of FI and its correlation with adverse outcomes. We found that a significant proportion of our patients developed FI during the first 10 ICU days. Similarly, Kaafarani et al. found a high prevalence of FI in their study, with 46% of the COVID-19 ICU patients having gastric feeding held for at least 24 h due to high gastric residuals.8 FI in our study correlated with significantly higher mortality in the Kaplan-Meier analysis almost to the same extent as the presence of high-grade AGI. Although FI is only one aspect of the AGI grading system, it has been shown that its presence within the first week of ICU stay is an independent factor of mortality and that it can incrementally increase the prognosticating value of AGI score.5,26
Our analysis suggests a relationship between gastrointestinal dysfunction and elevated D-dimer levels, which was previously established as a predictive factor of adverse outcomes in COVID-19 patients.9 An abnormal elevation of D-dimer was widespread among our patient cohort. However, those who developed AGI III/IV had higher mean D-dimer levels and higher D-dimer daily trends over 30 days than patients with AGI 0/I/II, which indirectly suggests they were in a relatively more hypercoagulable state. Our findings are consistent with Sun and colleagues’ conclusions. They found that patients with AGI III/IV had significantly higher D-dimer levels than those with AGI grades II, I, and 0.6
The pathogenetic mechanism behind the gastrointestinal dysfunction in critically ill COVID-19 patients is unknown so far. The two dominant theories suggest that this can be attributed either to direct viral injury to GI epithelia or the formation of microvasculature thrombi,8,27 the latter of which is a finding well documented by autopsy studies in multiple organ systems.12 Although the incidence of bowel ischemia necessitating emergent operative intervention was evident in the study by Kaafarani et al.,8 there were no patients in our study with clinical or imaging evidence of bowel ischemia. The absence of intestinal macrovascular thrombosis in our study could be partly explained by the implementation of aggressive anticoagulation in our hospital, driven by elevated D-dimers linked with improved outcomes.6,28 However, since we have no autopsy studies, we cannot establish whether this aggressive protocol also prevented microvascular intestinal thrombosis, which is hypothesized to be the cause of acute gastrointestinal dysfunction in critically ill COVID-19 patients.
Finally, our findings that the mean CRP levels and their daily trends were significantly higher in patients with AGI III/IV may reflect the cohort’s greater inflammatory state due to impaired gastrointestinal function. However, this could be caused by a generalized inflammatory response to SARS-CoV-2, affecting multiple organ systems. Consequently, we can conclude that AGI grade may be a surrogate for the degree of inflammation in this patient population. Conversely, high CRP could predict the development of acute gastrointestinal failure. The high mortality that was observed in patients with AGI III/IV could be due to the more intense inflammatory response as reflected by higher CRP levels, which have been shown to correlate with disease mortality.29 Up-trending D-dimer and CRP levels over the hospital course were considered prognostic factors of the poor outcome by our ICU teams as they reflected no improvement in the inflammation process30 and indicated a higher risk for multiorgan failure prompting escalation of anticoagulation and consideration for limiting enteral nutrition, respectively.
The overall incidence of bacterial bloodstream infections in our cohort was 14.5%, which was lower than other studies that found the risk to be as high as 50%.31 Patients with AGI 0/I/II had significantly lower risks of bacteremia and fungemia than those with AGI III/IV. This could reflect the fact that patients with higher AGI grades were sicker but could also indicate the importance of nutrition in the immune system’s function and the prevention of superinfections in those critically ill. The fungemia risk was 8.7%, which has been found to correlate with prolonged mechanical ventilation, presence of arterial and venous central lines, urinary catheters, and administration of broad-spectrum antibiotics and corticosteroids.32
Limitations
This study had several limitations. The exact relationship and pathogenetic mechanism between AGI and COVID-19 is unknown. This is especially true in critically ill patients who suffer from multiorgan failure and shock requiring high pressor management, which could affect gastrointestinal function. We did not investigate whether patients experienced any gastrointestinal symptoms before they were intubated, which could be an early manifestation of gastrointestinal injury. Furthermore, due to the study’s design, we could not establish a temporal relationship between the development of AGI and mortality because AGI could be part of the constellation of multiorgan system failure. The administration of different types of tube feeds, depending on the patients’ clinical condition, could affect the number of documented bowel movements which was one of the data we used to estimate the presence of diarrhea or ileus. Our study’s single-center retrospective design indicates that large-scale clinical prospective studies should confirm our results’ accuracy.
Conclusion
To our knowledge, this is the first study to correlate daily trending of known predictors of adverse outcomes in COVID-19, such as D-dimer and CRP levels, to the development of AGI and FI and to delineate the utility of AGI and FI development during the first 10 days of ICU stay as long-term prognosticating tools. We found a high prevalence of AGI and FI among critically ill COVID-19 patients. AGI grades III/IV was associated with a higher risk for prolonged mechanical ventilation and a higher mortality rate. The correlation of AGI III/IV to higher D-dimer and CRP levels could reflect the hypercoagulability and intense inflammatory response in these patients. FI was independently associated with higher mortality. The development of high-grade AGI and FI during the first days of ICU stay can serve as prognostic tools to predict critically ill COVID-19 patients’ outcomes.
Acknowledgements
The authors would like to thank the generous contributions of Theresa Gammel, Jordan Saadon, Susan Fiore, Drs. Apostolos Tassiopoulos, Nicos Labropoulos, Ali Sattari, Isadora Botwinick, Dan Rutigliano, and Nektarios Charisis. Special thanks to the Department of Biomedical Informatics, Department of Surgery, and the Department of Neurosurgery at Stony Brook University Hospital for supporting this research.
Author Contribution
• Konstantinos Spaniolas, M.D., Panagiotis Drakos, M.D., Panagiotis Volteas, M.D., and Sima Mofakham, Ph.D.: study conception and design.
• Panagiotis Drakos, M.D., Panagiotis Volteas, M.D., Mohsen Bannazadeh M.D., Konstantinos Spaniolas, M.D., and Sima Mofakham, Ph.D.: data analysis and making figures.
• Panagiotis Drakos, M.D., Panagiotis Volteas, M.D., Nathaniel A. Cleri, B.S., Leor N. Alkadaa, B.A., Anthony A. Asencio, B.S., Anthony Oganov, B.S., Aurora Pryor, M.D., Mark Talamini, M.D., Jerry Rubano, M.D., Mohsen Bannazadeh, M.D., Charles B. Mikell, M.D., Sima Mofakham, Ph.D., and Konstantinos Spaniolas, M.D.: planning the data analysis and data interpretation.
• Panagiotis Drakos, M.D., Panagiotis Volteas, M.D., Nathaniel A. Cleri, B.S., Leor N. Alkadaa, B.A., Anthony A. Asencio, B.S., and Anthony Oganov, B.S.: data acquisition.
• Panagiotis Drakos, M.D., Panagiotis Volteas, Jerry Rubano, M.D., Mohsen Bannazadeh, M.D., Charles B. Mikell, M.D., Sima Mofakham, Ph.D., and Konstantinos Spaniolas, M.D.: drafting the manuscript.
• All authors: critically revised the manuscripts. All the authors gave the final approval of the version to be published.
Funding
This work was supported by the SUNY Seed grant 1160738-1-87777.
Declarations
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Dr. Pryor received honoraria for speaking for Ethicon, Gore, Medtronic, Merck, and Stryker and is a consultant for Obalon. Dr. Spaniolas receives research support from Merck; he is a speaker for Gore. The remaining authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Panagiotis Drakos M.D. and Panagiotis Volteas M.D. contributed equally to this work as first authors. Sima Mofakham Ph.D. and Konstantinos Spaniolas M.D. contributed equally to this work as last authors.
Contributor Information
Konstantinos Spaniolas, Email: konstantinos.spaniolas@stonybrookmedicine.edu.
Sima Mofakham, Email: sima.mofakham@stonybrookmedicine.edu.
References
- 1.https://www.worldometers.info/coronavirus/
- 2.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 Mar 17;323(11):1061-1069. doi: 10.1001/jama.2020.1585. PMID: 32031570; PMCID: PMC7042881. [DOI] [PMC free article] [PubMed]
- 4.Reintam Blaser A, Malbrain ML, Starkopf J, Fruhwald S, Jakob SM, De Waele J, Braun JP, Poeze M, Spies C. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med. 2012 Mar;38(3):384-94. 10.1007/s00134-011-2459-y. Epub 2012 Feb 7. PMID: 22310869; PMCID: PMC3286505. [DOI] [PMC free article] [PubMed]
- 5.Hu B, Sun R, Wu A, Ni Y, Liu J, Guo F, Ying L, Ge G, Ding A, Shi Y, Liu C, Xu L, Jiang R, Lu J, Lin R, Zhu Y, Wu W, Xie B. Severity of acute gastrointestinal injury grade is a predictor of all-cause mortality in critically ill patients: a multicenter, prospective, observational study. Crit Care. 2017;21:188. doi: 10.1186/s13054-017-1780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun JK, Liu Y, Zou L, Zhang WH, Li JJ, Wang Y, Kan XH, Chen JD, Shi QK, Yuan ST. Acute gastrointestinal injury in critically ill patients with COVID-19 in Wuhan, China. World J Gastroenterol. 2020 Oct 21;26(39):6087-6097. 10.3748/wjg.v26.i39.6087.PMID:33132657;PMCID:PMC7584062. [DOI] [PMC free article] [PubMed]
- 7.Chang RW, Jacobs S, Lee B. Gastrointestinal dysfunction among intensive care unit patients. Crit Care Med. 1987;15(10):909–14. doi: 10.1097/00003246-198710000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kaafarani HMA, El Moheb M, Hwabejire JO, Naar L, Christensen MA, Breen K, Gaitanidis A, Alser O, Mashbari H, Bankhead-Kendall B, Mokhtari A, Maurer L, Kapoen C, Langeveld K, El Hechi MW, Lee J, Mendoza AE, Saillant NN, Parks J, Fawley J, King DR, Fagenholz PJ, Velmahos GC. Gastrointestinal Complications in Critically Ill Patients With COVID-19. Ann Surg. 2020 Aug;272(2):e61-e62. doi: 10.1097/SLA.0000000000004004. PMID: 32675498; PMCID: PMC7268843. [DOI] [PMC free article] [PubMed]
- 9.Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020 Jun;18(6):1324-1329. 10.1111/jth.14859. PMID: 32306492; PMCID: PMC7264730. [DOI] [PMC free article] [PubMed]
- 10.Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors AA, Osterhaus AD, van der Voort PH, Mulder DJ, van Goor H. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020 Jul;251(3):228-248. doi: 10.1002/path.5471. Epub 2020 Jun 10. PMID: 32418199; PMCID: PMC7276767. [DOI] [PMC free article] [PubMed]
- 11.Zhang H, Li HB, Lyu JR, Lei XM, Li W, Wu G, Lyu J, Dai ZM. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int J Infect Dis. 2020 Jul;96:19-24. 10.1016/j.ijid.2020.04.027. Epub 2020 Apr 18. PMID: 32311451; PMCID: PMC7165079. [DOI] [PMC free article] [PubMed]
- 12.Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, Thomas S, Adler NM, Charytan DM, Gasmi B, Hochman JS, Reynolds HR. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine. 2020 Jun 25;24:100434. 10.1016/j.eclinm.2020.100434. PMID: 32766543; PMCID: PMC7316051. [DOI] [PMC free article] [PubMed]
- 13.Ji P, Zhu J, Zhong Z, Li H, Pang J, Li B, Zhang J. Association of elevated inflammatory markers and severe COVID-19: A meta-analysis. Medicine (Baltimore). 2020 Nov 20;99(47):e23315. 10.1097/MD.0000000000023315. PMID: 33217868; PMCID: PMC7676531. [DOI] [PMC free article] [PubMed]
- 14.Falcone M, Tiseo G, Giordano C, Leonildi A, Menichini M, Vecchione A, Pistello M, Guarracino F, Ghiadoni L, Forfori F, Barnini S, Menichetti F; Pisa COVID-19 Study Group. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: a prospective observational study. J Antimicrob Chemother. 2020 Dec 29:dkaa530. 10.1093/jac/dkaa530. Epub ahead of print. PMID: 33374002; PMCID: PMC7799007. [DOI] [PMC free article] [PubMed]
- 15.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-1062. 10.1016/S0140-6736(20)30566-3. Epub 2020 Mar 11. Erratum in: Lancet. 2020 Mar 28;395(10229):1038. Erratum in: Lancet. 2020 Mar 28;395(10229):1038. PMID: 32171076; PMCID: PMC7270627. [DOI] [PMC free article] [PubMed]
- 16.Tassiopoulos AK, Mofakham S, Rubano JA, Labropoulos N, Bannazadeh M, Drakos P, Volteas P, Cleri NA, Alkadaa LN, Asencio AA, Oganov A, Hou W, Rutigliano DN, Singer AJ, Vosswinkel J, Talamini M, Mikell CB., Kaushansky K. D-Dimer-Driven Anticoagulation Reduces Mortality in Intubated COVID-19 Patients: A Cohort Study with a Propensity-Matched Analysis. Frontiers in Medicine. 2021 Feb, doi: 10.3389/fmed.2021.631335 [DOI] [PMC free article] [PubMed]
- 17.Moreno R, Vincent JL, Matos R, Mendonça A, Cantraine F, Thijs L, Takala J, Sprung C, Antonelli M, Bruining H, Willatts S. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working Group on Sepsis related Problems of the ESICM. Intensive Care Med. 1999;25(7):686–96. doi: 10.1007/s001340050931. [DOI] [PubMed] [Google Scholar]
- 18.Lambden S, Laterre PF, Levy MM, Francois B. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit Care. 2019 Nov 27;23(1):374. 10.1186/s13054-019-2663-7. PMID: 31775846; PMCID: PMC6880479. [DOI] [PMC free article] [PubMed]
- 19.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017 Mar;43(3):304-377. 10.1007/s00134-017-4683-6. Epub 2017 Jan 18. [DOI] [PubMed]
- 20.Zhang D, Li Y, Ding L, Fu Y, Dong X, Li H. Prevalence and outcome of acute gastrointestinal injury in critically ill patients: A systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e12970. doi: 10.1097/MD.0000000000012970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Zhang D, Wang Y, Zhao S. Association between acute gastrointestinal injury grading system and disease severity and prognosis in critically ill patients: A multicenter, prospective, observational study in China. J Crit Care. 2016;36:24–28. doi: 10.1016/j.jcrc.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Silva MA, Santos Sda G, Tomasi CD, Luz Gd, Paula MM, Pizzol FD, Ritter C. Enteral nutrition discontinuation and outcomes in general critically ill patients. Clinics (Sao Paulo). 2013;68(2):173-8. 10.6061/clinics/2013(02)oa09. PMID: 23525312; PMCID: PMC3584265. [DOI] [PMC free article] [PubMed]
- 23.Gul F, Lo KB, Peterson J, McCullough PA, Goyal A, Rangaswami J. Meta-analysis of outcomes of patients with COVID-19 infection with versus without gastrointestinal symptoms. Proc (Bayl Univ Med Cent). 2020 May 29;33(3):366-369. 10.1080/08998280.2020.1771164. PMID: 32669979; PMCID: PMC7265105. [DOI] [PMC free article] [PubMed]
- 24.Liu J, Cui M, Yang T, Yao P. Correlation between gastrointestinal symptoms and disease severity in patients with COVID-19: a systematic review and meta-analysis. BMJ Open Gastroenterol. 2020 Jul;7(1):e000437. 10.1136/bmjgast-2020-000437. PMID: 32665397; PMCID: PMC7359194. [DOI] [PMC free article] [PubMed]
- 25.Ramachandran P, Onukogu I, Ghanta S, Gajendran M, Perisetti A, Goyal H, Aggarwal A. Gastrointestinal Symptoms and Outcomes in Hospitalized Coronavirus Disease 2019 Patients. Dig Dis. 2020;38(5):373-379. 10.1159/000509774. Epub 2020 Jun 29. PMID: 32599601; PMCID: PMC7445385. [DOI] [PMC free article] [PubMed]
- 26.Reintam Blaser A, Starkopf L, Deane AM, Poeze M, Starkopf J. Comparison of different definitions of feeding intolerance: A retrospective observational study. Clin Nutr. 2015 Oct;34(5):956-61. doi: 10.1016/j.clnu.2014.10.006. Epub 2014 Oct 31. [DOI] [PubMed]
- 27.Giuffrè M, Di Bella S, Sambataro G, et al. COVID-19-Induced Thrombosis in Patients without Gastrointestinal Symptoms and Elevated Fecal Calprotectin: Hypothesis Regarding Mechanism of Intestinal Damage Associated with COVID-19. Trop Med Infect Dis. 2020;5(3):147. Published 2020 Sep 16. 10.3390/tropicalmed5030147 [DOI] [PMC free article] [PubMed]
- 28.Hsu A, Liu Y, Zayac AS, Olszewski AJ, Reagan JL. Intensity of anticoagulation and survival in patients hospitalized with COVID-19 pneumonia. Thromb Res. 2020 Dec;196:375-378. 10.1016/j.thromres.2020.09.030. Epub 2020 Sep 23. PMID: 32980620; PMCID: PMC7511207. [DOI] [PMC free article] [PubMed]
- 29.Sahu BR, Kampa RK, Padhi A, Panda AK. C-reactive protein: A promising biomarker for poor prognosis in COVID-19 infection. Clin Chim Acta. 2020 Oct;509:91-94. 10.1016/j.cca.2020.06.013. Epub 2020 Jun 5. PMID: 32511972; PMCID: PMC7274122. [DOI] [PMC free article] [PubMed]
- 30.Davis CJ, Sowa D, Keim KS, Kinnare K, Peterson S. The use of prealbumin and C-reactive protein for monitoring nutrition support in adult patients receiving enteral nutrition in an urban medical center. JPEN J Parenter Enteral Nutr. 2012 Mar;36(2):197-204. doi: 10.1177/0148607111413896. Epub 2011 Jul 28. [DOI] [PubMed]
- 31.Giacobbe DR, Battaglini D, Ball L, Brunetti I, Bruzzone B, Codda G, Crea F, De Maria A, Dentone C, Di Biagio A, Icardi G, Magnasco L, Marchese A, Mikulska M, Orsi A, Patroniti N, Robba C, Signori A, Taramasso L, Vena A, Pelosi P, Bassetti M. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020 Oct;50(10):e13319. 10.1111/eci.13319. Epub 2020 Aug 11. PMID: 32535894; PMCID: PMC7323143. [DOI] [PMC free article] [PubMed]
- 32.Bishburg E, Okoh A, Nagarakanti SR, Lindner M, Migliore C, Patel P. Fungemia in COVID-19 ICU patients, a single medical center experience. J Med Virol. 2020 Oct 27. doi: 10.1002/jmv.26633.Epubaheadofprint. [DOI] [PubMed]