Abstract
Background
Multiple guidelines for pancreatic ductal adenocarcinoma (PDAC) suggest that all stages of patients need to receive postoperative adjuvant chemotherapy. S-1 is a recently emerged oral antitumour agent recommended by the guidelines. However, which population would benefit from S-1 needs to be determined, and predictors of chemotherapy response are needed for personalized precision medicine. This pilot study aimed to initially identify whether whole-tumour evaluation with MRI and radiomics features could be used for predicting the efficacy of S-1 and to find potential predictors of the efficacy of S-1 as evidence to assist personalized precision treatment.
Methods
Forty-six patients with PDAC (31 in the primary cohort and 15 in the validation cohort) who underwent curative resection and subsequently adjuvant chemotherapy with S-1 were included. Pre-operative abdominal contrast-enhanced MRI was performed, and radiomics features of the whole PDAC were extracted from the primary cohort. After univariable analysis and radiomics features selection, a multivariable Cox regression model for survival analysis was subsequently used to select statistically significant factors associated with postoperative disease-free survival (DFS). Predictive capacities of the factors were tested on the validation cohort by using Kaplan–Meier method.
Results
Multivariable Cox regression analysis identified the probability of T1WI_NGTDM_Strength and tumour location as independent predictors of the efficacy of S-1 for adjuvant chemotherapy of PDAC (p = 0.005 and 0.013) in the primary cohort, with hazard ratios (HRs) of 0.289 and 0.293, respectively. Further survival analysis showed that patients in the low-T1WI_NGTDM_Strength group had shorter DFS (median = 5.1 m) than those in the high-T1WI_NGTDM_Strength group (median = 13.0 m) (p = 0.006), and patients with PDAC on the pancreatic head exhibited shorter DFS (median = 7.0 m) than patients with tumours in other locations (median = 20.0 m) (p = 0.016). In the validation cohort, the difference in DFS between patients with low-T1WI_NGTDM_Strength and high-T1WI_NGTDM_Strength and the difference between patients with PDAC on the pancreatic head and that in other locations were approved, with marginally significant (p = 0.073 and 0.050), respectively.
Conclusions
Whole-tumour radiomics feature of T1WI_NGTDM_Strength and tumour location were potential predictors of the efficacy of S-1 and for the precision selection of S-1 as adjuvant chemotherapy regimen for PDAC.
Keywords: Magnetic resonance imaging; Radiomics; Carcinoma, Pancreatic Ductal; Drug therapy; Survival analysis; Personalized medicine
Background
Pancreatic cancer is one of the most important causes of death in cancer patients [1], and pancreatic ductal adenocarcinoma (PDAC) is the most common pathological type of pancreatic cancer. Due to high-grade malignancy, even if the tumour is detected early and curative resection is performed, as many as 60% of patients experience recurrence and metastasis within a short period of time after the operation [2, 3]. Multiple diagnostic and treatment guidelines suggest that all stages of PDAC patients need to undergo postoperative adjuvant chemotherapy [4–6].
The regimens commonly suggested for adjuvant chemotherapy in the guidelines for PDAC include gemcitabine, 5-fluorouracil (5-FU)/leucovorin, S-1, etc. [4–6]. S-1 is a newly developed oral antitumour agent consisting of tegafur (a prodrug of 5-FU), gimeracil [a potent dihydropyrimidine dehydrogenase (DPD) inhibitor], and oteracil (an inhibitor of the phosphorylation of 5-FU in the gastrointestinal tract). Tegafur is transformed into 5-FU in the liver after oral administration [7]. It has been reported that monotherapy with S-1 demonstrates noninferiority to commonly used gemcitabine in overall survival for locally advanced and metastatic pancreatic cancer [8]. For postoperative adjuvant chemotherapy, S-1 significantly extended both the overall and relapse-free survival of patients with resected pancreatic cancer compared with gemcitabine and might contribute to the improvement of patients’ quality of life with fewer adverse reactions [9].
How can a suitable postoperative regimen be personalized from a variety of chemotherapy regimens listed in the PDAC guidelines? Because adverse reactions are common and the proportion of patients benefiting from chemotherapy is not high [10, 11], it is urgent to determine how to identify patients who are more likely to benefit from certain adjuvant chemotherapy regimens and how to maintain their quality of life while pursuing better clinical outcomes. However, the guidelines have not provided answers to these questions or solutions to these problems. Currently, there is no definite standard for the selection of drugs in the adjuvant chemotherapy of PDAC [4], and predictors of chemotherapy response are needed for personalized precision medicine.
The same questions and problems exist in the clinical application of S-1, especially which population can benefit from postoperative adjuvant chemotherapy with S-1. Radiomics technology, which has emerged in recent years, offers important advantages for the assessment of tumour biology. Radiomics analysis can aid in evidence-based clinical decision making in oncologic management and help achieve individualized precision medical care [12–14]. In PDAC, most prior radiomics studies represented by texture analysis were based on CT imaging, which have been associated with survival in patients who underwent surgeries [15–17], but none of these studies mentioned or analysed the effects of treatments on survival after the surgeries. Only a few radiomics analysis studies on MRI have been performed in PDAC. Whether MRI and radiomics features could be used for the prediction of therapy response to adjuvant chemotherapy in postoperative pancreatic cancer patients has not been reported in previous literature.
This pilot study aimed to find potential predictors of the efficacy of S-1 by analysing the associations among the clinical data, MRI findings, and whole-tumour radiomics features of PDAC patients with distinct responses to S-1 in postoperative adjuvant chemotherapy and to provide initial evidence as basis of further studies to assist personalized precision treatment in postoperative patients with PDAC.
Methods
Patients
General clinical data
Our institutional ethics review board approved this retrospective study (No. B2018-266), and the requirement for written informed consent was conditionally waived. From January 2012 to September 2017, the diagnosis of PDAC was confirmed in 91 patients by surgery and pathological examination at our institute among the patients who underwent an abdominal contrast-enhanced MRI examination with the same scanner (Magnetom Aera, Siemens Healthcare, Germany, 1.5 T). After curative resection, 31 of them who subsequently received adjuvant chemotherapy with S-1 were included in this study as the primary cohort. The follow-up period was from the time of surgery to November 2018. The inclusion and exclusion criteria of our study were as follows. Within the same study and follow-up period, a total of 15 patients were enrolled using the same criteria that used for the primary cohort besides that the MRI examinations were performed on Magnetom Avanto (Siemens Healthcare, Germany, 1.5 T) as the independent validation cohort.
Inclusion criteria:
Patients received abdominal contrast-enhanced MRI examination with Magnetom Aera (Siemens Healthcare, Germany, 1.5 T) at our institute and were suspected of having pancreatic cancer; the image quality was satisfactory for the study;
Patients underwent curative resection of the tumour at our institute, and the diagnosis of PDAC was confirmed by pathological examination;
The pre-operative laboratory tests and operation were within 1 month from the date of the MRI examination;
Patients received postoperative adjuvant chemotherapy with S-1 and follow-up;
Clinical information including demographic characteristics, laboratory tests, surgery, chemotherapy regimen, pathological findings, and follow-up were collected.
Exclusion criteria:
Poor image quality that was not acceptable;
Patients underwent percutaneous transhepatic cholangiodrainage (PTCD), biliary stent placement or antitumour treatments before the MRI examination;
PDAC failed to be resected;
The pre-operative laboratory test or operation was over 1 month from the date of the MRI examination;
Clinical information was incomplete;
Adjuvant chemotherapy other than S-1, follow-up only, or unknown treatments;
Other reasons that patients should be excluded.
S-1 regimen
Chemotherapy with S-1 started within 8 weeks after the operation. The dosage was determined based on the body surface at 40–60 mg per dose with 2 doses per day. S-1 was orally administered after breakfast and dinner for 28 days, followed by 14 days of rest. This administration of S-1 was repeated every 6 weeks for up to four cycles until the disease progressed or until patients were intolerant.
Factor to evaluate the efficacy of S-1 for adjuvant chemotherapy of PDAC: disease-free survival (DFS) time
DFS was defined as the time from the date of surgery to that of the first recurrence of the disease, the date last known to have no evidence of disease, or the date of the most recent follow-up with no disease. Follow-up information was garnered from the medical and follow-up records in the electronic medical records (EMR) system and radiological information system (RIS) of our institute. Patients who had no recurrence at the last follow-up (November 2018) or who were lost to follow-up were treated as censored in the analyses.
Kaplan–Meier analysis was used to calculate the median DFS of the patients in our study. The efficacy of S-1 for adjuvant chemotherapy of PDAC was evaluated by the median DFS. The patients were divided into the non-response group and the response group by the median DFS.
MRI protocol
All patients in the primary cohort underwent the abdominal contrast-enhanced MR examination on the same scanner (Magnetom Aera, Siemens Healthcare, Germany, 1.5 T). T1-weighted images with spoiled gradient-echo using volumetric interpolated breath-hold examination (VIBE) sequence, T2-weighted turbo spin-echo (TSE) sequence and DWI (b = 0, 500 s/mm2) using single-shot spin-echo echo-planar imaging were obtained before contrast was administrated. Dynamic contrast-enhanced MR images with T1-weighted images using VIBE sequences were obtained at the arterial phase (AP, 10 s after the trigger threshold reached), portal venous phase (PVP, 30 s after AP), and delayed phase (DP, 80–120 s after PVP) after injection of 0.1 mmol/kg Gadopentetate dimeglumine at a rate of 1–2 mL/s. Automatic tracking trigger scanning was applied for contrast enhancement, with the trigger points located in the abdominal aorta and the threshold set at a signal of 90. T1-weighted images using VIBE sequence performed on Magnetom Avanto (Siemens Healthcare, Germany, 1.5 T) was used for the validation. Detailed parameters of each sequence are shown in Table 1.
Table 1.
Parameters of MRI sequences
| Parameter | Primary cohort | Validation cohort | ||
|---|---|---|---|---|
| T1WI (VIBE) | T2WI | DWI | T1WI (VIBE) | |
| Repetition time (ms) | 3.47 | 2800 | 5100 | 5.04 |
| Echo time (ms) | 1.36 | 95 | 55 | 2.31 |
| Field of view (mm2) | 308 × 380 | 308 × 380 | 297 × 380 | 308 × 360 |
| Matrix | 320 × 240 | 384 × 273 | 192 × 154 | 256 × 167 |
| Section thickness (mm) | 3 | 5.5 | 6 | 3.5 |
| Fat suppression | Y | Y | Y | Y |
Extraction of whole-tumour radiomics features
Whole-tumour segmentation
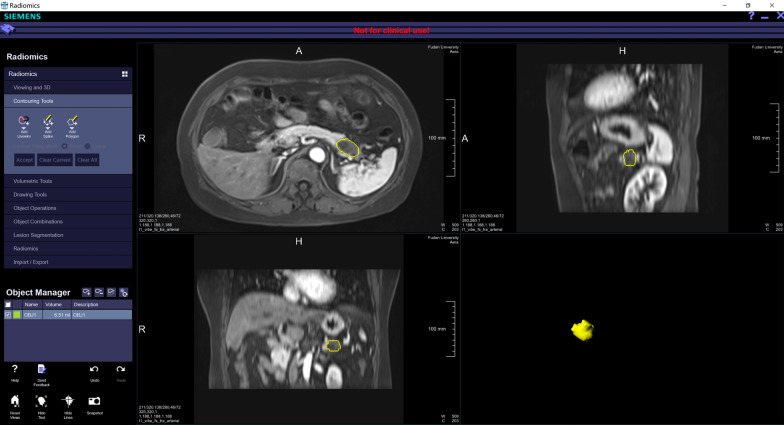
Whole-tumour segmentation was performed semi-automatically by a radiologist (with 11 years of experience in abdominal imaging), then checked and corrected by another radiologist (with 13 years of experience in abdominal imaging). A prototype software (Radiomics, Siemens Healthineers, Germany, not for commercial use) using a generic automatic segmentation algorithm based on a 3D domain in the workflow [18] was used for the segmentation. If the contour was not properly drawn, editing of the contour would be performed. A typical example of whole-tumour segmentation is presented in Fig. 1.
Fig. 1.
Whole-tumour radiomics analysis. Tumour segmentation was performed semi-automatically and whole-tumour radiomics features were extracted from the PDAC area (yellow overlay)
Extraction of radiomics features
All the segmentation data were subjected to radiomics feature extraction using the same prototype software interfacing with the Pyradiomics library [19]. A total of 110 radiomics features comprising 7 feature groups of whole-tumour were extracted from each studied sequence completely automatically: 19 first order statistics features, 16 contour-based features, 24 Grey Level Cooccurence Matrix (GLCM) features, 16 Grey Level Run Length Matrix (GLRLM) features, 16 Grey Level Size Zone Matrix (GLSZM) features, 5 Neighbouring Grey Tone Difference Matrix (NGTDM) features, 14 Grey Level Dependence Matrix (GLDM) features.
Image analysis
The pre-operative PDAC imaging were evaluated subjectively and quantitatively. Signal of tumour, morphology of tumour, tumour margins, peripheral or central delayed enhancement of tumour, necrosis of tumour, peripancreatic infiltration, peripancreatic blood vessel invasion (arterial and venous), pancreatic duct dilatation, atrophy of the upstream pancreas and the presence of retention cyst [20] were evaluated by 2 radiologists (with 11 years and 13 years of experience in abdominal imaging) who were blinded to the clinical information on the picture archiving communication system (PACS). Consensus was achieved through discussion to resolve the controversy. Whole-tumour quantitative evaluation including the tumour size, ADC value of the whole tumour, enhancement rates of the whole tumour on AP, PVP and DP, as well as enhancement rate differences of the whole tumour between different dynamic contrast-enhanced phases [20] was performed by using data obtained from radiomics analysis.
Statistical analysis
We performed the statistical analysis with SPSS statistical software (version 19.0.0, IBM, USA).
The demographic and clinical characteristics of the non-response group and the response group in the primary cohort were compared using Fisher's exact test (for categorical variables) or the Mann–Whitney U test (for continuous variables) to determine any relationship between the characteristics and the efficacy of S-1 for postoperative adjuvant chemotherapy of PDAC. The median DFS was calculated with the Kaplan–Meier method and compared by the Log Rank test. Univariable analysis with Fisher's exact test (for the subjective evaluation of pre-operative PDAC imaging, all of which were categorical variables) or the Mann–Whitney U test (for the whole-tumour quantitative evaluation of pre-operative PDAC imaging, all of which were continuous variables) was used for comparisons between the non-response group and the response group of the primary cohort to determine any association between pre-operative PDAC imaging findings and the efficacy of S-1 for postoperative adjuvant chemotherapy of PDAC.
Radiomics features selection: Univariable analysis with the Mann–Whitney U test method was adopted to select potentially valuable features to predict the efficacy of S-1 for postoperative adjuvant chemotherapy of PDAC. Features were selected from the whole-tumour radiomics features of T1WI (VIBE), T2WI, AP, PVP, and DP images from the primary cohort. The p value threshold was set at 0.05 for appropriate selection.
After univariable analysis and radiomics features selection, a multivariable Cox regression model (Cox proportional hazards model) of survival analysis was subsequently used to select statistically significant factors associated with postoperative DFS. Potentially significant variables with p value < 0. 05 in univariable analysis and selected radiomics features were enrolled in the analysis. A forward stepwise regression method based on maximum likelihood estimation (Forward: LR) was used for multivariable Cox regression analysis. Then, the patients were grouped by the factors selected through multivariable Cox regression analysis. Continuous variables were grouped by the cut-off value as the threshold, which was identified using receiver operating characteristic (ROC) curve analysis. Postoperative DFS between the subgroups was analysed using the Kaplan–Meier method and compared with the Log Rank test and survival curves to confirm the prediction performance of the selected factors. Predictive capacities of the selected factors were also tested on the validation cohort by using the Kaplan–Meier method and survival curves that evaluated by the Log Rank test.
p values < 0.05 were considered statistically significant and 0.05 ≤ p values < 0.1 were considered marginally significant in the study. In this pilot study we did not perform a correction for multiplicity given the small patient cohorts and exploratory nature of this study.
Results
Patient characteristics
The demographic information and clinical characteristics of the patients in the study are shown in Table 2. There were no differences found between the primary and validation cohorts, which enabled their use as primary and validation cohorts. The median DFS of the patients was 10.7 months in the primary cohort and 11.0 months in the validation cohort (p = 0.772). The patients in the primary cohort were divided into the non-response to S-1 group (DFS ≤ 10. 7 m, n = 16) and the response group (DFS > 10.7 m, n = 15) by the median DFS. The pre-operative CEA and CA19-9 levels in the non-response group were higher than those in the response group (p = 0.028 and 0.022). Tumour location was also different between the two groups (p = 0.032), i.e., the non-response group had a much higher proportion of tumours located on the head of the pancreas.
Table 2.
Baseline demographic and clinical characteristics of patients
| Primary cohort (n = 31) | Validation cohort (n = 15) | p value (Inter cohorts) | |||
|---|---|---|---|---|---|
| Non-response group (n = 16) | Response group (n = 15) | p value | |||
| Age (years)a | 64.25 ± 8.00 | 61.47 ± 8.63 | 0.259 | 63.40 ± 7.52 | 0.805 |
| Sex | 1.000 | 1.000 | |||
| Male | 8 (50.0%) | 8 (53.3%) | 7 (46.7%) | ||
| Female | 8 (50.0%) | 7 (46.7%) | 8 (53.3%) | ||
| CEA (ng/mL)b | 3.15 (2.55–5.73) | 2.00 (1.60–2.80) | 0.028 | 2.90 (2.30–4.70) | 0.489 |
| CA19-9 (U/mL)b | 328.40 (120.78–469.38) | 75.00 (45.80–166.30) | 0.022 | 164.80 (22.70–429.50) | 0.648 |
| Tumour location | 0.032 | 1.000 | |||
| Pancreatic head | 11 (68.8%) | 4 (26.7%) | 8 (53.3%) | ||
| Pancreatic neck, body, and tail | 5 (31.3%) | 11 (73.3%) | 7 (46.7%) | ||
| Grade of tumour | 1.000 | 1.000 | |||
| 2 | 5 (31.3%) | 5 (33.3%) | 5 (33.3%) | ||
| 3 | 11 (68.8%) | 10 (66.7%) | 10 (66.7%) | ||
| Stage of tumour | 0.585 | 0.848 | |||
| I | 6 (37.5%) | 8 (53.3%) | 6 (40.0%) | ||
| II | 7 (43.8%) | 4 (26.7%) | 5 (33.3%) | ||
| III | 3 (18.8%) | 3 (20.0%) | 4 (26.7%) | ||
| Median DFS (months) | 6.0 | 23.7 | < 0.001 | 11.0 | 0.772 |
Bold means statistically significant (p values < 0.05)
aMean ± standard deviation
bMedian (P25–P75)
Subjective evaluation of pre-operative MRI in the primary cohort
The findings of the subjective evaluation of pre-operative PDAC imaging are presented in Table 3. Two subjective factors of pre-operative MRI, irregular tumour morphology (43.8% vs. 6.7%) and peripancreatic tissue involvement (75.0% vs. 26.7%), were higher in the non-response group than in the response group of the primary cohort; the difference was statistically significant (p = 0.037 and 0.012). There seemed to be no differences in the other subjective evaluation variables of pre-operative MRI between the two groups in the primary cohort.
Table 3.
Univariable analysis of subjective evaluation of pre-operative PDAC imaging findings and therapy efficacy of S-1 in the primary cohort
| Non-response group (n = 16) | Response group (n = 15) | p value | |
|---|---|---|---|
| T1WI | 1.000 | ||
| Iso-intensity | 1 (6.3%) | 1 (6.7%) | |
| Mild hypo-intensity | 15 (93.8%) | 14 (93.3%) | |
| T2WI | 1.000 | ||
| Iso-/mild hypo-intensity | 7 (43.8%) | 7 (46.7%) | |
| Mild hyper-intensity | 9 (56.3%) | 8 (53.3%) | |
| Morphology | 0.037 | ||
| Regular | 9 (56.3%) | 14 (93.3%) | |
| Irregular | 7 (43.8%) | 1 (6.7%) | |
| Tumour margins on T2WI | 0.394 | ||
| Well defined | 2 (12.5%) | 4 (26.7%) | |
| Ill defined | 14 (87.5%) | 11 (73.3%) | |
| Tumour margins on T1WI | 0.473 | ||
| Well defined | 5 (31.3%) | 7 (46.7%) | |
| Ill defined | 11 (68.6%) | 8 (53.3%) | |
| Tumour margins after enhancement | 0.073 | ||
| Well defined | 6 (37.5%) | 11 (73.3%) | |
| Ill defined | 10 (62.5%) | 4 (26.7%) | |
| Peripheral delayed enhancement of tumour | 0.135 | ||
| Invisible | 13 (81.3%) | 8 (53.3%) | |
| Visible | 3 (18.8%) | 7 (46.7%) | |
| Central delayed enhancement of tumour | 0.473 | ||
| Invisible | 8 (50.0%) | 5 (33.3%) | |
| Visible | 8 (50.0%) | 10 (66.7%) | |
| Necrosis of tumour | 0.172 | ||
| Absence | 11 (68.8%) | 14 (93.3%) | |
| Presence | 5 (31.3%) | 1 (6.7%) | |
| Peripancreatic infiltration | 0.012 | ||
| Invisible | 4 (25.0%) | 11 (73.3%) | |
| Visible | 12 (75.0%) | 4 (26.7%) | |
| Peripancreatic blood vessel invasion | 1.000 | ||
| Invisible | 10 (62.5%) | 10 (66.7%) | |
| Visible | 6 (37.5%) | 5 (33.3%) | |
| Artery invasion | 0.654 | ||
| Invisible | 14 (87.5%) | 12 (80.0%) | |
| Visible | 2 (12.5%) | 3 (20.0%) | |
| Vein invasion | 0.704 | ||
| Invisible | 10 (62.5%) | 11 (73.3%) | |
| Visible | 6 (37.5%) | 4 (26.7%) | |
| Pancreatic duct dilatation | 0.704 | ||
| Absence | 6 (37.5%) | 4 (26.7%) | |
| Presence | 10 (62.5%) | 11 (73.3%) | |
| Atrophy of upstream pancreas | 0.458 | ||
| Absence | 9 (56.3%) | 11 (73.3%) | |
| Presence | 7 (43.8%) | 4 (26.7%) | |
| Retention cyst formation | – | ||
| Absence | 16 (100.0%) | 15 (100.0%) | |
| Presence | 0 (0.0%) | 0 (0.0%) |
Bold means statistically significant (p values < 0.05)
Whole-tumour quantitative evaluation of pre-operative MRI in the primary cohort
The results of the whole-tumour quantitative evaluation of pre-operative PDAC imaging are presented in Table 4. Univariable analysis showed that two factors of whole-tumour quantitative evaluation, the enhancement rate of DP, the enhancement rate difference between DP and AP, were significantly different between the non-response group and the response group in the primary cohort (p = 0.013 and 0.036), both of which were lower in the non-response group. There seemed to be no differences in the other variables of the whole-tumour quantitative evaluation of pre-operative MRI between the two groups in the primary cohort.
Table 4.
Univariable analysis of whole-tumour quantitative evaluation of pre-operative PDAC imaging and therapy efficacy of S-1 in the primary cohort
| Non-response group (n = 16) | Response group (n = 15) | p value | |
|---|---|---|---|
| Short diameter (cm) | 1.92 ± 0.71 | 1.84 ± 0.63 | 0.858 |
| Long diameter (cm) | 2.73 ± 1.17 | 3.16 ± 1.96 | 0.937 |
| ADC value (10–3 mm2/s) | 1.64 ± 0.53 | 1.85 ± 0.61 | 0.343 |
| Enhancement rate of AP (%) | 70.87 ± 26.78 | 88.98 ± 42.76 | 0.268 |
| Enhancement rate of PVP (%) | 141.08 ± 45.53 | 167.23 ± 50.48 | 0.114 |
| Enhancement rate of DP (%) | 158.29 ± 33.48 | 198.05 ± 45.97 | 0.013 |
| Enhancement rate difference between PVP and AP (%) | 70.22 ± 25.10 | 78.25 ± 31.96 | 0.607 |
| Enhancement rate difference between DP and AP (%) | 87.42 ± 26.08 | 109.07 ± 32.77 | 0.036 |
| Enhancement rate difference between DP and PVP (%) | 17.21 ± 29.44 | 30.82 ± 25.78 | 0.304 |
Bold means statistically significant (p values < 0.05)
Whole-tumour radiomics features selection from the primary cohort
A total of 110 radiomics features of the whole tumour were extracted from each studied sequence of pre-operative MRI, and a total of 550 features were analysed for each PDAC lesion of the primary cohort. The results of whole-tumour radiomics features selection are shown in Table 5. Two features, including Complexity of NGTDM from T1WI (T1WI_NGTDM_Complexity) and Strength of NGTDM from T1WI (T1WI_NGTDM_Strength), were selected as potentially valuable features to predict the efficacy of S-1 for postoperative adjuvant chemotherapy of PDAC (p = 0.044 and 0.030). The mathematical formulas of the selected features are listed in the “Appendix”.
Table 5.
Results of whole-tumour radiomics features selection from the primary cohort: based on therapy efficacy of S-1
| Feature | Non-response group (n = 16) | Response group (n = 15) | p value |
|---|---|---|---|
| T1WI_NGTDM_Complexitya | 0.00064 (0.00031–0.00116) | 0.00131 (0.00065–0.00480) | 0.044 |
| T1WI_NGTDM_Strengtha | 0.021 (0.013–0.040) | 0.038 (0.023–0.096) | 0.030 |
Bold means statistically significant (p values < 0.05)
aMedian (P25–P75)
Survival analysis: factors relevant to therapy efficacy of S-1
After univariable analysis and radiomics features selection, CEA, CA19-9, tumour location, morphology of tumour, peripancreatic infiltration, enhancement rate of DP, enhancement rate difference between DP and AP, and the whole-tumour radiomics features of T1WI_NGTDM_Complexity and T1WI_NGTDM_Strength were enrolled into the multivariable Cox regression analysis. According to the results of the analyses, whole-tumour radiomics feature of T1WI_NGTDM_Strength and tumour location were significantly associated with postoperative DFS (p = 0.005 and 0.013), with hazard ratios (HRs) of 0.289 and 0.293, respectively (Table 6). Thus, these two factors may potentially predict the efficacy of S-1 for postoperative adjuvant chemotherapy of PDAC.
Table 6.
Results of multivariable Cox regression analysis for factors relevant to therapy efficacy of S-1 in the primary cohort
| B | HR | 95% CI | p value | |
|---|---|---|---|---|
| T1WI_NGTDM_Strength | − 1.240 | 0.289 | 0.123–0.682 | 0.005 |
| Tumour location | − 1.227 | 0.293 | 0.112–0.769 | 0.013 |
Bold means statistically significant (p values < 0.05)
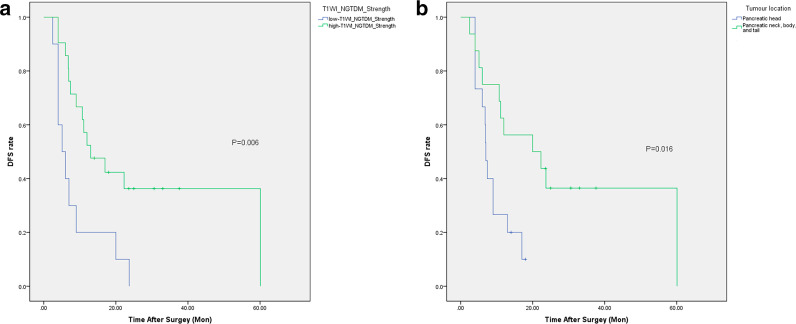
Further survival analysis with the Kaplan–Meier method and the Log Rank test showed that grouping by T1WI_NGTDM_Strength or tumour location prompted significantly different postoperative DFS between the respective subgroups in the primary cohort (p = 0.006 and 0.016). The optimum cut-off value of T1WI_NGTDM_Strength generated by ROC curve analysis was 0.021 (p = 0.030, 95% confidence interval [CI], 0.551–0.907). Using this threshold value, the patients were classified into a low-T1WI_NGTDM_Strength group (value of T1WI_NGTDM_Strength < 0.021) and a high-T1WI_NGTDM_Strength group (value of T1WI_NGTDM_Strength ≥ 0.021).
In the primary cohort, patients in the low-T1WI_NGTDM_Strength group had shorter DFS (median = 5.1 m) than those in the high-T1WI_NGTDM_Strength group (median = 13.0 m) (Table 7, Fig. 2a). Patients with PDAC on the pancreatic head exhibited shorter DFS (median = 7.0 m) than patients with PDAC in other tumour locations (median = 20.0 m) (Table 7, Fig. 2b). Whole-tumour radiomics feature of T1WI_NGTDM_Strength and tumour location showed significant value for predicting the efficacy of S-1 for postoperative adjuvant chemotherapy of PDAC independently.
Table 7.
Results of survival analysis in the primary cohort: grouping by predictive factors
| Median DFS (months) | 95% CI | p value | |
|---|---|---|---|
| T1WI_NGTDM_Strength | 0.006 | ||
| Low-T1WI_NGTDM_Strength (n = 10) | 5.1 | 2.0–8.2 | |
| High-T1WI_NGTDM_Strength (n = 21) | 13.0 | 4.5–21.5 | |
| Tumour location | 0.016 | ||
| Pancreatic head (n = 15) | 7.0 | 6.2–7.8 | |
| Pancreatic neck, body, and tail (n = 16) | 20.0 | 0.0–40.2 | |
| Total (n = 31) | 10.7 | 5.7–15.7 |
Bold means statistically significant (p values < 0.05)
Fig. 2.
Survival analysis (DFS) for postoperative patients using adjuvant chemotherapy with S-1 in the primary cohort (K–M method). Grouping by T1WI_NGTDM_Strength (a) and tumour location (b) both prompted significantly different postoperative DFS between the respective subgroups
Validation of the factors relevant to therapy efficacy of S-1
In the validation cohort, different subgroups of T1WI_NGTDM_Strength or tumour locations also prompted different postoperative DFS, with marginally significant (p = 0.073 and 0.050), respectively. Patients in the low-T1WI_NGTDM_Strength group had shorter DFS (median = 5.4 m) than those in the high-T1WI_NGTDM_Strength group (median = 28.0 m) (Table 8, Fig. 3a). Patients with PDAC on the pancreatic head exhibited shorter DFS (median = 7.0 m) than patients with PDAC in other tumour locations (median = 28.0 m) (Table 8, Fig. 3b).
Table 8.
Results of survival analysis in the validation cohort: grouping by predictive factors
| Median DFS (months) | 95% CI | p value | |
|---|---|---|---|
| T1WI_NGTDM_Strength | 0.073 | ||
| Low-T1WI_NGTDM_Strength (n = 8) | 5.4 | 2.6–8.2 | |
| High-T1WI_NGTDM_Strength (n = 7) | 28.0 | 13.7–42.3 | |
| Tumour location | 0.050 | ||
| Pancreatic head (n = 8) | 7.0 | 4.0–10.0 | |
| Pancreatic neck, body, and tail (n = 7) | 28.0 | 0.0–62.9 | |
| Total (n = 15) | 11.0 | 4.0–18.0 |
Fig. 3.
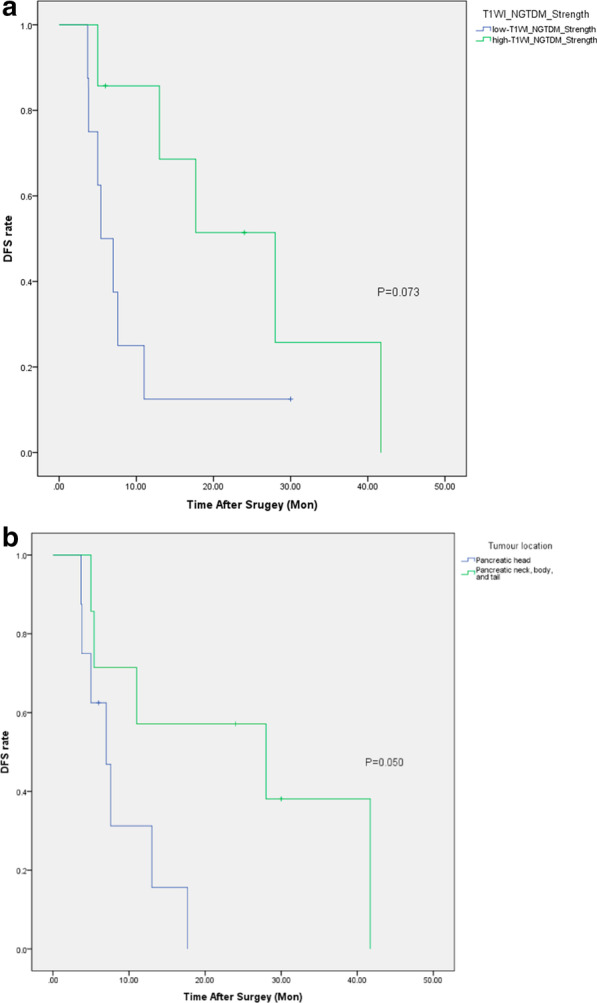
Survival analysis (DFS) for postoperative patients using adjuvant chemotherapy with S-1 in the validation cohort (K–M method). Grouping by T1WI_NGTDM_Strength (a) and tumour location (b) both prompted marginally different postoperative DFS between the respective subgroups
Discussion
Multiple diagnostic and treatment guidelines suggest that all stages of PDAC patients need to undergo postoperative adjuvant chemotherapy [4–6]. As mentioned above, two important clinical studies in Asian populations confirmed the efficacy of S-1 for PDAC [8, 9], especially the superiority of S-1 to gemcitabine as postoperative adjuvant chemotherapy for PDAC in terms of overall survival. Additionally, compared to gemcitabine, S-1 has the convenience of oral administration, no complications of intravenous chemotherapy, good tolerability with fewer adverse reactions and might contribute to improving patients’ quality of life [9]. Currently, S-1 is recommended as a first-line adjuvant chemotherapy drug in the diagnostic and treatment guidelines for PDAC [5, 6].
However, the guidelines do not clearly indicate how to select the optimal chemotherapy regimen for PDAC patients suitably and individualizedly, and which population can benefit from S-1 is still unknown; proper evidence-based guidance is still lacking. Some studies proposed that the intratumoural expression levels of DPD and thymidylate synthase (TS) might be related to therapeutic outcomes in PDAC patients receiving S-1 chemotherapy [21–23], but the conclusion remains controversial; some studies even had contradictory results [24, 25].
In our study, the non-response to S-1 group had a much higher proportion of PDAC located on the pancreatic head than the response group in the primary cohort. In the validation cohort, patients with PDAC on the pancreatic head also exhibited shorter DFS. Previous studies also indicated that pancreatic head cancers and pancreatic body/tail cancers had different overall survival rates and tumour-free survival [26, 27]. Their biological characteristics, such as the concentration of cell-of-origin and microRNA expression, were also different [27]. These differences may be related to the embryonic development of the pancreas. The pancreas develops from the formation of a ventral bud and a dorsal bud, each with its own duct originating from the primitive intestine, that finally fuse as the pancreas. Thus, the blood supply, lymphatic backflow, and innervations between the head and body/tail of the pancreas are significantly different [28]. This may be the reason why the biological behaviours and the treatment responses of PDAC in different locations also differ.
To date, there are limited data on the effect of tumour location on the benefit from adjuvant chemotherapy, as most studies have not yet analysed the different responses to chemotherapy of tumours in different locations [28, 29]. Our study has partially filled that research gap, even though the sample size was relatively small. In our study, S-1 used for postoperative adjuvant chemotherapy of PDAC was much more effective for patients with tumours located in areas other than the pancreatic head, suggesting that the selection of S-1 for PDAC located on the pancreatic head should be made more cautiously. Interestingly, another study found that gemcitabine improved overall survival in a subgroup of postoperative patients with pancreatic head tumours compared to 5-FU [30, 31].
Because of the strict requirements for the consistency of treatment regimen and follow-ups in the enrolment criteria, the number of patients included in this pilot study was relatively small. Consequently, we only performed radiomics features screening and selection; multi-factor fusion model construction, which could be carried out in radiomics analysis, was not performed in our study. However, the identified radiomics feature-T1WI_NGTDM_Strength-was confirmed by a multivariable Cox proportional hazards model and Kaplan–Meier analysis to be a potential predictor for the efficacy of S-1 for postoperative adjuvant chemotherapy of PDAC. NGTDM_Strength is one of the radiomics features from the group of Neighbouring Grey Tone Difference Matrix (NGTDM). As high-order statistical parameters, NGTDM examines the signal intensity and spatial interrelationship between neighbouring voxels between adjacent image planes, which describe the dynamic range of intensities at a local level and better quantify the heterogeneity within the tumour [32]. Strength is a measure of the primitives in the image. Radiomics features can uncover tumour characteristics that may fail to be appreciated by the naked eye [33]. From the results of our study, NGTDM_Strength from T1WI was independently related to the therapy response of PDAC, supporting that radiomics features could potentially reflect the biological information of tumours, including the intratumoural heterogeneity associated with the response to treatment and survival [14, 34, 35]. In another diffusion-weighted-MRI-derived radiomics study, 13 radiomics features were found to be important for predicting the gemcitabine-based chemotherapy response of PDAC [36]. NGTDM_Strength was not included, demonstrating the possible specificity of NGTDM_Strength for predicting the efficacy of S-1, but the relationship between the radiomics features and therapy response to S-1 still needs to be further analysed in larger cohorts.
A few studies have assessed the potential of imaging data, including radiomics features, for the prediction of survival in PDAC with different examination techniques. One study showed that not only for resectable PDAC but also for locally advanced and/or metastatic PDAC, specific CT radiomics feature was a significant prognostic factor [37]. Another study that first evaluated the prognostic value of FDG-PET radiomics in pancreatic cancer found that feature of GLZLM GLNU was the most relevant factor for predicting 1-year survival [38]. However, these studies did not analyse the treatment response of specific regimens. Compared with most previous texture analysis studies in PDAC that were based on CT imaging [15–17, 39, 40], we performed radiomics analysis based on MRI, which has a higher resolution in soft tissue than CT. Although based on different examination techniques, the selected features are mostly related to intratumoural heterogeneity [32, 37, 38]. However, performances have not yet been compared among CT, MRI, and PET/CT [41, 42]. It is difficult to determine which examination technique is better for radiomics analysis from previous studies. MRI-based radiomics analysis may likely be more predictive of tumour heterogeneity but might be more susceptible to variations in imaging parameters compared to CT [41]. We performed all the MRI examinations of the same cohort with the same scanner in our study to avoid the influences of equipment and parameter differences.
Additionally, the univariable analysis demonstrated that the non-response to S-1 group had more cases of irregular tumour morphology and peripancreatic tissue involvement on pre-operative MRI, which reflected the higher invasiveness of PDAC in this group than in the response group. Although the multivariable Cox proportional hazards model did not show an association of these imaging signs and postoperative DFS with adjuvant chemotherapy of S-1, if these signs are present in pre-operative image assessment, the selection of S-1 warrants great caution, and a more intensive adjuvant chemotherapy regimen may be more appropriate. The above recommendation still needs to be further validated by studying larger cohorts.
The region of interest (ROI) of the whole tumour was conducted during the analysis in this study. Compared with the largest cross-sectional area analysis that was performed in several previous studies [17, 39], whole-tumour analysis is more representative of intratumoural biological characteristics and could avoid selection bias. The results are also relatively more reliable [43]. Therefore, whole-tumour imaging analysis including radiomics features may obtain additional predictive factors about the outcomes of treatments noninvasively and no extra examinations and medical expenses will be added. To the best of our knowledge, there are no similar studies about the imaging evaluation of PDAC in the literature. The results of our study could be the feasible basis of evidence and further studies for personalized precision medicine to select the right treatment for the right patient at the right time, thereby reducing the adverse reactions of ineffective treatments and improving the quality of life as well as prognosis of PDAC patients.
Our study has several limitations. Due to the strict requirements for the consistency of treatment regimen and follow-ups, the number of enrolled patients was relatively small and our study was a pilot study. In addition, the follow-up period was relatively short. Despite these limitations, this pilot study found that whole-tumour radiomics feature of T1WI_NGTDM_Strength and tumour location had significant value in predicting the efficacy of S-1 for postoperative adjuvant chemotherapy of PDAC independently in the primary cohort. However, the differences of DFS between different subgroups in the validation cohort were marginally significant with the same survival trends as the primary cohort. The reason for this might be that the sample size was not large enough to make the selected factors achieve steady performance. Another cause could be the different MRI scanners used in image acquisition in two cohorts, since radiomics features are known to vary with scanner type. Prospective research with a large sample size and different institutes is needed to further verify the reliability and repeatability of the predictors. Further studies to identify predictors for the selection of S-1 as chemotherapy regimen for locally advanced and metastatic pancreatic cancer are also needed.
Conclusions
Our study has demonstrated that whole-tumour evaluation with MRI and radiomics features could be used for predicting the efficacy of S-1, along with that the whole-tumour radiomics feature of T1WI_NGTDM_Strength and tumour location were potential predictors of the efficacy of S-1 and for the precision selection of S-1 as adjuvant chemotherapy regimen of PDAC.
Acknowledgements
None.
Abbreviations
- PDAC
Pancreatic ductal adenocarcinoma
- DFS
Disease-free survival
- HR
Hazard ratio
- DPD
Dihydropyrimidine dehydrogenase
- PTCD
Percutaneous transhepatic cholangiodrainage
- EMR
Electronic medical record
- RIS
Radiological information system
- TSE
Turbo spin-echo
- VIBE
Volumetric interpolated breath-hold examination
- ADC
Apparent diffusion coefficient
- GLCM
Grey Level Cooccurence Matrix
- GLRLM
Grey Level Run Length Matrix
- GLSZM
Grey Level Size Zone Matrix
- NGTDM
Neighbouring Grey Tone Difference Matrix
- GLDM
Grey Level Dependence Matrix
- PACS
Picture archiving communication system
- AP
Arterial phase
- PVP
Portal venous phase
- DP
Delayed phase
- ROC
Receiver-operating characteristic
- TS
Thymidylate synthase
- ROI
Region of interest
Appendix
The mathematical formulas of the selected features in part 4 of the results are as follows:
where pi ≠ 0, pj ≠ 0
where pi ≠ 0, pj ≠ 0 (Only the mathematical formulas of the selected features are listed. For the mathematical formulas of the remaining studied radiomics features, see https://pyradiomics.readthedocs.io/en/latest/features.html).
Authors' contributions
LL designed the study, evaluated the imagings and wrote the original draft. YD evaluated the imagings and analysed the data. YY analysed and interpreted the clinical data of the patients. KL performed MRI examination. SR validated and interpreted the results of the study. YG provided technical support of the software. MZ designed the study, reviewed and revised the article. All authors read and approved the final manuscript.
Funding
This study was supported by the Special Program of Clinical Research in Health Industry, Shanghai Municipal Health Commission (Grant Number 201840343). The funding body assessed the feasibility of the study, provided possible costs for open access publication, but had no role in the data collection, analysis, interpretation of data, or in writing the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Ethics Committee of Zhongshan Hospital, Fudan University approved this retrospective study (No. B2018-266), and the requirement for written informed consent was conditionally waived. The Ethics Committee permitted the members of our team, all of which were familiar with Declaration of Helsinki, to use the raw data/samples for research. All patient data used in this study was anonymised before its use.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Tsai S, Erickson BA, Dua K, Ritch PS, Tolat P, Evans DB. Evolution of the management of resectable pancreatic cancer. J Oncol Pract. 2016;12:772–778. doi: 10.1200/JOP.2016.015818. [DOI] [PubMed] [Google Scholar]
- 3.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 4.Tempero M, Malafa M, Al-Hawary M, Asbun H, Behrman S, Benson III A, et al. Pancreatic adenocarcinoma, version 1.2020, NCCN clinical practice guidelines in oncology. National Comprehensive Cancer Network. 2020. http://www.nccn.org/. Accessed 2 Mar 2020.
- 5.Pancreatic Cancer Committee of Chinese Anti-Cancer Association Comprehensive guidelines for the diagnosis and treatment of pancreatic cancer(2018 version) Chin J Surg. 2018;56:481–94. doi: 10.3760/cma.j.issn.0529-5815.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Okusaka T, Nakamura M, Yoshida M, Kitano M, Uesaka K, Ito Y, et al. Clinical practice guidelines for pancreatic cancer 2019 from the japan pancreas society: a synopsis. Pancreas. 2020;49:326–335. doi: 10.1097/MPA.0000000000001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumour selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7:548–557. doi: 10.1097/00001813-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640–1648. doi: 10.1200/JCO.2012.43.3680. [DOI] [PubMed] [Google Scholar]
- 9.Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01) Lancet. 2016;388:248–257. doi: 10.1016/S0140-6736(16)30583-9. [DOI] [PubMed] [Google Scholar]
- 10.Adamska A, Domenichini A, Falasca M. Pancreatic ductal adenocarcinoma: current and evolving therapies. Int J Mol Sci. 2017;18:E1338. doi: 10.3390/ijms18071338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Springfeld C, Jäger D, Büchler MW, Strobel O, Hackert T, Palmer DH, et al. Chemotherapy for pancreatic cancer. Presse Med. 2019;48:e159–e174. doi: 10.1016/j.lpm.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Lubner MG, Smith AD, Sandrasegaran K, Sahani DV, Pickhardt PJ. CT texture analysis: definitions, applications, biologic correlates, and challenges. Radiographics. 2017;37:1483–1503. doi: 10.1148/rg.2017170056. [DOI] [PubMed] [Google Scholar]
- 13.Verma V, Simone CB, II, Krishnan S, Lin SH, Yang J, Hahn SM. The rise of radiomics and implications for oncologic management. J Natl Cancer Inst. 2017;109:055. doi: 10.1093/jnci/djx055. [DOI] [PubMed] [Google Scholar]
- 14.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures. They are data. Radiology. 2016;278:563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eilaghi A, Baig S, Zhang Y, Zhang J, Karanicolas P, Gallinger S, et al. CT texture features are associated with overall survival in pancreatic ductal adenocarcinoma—a quantitative analysis. BMC Med Imaging. 2017;17:38. doi: 10.1186/s12880-017-0209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Attiyeh MA, Chakraborty J, Doussot A, Langdon-Embry L, Mainarich S, Gönen M, et al. Survival prediction in pancreatic ductal adenocarcinoma by quantitative computed tomography image analysis. Ann Surg Oncol. 2018;25:1034–1042. doi: 10.1245/s10434-017-6323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yun G, Kim YH, Lee YJ, Kim B, Hwang JH, Choi DJ. Tumor heterogeneity of pancreas head cancer assessed by CT texture analysis: association with survival outcomes after curative resection. Sci Rep. 2018;8:7226. doi: 10.1038/s41598-018-25627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wels MG, Lades F, Muehlberg A, Suehling M. General purpose radiomics for multi-modal clinical research. Comput-Aided Diagn. 2019;10:1–12. doi: 10.1117/12.2511856. [DOI] [Google Scholar]
- 19.van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77:e104–e107. doi: 10.1158/0008-5472.CAN-17-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang L, Luo R, Ding Y, Liu K, Shen L, Zeng H, et al. S100A4 overexpression in pancreatic ductal adenocarcinoma: imaging biomarkers from whole-tumor evaluation with MRI and texture analysis. Abdom Radiol (NY) 2021;46:623–635. doi: 10.1007/s00261-020-02676-3. [DOI] [PubMed] [Google Scholar]
- 21.Yabushita Y, Mori R, Taniguchi K, Matsuyama R, Kumamoto T, Sakamaki K, et al. Combined analyses of hENT1, TS, and DPD predict outcomes of borderline-resectable pancreatic cancer. Anticancer Res. 2017;37:2465–2476. doi: 10.21873/anticanres.11587. [DOI] [PubMed] [Google Scholar]
- 22.Shimoda M, Kubota K, Shimizu T, Katoh M. Randomized clinical trial of adjuvant chemotherapy with S-1 versus gemcitabine after pancreatic cancer resection. Br J Surg. 2015;102:746–754. doi: 10.1002/bjs.9775. [DOI] [PubMed] [Google Scholar]
- 23.Kondo N, Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, et al. Prognostic impact of dihydropyrimidine dehydrogenase expression on pancreatic adenocarcinoma patients treated with S-1-based adjuvant chemotherapy after surgical resection. J Surg Oncol. 2011;104:146–154. doi: 10.1002/jso.21955. [DOI] [PubMed] [Google Scholar]
- 24.Oba A, Ban D, Kirimura S, Akahoshi K, Mitsunori Y, Matsumura S, et al. Clinical application of the biomarkers for the selection of adjuvant chemotherapy in pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat Sci. 2016;23:480–488. doi: 10.1002/jhbp.366. [DOI] [PubMed] [Google Scholar]
- 25.Murakawa M, Aoyama T, Miyagi Y, Atsumi Y, Kazama K, Yamaoku K, et al. Clinical implications of dihydropyrimidine dehydrogenase expression in patients with pancreatic cancer who undergo curative resection with S-1 adjuvant chemotherapy. Oncol Lett. 2017;14:1505–1511. doi: 10.3892/ol.2017.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau MK, Davila JA, Shaib YH. Incidence and survival of pancreatic head and body and tail cancers: a population-based study in the United States. Pancreas. 2010;39:458–462. doi: 10.1097/MPA.0b013e3181bd6489. [DOI] [PubMed] [Google Scholar]
- 27.Ling Q, Xu X, Ye P, Xie H, Gao F, Hu Q, et al. The prognostic relevance of primary tumour location in patients undergoing resection for pancreatic ductal adenocarcinoma. Oncotarget. 2017;8:15159–15167. doi: 10.18632/oncotarget.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ling Q, Xu X, Zheng SS, Kalthoff H. The diversity between pancreatic head and body/tail cancers: clinical parameters and in vitro models. Hepatobiliary Pancreat Dis Int. 2013;12:480–487. doi: 10.1016/S1499-3872(13)60076-4. [DOI] [PubMed] [Google Scholar]
- 29.Mukhija D, Sohal DPS, Khorana AA. Adjuvant treatment in potentially curable pancreatic cancer: need to include tumour location in the equation? Pancreas. 2018;47:e50–e52. doi: 10.1097/MPA.0000000000001125. [DOI] [PubMed] [Google Scholar]
- 30.Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 31.Regine WF, Winter KA, Abrams R, Safran H, Hoffman JP, Konski A, et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18:1319–1326. doi: 10.1245/s10434-011-1630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gourtsoyianni S, Doumou G, Prezzi D, Taylor B, Stirling JJ, Taylor NJ, et al. Primary rectal cancer: repeatability of global and local-regional MR imaging texture features. Radiology. 2017;284:552–561. doi: 10.1148/radiol.2017161375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yip SS, Aerts HJ. Applications and limitations of radiomics. Phys Med Biol. 2016;61:R150–R166. doi: 10.1088/0031-9155/61/13/R150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yip C, Landau D, Kozarski R, Ganeshan B, Thomas R, Michaelidou A, et al. Primary esophageal cancer: heterogeneity as potential prognostic biomarker in patients treated with definitive chemotherapy and radiation therapy. Radiology. 2014;270:141–148. doi: 10.1148/radiol.13122869. [DOI] [PubMed] [Google Scholar]
- 35.Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaissis G, Ziegelmayer S, Lohöfer F, Steiger K, Algül H, Muckenhuber A, et al. A machine learning algorithm predicts molecular subtypes in pancreatic ductal adenocarcinoma with differential response to gemcitabine-based versus FOLFIRINOX chemotherapy. PLoS ONE. 2019;14:e0218642. doi: 10.1371/journal.pone.0218642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salinas-Miranda E, Khalvati F, Namdar K, Deniffel D, Dong X, Abbas E, et al. Validation of prognostic radiomic features from resectable pancreatic ductal adenocarcinoma in patients with advanced disease undergoing chemotherapy. Can Assoc Radiol J. 2020. 10.1177/0846537120968782. [DOI] [PubMed]
- 38.Toyama Y, Hotta M, Motoi F, Takanami K, Minamimoto R, Takase K. Prognostic value of FDG-PET radiomics with machine learning in pancreatic cancer. Sci Rep. 2020;10:17024. doi: 10.1038/s41598-020-73237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandrasegaran K, Lin Y, Asare-Sawiri M, Taiyini T, Tann M. CT texture analysis of pancreatic cancer. Eur Radiol. 2019;29:1067–1073. doi: 10.1007/s00330-018-5662-1. [DOI] [PubMed] [Google Scholar]
- 40.Qiu W, Duan N, Chen X, Ren S, Zhang Y, Wang Z, et al. Pancreatic ductal adenocarcinoma: machine learning-based quantitative computed tomography texture analysis for prediction of histopathological grade. Cancer Manag Res. 2019;11:9253–9264. doi: 10.2147/CMAR.S218414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masokano IB, Liu W, Xie S, Marcellin DFH, Pei Y, Li W. The application of texture quantification in hepatocellular carcinoma using CT and MRI: a review of perspectives and challenges. Cancer Imaging. 2020;20:67. doi: 10.1186/s40644-020-00341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartoli M, Barat M, Dohan A, Gaujoux S, Coriat R, Hoeffel C, et al. CT and MRI of pancreatic tumors: an update in the era of radiomics. Jpn J Radiol. 2020;38:1111–24. doi: 10.1007/s11604-020-01057-6. [DOI] [PubMed] [Google Scholar]
- 43.Ng F, Kozarski R, Ganeshan B, Goh V. Assessment of tumour heterogeneity by CT texture analysis: can the largest cross-sectional area be used as an alternative to whole tumour analysis? Eur J Radiol. 2013;82:342–348. doi: 10.1016/j.ejrad.2012.10.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.