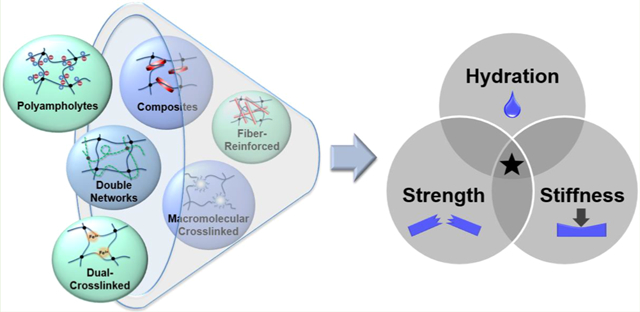
Abstract
Hydrogels are frequently used biomaterials due to their similarity in hydration and structure to biological tissues. However, their utility is limited by poor mechanical properties, namely, a lack of strength and stiffness that mimic that of tissues, particularly load-bearing tissues. Thus, numerous recent strategies have sought to enhance and tune these properties in hydrogels, including interpenetrating networks (IPNs), macromolecular cross-linking, composites, thermal conditioning, polyampholytes, and dual cross-linking. Individually, these approaches have achieved hydrogels with either high strength (σf > 10 MPa), high stiffness (E > 1 MPa), or, less commonly, both high strength and stiffness (σf > 10 MPa and E > 1 MPa). However, only certain unique combinations of these approaches have been able to synergistically achieve retention of a high, tissuelike water content as well as high strength and stiffness. Applying such methods to stimuli-responsive hydrogels has also produced robust, smart biomaterials. Overall, methods to achieve hydrogels that simultaneously mimic the hydration, strength, and stiffness of soft and load-bearing tissues have the potential to be used in a much broader range of biomedical applications.
Graphical Abstract
Although hydrogels have been extensively studied for decades, the enhancement of their mechanical properties to permit their use in broader applications, particularly biological tissue replacement, remains a challenge. Due to their high equilibrium water content (EWC) (up to >90%), most hydrogels exhibit strengths and moduli on the order of kPa’s,1 similar to that of soft tissues such as vasculature,2,3 skin,4,5 or muscle6 (Figure 1). This tissuelike hydration of hydrogels promotes their biocompatibility and, thus, their frequent use as tissue-contacting biomaterials. Currently, hydrogels are utilized in a multitude of biomedical applications,7 including drug delivery,8,9 wound dressings,10,11 soft contact lenses,12 and tissue engineering.13,14 However, replacement of load-bearing tissues, such as cartilage, tendons, and ligaments, requires hydrogels whose fracture strengths (σf) and moduli (E) are in the MPa range (Figure 1).15–18 In addition, other existing biomaterials could benefit from an enhancement in mechanical properties of hydrogels, such as vascular grafts,19,20 artificial muscles,21,22 flexible electronics,23,24 soft machines,25 and implantable biosensors.26,27 For many biomedical devices, maintaining high hydration while withstanding daily mechanical stresses, as well as potentially high peak stresses experienced during implantation and/or accidental impact, is essential.
Figure 1.
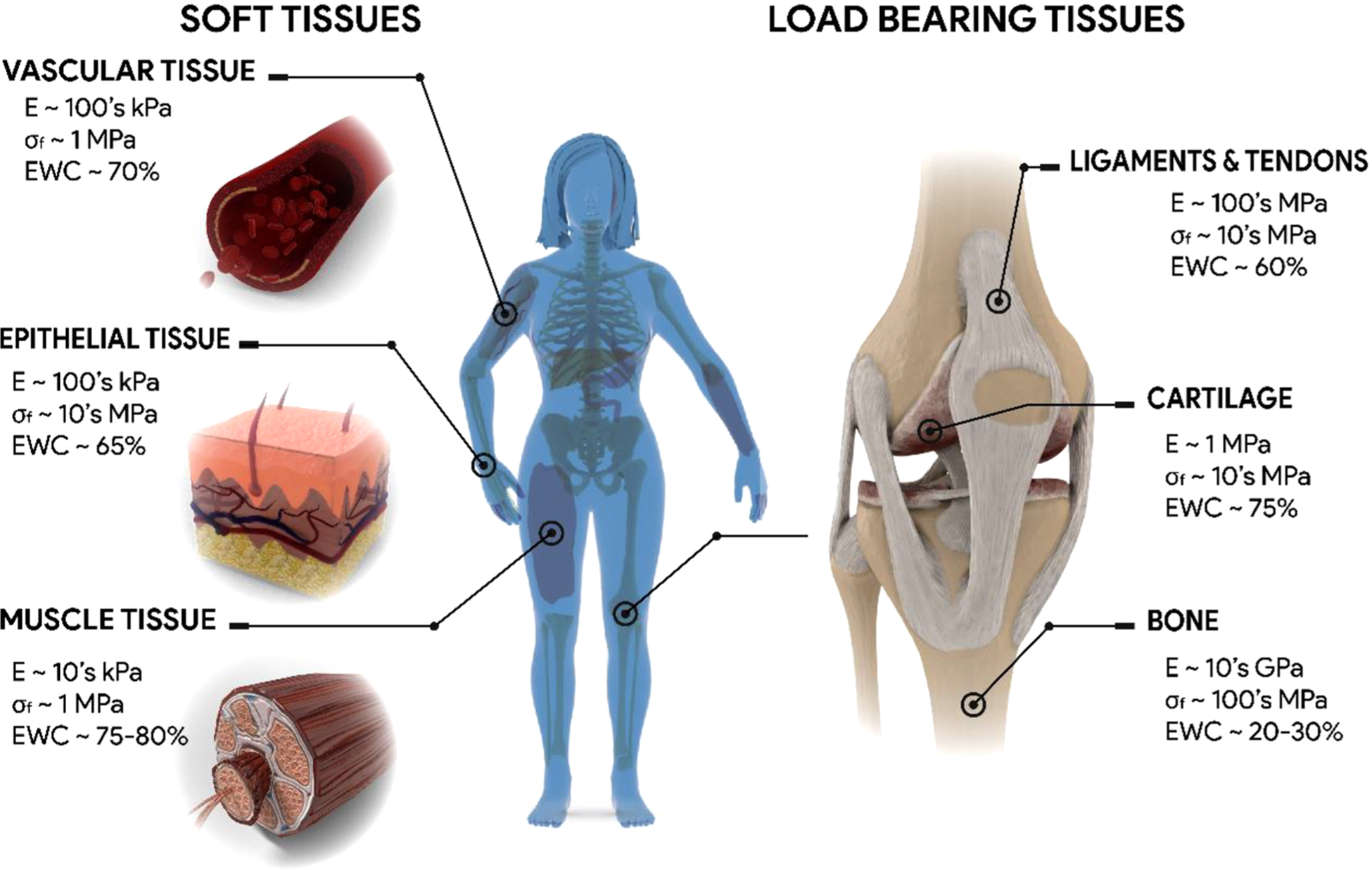
Summary of the modulus (E), fracture strength (σf), and equilibrium water content (EWC) values of biological tissues.2–6,15–18,41–43
Many factors can influence the mechanical properties of conventional hydrogels [i.e., single network (SN) hydrogels prepared with common cross-linkers and no fillers], including cross-link density, monomer concentration, homopolymer properties (e.g., hydrophilicity, electrostatic charge, backbone mobility, etc.), and copolymerization (Figure 2).1 In general, hydrogel networks can be physically or covalently cross-linked, with the latter typically exhibiting superior mechanical robustness. The direct correlation between increasing cross-link density and/or monomer concentration to an increase in stiffness is well-established.1 However, by creating a denser polymer network, the water content is inherently decreased. Alternatively, electrostatic charge has been shown to enhance the modulus through chain stiffening due to repulsive forces. Unlike the decrease in water content seen with increasing cross-link density or monomer concentration, the hydration of electrostatic hydrogels is increased with greater ionic content.28,29 However, the excessive chain stiffening produces an extremely brittle polymer network due to the highly extended state of the polymer chains which lack the ability to undergo deformation before fracture.29 Other inherent polymer properties, such as hydrophilicity and molecular mobility, can also influence the hydration and strength of hydrogels. Copolymerization can be utilized to combine desired attributes of more than one polymer to greatly broaden the properties of conventional hydrogels.30–32
Figure 2.
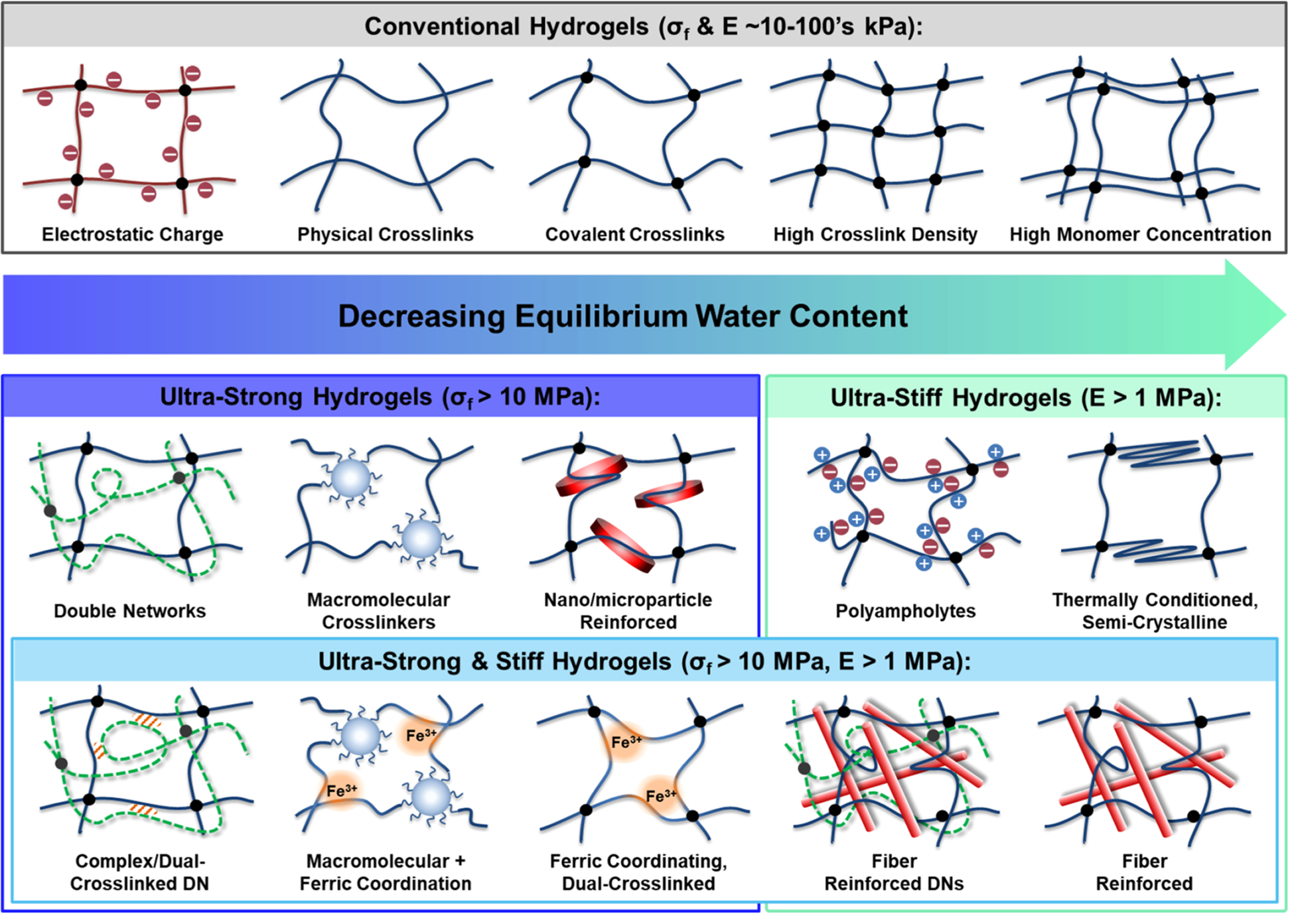
Strategies to enhance mechanical properties of hydrogels categorized by fracture strength (σf) and modulus (E) [denoted in boxes] and by decreasing equilibrium water content (EWC) [from left to right].
More recently, several strategies have been reported to improve the mechanical properties of hydrogels, including the use of interpenetrating networks (IPNs),28,33–40 macromolecular cross-linking,44–46 composites,47–54 thermal conditioning,55–59 polyampholytes,60–62 and dual cross-linking.63–67 Hydrogels produced from these methods can generally be categorized by their mechanical properties into the following groups: “conventional hydrogels” (sub-MPa σf and E), “ultrastrong hydrogels” (σf > 10 MPa), “ultrastiff hydrogels” (E > 1 MPa), and “ultrastrong and stiff hydrogels” (σf > 10 MPa and E > 1 MPa) (Figure 2). Although progress has been made toward enhancing hydrogel modulus and strength, it has proven difficult to concurrently maintain a high equilibrium water content (EWC > 70%).28,45,46,59,68–72 Most high-stiffness hydrogels exhibit moderate (EWC ~ 60–70%) or, more commonly, low (EWC < 60%) water contents. However, combinations of the aforementioned strategies have been successful in producing ultrastrong and stiff hydrogels with tissuelike hydration making them candidates for load-bearing applications.40,73–75 Herein, modern strategies to individually as well as simultaneously tune hydrogel hydration and mechanical properties are discussed. Additionally, examples of highly robust, stimuli-responsive hydrogels are highlighted to demonstrate the utility of these strategies to produce robust, smart biomaterials that can further broaden the use of hydrogels in biomedical applications.
Ultrastrong hydrogels—IPN hydrogels: One of the most studied methods to enhance hydrogel mechanical properties is the use of IPN designs. IPNs can be formed either as a non-cross-linked (i.e., thermoplastic) polymer cured within a cross-linked network (i.e., semi-IPNs) or as two interwoven, independently cross-linked networks (i.e., double networks, DNs). DN hydrogels were first reported by Gong et al. in which a tightly cross-linked, anionic network of poly(2-acrylamido-2-methylpropanesulfonic acid) (PAMPS) was combined with a loosely cross-linked, neutral network of poly(acrylamide) (PAAm).28 This hydrogel and a variety of other DN hydrogels can withstand high strains (>98%) and reach remarkable compressive fracture strengths (σf ~ 10s MPa, Figure 2) while maintaining high water contents (EWC > 80%).28,36,69,70,76–78 However, their moduli are typically only in the hundreds of kPa range, similar to that of soft tissues (Figure 1). DN hydrogels are most commonly fabricated sequentially in which a SN hydrogel is soaked in a second precursor solution and then cured to form the second polymer network within the pre-existing first network.34 Alternatively, two independent networks can be formed simultaneously if noncompeting cross-linking chemistries are used.78 For sequential DNs, the main contributing factors to their notable mechanical properties include the ratio of monomer concentration of the first to second network, cross-link density of each network, and ionic charge.34 For instance, it has been shown that the strength of PAMPS/PAAm DN hydrogels increases when the molar ratio of the second network to the first network increases.28 Additionally, the cross-linking density of the first network is typically greater than the second network to impart an asymmetric structure of extended and coiled polymer chains. Finally, the incorporation of a polyelectrolyte produces charge–charge repulsion and exaggerates the chain extension resulting in chain stiffening. Thus, the tightly cross-linked first network is commonly charged while the loosely cross-linked second network is neutral to remain relaxed and mobile.34 Ultimately, the asymmetric cross-linking structure of DN hydrogels permits stress dissipation through the fracture of the brittle first network while the ductile second network remains intact.79 The effects of additional independent networks have been evaluated as well from triple networks (TNs) to up to five individual networks.80,81 However, the benefits in mechanical properties achieved with more than two interpenetrating networks are typically due to the associated decrease in water content as overall polymer concentration increases. In general, versus conventional hydrogels, IPNs exhibit greatly improved fracture strength without diminishing their tissuelike water content, but their moduli remain limited to the sub-MPa range.
Ultrastrong hydrogels—macromolecular cross-linking: Macromolecular cross-linking also utilizes the regulation of polymer network structure to enhance hydrogel mechanical properties.44 Most conventional, synthetic hydrogels use low-molecular-weight, difunctional cross-linkers or difunctional prepolymers (i.e., macromers) to form a covalently cross-linked network with many inhomogeneities, such as kinks and loops, within a ladder type architecture. However, by using alternative macromolecular cross-linkers, network architectures with greater homogeneities can be achieved to improve their strength. A wide range of macromolecular cross-linkers have been developed, including complex prepolymers,82 micelles,83 polymer microspheres,46 and microgels.84 By forming defined prepolymers before cross-linking, the distance between cross-links and the number of branches stemming from each cross-linking point can be highly controlled. Multiarm, star-shaped prepolymers are predominantly used to enhance homogeneity via their highly symmetrical structure. Larger macromolecular cross-linkers, such as self-assembled micelles, polymer microspheres, and microgels, are less restrictive compared to typically rigid and brittle chemical cross-links. While the use of macromolecular cross-linkers provides a means of stress dissipation and enhances the compressive strength of resulting hydrogels (σf ~ tens of MPa) while retaining high water contents (EWC > 70%) (Figure 2), their moduli are generally lower than those of most DN hydrogels (E ~ tens of kPa).46,83–85
Ultrastrong hydrogels—nanocomposite hydrogels: Rather than altering network architecture and/or composition, a nanocomposite approach may be utilized in which inorganic fillers are used to reinforce the hydrogel matrix. Some of the most commonly used fillers range from nano- to microscale for clays,47,86,87 silicates,88–90 and carbon nanotubes.54,68 Many key factors determine their effects on the hydrogel properties, including size, charge, hydrophobicity, and surface functionality. These nanofillers typically serve as macro-cross-linkers through either covalent bonding or physical adsorption of polymer chains. Similar to organic macromolecular cross-linkers, nanocomposite hydrogels exhibit improved structural homogeneity and high compressive fracture strengths (σf > 10 MPa) without diminishing high water contents (EWC > 70%) (Figure 2).54,68,87,90 Additionally, the reversible adsorption/desorption of polymer chains during deformation allows for stress dissipation without permanently damaging the hydrogel network, greatly enhancing their fatigue resistance.88 In instances when fillers were incorporated within DN hydrogels,68,87 exceptional compressive strengths (σf > 50 MPa) were achieved. However, little enhancement in the modulus was observed which, thus, remained low (E ~ hundreds of kPa).
Ultrastiff hydrogels—thermally conditioned hydrogels: Although a number of hydrogels have achieved significant strengths, their low moduli limit their use in many load-bearing tissues (Figure 1). In attempts to increase stiffness in hydrogels, methods have been developed to incorporate crystalline domains within hydrogel networks. Those involving rigid, hydrophobic polymers typically require the use of surfactants91 or organic solvents58 to reduce phase separation, making them less desirable for most biomedical applications due to the concern of cytotoxicity. Furthermore, the hydrophobicity of the crystalline domains decreases hydrogel water content to as low as 50%.58 Alternatively, some hydrophilic polymers (e.g., poly(vinyl alcohol), PVA) can form crystallites through thermal conditioning methods (e.g., dry-annealing56,92 and freeze–thawing55,59) that serve as physical cross-links. Although dry-annealing can produce hydrogels with moduli in the MPa range, their water contents are greatly diminished (EWC ~ 40–60%).56,92 On the other hand, freeze–thawing can maintain hydration levels similar to cartilage and tendon/ligament tissues (EWC ~ 60–80%) while exhibiting high moduli (E > 1 MPa) (Figure 2) and moderate fracture strengths (σf ~ 1–2 MPa).55,59,93 Additionally, the degree of crystallite formation can be highly controlled by the freezing temperature and rate, number of cycles, and direction of freezing,55 allowing for high tunability of their mechanical and hydration properties. Thus, freeze–thawed PVA hydrogels have been utilized in low-impact connective tissues. For example, Cartiva is a PVA hydrogel that is an FDA-approved cartilage replacement for the first metatarsophalangeal joint. These implants have notable moduli ~1 MPa; however, their compressive fracture strength is relatively low (σf ~ 2 MPa), limiting them to non-load-bearing joints.59 In general, the incorporation of crystalline domains into hydrogels has the ability to enhance their stiffness, but water content or strength is diminished in return.
Ultrastiff hydrogels—polyampholytes and polyion complexes (PICs): Polyampholytes, polymers with both anionic and cationic charges, have the potential to impart stiffness in hydrogels due to intra- and interchain electrostatic interactions that produce ionic complexes that serve as cross-linking points.62,94 Likewise, two oppositely charged polymers, one anionic and one cationic, can form polyion complexes (PICs).60,61 Despite being physically cross-linked hydrogels, polyampholyte and PIC hydrogels simultaneously exhibit high moduli (E up to ~8 MPa) and moderately high tensile strengths (σf up to ~5 MPa) (Figure 2).60–62 It has been shown that strong ionic complexing can serve as pseudopermanent cross-links at most strains, whereas weaker ionic interactions serve as reversible sacrificial bonds allowing for stress dissipation.61 However, the excellent mechanical properties of these types of hydrogels are primarily associated with their low water contents (EWC ~ 40–60%).60–62 In summary, similar to thermally conditioned hydrogels, polyampholyte and PIC hydrogels have demonstrated remarkably high moduli but lack the proper hydration and/or high strength to serve as load-bearing tissue replacements.
Ultrastrong and stiff hydrogels—dual-cross-linked hydrogels: To obtain hydrogels with both high strength and stiffness, the combination of multiple cross-linking types appears key. Particularly effective are dual-cross-linked hydrogels that utilize permanent covalent cross-links combined with reversible physical cross-links which act as sacrificial bonds to dissipate stress. These reversible cross-links can be formed through a variety of physical interactions such as hydrogen bonding,57,65 hydrophobic interactions,58,91 host–guest interactions,67 and ionic interactions.30,63,64,66 During deformation, these sacrificial physical bonds provide the rigidity of a highly cross-linked network under low strains without imparting the brittleness typically seen at high strains due to their ability to break and reform repeatedly.95 This unique character has produced hydrogels with both high strength and high stiffness as well as good self-healing.63,66,91 However, these mechanical properties are directly related to their low water contents (EWC ~ 40–60%) stemming from their high apparent cross-link densities and/or increased hydrophobicity.57,58,63–66,91 One of the most predominant dual-cross-linking mechanisms used in the recent literature utilizes ferric coordination with a covalently cross-linked, ionic polymer network to form secondary, noncovalent cross-links. For example, Lin et al. have pioneered the use of Fe3+ coordination with a covalently cross-linked poly(acrylic acid) (PAAc) network through which mechanically robust hydrogels with excellent tensile strengths (σf ~ tens of MPa) and moduli (E ~ tens of MPa) (Figure 2); however, these exhibit low water contents (EWC < ~60%).63,64 Despite lacking proper hydration, this dual-cross-linking strategy is one of the most promising approaches to enhancing hydrogel mechanical properties toward load-bearing biomaterials, such as cartilage, ligaments, and tendons.
Ultrastrong and stiff hydrogels—fiber reinforced hydrogels: Another method to enhance both the strength and modulus of hydrogels involves their reinforcement with rigid, nonhydrated fibers. Typically, these fibers are highly organized through electrospinning,96,97 3D printing,53,98,99 or standard weaving techniques (e.g., gauze and fabrics)50,100 to enhance their durability. Others have utilized naturally occurring fibers such as silk51 and nanocellulose101 which are evenly dispersed in the hydrogel precursor solution before curing. Many studies have demonstrated an increase in hydrogel stiffness with the incorporation of fibers; however, most exhibit low fracture strengths (σf < ~2 MPa) due to the poor hydrogel–fiber interactions.51,96,98,99 Recently, advancements have been made in fiber-reinforced hydrogels through their combination with additional strengthening methods, including DNs,102,103 thermal conditioning,101 and polyampholyte interactions.50 For example, Yang et al. produced cellulose nanofiber/PVA composite hydrogels with a high modulus (E ~ 50 MPa) and high tensile fracture strength (σf ~ 16 MPa) through the formation of strong hydrogen bonds between the cellulose nanofibers and PVA during dry-annealing.101 Still, while similar to that of epithelial tissue (EWC ~ 65%), the hydration of these hydrogels was not particularly high. Arguably the strongest and stiffest fiber-reinforced hydrogels have been developed by King et al., in which polyampholyte hydrogels were formed around woven glass fibers.50 Inter- and intrachain ionic bonding interactions force the polyampholyte hydrogel to deswell onto the glass fibers, producing an exceptional modulus (E ~ 600 MPa) and high tensile fracture strength (σf ~ 17 MPa). However, this deswelling produces hydrogels with low water contents (EWC ~ 50%). In addition, fiber-reinforced hydrogels show extremely high tear resistance and the ability to exhibit anisotropic properties, making them highly desirable for many biological applications. Such hydrogels could serve as synthetic tendons and ligaments (σf ~ tens of MPa, E ~ hundreds of MPa, EWC ~ 60%) (Figure 1).
Ultrastrong and stiff hydrogels—complex/dual-cross-linked DN and other hydrogels formed with combined strategies: Several combinations of strategies have been successfully used to form a wide range of complex hydrogels with exceptional mechanical properties. For example, two physically cross-linked hydrogels, through the use of reversible cross-linking mechanisms, have attained strength and moduli in the MPa range. Li et al. applied thermal conditioning to a dual-cross-linked network to form a physical PVA/hyaluronic acid–Fe3+ DN hydrogel.56 Zhang et al. reported DNs that employed hydrogen bonding and hydrophobic interactions as secondary, reversible interactions to attain a physically cross-linked DN hydrogel from an amphiphilic triblock copolymer and linear PAAm.104 Although both hydrogel systems exhibited impressive moduli (E ~ 1–5 MPa) and tensile strengths (σf ~ 10 MPa), their water content values remained relatively low (EWC ~ 45%).56,104 Additional studies have paired ferric coordination with macromolecular cross-linking45,105 or DN72,106 structures to achieve strong, rigid hydrogels (σf > 10 MPa and E > 1 MPa) (Figure 2) with slightly higher water contents (EWC ~ 50–65%) on average versus purely dual-cross-linked hydrogels (EWC ~ 40–60%). While these complex/dual-cross-linked hydrogels exhibit mechanical properties on the same order as connective tissues, their water contents typically remain <70%.
Ultrastrong and stiff hydrogels—maintaining hydration in strong and stiff hydrogels: Hydrogels with high water contents (EWC > 70%) that also possess the high strength (σf > ~10 MPa) and high rigidity (E > ~1 MPa) of load-bearing tissues are challenging to produce due to the inverse relationship of these properties. However, a few hydrogel systems have achieved this through unique combinations of strategies (Figure 3). Huang et al. reported a chemically cross-linked P(AAm-co-AAc) hydrogel with embedded cellulose nanofibers wherein the nanofibers formed ionic complexes with AAc blocks through ferric coordination. With a minimal fiber content (~0.6%), these hydrogels exhibited a high tensile strength (~10 MPa) and high modulus (E ~ 3 MPa) as well as retained a high water content, near that of cartilage (EWC ~ 70–80%).73 While interactions between stiff fibers and soft, hydrated networks are typically poor, this Fe3+ dual-cross-linking strategy was able to overcome this challenge. Other hydrogels that have achieved high strength, rigidity, and hydration have all been based on DNs that incorporated secondary reversible interactions to serve as sacrificial bonds that increased apparent cross-linking density at low strains but provided stress dissipation at high strains. For instance, Argun et al. reported a nonionic TN hydrogel composed of a chemically cross-linked poly(N,N-dimethylacrylamide) (PDMA) first network and linear, non-cross-linked PDMA second and third networks. These high-water-content hydrogels (EWC ~ 90%) exhibited both high compressive fracture strengths (σf ~ 20 MPa) and high moduli (E ~ 2 MPa). Their exceptional mechanical properties were attributed to the hydrophobic interactions between sequential network components allowing for a high molar ratio of the second and third networks to the first network.40 Our group reported the combination of an anionic PAMPS first network with a second network composed of NIPAAm copolymerized with a zwitterionic comonomer to produce a DN hydrogel with high compressive strength (σf ~ 23 MPa) and high modulus (E ~ 1.5 MPa) as well as high hydration (EWC ~ 83%).74 Beyond the double network structure, this unique combination of mechanical properties was attributed to a stiffening phenomenon in which physical interactions between the PNIPAAm chains increase the apparent cross-link density, an observation previously made for PNIPAAm-based semi-IPN hydrogels.107 Additionally, the zwitterionic comonomer, [2-(methacryloyloxy)ethyl] dimethyl-(3-sulfopropyl) ammonium hydroxide (MEDSAH), induced reversible, inter- and intra-network ionic interactions with itself and the PAMPS first network. This zwitterionic DN hydrogel retained the characteristic thermoresponsiveness of PNIPAAm hydrogels, exhibiting a volume phase transition temperature (VPTT) of ~35 °C.
Figure 3.
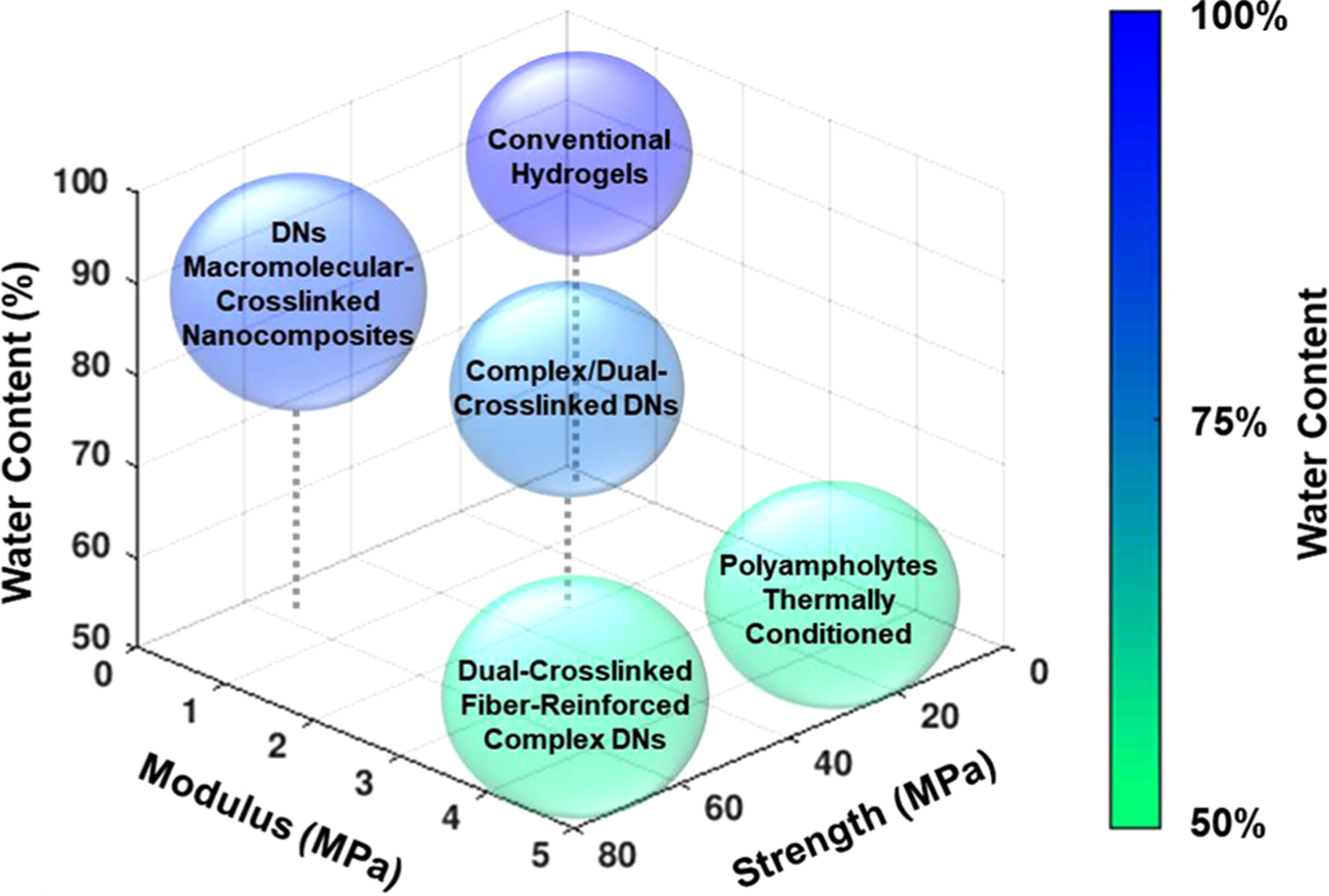
Visual comparison of the fracture strength (σf), modulus (E), and equilibrium water content (EWC) of hydrogels produced with different strategies, demonstrating the rarity of enhancing all three simultaneously.
In a second study by our group, an anionic PAMPS first network was combined with a second network composed of NIPAAm copolymerized with a neutral, hydrophilic comonomer (AAm).75 This effectively tuned the VPTT out of the physiological range (>40 °C) such that the DN hydrogel would not be subjected to deswelling/reswelling due to body temperature fluctuations, an ability which may be preferred in some applications. This DN also achieved high compressive strength (σf ~ 26 MPa) and a high modulus (E ~ 1.1 MPa) while maintaining a high water content (EWC ~ 84%). These collective studies demonstrate the ability of complex structured hydrogels to achieve robust mechanical properties with excellent hydration as well as ways to tailor their properties by regulating the amount of secondary bonding present. Thus, these complex cross-linking strategies permit the use of hydrogels in load-bearing tissue applications that require high water contents, stiffness, and strength simultaneously.
Incorporating stimuli-responsiveness—stimuli-responsive, robust hydrogels: These modern strategies to obtain mechanically robust hydrogels have been applied to stimuli-responsive materials to permit their use in a variety of biomedical applications, such as artificial muscles,21,108 soft robotics,109,110 drug delivery,111,112 and regulation of cell adhesion/detachment.27,113 A range of robust, smart hydrogels have been reported, including thermoresponsive,74,114–117 pH-responsive,84,118–120 photoresponsive,113,121 and others108,112 (Figure 4). Nanocomposite reinforcement and macromolecular cross-linking are frequently utilized in stimuli-responsive hydrogels due to their exceptional swelling kinetics. Their rapid swelling rates are attributed to their less restrictive cross-linking mechanisms versus more rigid, chemical cross-links, allowing the network chains to behave similar to that of linear polymers.86,115,116,118,122,123 Other complex cross-linked hydrogels including polyampholytes,84,124 DNs,74,76,120,122 and dual-cross-linked109,113,114,119,121 hydrogels have been developed as robust, stimuli-responsive membranes. In our group, various thermoresponsive, DN hydrogels have been designed with polysiloxane nanoparticles to enhance swelling kinetics123 and polyampholyte copolymers to enhance mechanical properties without diminishing thermosensitivity.74 Thus, by combining unique strengthening approaches to stimuli-responsive hydrogels, these smart, tissue-mimetic materials could be used in a broad range of load-bearing applications that require a response to their external environment.
Figure 4.
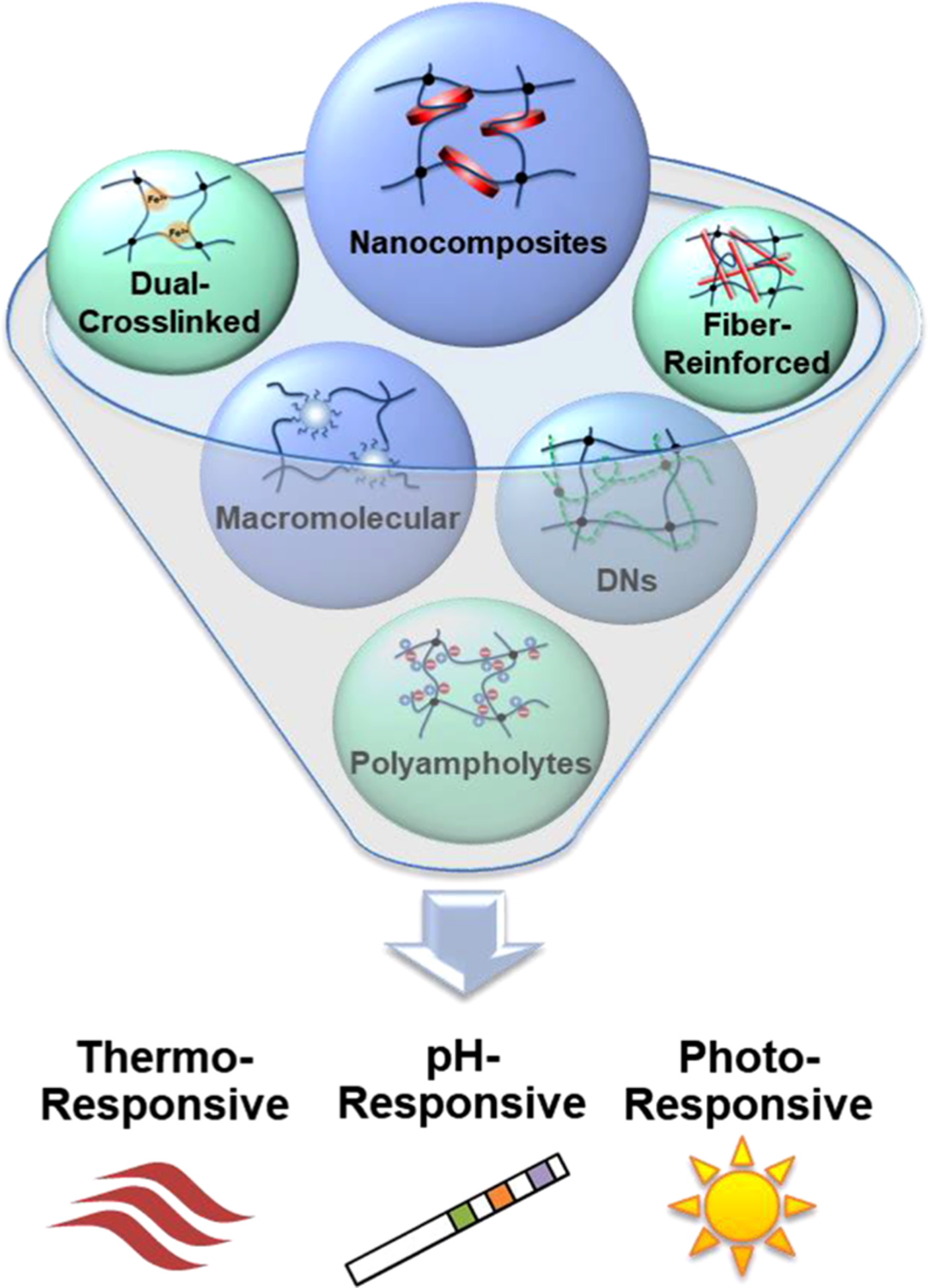
Robust, stimuli-responsive hydrogels can be developed through the application of modern strategies to enhance the mechanical properties on conventional stimuli-responsive hydrogels.
In summary, the development of numerous complex cross-linking structures has progressed hydrogel biomaterials toward mimicking both the mechanics and hydration of tissues. Compared to conventional hydrogels with sub-MPa moduli and strengths, these membranes have achieved ultrahigh strength (σf > 10 MPa), high stiffness (E > 1 MPa), and even ultrahigh strength and stiffness together (σf > 10 MPa and E > 1 MPa) in some, although many suffer from a reduction in water content. In general, most DN, macromolecular cross-linked, and nanocomposite hydrogels are able to withstand high strains and reach ultrahigh fracture strengths while maintaining >70% water content but exhibit sub-MPa moduli. On the other hand, thermally conditioned and polyampholyte hydrogels have shown high stiffness but typically suffer from moderately low fracture strengths and poor hydration. Dual-cross-linked and fiber-reinforced hydrogels have been able to simultaneously achieve high fracture strengths and moduli but are limited to ~40–60% water content. Thus far, few hydrogels have been able to retain tissue-mimetic hydration while enhancing both their stiffness and strength. By using a combination of synergistic strategies, such as dual-cross-linking incorporated into a DN structure, exceptional strengths, moduli, and water contents can be attained concurrently in a single hydrogel. Finally, these cross-linking structures can be applied to stimuli-responsive hydrogels (i.e., thermoresponsive, pH-responsive, photoresponsive, and others) to produce smart hydrogels with exceptional mechanical properties. By employing these complex cross-linking strategies to match the hydration and mechanics of tissues, the utility of hydrogels can be greatly expanded, even to load-bearing regions of the body.
ACKNOWLEDGMENTS
Funding from the NIH/NIDDK (1R01DK095101-01A1) and the Texas A&M Engineering and Experiment Station (TEES) is gratefully acknowledged. A.K.M. thanks the National Science Foundation (NSF) Graduate Research Fellowship Program (NSF GRFP M1703014). We thank Don Mai for his use of the open source project Blender.org.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Anseth KS; Bowman CN; Brannon-Peppas L Mechanical properties of hydrogels and their experimental determination. Biomaterials 1996, 17 (17), 1647–1657. [DOI] [PubMed] [Google Scholar]
- (2).Bergel DH The static elastic properties of the arterial wall. J. Physiol 1961, 156 (3), 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Stemper BD; Yoganandan N; Pintar FA Methodology to study intimal failure mechanics in human internal carotid arteries. J. Biomech 2005, 38 (12), 2491–2496. [DOI] [PubMed] [Google Scholar]
- (4).Li C; Guan G; Reif R; Huang Z; Wang RK Determining elastic properties of skin by measuring surface waves from an impulse mechanical stimulus using phase-sensitive optical coherence tomography. J. R. Soc., Interface 2012, 9 (70), 831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).NíAnnaidh A; Bruyère K; Destrade M; Gilchrist MD; Otténio M Characterization of the anisotropic mechanical properties of excised human skin. J. Mech Behav Biomed Mater 2012, 5 (1), 139–148. [DOI] [PubMed] [Google Scholar]
- (6).Schleifenbaum S; Schmidt M; Möbius R; Wolfskämpf T; Schröder C; Grunert R; Hammer N; Prietzel T Load and failure behavior of human muscle samples in the context of proximal femur replacement. BMC Musculoskeletal Disord. 2016, 17 (1), 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Hoffman AS Hydrogels for biomedical applications. Adv. Drug Delivery Rev 2012, 64, 18–23. [DOI] [PubMed] [Google Scholar]
- (8).Qiu Y; Park K Environment-sensitive hydrogels for drug delivery. Adv. Drug Delivery Rev 2001, 53 (3), 321–339. [DOI] [PubMed] [Google Scholar]
- (9).Hoare TR; Kohane DS Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49 (8), 1993–2007. [Google Scholar]
- (10).Boateng JS; Matthews KH; Stevens HNE; Eccleston GM Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci 2008, 97 (8), 2892–2923. [DOI] [PubMed] [Google Scholar]
- (11).Kamoun EA; Kenawy E-RS; Chen X A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res 2017, 8 (3), 217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Nicolson PC; Vogt J Soft contact lens polymers: an evolution. Biomaterials 2001, 22 (24), 3273–3283. [DOI] [PubMed] [Google Scholar]
- (13).Lee KY; Mooney DJ Hydrogels for tissue engineering. Chem. Rev 2001, 101 (7), 1869–1880. [DOI] [PubMed] [Google Scholar]
- (14).Nicodemus GD; Bryant SJ Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng., Part B 2008, 14 (2), 149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Boschetti F; Pennati G; Gervaso F; M Peretti G; Dubini G Biomechanical properties of human articular cartilage under compressive loads. Biorheology 2004, 41, 159–166. [PubMed] [Google Scholar]
- (16).Kerin A; Wisnom M; Adams M The compressive strength of articular cartilage. Proc. Inst. Mech. Eng., Part H 1998, 212 (4), 273–280. [DOI] [PubMed] [Google Scholar]
- (17).Maganaris CN; Paul JP In vivo human tendon mechanical properties. J. Physiol 1999, 521 (1), 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Smeets K; Slane J; Scheys L; Claes S; Bellemans J Mechanical analysis of extra-articular knee ligaments. Part one: Native knee ligaments. Knee 2017, 24 (5), 949–956. [DOI] [PubMed] [Google Scholar]
- (19).Skardal A; Zhang J; Prestwich GD Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials 2010, 31 (24), 6173–6181. [DOI] [PubMed] [Google Scholar]
- (20).Hahn MS; McHale MK; Wang E; Schmedlen RH; West JL Physiologic pulsatile flow bioreactor conditioning of poly(ethylene glycol)-based tissue engineered vascular grafts. Ann. Biomed. Eng 2007, 35 (2), 190–200. [DOI] [PubMed] [Google Scholar]
- (21).Liu Z; Calvert P Multilayer hydrogels as muscle-like actuators. Adv. Mater 2000, 12 (4), 288–291. [Google Scholar]
- (22).Takashima Y; Hatanaka S; Otsubo M; Nakahata M; Kakuta T; Hashidzume A; Yamaguchi H; Harada A Expansion-contraction of photoresponsive artificial muscle regulated by h ost-guest interactions. Nat. Commun 2012, 3, 1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Xu Y; Sheng K; Li C; Shi G Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 2010, 4 (7), 4324–4330. [DOI] [PubMed] [Google Scholar]
- (24).Lin S; Yuk H; Zhang T; Parada GA; Koo H; Yu C; Zhao X Stretchable hydrogel electronics and devices. Adv. Mater 2016, 28 (22), 4497–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Calvert P Hydrogels for soft machines. Adv. Mater 2009, 21 (7), 743–756. [Google Scholar]
- (26).Abraham AA; Means AK; Clubb FJ; Fei R; Locke AK; Gacasan EG; Coté GL; Grunlan MA Foreign body reaction to a subcutaneously implanted self-cleaning, thermoresponsive hydrogel membrane for glucose biosensors. ACS Biomater. Sci. Eng 2018, 4 (12), 4104–4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Fei R; Means AK; Abraham AA; Locke AK; Coté GL; Grunlan MA Self-cleaning, thermoresponsive P(NIPAAm-co-AM PS) double network membranes for implanted glucose biosensors. Macromol. Mater. Eng 2016, 301 (8), 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Gong JP; Katsuyama Y; Kurokawa T; Osada Y Double-network hydrogels with extremely high mechanical strength. Adv. Mater 2003, 15 (14), 1155–1158. [Google Scholar]
- (29).Okay O; Durmaz S Charge density dependence of elastic modulus of strong polyelectrolyte hydrogels. Polymer 2002, 43 (4), 1215–1221. [Google Scholar]
- (30).Henderson KJ; Zhou TC; Otim KJ; Shull KR Ionically cross-linked triblock copolymer hydrogels with high strength. Macromolecules 2010, 43 (14), 6193–6201. [Google Scholar]
- (31).Yildiz B; Isik B; Kis M Synthesis and characterization of thermoresponsive isopropylacrylamide-acrylamide hydrogels. Eur. Polym. J 2002, 38 (7), 1343–1347. [Google Scholar]
- (32).Ning J; Kubota K; Li G; Haraguchi K Characteristics of zwitterionic sulfobetaine acrylamide polymer and the hydrogels prepared by free-radical polymerization and effects of physical and chemical crosslinks on the U CST. React. Funct. Polym 2013, 73 (7), 969–978. [Google Scholar]
- (33).Yasuda K; Ping Gong J; Katsuyama Y; Nakayama A; Tanabe Y; Kondo E; Ueno M; Osada Y Biomechanical properties of high-toughness double network hydrogels. Biomaterials 2005, 26 (21), 4468–4475. [DOI] [PubMed] [Google Scholar]
- (34).Haque MA; Kurokawa T; Gong JP Super tough double network hydrogels and their application as biomaterials. Polymer 2012, 53 (9), 1805–1822. [Google Scholar]
- (35).Milner PE; Parkes M; Puetzer JL; Chapman R; Stevens MM; Cann P; Jeffers JRT A low friction, biphasic and boundary lubricating hydrogel for cartilage replacement. Acta Biomater. 2018, 65, 102–111. [DOI] [PubMed] [Google Scholar]
- (36).Chen Q; Wei D; Chen H; Zhu L; Jiao C; Liu G; Huang L; Yang J; Wang L; Zheng J Simultaneous enhancement of stiffness and toughness in hybrid double-network hydrogels via the first, physically linked network. Macromolecules 2015, 48 (21), 8003–8010. [Google Scholar]
- (37).Sun J-Y; Zhao X; Illeperuma WRK; Chaudhuri O; Oh KH; Mooney DJ; Vlassak JJ; Suo Z Highly stretchable and tough hydrogels. Nature 2012, 489, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Chen F; Lu S; Zhu L; Tang Z; Wang Q; Qin G; Yang J; Sun G; Zhang Q; Chen Q Conductive regenerated silk-fibroin-based hydrogels with integrated high mechanical performances. J. Mater. Chem. B 2019, 7, 1708. [DOI] [PubMed] [Google Scholar]
- (39).Kaneko D; Tada T; Kurokawa T; Gong JP; Osada Y Mechanically strong hydrogels with ultra-low frictional coefficients. Adv. Mater 2005, 17 (5), 535–538. [Google Scholar]
- (40).Argun A; Can V; Altun U; Okay O Nonionic double and triple network hydrogels of high mechanical strength. Macromolecules 2014, 47 (18), 6430–6440. [Google Scholar]
- (41).Woodard HQ; White DR The composition of body tissues. Br. J. Radiol 1986, 59 (708), 1209–1218. [DOI] [PubMed] [Google Scholar]
- (42).Gong JK; Arnold JS; Cohn SH Composition of trabecular and cortical bone. Anat. Rec 1964, 149 (3), 325–331. [DOI] [PubMed] [Google Scholar]
- (43).Reilly DT; Burstein AH The elastic and ultimate properties of compact bone tissue. J. Biomech 1975, 8 (6), 393–405. [DOI] [PubMed] [Google Scholar]
- (44).Fu J Strong and tough hydrogels crosslinked by multi-functional polymer colloids. J. Polym. Sci., Part B: Polym. Phys 2018, 56 (19), 1336–1350. [Google Scholar]
- (45).Li J; Yang J; Liu W A mechanically robust, stiff, and tough hyperbranched supramolecular polymer hydrogel. Macromol. Rapid Commun 2019, 40 (6), 1800819. [DOI] [PubMed] [Google Scholar]
- (46).Huang T; Xu HG; Jiao KX; Zhu LP; Brown HR; Wang HL A novel hydrogel with high mechanical strength: A macromolecular microsphere composite hydrogel. Adv. Mater 2007, 19 (12), 1622–1626. [Google Scholar]
- (47).Haraguchi K; Li H-J Mechanical properties and structure of polymer–clay nanocomposite gels with high clay content. Macromolecules 2006, 39 (5), 1898–1905. [Google Scholar]
- (48).Gaharwar AK; Dammu SA; Canter JM; Wu C-J; Schmidt G Highly extensible, tough, and elastomeric nanocomposite hydrogels from poly(ethylene glycol) and hydroxyapatite nanoparticles. Biomacromolecules 2011, 12 (5), 1641–1650. [DOI] [PubMed] [Google Scholar]
- (49).Tanpichai S; Oksman K Cross-linked nanocomposite hydrogels based on cellulose nanocrystals and PVA: Mechanical properties and creep recovery. Composites, Part A 2016, 88, 226–233. [Google Scholar]
- (50).King DR; Sun TL; Huang Y; Kurokawa T; Nonoyama T; Crosby AJ; Gong JP Extremely tough composites from fabric reinforced polyampholyte hydrogels. Mater. Horiz 2015, 2 (6), 584–591. [Google Scholar]
- (51).Kundanati L; Singh SK; Mandal BB; Murthy TG; Gundiah N; Pugno NM Fabrication and mechanical characterization of hydrogel infused network silk scaffolds. Int. J. Mol. Sci 2016, 17 (10), 1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Huang Y; King DR; Sun TL; Nonoyama T; Kurokawa T; Nakajima T; Gong JP Energy-dissipative matrices enable synergistic toughening in fiber reinforced soft composites. Adv. Funct. Mater 2017, 27 (9), 1605350. [Google Scholar]
- (53).Castilho M; Hochleitner G; Wilson W; van Rietbergen B; Dalton PD; Groll J; Malda J; Ito K Mechanical behavior of a soft hydrogel reinforced with three-dimensional printed microfibre scaffolds. Sci. Rep 2018, 8 (1), 1245–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Feng H; Zheng T; Wang X; Wang H Poly(acrylamide)-MW NTs hybrid hydrogel with extremely high mechanical strength. Open Chem. 2016, 14, 150. [Google Scholar]
- (55).Peppas NA; Stauffer SR Reinforced uncrosslinked poly (vinyl alcohol) gels produced by cyclic freezing-thawing processes: a short review. J. Controlled Release 1991, 16 (3), 305–310. [Google Scholar]
- (56).Li A; Si Y; Wang X; Jia X; Guo X; Xu Y Polyvinyl alcohol nanocrystal assisted hydrogels with high toughness and elastic modulus for 3D printing. ACS Appl Nano Mater. 2019, 2, 707. [Google Scholar]
- (57).Liu T; Jiao C; Peng X; Chen Y-N; Chen Y; He C; Liu R; Wang H Super-strong and tough poly(vinyl alcohol)/poly(acrylic acid) hydrogels reinforced by hydrogen bonding. J. Mater. Chem. B 2018, 6 (48), 8105–8114. [DOI] [PubMed] [Google Scholar]
- (58).Mredha MTI; Pathak SK; Tran VT; Cui J; Jeon I Hydrogels with superior mechanical properties from the synergistic effect in hydrophobic-hydrophilic copolymers. Chem. Eng. J 2019, 362, 325–338. [Google Scholar]
- (59).Stammen JA; Williams S; Ku DN; Guldberg RE Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Biomaterials 2001, 22 (8), 799–806. [DOI] [PubMed] [Google Scholar]
- (60).Luo F; Sun TL; Nakajima T; King DR; Kurokawa T; Zhao Y; Ihsan AB; Li X; Guo H; Gong JP Strong and tough polyion-complex hydrogels from oppositely charged polyelectrolytes: A comparative study with polyampholyte hydrogels. Macromolecules 2016, 49 (7), 2750–2760. [Google Scholar]
- (61).Luo F; Sun TL; Nakajima T; Kurokawa T; Zhao Y; Sato K; Ihsan AB; Li X; Guo H; Gong JP Oppositely charged polyelectrolytes form tough, self-healing, and rebuildable hydrogels. Adv. Mater 2015, 27 (17), 2722–2727. [DOI] [PubMed] [Google Scholar]
- (62).Sun TL; Kurokawa T; Kuroda S; Ihsan AB; Akasaki T; Sato K; Haque MA; Nakajima T; Gong JP Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater 2013, 12, 932. [DOI] [PubMed] [Google Scholar]
- (63).Lin P; Ma S; Wang X; Zhou F Molecularly engineered dual-crosslinked hydrogel with ultrahigh mechanical strength, toughness, and good self-recovery. Adv. Mater 2015, 27 (12), 2054–2059. [DOI] [PubMed] [Google Scholar]
- (64).Lin P; Zhang T; Wang X; Yu B; Zhou F Freezing molecular orientation under stretch for high mechanical strength but anisotropic hydrogels. Small 2016, 12 (32), 4386–4392. [DOI] [PubMed] [Google Scholar]
- (65).Zhang J; Wang N; Liu W; Zhao X; Lu W Intermolecular hydrogen bonding strategy to fabricate mechanically strong hydrogels with high elasticity and fatigue resistance. Soft Matter 2013, 9 (27), 6331–6337. [Google Scholar]
- (66).Liang Y; Xue J; Du B; Nie J Ultrastiff, tough, and healable ionic-hydrogen bond cross-linked hydrogels and their uses as building blocks to construct complex hydrogel structures. ACS Appl. Mater. Interfaces 2019, 11, 5441. [DOI] [PubMed] [Google Scholar]
- (67).Wang Z; An G; Zhu Y; Liu X; Chen Y; Wu H; Wang Y; Shi X; Mao C 3D-printable self-healing and mechanically reinforced hydrogels with host-guest non-covalent interactions integrated into covalently linked networks. Mater. Horiz 2019, 6, 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Dong W; Huang C; Wang Y; Sun Y; Ma P; Chen M Superior mechanical properties of double-network hydrogels reinforced by carbon nanotubes without organic modification. Int. J. Mol. Sci 2013, 14 (11), 22380–22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Du G; Gao G; Hou R; Cheng Y; Chen T; Fu J; Fei B Tough and fatigue resistant biomimetic hydrogels of interlaced self-assembled conjugated polymer belts with a polyelectrolyte network. Chem. Mater 2014, 26 (11), 3522–3529. [Google Scholar]
- (70).Li Z; Su Y; Xie B; Liu X; Gao X; Wang D A novel biocompatible double network hydrogel consisting of konjac glucomannan with high mechanical strength and ability to be freely shaped. J. Mater. Chem. B 2015, 3 (9), 1769–1778. [DOI] [PubMed] [Google Scholar]
- (71).Wang J; Wei J; Su S; Qiu J; Wang S Ion-linked double-network hydrogel with high toughness and stiffness. J. Mater. Sci 2015, 50 (16), 5458–5465. [Google Scholar]
- (72).Xiang S; Qian W; Li T; Wang Y; Chen M; Ma P; Dong W Hierarchical structural double network hydrogel with high strength, toughness, and good recoverability. New J. Chem 2017, 41 (23), 14397–14402. [Google Scholar]
- (73).Huang S; Zhao Z; Feng C; Mayes E; Yang J Nanocellulose reinforced P(AAm-co-AAc) hydrogels with improved mechanical properties and biocompatibility. Composites, Part A 2018, 112, 395–404. [Google Scholar]
- (74).Means AK; Ehrhardt DA; Whitney LV; Grunlan MA Thermoresponsive double network hydrogels with exceptional compressive mechanical properties. Macromol. Rapid Commun 2017, 38 (20), 1700351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Means AK; Shrode CA; Whitney LV; Ehrhardt DA; Grunlan MA Double network hydrogels that mimic the modulus, strength and lubricity of cartilage. Biomacromolecules 2019, 20 (5), 2034–2042. [DOI] [PubMed] [Google Scholar]
- (76).Fei R; George JT; Park J; Means AK; Grunlan MA Ultra-strong thermoresponsive double network hydrogels. Soft Matter 2013, 9 (10), 2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Wang L; Shan G; Pan P A strong and tough interpenetrating network hydrogel with ultrahigh compression resistance. Soft Matter 2014, 10 (21), 3850–3856. [DOI] [PubMed] [Google Scholar]
- (78).Chen P; Wu R; Wang J; Liu Y; Ding C; Xu S One-pot preparation of ultrastrong double network hydrogels. J. Polym. Res 2012, 19 (3), 1–4. [Google Scholar]
- (79).Gong JP Why are double network hydrogels so tough? Soft Matter 2010, 6 (12), 2583–2590. [Google Scholar]
- (80).Panteli PA; Patrickios CS Complex hydrogels based on multiply interpenetrated polymer networks: Enhancement of mechanical properties via network multiplicity and monomer concentration. Macromolecules 2018, 51 (19), 7533–7545. [Google Scholar]
- (81).Shams Es-haghi S; Weiss RA Fabrication of Tough Hydrogels from Chemically Cross-Linked Multiple Neutral Networks. Macromolecules 2016, 49 (23), 8980–8987. [Google Scholar]
- (82).Groll J Mechanically strong microstructured hydrogels based on reactive star-shaped prepolymers. Macromol Symp. 2015, 358 (1), 148–156. [Google Scholar]
- (83).Sun Y; Liu S; Du G; Gao G; Fu J Multi-responsive and tough hydrogels based on triblock copolymer micelles as multi-functional macro-crosslinkers. Chem. Commun 2015, 51 (40), 8512–8515. [DOI] [PubMed] [Google Scholar]
- (84).Wang H; Li P; Xu K; Tan Y; Lu C; Li Y; Liang X; Wang P Synthesis and characterization of multi-sensitive microgel-based polyampholyte hydrogels with high mechanical strength. Colloid Polym. Sci 2016, 294 (2), 367–380. [Google Scholar]
- (85).Sakai T; Akagi Y; Matsunaga T; Kurakazu M; Chung U.-i.; Shibayama M Highly Elastic and Deformable Hydrogel Formed from Tetra-arm Polymers. Macromol. Rapid Commun 2010, 31 (22), 1954–1959. [DOI] [PubMed] [Google Scholar]
- (86).Haraguchi K; Takehisa T Nanocomposite hydrogels: A unique organic-inorganic network structure with extraordinary mechanical, optical, and swelling/de-swelling properties. Adv. Mater 2002, 14 (16), 1120–1124. [Google Scholar]
- (87).Gao G; Du G; Cheng Y; Fu J Tough nanocomposite double network hydrogels reinforced with clay nanorods through covalent bonding and reversible chain adsorption. J. Mater. Chem. B 2014, 2 (11), 1539–1548. [DOI] [PubMed] [Google Scholar]
- (88).Rose S; Dizeux A; Narita T; Hourdet D; Marcellan A Time dependence of dissipative and recovery processes in nanohybrid hydrogels. Macromolecules 2013, 46 (10), 4095–4104. [Google Scholar]
- (89).Lin W-C; Fan W; Marcellan A; Hourdet D; Creton C Large strain and fracture properties of poly(dimethylacrylamide)/silica hybrid hydrogels. Macromolecules 2010, 43 (5), 2554–2563. [Google Scholar]
- (90).Wang Q; Hou R; Cheng Y; Fu J Super-tough double-network hydrogels reinforced by covalently compositing with silica-nanoparticles. Soft Matter 2012, 8 (22), 6048–6056. [Google Scholar]
- (91).Bilici C; Can V; Nochel U; Behl M; Lendlein A; Okay O Melt-processable shape-memory hydrogels with self-healing ability of high mechanical strength. Macromolecules 2016, 49 (19), 7442–7449. [Google Scholar]
- (92).Li J; Suo Z; Vlassak JJ Stiff, strong, and tough hydrogels with good chemical stability. J. Mater. Chem. B 2014, 2 (39), 6708–6713. [DOI] [PubMed] [Google Scholar]
- (93).Sun Y; Xiang N; Jiang X; Hou L Preparation of high tough poly(vinyl alcohol) hydrogel by soaking in NaCl aqueous solution. Mater. Lett 2017, 194, 34–37. [Google Scholar]
- (94).Chen L; Honma Y; Mizutani T; Liaw DJ; Gong JP; Osada Y Effects of polyelectrolyte complexation on the UCST of zwitterionic polymer. Polymer 2000, 41 (1), 141–147. [Google Scholar]
- (95).Haque MA; Kurokawa T; Kamita G; Gong JP Lamellar bilayers as reversible sacrificial bonds to toughen hydrogel: Hysteresis, self-recovery, fatigue resistance, and crack blunting. Macromolecules 2011, 44 (22), 8916–8924. [Google Scholar]
- (96).Eslami M; Vrana NE; Zorlutuna P; Sant S; Jung S; Masoumi N; Khavari-Nejad RA; Javadi G; Khademhosseini A Fiber-reinforced hydrogel scaffolds for heart valve tissue engineering. J. Biomater. Appl 2014, 29 (3), 399–410. [DOI] [PubMed] [Google Scholar]
- (97).Coburn J; Gibson M; Bandalini PA; Laird C; Mao H-Q; Moroni L; Seliktar D; Elisseeff J Biomimetics of the extracellular matrix: An integrated three-dimensional fiber-hydrogel composite for cartilage tissue engineering. Smart Struct Syst 2011, 7 (3), 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Agrawal A; Rahbar N; Calvert PD Strong fiber-reinforced hydrogel. Acta Biomater. 2013, 9 (2), 5313–5318. [DOI] [PubMed] [Google Scholar]
- (99).Lin S; Cao C; Wang Q; Gonzalez M; Dolbow JE; Zhao X Design of stiff, tough and stretchy hydrogel composites via nanoscale hybrid crosslinking and macroscale fiber reinforcement. Soft Matter 2014, 10 (38), 7519–7527. [DOI] [PubMed] [Google Scholar]
- (100).Young C-D; Wu J-R; Tsou T-L High-strength, ultra-thin and fiber-reinforced pHEMA artificial skin. Biomaterials 1998, 19 (19), 1745–1752. [DOI] [PubMed] [Google Scholar]
- (101).Yang X; Abe K; Biswas SK; Yano H Extremely stiff and strong nanocomposite hydrogels with stretchable cellulose nanofiber/poly(vinyl alcohol) networks. Cellulose 2018, 25, 6571–6580. [Google Scholar]
- (102).Hagiwara Y; Putra A; Kakugo A; Furukawa H; Gong JP Ligament-like tough double-network hydrogel based on bacterial cellulose. Cellulose 2010, 17 (1), 93–101. [Google Scholar]
- (103).Nakayama A; Kakugo A; Gong JP; Osada Y; Takai M; Erata T; Kawano S High mechanical strength double-network hydrogel with bacterial cellulose. Adv. Funct. Mater 2004, 14 (11), 1124–1128. [Google Scholar]
- (104).Zhang HJ; Sun TL; Zhang AK; Ikura Y; Nakajima T; Nonoyama T; Kurokawa T; Ito O; Ishitobi H; Gong JP Tough physical double-network hydrogels based on amphiphilic triblock copolymers. Adv. Mater 2016, 28 (24), 4884–4890. [DOI] [PubMed] [Google Scholar]
- (105).Xu Z; Li J; Gao G; Wang Z; Cong Y; Chen J; Yin J; Nie L; Fu J Tough and self-recoverable hydrogels crosslinked by triblock copolymer micelles and Fe3+ coordination. J. Polym. Sci., Part B: Polym. Phys 2018, 56 (11), 865–876. [Google Scholar]
- (106).Li H; Wang H; Zhang D; Xu Z; Liu W A highly tough and stiff supramolecular polymer double network hydrogel. Polymer 2018, 153, 193–200. [Google Scholar]
- (107).Muniz EC; Geuskens G Compressive elastic modulus of polyacrylamide hydrogels and semi-IPNs with poly(N-isopropylacry-lamide). Macromolecules 2001, 34 (13), 4480–4484. [Google Scholar]
- (108).Moschou EA; Madou MJ; Bachas LG; Daunert S Voltage-switchable artificial muscles actuating at near neutral pH. Sens. Actuators, B 2006, 115 (1), 379–383. [Google Scholar]
- (109).Jiao C; Chen Y; Liu T; Peng X; Zhao Y; Zhang J; Wu Y; Wang H Rigid and strong thermoresponsive shape memory hydrogels transformed from poly(vinylpyrrolidone-co-acryloxy aceto-phenone) organogels. ACS Appl. Mater. Interfaces 2018, 10 (38), 32707–32716. [DOI] [PubMed] [Google Scholar]
- (110).Liu S; Gao G; Xiao Y; Fu J Tough and responsive oppositely charged nanocomposite hydrogels for use as bilayer actuators assembled through interfacial electrostatic attraction. J. Mater. Chem. B 2016, 4 (19), 3239–3246. [DOI] [PubMed] [Google Scholar]
- (111).Chen Y; Kang S; Yu J; Wang Y; Zhu J; Hu Z Tough robust dual responsive nanocomposite hydrogel as controlled drug delivery carrier of asprin. J. Mech Behav Biomed Mater 2019, 92, 179–187. [DOI] [PubMed] [Google Scholar]
- (112).Tang J; Qiao Y; Chu Y; Tong Z; Zhou Y; Zhang W; Xie S; Hu J; Wang T Magnetic double-network hydrogels for tissue hyperthermia and drug release. J. Mater. Chem. B 2019, 7, 1311. [DOI] [PubMed] [Google Scholar]
- (113).Wang N; Li Y; Zhang Y; Liao Y; Liu W High-strength photoresponsive hydrogels enable surface-mediated gene delivery and light-induced reversible cell adhesion/detachment. Langmuir 2014, 30 (39), 11823–11832. [DOI] [PubMed] [Google Scholar]
- (114).Zhang H; Sun L; Yang B; Zhang Y; Zhu S A thermoresponsive dual-crosslinked hydrogel with ultrahigh mechanical strength. RSC Adv. 2016, 6 (68), 63848–63854. [Google Scholar]
- (115).Zhao J; Jiao K; Yang J; He C; Wang H Mechanically strong and thermosensitive macromolecular microsphere composite poly(N-isopropylacrylamide) hydrogels. Polymer 2013, 54 (6), 1596–1602. [Google Scholar]
- (116).Sun Y.-n.; Gao G.-r.; Du G.-l.; Cheng Y.-j.; Fu J Super tough, ultrastretchable, and thermoresponsive hydrogels with functionalized triblock copolymer micelles as macro-cross-linkers. ACS Macro Lett. 2014, 3 (5), 496–500. [DOI] [PubMed] [Google Scholar]
- (117).Kamata H; Akagi Y; Kayasuga-Kariya Y; Chung U.-i.; Sakai T Nonswellable” Hydrogel Without Mechanical Hysteresis. Science 2014, 343 (6173), 873–875. [DOI] [PubMed] [Google Scholar]
- (118).Shin MK; Spinks GM; Shin SR; Kim SI; Kim SJ Nanocomposite hydrogel with high toughness for bioactuators. Adv. Mater 2009, 21 (17), 1712–1715. [Google Scholar]
- (119).Gao H; Wang N; Hu X; Nan W; Han Y; Liu W Double hydrogen-bonding pH-sensitive hydrogels retaining high-strengths over a wide pH range. Macromol Rapid Commun. 2013, 34 (1), 63–68. [DOI] [PubMed] [Google Scholar]
- (120).Naficy S; Razal JM; Whitten PG; Wallace GG; Spinks GM A pH-sensitive, strong double-network hydrogel: Poly(ethylene glycol) methyl ether methacrylates-poly(acrylic acid). J. Polym. Sci., Part B: Polym. Phys 2012, 50 (6), 423–430. [Google Scholar]
- (121).Giammanco GE; Carrion B; Coleman RM; Ostrowski AD Photoresponsive polysaccharide-based hydrogels with tunable mechanical properties for cartilage tissue engineering. ACS Appl. Mater. Interfaces 2016, 8 (23), 14423–14429. [DOI] [PubMed] [Google Scholar]
- (122).Li Z; Shen J; Ma H; Lu X; Shi M; Li N; Ye M Preparation and characterization of pH- and temperature-responsive nanocomposite double network hydrogels. Mater. Sci. Eng. C 2013, 33 (4), 1951–1957. [DOI] [PubMed] [Google Scholar]
- (123).Fei R; George JT; Park J; Grunlan MA Thermoresponsive nanocomposite double network hydrogels. Soft Matter 2012, 8 (2), 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Xu K; Tan Y; Chen Q; An H; Li W; Dong L; Wang P A novel multi-responsive polyampholyte composite hydrogel with excellent mechanical strength and rapid shrinking rate. J. Colloid Interface Sci 2010, 345 (2), 360–368. [DOI] [PubMed] [Google Scholar]


