Abstract
Artemisia sp., especially A. annua and A. afra, have been used for centuries to treat many ailments. While artemisinin is the main therapeutically active component, emerging evidence demonstrates that the other phytochemicals in this genus are also therapeutically active. Those compounds include flavonoids, other terpenes, coumarins, and phenolic acids. Artemisia sp. phytochemicals also improve bioavailability of artemisinin and synergistically improve artemisinin therapeutic efficacy, especially when delivered as dried leaf Artemisia as a tea infusion or as powdered dry leaves in a capsule or compressed into a tablet. Here results from in vitro, and in vivo animal and human studies are summarized and critically discussed for mainly malaria, but also other diseases susceptible to artemisinin and Artemisia sp. including schistosomiasis, leishmaniasis, and trypanosomiasis.
Keywords: Artemisia annua and Artemisia afra, Artemisinin resistance, Bioavailability, Leishmaniasis, Malaria, Schistosomiasis, Trypanosomiasis
Introduction
For centuries Artemisia annua L. and Artemisia afra Jacq. ex Willd. were used as medicinal herb infusions or powders to treat fever/malaria and worms (Liu et al. 2010; FAO 1986; van der Kooy and Sullivan 2013; Weathers et al. 2014b; Willcox et al. 2004). Both are commonly called “wormwoods”, and showed in vitro efficacy against Plasmodium falciparum (Gathirwa et al. 2008; Kraft et al. 2003; Liu et al. 2010; Moyo et al. 2016). Both species have long traditions in their respective ethnopharmacopeia of southeast Asia (A. annua) and southern Africa (A. afra). While A. annua cultivars have been cultivated and selected for their high artemisinin content, A. afra is nearly devoid of this potent sesquiterpene lactone (van der Kooy and Sullivan 2013), having at most 0.05 mg/g DW (Munyangi et al. 2018, 2019) and, thus, this species also functions as a control herb for studies using artemisinin-rich A. annua.
A preliminary study suggested that ingestion of capsules containing leaf powder of either A. afra or A. annua were both effective in eliminating malaria parasites from patients (Tchandema and Lutgen 2016). Based on those encouraging results, two large-scale studies compared the efficacy of Artemisia annua and Artemisia afra tea infusions against the standard drugs for malaria and schistosomiasis. Current treatment for malaria is artemisinin combination therapy (ACT), and for schistosomiasis, praziquantel (PZQ). The ACTs contain usually either the artemisinin derivative, artemether or artesunate, and a co-drug such as lumefantrine or amodiaquine. Two common ACTs are artemether-lumefantrine (AL) and artesunate-amodiaquine (ASAQ). ACTs are used to minimize emergence of artemisinin drug resistance (WHO 2014).
Doing human clinical trials using dried herb material is challenging. A good study should be double-blinded and include placebo and, if available, the current best therapy. Following recommendations of the WHO (2000), establishing a placebo for herbal clinical trials involved using very dilute Artemisia tea infusion and glucose tablets similar in appearance to the standard ACT or PZQ drugs. Thus, patients in both trials were treated with either a high dose tea infusion (5 g leaves + twigs/L) or a very low dose (0.2 g leaves + twigs/L, considered an appropriate amount for a tea infusion placebo), and each trial arm received placebo sugar tablets. All treatments included pre-weighed dried leaves + twigs, and tablets were placed in labeled envelopes prepared for each of the 7 days of treatment. All patients and investigators were double-blinded to the delivered treatments (Cornet-Vernet et al. 2019a, b; Munyangi et al. 2018, 2019).
Here summarized are the results of two superiority clinical trials conducted in 2015 in the Kalima district, Democratic Republic of Congo, comparing A. annua and A. afra tea infusions against the current standard drugs for treating malaria and schistosomiasis. Preliminary results for treating leishmaniasis and trypanosomiasis are also reviewed. In an effort to provide a scientific foundation for empirical in vivo observations, information about the mechanism of action of these complex herbal remedies follows.
Artemisia annua and Artemisia afra efficacy in clinical trials
Malaria
A double-blind malaria trial (Munyangi et al. 2019) was instigated following a number of smaller trials by others (summarized in Weathers et al. 2014a; Tchandema and Lutgen 2016) and had three treatment arms: 472 for ASAQ, 248 for A. annua, and 223 for A. afra. Figure 1 shows the mix of leaves and twigs used in the study; in the A. annua samples, the large white pieces are from the large thick stems characteristic of this species at harvest and that were chopped along with leaves to prepare the dried material for infusion. Artemisinin content of the A. afra and A. annua material was 0.036 and 1.34–1.70 mg/g DW, respectively. Patients receiving either Artemisia sp. drank 1 L/day of an Artemisia infusion, divided into 3, 330 mL aliquots per day (TID) for 7 days. Infusion was 5 g of dried plant material added to 1 L boiling water (0.2 g for placebo infusion), steeped for 10 min, and then strained through a sterilized 1 mm mesh to retain large particulates. Patients receiving Artemisia also received the artesunate-amodiaquine (ASAQ) sugar tablet placebo for all 7 days. Patients receiving ASAQ (Winthrop, Sanofi-Aventis) followed manufacturer’s posology of 4 mg of artesunate-10 mg of amodiaquine/kg, once daily for 3 days, followed by ASAQ placebo tablets for the remaining 4 days to follow the 7 day treatment equal to that of Artemisia sp. ASAQ patients also received dilute (0.2 g/L) Artemisia tea infusion all 7 days to parallel the therapeutic Artemisia tea infusion treatments. All patients had regular follow-up to 28 days. Trophozoites and gametocytes in blood samples were micro-scopically counted over 28 days. Side effects were documented.
Fig. 1.

A. annua (left) and A. afra (right) leaves and twigs as prepared for therapeutic tea infusions
Despite the negligible artemisinin content of A. afra, its therapeutic response was similar to patients treated with A. annua; after 24 h, trophozoites cleared, but took up to 14 days to clear in ASAQ-treated patients. Day 28 cure rates (no detectable parasitemia) overall are shown in Table 1; for adults cure rates were 91, 100, and 30% for A. afra, A. annua and ASAQ, respectively (Table 1), with 82, 91, and 50% for pediatrics. Fever clearance took 24 h for Artemisia, but 48 h for ASAQ. Although from days 14 to 28 there were no detectable gametocytes in Artemisia-treated patients, gametocytes remained in 10 ASAQ-treated patients at day 28, suggesting that both Artemisia sp. could break the cycle of malaria by inhibiting parasite transmission back to the mosquito vector. Eightfold more ASAQ patients reported adverse effects than Artemisia-treated patients, so Artemisia was more benign than ASAQ for sick patients. Although compelling, this trial recently received criticism from the scientific and medical establishment (Danis and Buisson 2019; Gillibert et al. 2019) to which a published rebuttal was made (Cornet-Vernet et al. 2019b). This trial corroborates the results of earlier tea trials (summarized in Weathers et al. 2014a) and also a set of recently published case studies where compassionate administration of dried A. annua leaf tablets successfully saved the lives of 18 patients with severe malaria who did not respond to therapy with ACT (artemether-lumefantrine) and i.v. artesunate (Daddy et al. 2017).
Table 1.
Comparative antimalarial results among A. annua, A. afra and ASAQ
| Treatment | Average parasite clearance (day) | Artemisinin dose (mg/day) | Total artemisinin delivered in 7 days (mg) | % Curec | % Gametocyte carriage at day 28d |
|---|---|---|---|---|---|
| A. annua | 1 | 6.7–8.5b | 33.5–59.5b | 96.4 | 0 |
| A. afra | 1 | 0.18 | 1.26 | 88.8 | 0 |
| ASAQa | ≤ 14 | 80–696 | 240–2088 | 34.3 | 2 |
Dose was 4 mg artesunate/kg per manufacturer posology
There were two different cultivars of A. annua used with different levels of artemisinin in the trial at each of the five test sites
Based on parasitemia in the blood
Based on microscopic analysis
Schistosomiasis
Schistosomiasis, also known as bilharzia, affects millions globally with few cost-effective treatments (WHO 2018b). It is caused by members of the Schistosome family, e.g. Schistosoma mansoni. Based on an informal observation by Sister Elke Stienacher in Degana, Senegal, people cured of their malaria by taking Artemisia tea also seemed cured of their bilharzia when they were co-infected. Malaria and schistosomiasis co-infection is common (Keiser and Utzinger 2012; Moriyasu et al. 2018), so in 2015 A. annua and A. afra tea infusions were compared to PZQ treatment in 800 patients in the Democratic Republic of Congo. There were three treatment arms in this double-blind trial: 400 for PZQ, 200 for A. annua, and 200 for A. afra; 780 completed the trial (Munyangi et al. 2018). The source of both Artemisia sp. was identical to that used for the above malaria trial. Patients receiving either Artemisia sp. drank 1 L/day of an Artemisia infusion, prepared and administered as described for the malaria trial. Patients receiving Artemisia also received sugar tablet placebo replacing PZQ for all 7 days. Patients receiving PZQ (Bayer) followed manufacturer’s posology, receiving PZQ tablets (60 mg/kg daily) for three consecutive days, and PZQ placebo (sugar tablets) for four more days to follow the 7-day treatment equal to that of the Artemisia sp. PZQ patients also received dilute (0.2 g/L) Artemisia tea infusion all 7 days to parallel the therapeutic Artemisia tea infusion treatments. All patients had 28-day follow-up. All drugs were administered orally under investigator supervision who were blinded to identity of drug delivered via use of sealed envelopes. Side effects were documented every time patients visited the clinic to receive treatment.
Patients enrolled in the trial had an average of > 700 S. mansoni eggs per fecal sample on entry. At 14 days post treatment, there were no detectable eggs in fecal smears in any Artemisia-treated patients. In contrast it took > 21 days for eggs to fully disappear in all PZQ patients. There were significantly fewer adverse effects in the Artemisia-treated patients for both pediatrics and adults. Patients treated with either Artemisia sp. reported 95% fewer adverse events compared to those treated with PZQ, 69 versus 1411 events, respectively (Munyangi et al. 2018). As with the malaria trial, the schistosomiasis trial also received a published critique (Argemi et al. 2019) to which a full rebuttal was published (Cornet-Vernet et al. 2019a).
Leishmaniasis
Other diseases are also showing positive clinical responses to Artemisia leaf powders. Leishmaniasis is a neglected tropical disease endemic in 98 countries on 5 continents caused by > 20 species of an intracellular protozoan parasite, Leishmania sp., transmitted by sandflies. There are three clinical forms of the disease: cutaneous, mucosal, and visceral. About 12 million people are affected with 350 million at risk of infection (WHO 2018a). Cutaneous leishmania is most prevalent, and while the standard treatments (including a pentavalent antimonial, miltefosine, pen-tamidine isethionate, and amphotericin B) are still effective, these drugs have serious side effects, so safer effective new drugs are needed (den Boer et al. 2011).
One study encompassed using powdered A. annua leaves in in vitro and in vivo (hamsters) systems, and with two human subjects (Mesa et al. 2017). The leaves had an in vitro EC50 of 48.07 μg/mL against amastigotes of L. (V) panamensis with a therapeutic index of 8.73. Amphotericin B had a therapeutic index of 625 and an EC50 of 0.06 μg/mL. In vivo results using 500 mg/kg/day in hamsters showed an 83.3% cure by 30 days. After these studies, two human males were treated with powdered encapsulated A. annua (voucher MNHNL17732 Herbarium Luxembourg) containing about 0.1% artemisinin (Mesa et al. 2017). Using Giemsa-stained smears and PCR analysis, both patients were validated as positive for L. (V) panamensis leishmaniasis. One patient had 4 lesions ranging from 0.90 to 20.25 cm2 and the other had a single lesion 20 cm2. Both patients were given A. annua powder as follows: from days 1–3, 3 g/day; days 4–7, 2 g/day; days 8–20, 1 g/day. Total A. annua delivered was 30 g per patient over 20 days. By end of treatment, ulcers had shrunk 20–35% with complete closure of the ulcer 45 days after end of treatment. Patient ulcers remained healed 2 years post-treatment (Mesa et al. 2017). During and after treatment neither patient experienced any adverse reactions, suggesting that Artemisia leaf powder also could prove useful in treating leishmaniasis. Clearly larger human trials are required.
Trypanosomiasis
American trypanosomiasis (Trypanosoma cruzi; Chagas disease) and African trypanosomiasis (T. brucei; sleeping sickness) are two other major neglected tropical diseases that are transmitted through the triatomine bug that defecates after biting thereby transmitting disease, or the bite of the tsetse fly, respectively. Symptomatic treatment against both infections is restricted to drugs with severe side effects, mammalian toxicity, and limited efficacy, so better therapies are urgently needed (Naß and Efferth 2018).
Therapeutic herbal treatments are a possible alternative. In vitro and in vivo animal studies showed extracts (Efferth et al. 2011) or infusions (Berrizbeitia de Morgado et al. 2017; Naß and Efferth 2018) of A. annua to be effective in inhibiting parasite growth in both diseases. While there are to our knowledge no major human trials, A. annua infusions exerted dose-dependent antiproliferative effects on the T. cruzi epimastigotes, and results depended on the concentration of the infusions and time of exposure (Naß and Efferth 2018).
Other Artemisia species besides A. annua also have inhibitory activity towards trypanosomes (Naß and Efferth 2018). These species included, A. absinthium, A. abyssinica, A. afra, A. douglasia, A. elegantissima, A. maciverae, A. mexicana, and A. roxburghiana, which were active against T. brucei, T. cruzi, and T. congolense. This field of research is, however, still in its infancy and more research on pharmacokinetics, bioavailability, and synergism among A. annua phytochemicals and with current commercial drugs is needed to explore the full potential of diverse Artemisia species and their phytochemicals for erad-ication of trypanosomal infections. Given the previously described efficacy and safety of A. annua tea infusions against malaria, schistosomiasis and leish-maniaisis, human trials are also warranted.
Artemisia annua as prophylaxis?
Ogwang et al. (2011, 2012) documented 80% reduction in malaria cases among Ugandan adults who drank a weekly dose of A. annua tea. Those individuals previously had malaria. Neither this review nor any of the authors’ prior studies recommend or suggests prophylactic use of A. annua to thwart malaria. Indeed, there were two case studies where French travelers, with no medical history of malaria, used A. annua prophylactically to avoid malaria. They returned with active and severe cases of malaria (Lagarce et al. 2016). We urge caution, especially for those who are medically naïve to malaria.
What in these Artemisia sp. is therapeutically effective?
Most studies focused on antimalarial activity. Many other compounds found in A. annua besides artemisinin have therapeutic efficacy against P. falciparum, and some also improved the IC50 when used in conjunction with artemisinin, indicating a synergistic action (Liu et al. 1992; Suberu et al. 2013). Table 2 lists a number of phytochemicals along with their IC50 values alone and in combination with artemisinin, if known, against various P. falciparum strains. Flavonoids in particular have shown importance with respect to efficacy of whole-plant treatment regimens (dried leaves or tea infusion, for example). Since flavonoids can persist in the body for more than 5 days, they could be helpful in promoting prophylactic antimalarial action (Ogwang et al. 2011, 2012 and Table 2).
Table 2.
Phytochemicals in A. annua with antimalarial activity
| Compound | Compound IC50 (μM) | Compound + artemisinin IC50 (μM) | References |
|---|---|---|---|
| Artemisinin | 0.033 | na | Liu et al. (1992) |
| 0.022, 0.023a | na | Suberu et al. (2013) | |
| Artemisinic acid | 77.8, 61.6a | Varies with compound concentration | |
| Arteannuin B | 3.2, 4.8a | ||
| Dihydroartemisinic acid | 21.1, 17.7a | ||
| Chlorogenic acid | 69.4, 61.4a | ||
| Rosmarinic acid | 65.1, 65.0a | ||
| Isovitexin | 72.5, 48.1a | ||
| Artemetin | 26.0 | 0.026 | Liu et al. (1992) |
| Casticin | 24.0 | 0.026 | |
| Cirsilineol | 23.0 | 0.023 | |
| Chrysoplenol-d | 32.0 | 0.015 | |
| Chrysoplenetin | 36.0 | 0.016 | |
| Eupatorin | 65.0 | 0.030 | |
| Apigenin | 20.0, 13.0b | Not determined | Lehane and Saliba (2008) |
| Luteolin | 11.0, 12.0b | ||
| Kaempferol | 33.0, 25.0b | ||
| Myricetin | 40.0, 76.0b | ||
| Quercetin | 15.0, 14.0b | ||
| 14.7, 4.1, 2.9c | Ganesh et al. (2012) | ||
| 13.0d | Penna-Coutinho et al. (2018)i | ||
| Rutin | 7.1, 3.5, 10.4c | Ganesh et al. (2012) | |
| a-Pinene | 1.0e | Van Zyl et al. (2006) | |
| 1,8-Cineole (eucalyptol) | 70.0e | ||
| Limonene | 533.0e | ||
| Nerolidol | 9.0e | ||
| 0.76f | Rodriguez-Rodrigues-Goulart et al. (2004) | ||
| Phytol | 18.9g | Grace et al. (2012) | |
| Scopoletin | 128.0, 121.5h | Zaid et al. (2016) |
Na not applicable, CQ chloroquine
CQ-sensitive HB3 and CQ-resistant Dd2 strains, respectively
CQ-sensitive 3D7 and CQ-resistant 7G8 strains, respectively
Fresh blood isolates (Bangladesh), CQ-sensitive 3D7, and CQ-resistant K1 strains, respectively
CQ-resistant W2 strain
CQ-resistant FCR-3 strain
CQ-sensitive 3D7
CQ-sensitive D10 strain
CQ-resistant K1 and CQ-sensitive 3D7 strains, respectively
Test in vitro and also in vivo in mice
Artemisinin still seems to be the main antimalarial constituent in A. annua as shown recently by Czechowski et al. (2019). Artemisinin-null mutants that still had high flavonoid content were not as effective as the wild type against in vitro P. falciparum. Although compelling, their results contrasted with the previously discussed in vivo animal and human clinical results. They also seem to contradict the A. afra clinical results (Munyangi et al. 2019) that showed near equivalency with A. annua. Using the Rath et al. (2004) pharmacokinetic study of an A. annua tea infusion, however, we estimated that since a minimum of 9–10 μg artemisinin/L blood is needed to kill the parasite (Alin and Bjorkman 1994), there was not enough artemisinin at 51.6 μg/dose (TID) in A. afra to account for its reported efficacy. Clearly more studies are required of DLA and A. annua tea infusions to clarify in vitro and in vivo discrepancies.
Nevertheless, flavonoids have been shown to function as antimalarials by inhibiting various stages of the malaria growth cycle. While monoterpenes make up a large proportion of the essential oil content of A. annua (Desrosiers and Weathers 2018; Desrosiers et al. 2019), and some have been shown to have antimalarial potential (Table 2) or enhance the activity of other molecules, most are too volatile to persist after drying and processing the plant material for tablets (Weathers and Towler 2014). Like flavonoids, monoterpenes assist in treatment of malaria by arresting parasite development (Moura et al. 2001; Rodrigues-Goulart et al. 2004; Su et al. 2008; Weathers et al. 2017). Eucalyptol in particular also inhibits several proinflammatory cytokines (Juergens et al. 2004), and chlorogenic and rosmarinic acid exhibit similar effects in vitro (de Magalhães et al. 2012). For further details, see Weathers et al. (2017).
Table 3 shows artemisinin and total flavonoid contents of clonal A. annua (SAM, voucher MASS 00317314) over the past 7 years. While growth conditions and time of harvest affect artemisinin levels, the amount is relatively consistent at 1.28 ± 0.23% (w/w) and this clonal line propagated solely through rooted cuttings remains a high-artemisinin producer (average 1.28% DW), results consistent with Simonnet et al. 2009. Total flavonoid content is more sensitive to variations in processing of harvested leaves, but is still relatively predictable over time at 4.63 ± 0.90 mg/g DW (Table 3).
Table 3.
Artemisinin and total flavonoid content of clonal crops of A. annua
| Lab versus field/garden cultivation | Harvest date | Artemisinin (mg/g DW) | Total flavonoidsa (mg/g DW) |
|---|---|---|---|
| Lab 2011 | 4/2011 | 14.55 | 5.75 |
| Lab May 2011 | 5/2011 | 11.92 | 3.72 |
| Lab December 2011 | 12/2011 | 8.71 | 3.05 |
| Lab February 2012 | 2/2012 | 13.91 | 5.34 |
| Lab March 2012 | 3/2012 | 14.79 | 4.35 |
| Field 2012 | 7/2012 | 13.96 | 4.17 |
| Garden 2012 | 9/2012 | 11.96 | 4.16 |
| Lab 2012 | 12/2012 | 15.78 | 5.44 |
| Lab 2013 | 1/2013 | 14.19 | 5.89 |
| Lab July 2013 | 7/2013 | 15.13 | 4.82 |
| Field 2013 | 9/2013 | 11.23 | 3.02 |
| Garden 2013 | 9/2013 | 13.70 | 4.82 |
| Lab 2014 | 6/2014 | 9.44 | 3.59 |
| Garden 2015 | 9/2015 | 8.33 | 5.24 |
| Garden 2016 | 9/2016 | 10.80 | na |
| Garden 2017 | 9/2017 | 15.12 | 5.34 |
| Garden 2018 | 9/2018 | 13.46 | 5.39 |
| Average over 7 years | 12.76 ± 2.28 | 4.63 ± 0.90 |
na not available
Quercetin equivalents
After harvesting and drying Artemisia leaves, the stability of the non-artemisinin phytochemicals is important if the leaves are to have future therapeutic efficacy. Similarly, content stability should be verified after processing steps. Many of the essential oils (EOs) in Artemisia sp. are therapeutically active against malaria, albeit with a lower efficacy than artemisinin (Table 2). Although artemisinin is very stable over many years in harvested leaves (Simonnet et al. 2009), many of the EOs are volatile and decline with processing (Weathers and Towler 2014) and long-term storage. Total flavonoid content, however, of harvested dried A. annua was quite stable for at least 4.5 years (Fig. 2).
Fig. 2.
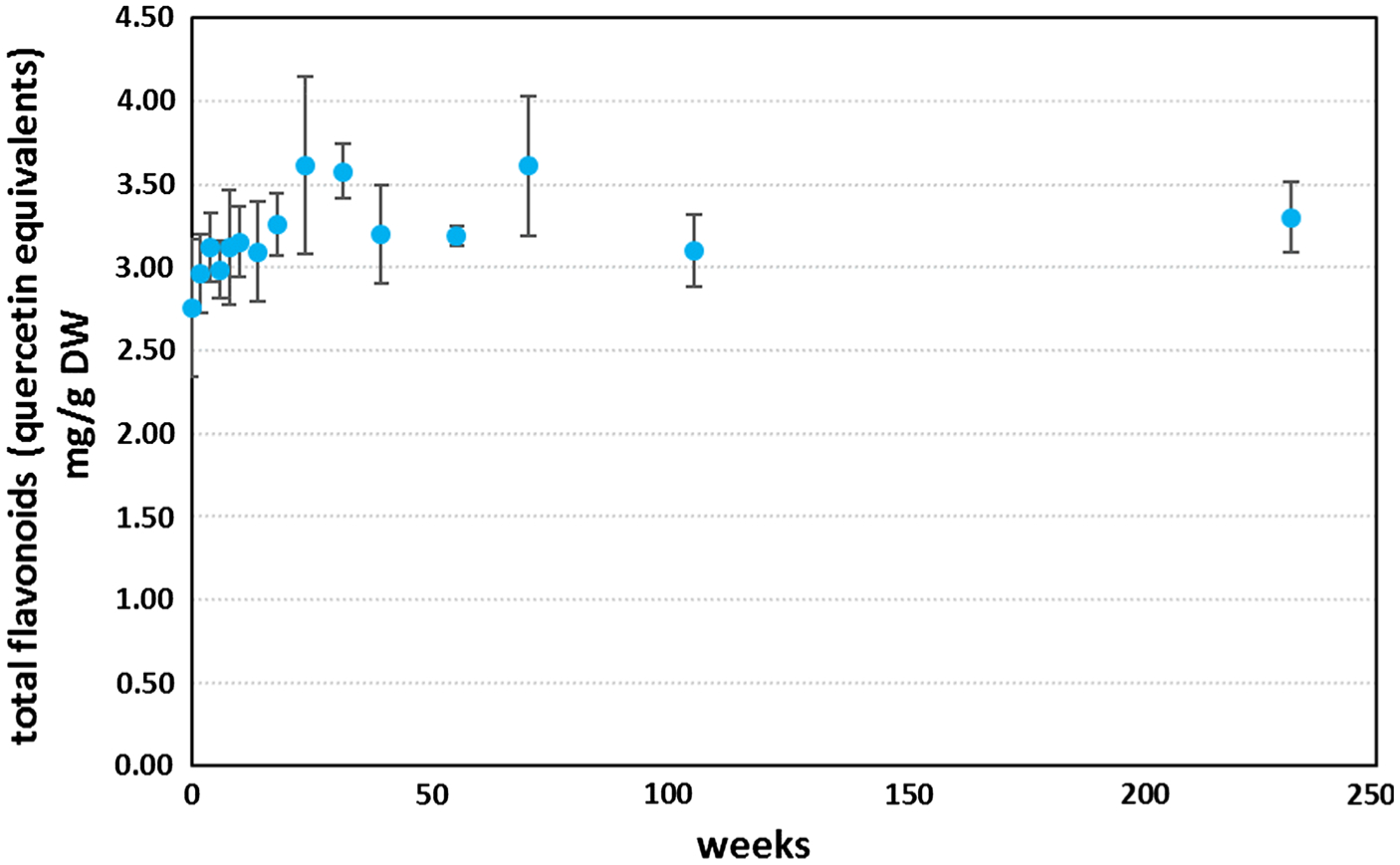
Long-term stability of total flavonoid content of dried A. annua leaves kept in a sealed clear plastic bag at room temperature under dim light for nearly 4.5 years
How does Artemisia annua work?
Bioavailability
Artemisinin, as a pure drug, has notoriously low bioavailability owed to its low aqueous solubility (van der Kooy and Verpoorte 2011; Jessing et al. 2009; Titulaer et al. 1990) and drove development of artemisinic derivatives such as artesunate and artemether, which solubilize more readily in water and oil, respectively. These derivatives are now commonly used in ACTs in the worldwide fight to eradicate malaria. However, if artemisinin’s low bioavailability prevents its use as a pure drug to treat malarial infections, then how has A. annua been seemingly so effective at treating fever (associated with malaria) over the course of history? One likely reason is that artemisinin bioavailability is significantly increased when it is delivered in whole plant form as opposed to a purified singular drug (Weathers et al. 2011, 2014b).
In mice, to reach similar artemisinin levels in the blood, about 45 × more artemisinin had to be delivered as pure drug, compared to powdered A. annua leaves (dried leaf Artemisia, DLA) (Weathers et al. 2011). Furthermore, the pharmacokinetics of artemisinin in healthy and Plasmodium chabaudi-infected mice when given A. annua were significantly different (Weathers et al. 2014b). Artemisinin metabolism was significantly reduced in infected mice, indicating that the diseased state altered artemisinin pharmacology. The same study also confirmed higher bioavailability of artemisinin when delivered as plant material. Artemisinin delivered as pure drug at 100 mg/kg was undetectable in the serum at 1 h while artemisinin delivered as A. annua reached a concentration of 4.33 mg/L (Weathers et al. 2014b). Interestingly, when pure artemisinin at 100 mg/kg was delivered combined with mouse chow (~ 0.015 mg artemisinin/mg mouse chow, largely made up of plant material, including corn, soybean, beet, oats, molasses, alfalfa, and wheat), artemisinin was 2.44 mg/L in the serum at 1 h, demonstrating that the general plant matrix (i.e. phytochemicals, oils, fiber etc.) aided artemisinin absorption (Weathers et al. 2014b).
Using an in vitro digestion system in which pure artemisinin or DLA was digested through the intestinal stage, the resulting intestinal digestate liquid phase of the leaves contained about fourfold more solubilized artemisinin than from pure digested artemisinin. This was partially explained by the essential oils (EOs) in the plant. When digested with EOs extracted from A. annua, pure artemisinin was 250% more soluble (Desrosiers and Weathers 2016). These results were unsurprising as artemisinin is stored in the glandular trichomes of A. annua in solution with the volatile oils produced by the plant (Duke and Paul 1993; Ferreira and Janick 1995; Tellez et al. 1999). For a more complete review of the therapeutic effects of EOs from Artemisia species, see Desrosiers et al. (2019). TheEO enhanced solubility of artemisinin partially explains the greater bioavailability afforded by delivery as DLA than as a pure drug; artemisinin is better absorbed through the intestine when in solution.
Independent of solubility, artemisinin delivered as DLA crosses the intestinal wall about 37% more efficiently than pure artemisinin (Desrosiers and Weathers 2018). This enhanced bioavailability also impacts different organs. In rats, artemisinin delivered as DLA vs. as pure artemisinin distributes in higher concentrations to many internal organs (results as yet unpublished from Weathers lab). A combination of artemisinin, arteannuin B, arteannuic acid, and scopoletin, all common components of A. annua, also increased the pharmacological properties of artemisinin (Li et al. 2018). This combination increased the area under the curve (AUC0→∞), Cmax, and t1/2 of artemisinin by 3.78-, 3.47-, and 1.13-fold respectively in healthy mice, while these same values increased by 2.62-, 1.82-, 1.22-fold in Plasmodium yoelii-infected mice, respectively, confirming the earlier studies concluding that infection affects artemisinin pharmacokinetics (Weathers et al. 2014b). The next steps in understanding how DLA alters bioavailability of artemisinin will involve the role of the liver and how DLA and its diverse phytochemicals impact hepatic cytochrome P450 (CYP450) enzymes in the liver. CYP3A4 and CYP2B6 are the major artemisinin-metabolizing enzymes present in the liver (Svensson and Ashton 1999). It is posited that A. annua small molecule phytochemicals such as flavonoids, coumarins, and terpenes may inhibit one or both of these hepatic P450 s, thereby increasing the amount of artemisinin that passes into the bloodstream. For example, one flavonoid found in DLA, chrysoplenetin, inhibited CYP3A activity and enhanced bioavailability of artemisinin in vivo (Wei et al. 2015).
Therapeutic activity of DLA secondary compounds
While some secondary compounds in DLA enhance bioavailability of artemisinin, others have synergistic or additive effects against the Plasmodium parasite. For example, the same combination of secondary compounds shown by Li et al. (2018) to enhance artemisinin bioavailability also showed a 93% reduction in parasitemia compared to 31% reduction in mice given pure artemisinin, indicating that artemisinin is not the only important component in A. annua. Chrysoplenetin, when given in combination with artemisinin, reduced parasitemia of Plasmodium berghei-infected mice more effectively than artemisinin alone (Wei et al. 2015). Besides these two examples, several secondary compounds found in DLA have their own inherent antimalarial efficacy or synergize with artemisinin to enhance its antiplasmodial affect. A comprehensive review and table of these phytochemicals is available in Weathers et al. 2017 (Artemisinin the Nobel Molecule Book Chapter); see also a review by Ferreira et al. (2010). In addition to treating malaria, DLA was shown recently to be efficacious against schistosomiasis (Munyangi et al. 2018). Interestingly, A. annua, which contains artemisinin, but also A. afra with negligible artemisinin, cured all patients significantly faster than praziquantel, the current standard therapy (Munyangi et al. 2018). This strongly suggested that artemisinin was not the only therapeutic compound found in the Artemisia species and these plants should be further studied to discover more therapeutic compounds.
Anti-inflammatory properties of Artemisia
Inflammation is a response to many infectious diseases (He et al. 2015) including malaria (Clark et al. 2006; Erdman et al. 2008). Besides its powerful antimalarial role, artemisinin and several other phytochemicals produced by the Artemisia species have immunomodulatory effects. Artemisinin alone has been extensively studied for its potent anti-inflammatory activity (Kim et al. 2015; Shi et al. 2015; Wang et al. 2017; Zhu et al. 2012). This anti-inflammatory capacity is owed to artemisinin’s ability to inhibit the expression of pro-inflammatory cytokines such as TNF-α and IL-6 by blocking the NF-κB and MAPK signaling cascades (Fig. 3) (Wang et al. 2017; Zhu et al. 2012). Artemisinin is a well-studied anti-inflammatory agent and the Artemisia species produce numerous secondary phytochemicals with anti-inflammatory efficacy. For example, several flavonoids reduced inflammation in both in vitro and in vivo experiments. Two flavonoids produced by A. annua, casticin and chrysoplenol-D, reduced inflammatory cytokine production in vitro and in a mouse model of systemic inflammatory response syndrome by inhibiting NF-κB and MAPK signaling pathways (Li et al. 2015).
Fig. 3.
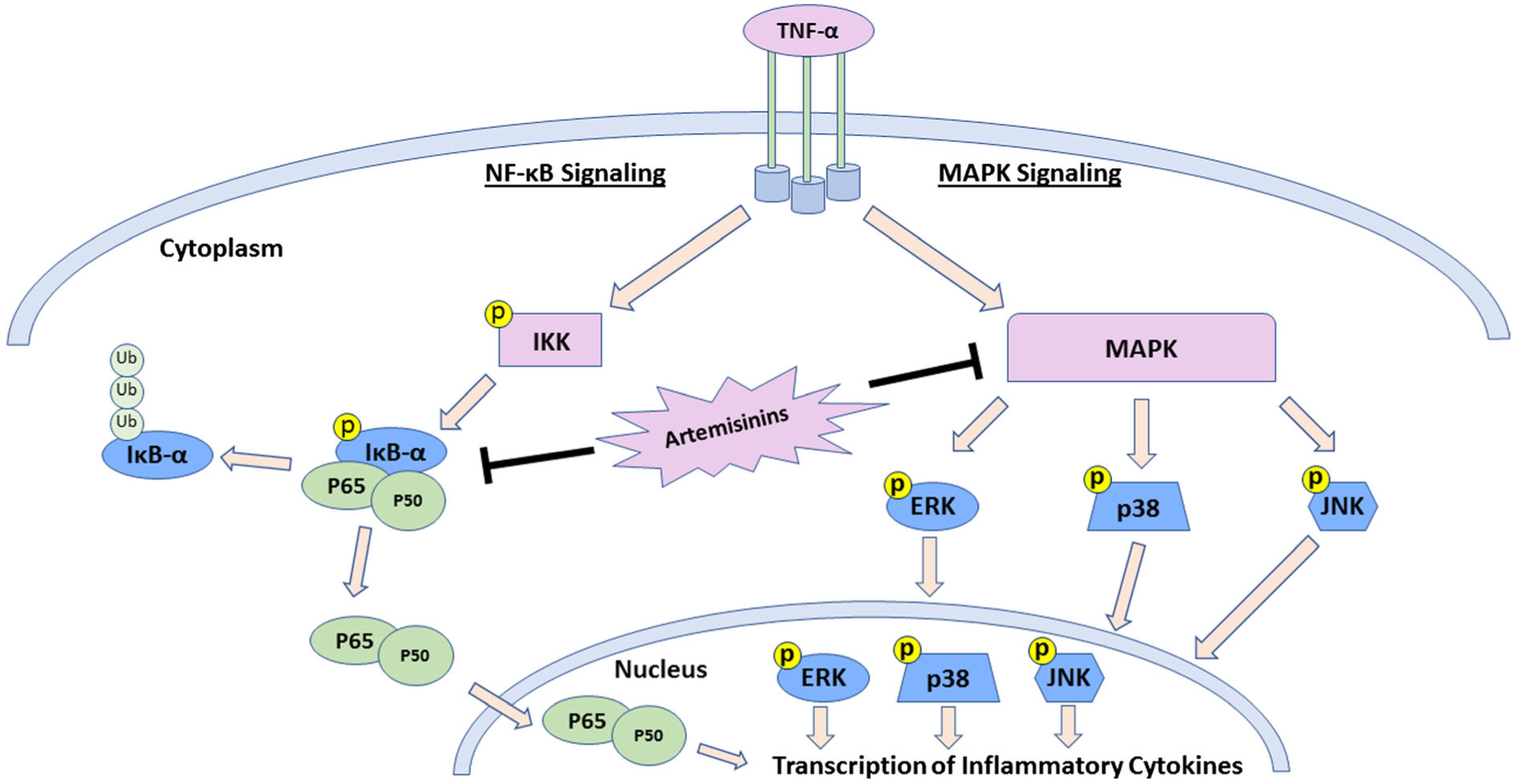
Artemisinin inhibition of inflammation through NF-κ B and MAPK signaling cascades. Abbreviations: TNF-α tumor necrosis factor alpha, NF-kB nuclear factor kappa-light-chain-enhancer of activated B cells, MAPK mitogen-activated protein kinases, IKK I kappa B kinase, IkB-alpha inhibitor of kappa B alpha, Ub ubiquitin, Yellow p’s indicate phosphorylation of protein, ERK extracellular signal-regulated kinases, JNK c-Jun N-terminal kinases
There are several good reviews that outline the anti-inflammatory effects and mechanisms of action of flavonoids from a variety of plant species, not just A. annua (Rathee et al. 2009; Serafini et al. 2010; Spagnuolo et al. 2018). Along with flavonoids, some monoterpenes produced by the Artemisia species have anti-inflammatory ability. As an example, 1,8-cineole (eucalyptol) inhibits the in vitro production of several pro-inflammatory cytokines including TNF-α, IL-1β, IL-4, IL-5, IL-6, and IL-8 (Juergens et al. 2004). A more comprehensive review of the effects of monoterpenes on inflammation is available (de Cássia da Silveira e Sá et al. 2013). Other phytochemicals found in Artemisia species with anti-inflammatory activities include rosmarinic acid, chlorogenic acid, and scopoletin (de Magalhães et al. 2012; Malik et al. 2011). Many Artemisia phytochemicals have anti-inflammatory activity, so extracts and teas have been tested for their overall anti-inflammatory activity. A. annua teas reduced IL-6 and IL-8 in activated Caco-2 cells (de Magalhães et al. 2012). Kim et al. (2015) showed extracts of A. annua reduced nitric oxide, prostaglandin E2, IL-1β, IL-6, and IL-10 compared to induced controls (Kim et al. 2015). In another study, leaf extracts made from A. argyi and A. princeps reduced inflammation in a mouse model of contact dermatitis and inhibited expression of inducible nitric oxide synthase (iNOS), nitric oxide (NO), cyclooxygenase-2 (COX-2), and prostaglandin E2 (PGE2) in vitro (Yun et al. 2016). To our knowledge, however, the anti-inflammatory efficacy of DLA from A. annua and A. afra has not yet been studied in vivo. In an as yet unpublished study, we recently showed in male rats that A. annua DLA treatment reduced production of TNF-α and IL-6 in comparison to control animals; pure artemisinin had no significant effect. Results were different in female rats. While DLA significantly reduced TNF-α production in female rats, so did pure artemisinin. Neither DLA nor pure artemisinin had an effect on IL-6 production, suggesting that gender plays a role in determining the efficacy of artemisinin and DLA on inflammation.
Will Artemisia sp. consumption induce artemisinin drug resistance?
Artemisinin drug resistance—current evidence
Artemisinin drug resistance was first observed in Southeast Asia as a prolonged parasite clearance half-time in patients (Dondorp et al. 2009). Artemisinin drug resistance likely was hastened by the conditions in Southeast Asia, including a susceptible subpopulation of migrant workers, an abundance of counterfeit medicines that amounted to insufficient and single-drug therapies, and a relatively high level of selective pressure from artemisinin monotherapy (Eastman and Fidock 2009; Packard 2014). A search for the physical mechanisms of activity soon followed. Early ring stages of resistant patient isolates and laboratory strains cultured under continuous artemisinin pressure enter a dormant state when under drug pressure (Witkowski et al. 2010, 2013). Eventually, Genome-Wide Association Studies identified a region of chromosome 13 as the region most closely associated with resistance in patient isolates (Cheeseman et al. 2012). Further studies revealed that single-nucleotide polymorphisms in the kelch13 gene were present in resistant isolates and were sufficient to induce artemisinin drug resistance in vitro (Ariey et al. 2014; Ménard et al. 2016; Straimer et al. 2015). Currently, in vitro artemisinin resistance is most commonly assessed using the Ring-Stage Survival Assay (RSA), wherein the degree of survival of a given isolate of P. falciparum is assessed by comparing parasite growth in a solvent control to growth following a brief, intense treatment with endoperoxides. Most commonly, this procedure uses 700 nM dihydroartemisinin for 6 h followed by 66 h of recovery and growth (Ariey et al. 2014; Mbengue et al. 2015; Rocamora et al. 2018; Straimer et al. 2015; Witkowski et al. 2013). Generally, these experiments find that mutant kelch13 strains have RSA survival rates greater than an order of magnitude higher than wild-type (Straimer et al. 2015).
The kelch13 protein appears to be an ubiquitin-ligase adaptor for the phosphatidylinositol-3-kinase protein (PI3 K). When mutated, kelch13 less effectively ubiquitinates PI3 K, reducing its rate of degradation that compensates for reduction of active PI3 K when the intracellular protein pool is targeted by artemisinins (Fig. 4) (Mbengue et al. 2015; Wang et al. 2015). The cellular synthesis of phosphatidylinositol-3-phosphate subsequently increases. Increased PI3P activates protein kinase B (Akt) in humans, reducing cell growth and division while increasing survival mechanisms such as autophagy and the unfolded protein response (UPR) (Blaustein et al. 2013). Evidence of PI3P-induced Akt activation in P. falciparum is not definitive. However, overexpression of Akt induces some artemisinin resistance in plasmodia, implicating it as a potential downstream effector of artemisinin resistance (Blaustein et al. 2013; Mbengue et al. 2015). Whether through Akt-dependent or-independent mechanisms, UPR and antioxidant pathways are upregulated in plasmodia placed under artesunate stress and are especially upregulated in clinical isolates of resistant P. falciparum (Mok et al. 2015; Natalang et al. 2008). However, transgenic upregulation of genes implicated in this P. falciparum stress response did not induce levels of artemisinin resistance high enough to account for the survival seen in kelch13 mutants (Rocamora et al. 2018). Instead, they suggest a marginal role in resistance for the UPR and ROS responses.
Fig. 4.
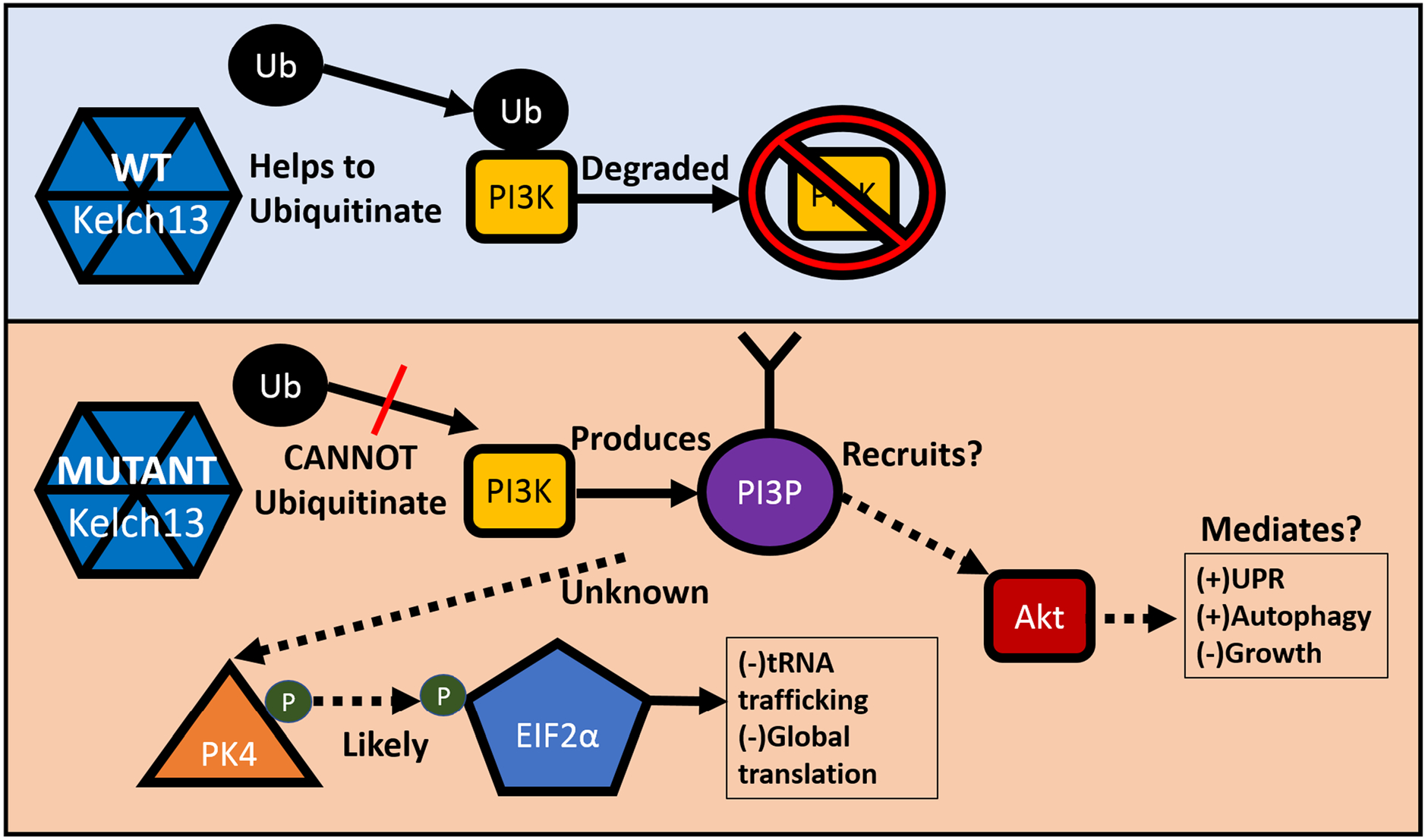
Proposed molecular mechanism of artemisinin resistance. Solid lines represent mechanisms with experimental evidence, while dashed lines represent postulated mechanisms. Abbreviations: PI3P phosphatidylinositol triphosphate, PI3 K phosphatidylinositol-3-kinase, Ub ubiquitin, UPR unfolded protein response
An alternate pathway that appears to be involved in artemisinin resistance is the PK4-eIF2α pathway. Under this model, the kelch13 mutant leads to a constitutive phosphorylation and deactivation of eIF2α in the early ring stage of the life cycle, likely by PK4 kinase. Because the primary function of eIF2α is tRNA trafficking, this leads to a global decrease in translation, thereby allowing the parasite to better maintain proteostasis under stress conditions (Zhang et al. 2017). This model better explains the ultimate downstream effects of kelch13 mutants, as phosphorylation status of eIF2α is affected by kelch13 mutation and pharmacological modulation of eIF2α phosphorylation appears to powerfully alter the rate of recrudescence in P. berghei-infected mice treated with artemisinins (Zhang et al. 2017). The intermediate effectors between kelch13 function and eIF2α phosphorylation have not been experimentally determined, but based on the homologous human pathway, the PK4-binding protein BiP has been conjectured to play a role (Paloque et al. 2016).
In humans, both the UPR/stress response pathways and the PK4-eIF2α pathways are interlinked, wherein the UPR ultimately enhances phosphorylation of eIF2α. However, in P. berghei-infected mice, kelch13-mutant plasmodia did not increase phosphorylation of eIF2α in the face of artemisinin stress, but instead maintained a steady moderate level of phosphorylation (Zhang et al. 2017). This suggested that the conventional stress response is separate from the global downregulation of translation in artemisinin-treated plasmodia. The major strategies used by mutant P. falciparum to resist artemisinin treatment are known, but the entire response pathway has not yet been experimentally elucidated. Unfortunately, artemisinin resistance now has spread well beyond Southeast Asia and into Africa (Naß and Efferth 2019).
Artemisia annua and artemisinin drug resistance
The current most common treatment for malaria is with artemisinin combination therapies (ACTs), which combine an artemisinin-based derivative with a second antimalarial that persists in the patient (Eastman and Fidock 2009; WHO 2012, 2017). ACTs include, but are not limited to, artemether + lumefantrine, artesunate + amodiaquine, dihydroartemisinin + piperaquine (Gbotosho et al. 2012; Lwin et al. 2012; Niaré et al. 2016; Sowunmi et al. 2017), and several other drug combinations. The rationale behind this treatment is to combine the fast parasite-clearing activity of an artemisinin with the long-term activity of a partner drug, but still kill parasites resistant to either of the two drugs. This strategy was expected to prevent the emergence and spread of resistant strains (Eastman and Fidock 2009). For these reasons, the use of artemisinin monotherapies is contraindicated. The World Health Organization officially extended this policy to use of A. annua-based therapies (WHO 2012). The WHO instituted this policy based on the findings that Artemisia therapies contain less artemisinin than pharmaceutical therapies and that weak artemisinin monotherapies would lead to high rates of recrudescence in patients and potentiate the development of plasmodia artemisinin resistance. In its 2012 white paper, the WHO assumed A. annua therapy was a monotherapy. It is not. As documented in Table 2, A. annua contains a plethora of antimalarial phytochemicals. The WHO (2012) assumed there was too little artemisinin delivered from per os A. annua (DLA). That is not the case. Weathers et al. (2011) showed that > 40 × more artemisinin entered the serum from per os A. annua than from per os artemisinin. Rath et al. 2004 also showed that 5 g DW of A. annua leaves (1% artemisinin) administered as a tea in humans resulted in 240 μg/L of artemisinin in the bloodstream. A. annua therefore delivers a large amount of artemisinin into the blood and acts as a polytherapy.
Despite the WHO’s official policy, there is evidence that A. annua dried leaves (DLA) and/or tea infusions would be an effective antimalarial therapy. In mouse models of malaria, A. annua more effectively reduced P. chabaudi parasitemia than artemisinin (Elfawal et al. 2012). A. annua as DLA also treated artemisinin-resistant mouse malaria (P. yoellii) while artemisinin did not (Elfawal et al. 2015). Most importantly, when cultured under artemisinin or DLA drug pressure, DLA proved at least three times more resilient against the evolution of artemisinin drug resistance in P. chabaudi (Elfawal et al. 2015). In case studies of patients with severe malaria, treatment with A. annua DLA also reversed P. falciparum infections that had not responded to ACTs or intravenous artesunate (Daddy et al. 2017). Together these studies suggest that A. annua DLA treatment is a promising solution to the challenges of artemisinin resistant P. falciparum containment and treatment. Based on all the evidence published since the 2012 WHO whitepaper, there is now a growing body of in vitro, animal, and clinical evidence arguing for the WHO to reconsider its position on use of A. annua as a malaria therapy.
Non-artemisinin phytochemical activity against artemisinin-resistant malaria and other microbes
As described above, there is empirical evidence that there is antimalarial activity in A. annua leaves beyond their artemisinin content. Artemisinin content fails to explain its efficacy as an antimalarial prophylactic well beyond the persistence time of artemisinin in the patient (Birgersson et al. 2016; Gordi et al. 2000; Hien et al. 2011; Ogwang et al. 2012). That conclusion is further supported by the antimalarial efficacy of A. afra, which contains at best traces of artemisinin (Munyangi et al. 2018). The most reasonable explanation for this phenomenon is that, like in ACTs, there are other antimalarial agents present in the plants that persist in the host. Examples include quercetin, which has a biological half-life of over 20 h when consumed from some fruits (Hollman et al. 1997), and a-pinene with a biological half-life of about 10 h (Kohlert et al. 2000).
Flavonoids present in A. annua are implicated as potential inhibitors of malaria. In an in vitro study, many flavonoids, including fisetin, kaempferol, luteolin, myricetin, and quercetin, inhibited FabI, FabZ, and FabG enzymes (Tasdemir et al. 2006). The Fab enzymes are important in parasite fatty acid synthesis. Those flavonoids inhibited parasite growth with an IC50 in the μM range (Tasdemir et al. 2006). Some flavonoids also inhibit hemoglobin digestion. For example, myricetin and fisetin inhibit both plas-mepsins and falcipains in vitro, while a variety of other flavonoids have a lesser activity towards the enzymes (Jin et al. 2014).
Other nonartemisinic terpenes within the plant also have antimalarial activity. For example, nerolidol, found in A. annua, has a sub-micromolar IC50 against P. falciparum (Rodrigues-Goulart et al. 2004), inhibiting Plasmodium isoprene synthesis. Apart from being a necessary component of a variety of biological compounds, isoprene may help protect against reactive oxygen species (ROS). Isoprene, for example, protects against ozone oxidative stress in tobacco plants, possibly through direct action as an antioxidant (Vickers et al. 2009). Inhibition of isoprene synthesis may benefit antimalarial drugs whose action is ROS-dependent.
Polyphenolic acids present in the extracts of A. annua also play a role in parasite inhibition. Chlorogenic acid inhibits drug efflux pump activity in Staphylococcus aureus (Fiamegos et al. 2011; Suberu et al. 2013). It is unclear, however, whether chlorogenic acid affects the P. falciparum multi-drug resistance transporter that confers resistance to many antimalarials (Petersen et al. 2011).
A. annua and A. afra teas also inhibited HIV in vitro (Lubbe et al. 2012). A. annua efficacy was not entirely explained by its artemisinin content, and A. afra efficacy was comparable to A. annua despite a much lower artemisinin content. That response was similar to results of recent clinical trials with these two Artemisia species against schistosomiasis (Munyangi et al. 2018) and malaria (Munyangi et al. 2019). While the specific chemicals are not yet known, caffeoylquinic acids in Artemisia species have antiretro-viral activity (Lubbe et al. 2012; McDougall et al. 1998). These positive effects suggest that DLA is an effective therapy for infectious diseases and most likely contain multiple active phytochemical components.
Taken together there is evidence that many compounds in Artemisia sp. inhibit diverse parasitic pathways thereby providing additional antimalarial activity that could add to or synergize with the effects of the artemisinin content in the plant. In vitro studies have probed the question of whether tea preparations of A. annua have more potent antimalarial activity than would be expected based solely on their artemisinin content. In some cases, the apparent IC50 of the tea was threefold lower or less than the equivalent concentration of pure artemisinin (De Donno et al. 2012; Suberu et al. 2013); this finding, however, is not consistent across the literature (Mouton et al. 2013; Silva et al. 2012). Such discrepancies may be attributed to use of different cultivars with different phytochemical content. Further study is needed to resolve these discrepancies and parse out the critical factors that would make DLA a robust combination therapy and a powerful tool in the treatment and containment of drug-resistant malaria and other diseases.
Conclusions
In 2012 WHO published a white paper stating that
WHO does not recommend the use A. annua plant material, in any form, including tea, for the treatment or the prevention of malaria. … extensive fundamental and clinical research would be required to demonstrate that non-pharmaceutical forms of A. annua, including tea bag, are safe and effective to treat malaria and that their dissemination would not promote the development of artemisinin-resistant parasites.
(WHO 2012)
Despite the absence of artemisinin in A. afra, it has proved nearly as effective as A. annua in treating malaria and equally effective in treating schistosomiasis. Accumulating studies show that, with respect to artemisinin, A. annua and A. afra infusions are not monotherapies, but instead polytherapies with better outcomes than the disease standard drugs against malaria and schistosomiasis and merit further investigation for possible inclusion of A. annua and A. afra preparations as a global alternative for fighting and eradicating malaria, schistosomiasis and other Artemisia-susceptible diseases.
Acknowledgements
Authors thank Impala Avenir Foundation, Mme Héléne de Cossé Brissac, Mr. Jean-Louis Bouchard, and the Ararat Fund at WPI for generously funding some of the studies herein. We are also grateful for Award Number NIH-2R15AT008277-02 from the National Center for Complementary and Integrative Health that enabled PJW, MD and MJT for funding artemisinin bioavailability studies, and phytochemical analyses of the plant material used in treating patients. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Complementary and Integrative Health or the National Institutes of Health.
Abbreviations
- ACT
Artemisinin combination therapy
- ASAQ
Artesunate and amodiaquine
- CQ
Chloroquine
- DLA
Dried leaf Artemisia
- EIF2α
Eukaryotic translation initiation factor 2α
- ERK
Extracellular signal-regulated kinases
- EOs
Essential oils
- IC50
Half-maximal inhibitory concentration
- IkB-alpha
Inhibitor of kappa B alpha
- IKK
I kappa B kinase
- JNK
c-Jun N-terminal kinases
- MAPK
Mitogen-activated protein kinases
- NF-kB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- PI3P
Phosphatidylinositol triphosphate
- PI3K
Phosphatidylinositol-3-kinase
- PK4
Protein kinase 4
- PZQ
Praziquantel
- RSA
Ring-stage survival assay
- TID
ter in die, three times/day
- TNF-α
Tumor necrosis factor alpha
- Ub
Ubiquitin
- UPR
Unfolded protein response
- WHO
World Health Organization
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
B. M. Gruessner, Department of Biology and Biotechnology, Worcester Polytechnic Institute, 100 Institute Road, Worcester, MA 01609, USA
L. Cornet-Vernet, More for Less-Maison de l’Artemisia, Paris, France
M. R. Desrosiers, Department of Biology and Biotechnology, Worcester Polytechnic Institute, 100 Institute Road, Worcester, MA 01609, USA
P. Lutgen, IFVB-BELHERB, Niederanven, Luxembourg
M. J. Towler, Department of Biology and Biotechnology, Worcester Polytechnic Institute, 100 Institute Road, Worcester, MA 01609, USA
P. J. Weathers, Department of Biology and Biotechnology, Worcester Polytechnic Institute, 100 Institute Road, Worcester, MA 01609, USA
References
- Alin MH, Bjorkman A (1994) Concentration and time dependency of artemisinin efficacy against Plasmodium falciparum in vitro. Am J Trop Med Hyg 50:771–776 [DOI] [PubMed] [Google Scholar]
- Argemi X, Hansmann Y, Gaudart J, Gillibert A, Caumes E, Jaureguiberry S, Meyer N (2019) Comment on “Effect of Artemisia annua and Artemisia afra tea infusions on schistosomiasis in a large clinical trial”. Phytomedicine 62:152804. [DOI] [PubMed] [Google Scholar]
- Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Ménard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Ménard D (2014) A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505:50–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrizbeitia de Morgado M, Cariaco Sifontes Y, Imery Buiza J, Lutgen P (2017) Activity of Artemisia annua infusions on epimastigotes of Trypanosoma cruzi. Enferm Infecc Microbiol Clin 35:390–392 [DOI] [PubMed] [Google Scholar]
- Birgersson S, Van Toi P, Truong NT, Dung NT, Ashton M, Hien TT, Abelö A, Tarning J (2016) Population pharmacokinetic properties of artemisinin in healthy male Vietnamese volunteers. Malar J 15:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff O, Natalang E, Deplaine G, Proux C, Dillies MA, Sismeiro O, Guigon G, Bonnefoy S, Patarapotikul J, Mercereau-Puijalon O, Coppeé JY, David PH (2008) Dynamic RNA profiling in Plasmodium falciparum synchronized blood stages exposed to lethal doses of artesunate. BMC Genom 9:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M, Pérez-Munizaga D, Sanchez MA, Urrutia C, Grande A, Risso G, Srebrow A, Alfaro J, Colman-Lerner A (2013) Modulation of the Akt pathway reveals a novel link with PERK/eIF2α, which is relevant during hypoxia. PLoS ONE 8:e69668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman IH, Miller BA, Nair S, Nkhoma S, Tan A, Tan JC, Al Saai S, Phyo AP, Moo CL, Lwin KM, McGready R, Ashley E, Imwong M, Stepniewska K, Yi P, Dondorp AM, Mayxay M, Newton PN, White NJ, Nosten F, Ferdig MT, Anderson TJ (2012) A major genome region underlying artemisinin resistance in malaria. Science 336:79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IA, Budd AC, Alleva LM, Cowden WB (2006) Human malarial disease: a consequence of inflammatory cytokine release. Malar J 5:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet-Vernet L, Munyangi J, Chen L, Towler M, Weathers P (2019a) Response to Argemi et al. 2019. Phytomedicine 62:152943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet-Vernet L, Munyangi J, Chen L, Towler M, Weathers P (2019b) Correspondence: response to Gillibert et al. 2019. Phytomedicine. In Press, Accepted Manuscript 152980 [DOI] [PubMed] [Google Scholar]
- Czechowski T, Rinaldi MA, Famodimu MT, Van Veelen M, Larson TR, Winzer T, Rathbone DA, Harvey D, Horrocks P, Graham IA (2019) Flavonoid versus artemisinin anti-malarial activity in Artemisia annua whole-leaf extracts. Front Plant Sci 10:984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daddy NB, Kalisya LM, Bagire PG, Watt RL, Towler MJ, Weathers PJ (2017) Artemisia annua dried leaf tablets treated malaria resistant to ACT and iv artesunate: case reports. Phytomedicine 32:37–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danis M, Buisson Y (2019) Tisane magique ou bouillon de onze heures? Bull Soc Pathol Exot 112:3–4 [DOI] [PubMed] [Google Scholar]
- de Cássia da Silveira e Sá R, Andrade LN, de Sousa DP (2013) A review on anti-inflammatory activity of monoterpenes. Molecules 18:1227–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Donno A, Grassi T, Idolo A, Guido M, Papadia P, Cac-cioppola A, Villanova L, Merendino A, Bagordo F, Fanizzi FP (2012) First-time comparison of the in vitro antimalarial activity of Artemisia annua herbal tea and artemisinin. Trans R Soc Trop Med Hyg 106:696–700 [DOI] [PubMed] [Google Scholar]
- de Magalhães MP, Dupont I, Hendrickx A, Joly A, Raas T, Dessy S, Sergent T, Schneider YJ (2012) Anti-inflammatory effect and modulation of cytochrome P450 activities by Artemisia annua tea infusions in human intestinal Caco-2 cells. Food Chem 134:864–871 [DOI] [PubMed] [Google Scholar]
- den Boer M, Argaw D, Jannin J, Alvar J (2011) Leishmaniasis impact and treatment access. Clin Microbiol Infect 17:1471–1477 [DOI] [PubMed] [Google Scholar]
- Desrosiers MR, Weathers PJ (2016) Effect of leaf digestion and artemisinin solubility for use in oral consumption of dried Artemisia annua leaves to treat malaria. J Ethnopharmacol 190:313–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers MR, Weathers PJ (2018) Artemisinin permeability via Caco-2 cells increases after simulated digestion of Artemisia annua leaves. J Ethnopharmacol 210:254–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers MR, Towler MJ, Weathers PJ (2019) Artemisia annua and Artemisia afra essential oils and their therapeutic potential. In: Malik S (ed) Essential oil research. Springer, Cham, pp 197–209 [Google Scholar]
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ (2009) Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke SO, Paul RN (1993) Development and fine structure of the glandular trichomes of Artemisia annua L. Int J Plant Sci 154:107–118 [Google Scholar]
- Eastman RT, Fidock DA (2009) Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat Rev Microbiol 7:864–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efferth T, Herrmann F, Tahrani A, Wink M (2011) Cytotoxic activity of secondary metabolites derived from Artemisia annua L. towards cancer cells in comparison to its designated active constituent artemisinin. Phytomedicine 18:959–969 [DOI] [PubMed] [Google Scholar]
- Elfawal MA, Towler MJ, Reich NG, Golenbock D, Weathers PJ, Rich SM (2012) Dried whole plant Artemisia annua as an antimalarial therapy. PLoS ONE 7:e52746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfawal MA, Towler MJ, Reich NG, Weathers PJ, Rich SM (2015) Dried whole-plant Artemisia annua slows evolution of malaria drug resistance and overcomes resistance to artemisinin. Proc Natl Acad Sci USA 112:821–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdman LK, Finney CAM, Liles WC, Kain KC (2008) Inflammatory pathways in malaria infection: TLRs share the stage with other components of innate immunity. Mol Biochem Parasitol 162(2):105–111 [DOI] [PubMed] [Google Scholar]
- Ferreira JFS, Janick J (1995) Floral morphology of Artemisia annua with special reference to trichomes. Int J Plant Sci 156(6):807–815 [Google Scholar]
- Ferreira JF, Luthria DL, Sasaki T, Heyerick A (2010) Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 15:3135–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiamegos YC, Kastritis PL, Exarchou V, Han H, Bonvin AM, Vervoort J, Lewis K, Hamblin MR, Tegos GP (2011) Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against gram-positive pathogenic bacteria. PLoS ONE 6:e18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agricultural Organization of the UN (1986) Forestry paper 67: Some medicinal forest plants of Africa and Latin America. http://www.fao.org/docrep/015/an797e/an797e00.pdf. Accessed Dec 29, 2018
- Ganesh D, Fuehrer HP, Starzengrüber P, Swoboda P, Khan WA, Reismann JA, Mueller MS, Chiba P, Noedl H (2012) Antiplasmodial activity of flavonol quercetin and its analogues in Plasmodium falciparum: evidence from clinical isolates in Bangladesh and standardized parasite clones. Parasitol Res 110:2289–2295 [DOI] [PubMed] [Google Scholar]
- Gathirwa JW, Rukunga GM, Njagi EN, Omar SA, Mwitari PG, Guantai AN, Tolo FM, Kimani CW, Muthaura CN, Kirira PG, Ndunda TN, Amalemba G, Mungai GM, Ndiege IO (2008) The in vitro anti-plasmodial and in vivo anti-malarial efficacy of combinations of some medicinal plants used traditionally for treatment of malaria by the Meru community in Kenya. J Ethnopharmacol 115:223–231 [DOI] [PubMed] [Google Scholar]
- Gbotosho GO, Sowunmi A, Okuboyejo TM, Happi CT (2012) Oral artesunate-amodiaquine and artemether-lumefantrine in the treatment of uncomplicated hyperpara-sitaemic Plasmodium falciparum malaria in children. J Trop Pediatr 58:151–153 [DOI] [PubMed] [Google Scholar]
- Gillibert A, Jauréguiberry S, Hansmann Y, Argemi X, Landier J, Caumes E, and Gaudart J (2019) Comment on “A. annua and A. afra infusions vs Artesunate-amodiaquine (ASAQ) in treating Plasmodium falciparum malaria in a large scale, double blind, randomized clinical trial”. Phytomedicine In Press, Accepted Manuscript: 152981 [DOI] [PubMed] [Google Scholar]
- Gordi T, Hai TN, Hoai NM, Thyberg M, Ashton M (2000) Use of saliva and capillary blood samples as substitutes for venous blood sampling in pharmacokinetic investigations of artemisinin. Eur J Clin Pharmacol 56:561–566 [DOI] [PubMed] [Google Scholar]
- Grace MH, Lategan C, Graziose R, Smith PJ, Raskin I, Lila MA (2012) Antiplasmodial activity of the ethnobotanical plant Cassia fistula. Nat Prod Commun 7:1263–1266 [PubMed] [Google Scholar]
- He Y, Yue Y, Zheng X, Zhang K, Chen S, Du Z (2015) Curcumin, inflammation, and chronic diseases: how are they linked? Molecules 20:9183–9213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien TT, Hanpithakpong W, Truong NT, Dung NT, Toi PV, Farrar J, Lindegardh N, Tarning J, Ashton M (2011) Orally formulated artemisinin in healthy fasting Vietnamese male subjects: a randomized, four-sequence, open-label, pharmacokinetic crossover study. Clin Ther 33:644–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollman PC, van Trijp JM, Buysman MN, van der Gaag MS, Mengelers MJ, de Vries JH, Katan MB (1997) Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett 418:152–156 [DOI] [PubMed] [Google Scholar]
- Jessing KK, Cedergreen N, Jensen J, Hansen HCB (2009) Degradation and ecotoxicity of the biomedical drug artemisinin in soil. Environ Chem 28(4):701–710 [DOI] [PubMed] [Google Scholar]
- Jin H, Xu Z, Cui K, Zhang T, Lu W, Huang J (2014) Dietary flavonoids fisetin and myricetin: dual inhibitors of Plasmodium falciparum falcipain-2 and plasmepsin II. Fitoterapia 94:55–61 [DOI] [PubMed] [Google Scholar]
- Juergens UR, Engelen T, Racké K, Stöber M, Gillissen A, Vetter H (2004) Inhibitory activity of 1,8-cineol (eucalyptol) on cytokine production in cultured human lymphocytes and monocytes. Pulm Pharmacol Ther 17:281–287 [DOI] [PubMed] [Google Scholar]
- Keiser J, Utzinger J (2012) Antimalarials in the treatment of schistosomiasis. Curr Pharm Des 18:3531–3538 [PubMed] [Google Scholar]
- Kim WS, Choi WJ, Lee S, Kim WJ, Lee DC, Sohn UD, Shin HS, Kim W (2015) Anti-inflammatory, antioxidant and antimicrobial effects of artemisinin extracts from Artemisia annua L. Korean J Physiol Pharmacol 19:21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlert C, van Rensen I, Marz R, Schindler G, Graefe EU, Veit M (2000) Bioavailability and pharmacokinetics of natural volatile terpenes in animals and humans. Planta Med 66:495–505 [DOI] [PubMed] [Google Scholar]
- Kraft C, Jenett-Siems K, Siems K, Jakupovic J, Mavi S, Bienzle U, Eich E (2003) In vitro antiplasmodial evaluation of medicinal plants from Zimbabwe. Phytother Res 17:123–128 [DOI] [PubMed] [Google Scholar]
- Lagarce L, Lerolle N, Asfar P, Le Govic Y, Lainé-Cessac P, de Gentile L (2016) A non-pharmaceutical form of Artemisia annua is not effective in preventing Plasmodium falciparum malaria. J Travel Med 23(5):1–3 [DOI] [PubMed] [Google Scholar]
- Lehane AM, Saliba KJ (2008) Common dietary flavonoids inhibit the growth of the intraerythrocytic malaria parasite. BMC Res Notes 1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJ, Guo Y, Yang Q, Weng XG, Yang L, Wang YJ, Chen Y, Zhang D, Li Q, Liu XC, Kan XX, Chen X, Zhu XX, Kmoniekova E, Zidek Z (2015) Flavonoids casticin and chrysosplenol D from Artemisia annua L inhibit inflammation in vitro and in vivo. Toxicol Appl Pharmacol 286:151–158 [DOI] [PubMed] [Google Scholar]
- Li J, Zhang C, Gong M, Wang M (2018) Combination of artemisinin-based natural compounds from Artemisia annua L for the treatment of malaria: pharmacodynamic and pharmacokinetic studies. Phytother Res 32:1415–1420 [DOI] [PubMed] [Google Scholar]
- Liu KC, Yang SL, Roberts MF, Elford BC, Phillipson JD (1992) Antimalarial activity of Artemisia annua flavonoids from whole plants and cell cultures. Plant Cell Rep 11:637–640 [DOI] [PubMed] [Google Scholar]
- Liu NQ, Cao M, Frédérich M, Choi YH, Verpoorte R, van der Kooy F (2010) Metabolomic investigation of the ethnopharmacological use of Artemisia afra with NMR spectroscopy and multivariate data analysis. J Ethnopharmacol 128:230–23520079415 [Google Scholar]
- Lubbe A, Seibert I, Klimkait T, van der Kooy F (2012) Ethnopharmacology in overdrive: the remarkable anti-HIV activity of Artemisia annua. J Ethnopharmacol 141:854–859 [DOI] [PubMed] [Google Scholar]
- Lwin KM, Phyo AP, Tarning J, Hanpithakpong W, Ashley EA, Lee SJ, Cheah P, Singhasivanon P, White NJ, Lindegardh N, Nosten F (2012) Randomized, double-blind, placebo-controlled trial of monthly versus bimonthly dihydroartemisinin-piperaquine chemoprevention in adults at high risk of malaria. Antimicrob Agents Chemother 56:1571–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A, Kushnoor A, Saini V, Singhal S, Kumar S, Yadav YC (2011) In vitro antioxidant properties of scopoletin. J Chem Pharm Res 3:659–665 [Google Scholar]
- Mbengue A, Bhattacharjee S, Pandharkar T, Liu H, Estiu G, Stahelin RV, Rizk SS, Njimoh DL, Ryan Y, Chotivanich K, Nguon C, Ghorbal M, Lopez-Rubio JJ, Pfrender M, Emrich S, Mohandas N, Dondorp AM, Wiest O, Haldar K (2015) A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature 520:683–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall B, King PJ, Wu BW, Hostomsky Z, Reinecke MG, Robinson WE (1998) Dicaffeoylquinic and dicaffeoyltartaric acids are selective inhibitors of human immunodeficiency virus type 1 integrase. Antimicrob Agents Chemother 42:140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard D, Khim N, Beghain J, Adegnika AA, Shafiul-Alam M, Amodu O, Rahim-Awab G, Barnadas C, Berry A, Boum Y, Bustos MD, Cao J, Chen JH, Collet L, Cui L, Thakur GD, Dieye A, Djallé D, Dorkenoo MA, Eboumbou-Moukoko CE, Espino FE, Fandeur T, Ferreira-da-Cruz MF, Fola AA, Fuehrer HP, Hassan AM, Herrera S, Hongvanthong B, Houzé S, Ibrahim ML, Jahirul-Karim M, Jiang L, Kano S, Ali-Khan W, Khanthavong M, Kremsner PG, Lacerda M, Leang R, Leelawong M, Li M, Lin K, Mazarati JB, Ménard S, Morlais I, Muhindo-Mavoko H, Musset L, Na-Bang-chang K, Nambozi M, Niaré K, Noedl H, Ouédraogo JB, Pillai DR, Pradines B, Quang-Phuc B, Ramharter M, Randrianarivelojosia M, Sattabongkot J, Sheikh-Omar A, Silué KD, Sirima SB, Sutherland C, Syafruddin D, Tahar R, Tang LH, Touré OA, Tshibangu-wa-Tshibangu P, Vigan-Womas I, Warsame M, Wini L, Zakeri S, Kim S, Eam R, Berne L, Khean C, Chy S, Ken M, Loch K, Canier L, Duru V, Legrand E, Barale JC, Stokes B, Straimer J, Witkowski B, Fidock DA, Rogier C, Ringwald P, Ariey F, Mercereau-Puijalon O, KARMA Consortium (2016) A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med 374:2453–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa LE, Vasquez D, Lutgen P, Velez ID, Restrepo AM, Ortiz I, Robledo SM (2017) In vitro and in vivo antileishmanial activity of Artemisia annua L. leaves powder and its potential usefulness in the treatment of uncomplicated cutaneous leishmaniasis in humans. Rev Soc Bras Med Trop 50:52–60 [DOI] [PubMed] [Google Scholar]
- Mok S, Ashley EA, Ferreira PE, Zhu L, Lin Z, Yeo T, Chotivanich K, Imwong M, Pukrittayakamee S, Dhorda M, Nguon C, Lim P, Amaratunga C, Suon S, Hien TT, Htut Y, Faiz MA, Onyamboko MA, Mayxay M, Newton PN, Tripura R, Woodrow CJ, Miotto O, Kwiatkowski DP, Nosten F, Day NP, Preiser PR, White NJ, Dondorp AM, Fairhurst RM, Bozdech Z (2015) Drug resistance population transcriptomics of human malaria parasites reveals the mechanism of artemisinin resistance. Science 347:431–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyasu T, Nakamura R, Deloer S, Senba M, Kubo M, Inoue M, Culleton R, Hamano S (2018) Schistosoma mansoni infection suppresses the growth of Plasmodium yoelii parasites in the liver and reduces gametocyte infectivity to mosquitoes. PLoS Negl Trop Dis 12(1):e0006197. 10.1371/journal.pntd.0006197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura IC, Wunderlich G, Uhrig ML, Couto AS, Peres VJ, Katzin AM, Kimura EA (2001) Limonene arrests parasite development and inhibits isoprenylation of proteins in Plasmodium falciparum. Antimicrob Agents Chemother 45:2553–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton J, Jansen O, Frédérich M, van der Kooy F (2013) Is artemisinin the only antiplasmodial compound in the Artemisia annua tea infusion? An in vitro study. Planta Med 79:468–470 [DOI] [PubMed] [Google Scholar]
- Moyo P, Botha ME, Nondaba S, Niemand J, Maharaj VJ, Eloff JN, Louw AI, Birkholtz L (2016) In vitro inhibition of Plasmodium falciparum early and late stage gametocyte viability by extracts from eight traditionally used South African plant species. J Ethnopharmacol 185:235–242 [DOI] [PubMed] [Google Scholar]
- Munyangi J, Cornet-Vernet L, Idumbo M, Lu C, Lutgen P, Perronne C, Ngombe N, Bianga J, Mupenda B, Lalukala P, Mergeai G, Mumba D, Towler M, Weathers P (2018) Effect of Artemisia annua and Artemisia afra tea infusions on schistosomiasis in a large clinical trial. Phytomedicine 51:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Munyangi J, Cornet-Vernet L, Idumbo M, Lu C, Lutgen P, Perronne C, Ngombe N, Bianga J, Mupenda B, Lalukala P, Mergeai G, Mumba D, Towler M, Weathers P (2019) Artemisia annua and Artemisia afra tea infusions vs artesunate-amodiaquine (ASAQ) in treating Plasmodium falciparum malaria in a large scale, double blind, randomized clinical trial. Phytomedicine 57:49–56 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Naß J, Efferth T (2018) The activity of Artemisia spp and their constituents against trypanosomiasis. Phytomedicine 47:184–191 [DOI] [PubMed] [Google Scholar]
- Naß J, Efferth T (2019) Development of artemisinin resistance in malaria therapy. Pharmacol Res 146:104275. 10.1016/j.phrs.2019.104275 [DOI] [PubMed] [Google Scholar]
- Niaré K, Dara A, Sagara I, Sissoko MS, Guindo CO, Cissé NH, Coulibaly CO, Ringwald P, Benoit-Vical F, Berry A, Djimdé AA, Doumbo OK (2016) In vivo efficacy and parasite clearance of artesunate + sulfadoxine-pyrimethamine versus artemether-lumefantrine in Mali. Am J Trop Med Hyg 94:634–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogwang PE, Ogwal JO, Kasasa S, Ejobi F, Kabasa D, Obua C (2011) Use of Artemisia annua L. infusion for malaria prevention: mode of action and benefits in a Ugandan community. Br J Pharm Res 1:e124–e132 [Google Scholar]
- Ogwang PE, Ogwal JO, Kasasa S, Olila D, Ejobi F, Kabasa D, Obua C (2012) Artemisia annua L. infusion consumed once a week reduces risk of multiple episodes of malaria: a randomised trial in a Ugandan community. Trop J Pharm Res 11:445–453 [Google Scholar]
- Packard RM (2014) The origins of antimalarial-drug resistance. N Engl J Med 371:397–399 [DOI] [PubMed] [Google Scholar]
- Paloque L, Ramadani AP, Mercereau-Puijalon O, Augereau JM, Benoit-Vical F (2016) Plasmodium falciparum: multifaceted resistance to artemisinins. Malar J 15:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna-Coutinho J, Aguiar AC, Krettli AU (2018) Commercial drugs containing flavonoids are active in mice with malaria and in vitro against chloroquine-resistant Plasmodium falciparum. Mem Inst Oswaldo Cruz 113:e180279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen I, Eastman R, Lanzer M (2011) Drug-resistant malaria: molecular mechanisms and implications for public health. FEBS Lett 585:1551–1562 [DOI] [PubMed] [Google Scholar]
- Rath K, Taxis K, Walz G, Gleiter CH, Li SM, Heide L (2004) Pharmacokinetic study of artemisinin after oral intake of a traditional preparation of Artemisia annua L. (Annual Wormwood). Am J Trop Med Hyg 70:128–132 [PubMed] [Google Scholar]
- Rathee P, Chaudhary H, Rathee S, Rathee D, Kumar V, Kohli K (2009) Mechanism of action of flavonoids as anti-inflammatory agents: a review. Inflamm Allergy Drug Targets 8:229–235 [DOI] [PubMed] [Google Scholar]
- Rocamora F, Zhu L, Liong KY, Dondorp A, Miotto O, Mok S, Bozdech Z (2018) Oxidative stress and protein damage responses mediate artemisinin resistance in malaria parasites. PLoS Pathog 14:e1006930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Goulart H, Kimura EA, Peres VJ, Couto AS, Aquino-Duarte FA, Katzin AM (2004) Terpenes arrest parasite development and inhibit biosynthesis of isoprenoids in Plasmodium falciparum. Antimicrob Agents Chemother 48:2502–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini M, Peluso I, Raguzzini A (2010) Antioxidants and the immune system: flavonoids as anti-inflammatory agents. Proc Nutr Soc 69:273–278 [DOI] [PubMed] [Google Scholar]
- Shi C, Li H, Yang Y, Hou L (2015) Anti-inflammatory and immunoregulatory functions of artemisinin and its derivatives. Med Inflamm 2015:435713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva LF, Magalhães PM, Costa MR, Alecrim M, Chaves FC, Hidalgo AF, Pohlit AM, Vieira PP (2012) In vitro susceptibility of Plasmodium falciparum Welch field isolates to infusions prepared from Artemisia annua L. cultivated in the Brazilian Amazon. Mem Inst Oswaldo Cruz 107:859–866 [DOI] [PubMed] [Google Scholar]
- Simonnet X, Grogg AF, Cutler M, Bathurst I (2009) Artemisia annua L. drying and storage. Artemisinin Forum 2008-joint meeting on ensuring sustainable artemisinin production: meeting global demand. Guilin, China, 24–26 November 2008 [Google Scholar]
- Sowunmi A, Akano K, Ntadom G, Ayede A, Oguche S, Agomo C, Okafor H, Watila I, Meremikwu M, Ogala W, Agomo P, Adowoye E, Fatunmbi B, Aderoyeje T, Happi C, Gbotosho G, Folarin O (2017) Anaemia following artemisinin-based combination treatments of uncomplicated Plasmodium falciparum malaria in children: temporal patterns of haematocrit and the use of uncomplicated hyperpara-sitaemia as a model for evaluating late-appearing anaemia. Chemotherapy 62:231–238 [DOI] [PubMed] [Google Scholar]
- Spagnuolo C, Moccia S, Russo GL (2018) Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur J MedChem 153:105–115 [DOI] [PubMed] [Google Scholar]
- Straimer J, Gnädig NF, Witkowski B, Amaratunga C, Duru V, Ramadani AP, Dacheux M, Khim N, Zhang L, Lam S, Gregory PD, Urnov FD, Mercereau-Puijalon O, Benoit-Vical F, Fairhurst RM, Ménard D, Fidock DA (2015) Drug resistance K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science 347:428–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su V, King D, Woodrow I, McFadden G, Gleadow R (2008) Plasmodium falciparum growth is arrested by monoterpenes from eucalyptus oil. Flavor Fragr J 23:315–318 [Google Scholar]
- Suberu JO, Gorka AP, Jacobs L, Roepe PD, Sullivan N, Barker GC, Lapkin AA (2013) Anti-plasmodial polyvalent interactions in Artemisia annua L. aqueous extract—possible synergistic and resistance mechanisms. PLoS ONE 8:e80790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson US, Ashton M (1999) Identification of the human cytochrome P450 enzymes involved in the in vitro metabolism of artemisinin. Br J Clin Pharmacol 48:528–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasdemir D, Lack G, Brun R, Rüedi P, Scapozza L, Perozzo R (2006) Inhibition of Plasmodium falciparum fatty acid biosynthesis: evaluation of FabG, FabZ, and FabI as drug targets for flavonoids. J Med Chem 49:3345–3353 [DOI] [PubMed] [Google Scholar]
- Tchandema CK, Lutgen P (2016) In vivo trials on the therapeutic effects of encapsulated Artemisia annua and Artemisia afra. Global J Res Anal 5(6):228–234 [Google Scholar]
- Tellez MR, Camilo C, Rimando AM, Duke SO (1999) Differential accumulation of isoprenoids in glanded and glandless Artemisia annua L. Phytochemistry 52(6):1035–1040 [Google Scholar]
- Titulaer HA, Zuidema J, Kager PA, Wetsteyn JC, Lugt CB, Merkus FW (1990) The pharmacokinetics of artemisinin after oral, intramuscular and rectal administration to volunteers. J Pharm Pharmacol 42:810–813 [DOI] [PubMed] [Google Scholar]
- Van der Kooy F, Sullivan SE (2013) The complexity of medicinal plants: the traditional Artemisia annua formulation, current status and future perspectives. J Ethnopharmacol 150:1–13 [DOI] [PubMed] [Google Scholar]
- Van der Kooy F, Verpoorte R (2011) The content of artemisinin in the Artemisia annua tea infusion. Planta Medica 77:1754–1756 [DOI] [PubMed] [Google Scholar]
- Van Zyl R, Seatlholo S, Van Vuuren S, Viljoen A (2006) The biological activities of 20 nature identical essential oil constituents. J Essent Oils Res 18:e129–e133 [Google Scholar]
- Vickers CE, Possell M, Cojocariu CI, Velikova VB, Laotha-wornkitkul J, Ryan A, Mullineaux PM, Nicholas Hewitt C (2009) Isoprene synthesis protects transgenic tobacco plants from oxidative stress. Plant Cell Environ 32:520–531 [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang Y, Cabrera M, Zhang Y, Gupta B, Wu Y, Kemirembe K, Hu Y, Liang X, Brashear A, Shrestha S, Li X, Miao J, Sun X, Yang Z, Cui L (2015) Artemisinin resistance at the China-Myanmar border and association with mutations in the K13 propeller gene. Antimicrob Agents Chemother 59:6952–6959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KS, Li J, Wang Z, Mi C, Ma J, Piao LX, Xu GH, Li X, Jin X (2017) Artemisinin inhibits inflammatory response via regulating NF-κ B and MAPK signaling pathways. Immunopharmacol Immunotoxicol 39:28–36 [DOI] [PubMed] [Google Scholar]
- Weathers PJ, Towler MJ (2014) Changes in key constituents of clonally propagated Artemisia annua L. during preparation of compressed leaf tablets for possible therapeutic use. Ind Crops Prod 62:173–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers PJ, Arsenault PR, Covello PS, McMickle A, Teoh KH, Reed DW (2011) Artemisinin production in Artemisia annua: studies in planta and results of a novel delivery method for treating malaria and other neglected diseases. Phytochem Rev 10:173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers PJ, Towler M, Hassanali A, Lutgen P, Engeu PO (2014a) Dried-leaf Artemisia annua: a practical malaria therapeutic for developing countries? World J Pharmacol 3:39–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers PJ, Elfawal MA, Towler MJ, Acquaah-Mensah GK, Rich SM (2014b) Pharmacokinetics of artemisinin delivered by oral consumption of Artemisia annua dried leaves in healthy vs. Plasmodium chabaudi-infected mice. J Ethnopharmacol 153:732–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers PJ, Cambra HM, Desrosiers MR, Rassias D, Towler MJ (2017) Artemisinin the noble molecule: from plant to patient. Stud Nat Prod Chem 52:193–229 [Google Scholar]
- Wei S, Ji H, Yang B, Ma L, Bei Z, Li X, Dang H, Yang X, Liu C, Wu X, Chen J (2015) Impact of chrysosplenetin on the pharmacokinetics and anti-malarial efficacy of artemisinin against Plasmodium berghei as well as in vitro CYP450 enzymatic activities in rat liver microsome. Malar J 14:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox M, Bodeker G, Bourdy G, Dhingra V, Falquet J, Ferreira JFS, Graz B, Hirt H-M, Hsu E, de Magalhães P, Provendier D, Wright C (2004) Artemisia annua as a traditional herbal antimalarial. In: Willcox ML, Bodeker G, Rasoanaivo P (eds) Traditional medicinal plants and malaria. CRC Press, Boca Raton, pp 43–59 [Google Scholar]
- Witkowski B, Lelièvre J, Barragan MJ, Laurent V, Su XZ, Berry A, Benoit-Vical F (2010) Increased tolerance to artemisinin in Plasmodium falciparum is mediated by a quiescence mechanism. Antimicrob Agents Chemother 54:1872–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski B, Khim N, Chim P, Kim S, Ke S, Kloeung N, Chy S, Duong S, Leang R, Ringwald P, Dondorp AM, Tripura R, Benoit-Vical F, Berry A, Gorgette O, Ariey F, Barale JC, Mercereau-Puijalon O, Menard D (2013) Reduced artemisinin susceptibility of Plasmodium falciparum ring stages in western Cambodia. Antimicrob Agents Chemother 57:914–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2000) General guidelines for methodologies on research and evaluation of traditional medicine. http://apps.who.int/iris/bitstream/handle/10665/66783/WHO_EDM_TRM_2000.1.pdf;jsessionid=58426E99-AFB3D5CEDC7DF3E3686E4D87?sequence=1
- World Health Organization (2012) Effectiveness of non-pharmaceutical forms of Artemisia annua L. against malaria. https://www.homt/malaria/position_statement_herbal_remedy_artemWoSa_annua_lpdf. Cited 27 Dec 2018
- World Health Organization (2014) Emergence and spread of artemisinin resistance calls for intensified efforts to with-draw oral artemisinin-based monotherapy from the market. https://www.who.int/malaria/publications/atoz/oral-artemisinin-based-monotherapies-1may2014.pdf?ua=1. Cited June 25, 2019
- World Health Organization (2017) Artemisinin and artemisinin-based combination therapy resistance-April 2017. http://apps.who.int/iris/bitstream/handle/10665/255213/WHO-HTM-GMP-2017.9-eng.pdf?sequence=1. Cited 27 Dec 2018
- World Health Organization (2018a) Leishmaniasis. https://www.hoint/news-room/fact-sheets/detail/leishmaniasis. Cited 27 Dec 2018
- World Health Organization (2018b) Schistosomiasis. https://www.hoint/news-room/fact-sheets/detail/schistosomiasis. Cited 27 Dec 2018
- Yun C, Jung Y, Chun W, Yang B, Ryu J, Lim C, Kim JH, Kim H, Cho SI (2016) Anti-inflammatory effects of Artemisia leaf extract in mice with contact dermatitis in vitro and in vivo. Med Inflamm 2016:8027537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid O, Abd MR, Hasidah M, Sabariah M, Rahi S, Basir R (2016) Antiplasmodial and chloroquine chemosensitizing and resistance reversal effects of coumarin derivatives against Plasmodium falciparum 3D7 and K1. Trop Biomed 33:14–26 [PubMed] [Google Scholar]
- Zhang M, Gallego-Delgado J, Fernandez-Arias C, Waters NC, Rodriguez A, Tsuji M, Wek RC, Nussenzweig V, Sullivan WJ (2017) Inhibiting the plasmodium eIF2α Kinase PK4 prevents artemisinin-induced latency. Cell Host Microbe 22:766–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Xiong Z, Chen X, Peng F, Hu X, Chen Y, Wang Q (2012) Artemisinin attenuates lipopolysaccharide-stimulated proinflammatory responses by inhibiting NF-κ B pathway in microglia cells. PLoS ONE 7:e35125. [DOI] [PMC free article] [PubMed] [Google Scholar]


