Abstract
Objective:
To determine whether higher membrane capacitance (CM), a bioelectrical measure of cell membrane function, is associated with insulin resistance (IR) and/or metabolic syndrome (MetS).
Methods:
Cross-sectional analyses were performed on 2,191 relatively healthy adults from the National Health and Nutrition Examination Survey. The CM of those with low/no disease risk was compared to those with IR, MetS, or both IR and MetS using analysis of covariance. The associations between CM and related clinical measures were assessed with multiple linear regression.
Results:
Compared to those with low/no risk, women and men with IR (p < 0.001) and IR + MetS (p < 0.001) had higher CM, whereas CM was similar in women (p = 0.4526) and men (p =0.1126) with MetS alone. Positive associations with CM were seen with waist circumference (women and men STD-β = 0.18, p<0.0001) and fasting insulin (women STD-β = 0.15 p<0.0001; men STD-β = 0.12, p<0.0001).
Conclusion:
Higher CM was associated with IR in relatively healthy adults. Higher CM was not associated with MetS as defined by its clinical diagnostic criteria in the absence of IR. This study suggests that with further investigation, CM may be a potential tool to detect IR-related cell membrane dysfunction.
Keywords: Bioimpedance Spectroscopy, Membrane Capacitance, Metabolic Syndrome, Insulin Resistance
Introduction
Metabolic syndrome (MetS) is a group of metabolic abnormalities that represents a significant public health concern due to its association with increased risk for cardiometabolic disease (CMD) (e.g., diabetes, cardiovascular disease, and cancer).1 MetS includes insulin resistance (IR), hyperinsulinemia, hyperglycemia, abdominal obesity, hypertension, dyslipidemia, and inflammation. Although IR has been removed from clinical guidelines to simplify assessments, the consensus remains that IR is the central component of MetS and that IR is a principal determinant of CMD risk.1–3 Although early detection of IR might mitigate disease risk, current practices often fail to detect IR and related conditions due to a lack of clinically-suitable screening methods for IR. Direct measures of insulin sensitivity (i.e., the inverse of IR) come from the hyperinsulinemic-euglycemic clamp, which takes several hours to perform and requires repeated intravenous sampling that is too expensive and impractical for wide-spread screening applications.4 Further, MetS screening is often not performed or performed too late to prevent progression to CMD, due to rising costs and limited access.4,5 Thus, widely-accessible methods to identify MetS and IR are needed and will become more critical as the prevalence of obesity, MetS, and IR continue to rise.3
Bioelectrical impedance spectroscopy (BIS) may provide a non-invasive approach to detect underlying cellular dysfunction in those with MetS and IR. BIS is a widely-accepted approach that has several practical advantages such as low cost, non-invasiveness, and suitability for high-throughput use.6 BIS passes alternating current through the body’s water- and electrolyte-rich tissues over a range of frequencies (1 kilohertz (kHz) to 1000 kHz).7 By fitting frequency-specific measures of resistance (R) and reactance (X) to the Cole bioelectrical model,8,9 BIS can characterize the health of cells and tissues within the current’s path using estimates of membrane capacitance (CM) in nanoFarads (nF) and resistivity (i.e., opposition to the current) of the intracellular (RI) and extracellular (RE) fluid in ohms. Recent interest in using BIS as an approach to assess health and disease has grown,6,10–14 however, BIS has not been thoroughly examined in the context of IR or MetS.
CM might capture MetS and IR related disturbances to the structural integrity and functional health of cell membranes. CM represents an aggregate measure of the frequency-dependent responses that are exhibited by the healthy cell membrane’s function as an imperfect capacitor that briefly stores the current as it passes through the fluid-membrane-fluid cell-matrix within tissues. Evidence suggests that membrane lipid and protein composition, membrane thickness/saturation, and transmembrane potential influence CM.9,15 Hence, CM is lower in several advanced disease states where catabolism occurs, including diabetes,13,14 cancer,12 and cardiovascular diseases.10,16 However, unlike in advanced disease where cell membranes are catabolized, in MetS and IR, chronic shifts in glucose, insulin, lipids, body water, and electrolytes lead to membrane lipid restructuring and abnormal membrane-ion interactions, which can increase membrane thickness and transmembrane potential.17–21 Therefore, we hypothesize that CM would be higher in those with IR and MetS.
The purpose of this study was to determine whether higher CM is associated with IR, MetS, and related clinical measures. We compared the CM of women and men with low/no CMD risk to CM among those with higher CMD risk: IR, MetS, or both conditions. Also, we evaluated the linear association of CM with clinical measures related to IR and MetS. Analyses controlled for the influence of age, sex, race/ethnicity, height, fat mass, lean mass, smoking, alcohol, and medication use.
Methods
Study Design and Population
This cross-sectional study was designed to analyze data from the 1999 – 2004 National Health and Nutrition Examination Survey (NHANES). We included all Non-Hispanic White, Non-Hispanic Black, or Mexican American participants from the morning (fasted) subgroup that were 18 – 49 years old with completed data for relevant laboratory, sociodemographic, BIS, and dual-x-ray absorptiometry (DXA) variables.
We excluded those with a medical history, current diagnosis, or laboratory values indicating human immunodeficiency virus, cancer, diabetes (type 1 or type 2), and liver, thyroid, cardiovascular, or cerebrovascular disease. We excluded those using diuretics, or using anti-diabetes, chemotherapy, kidney disease, anti-hypertensive, cardiac, lipid-lowering (e.g., statins), and hormone therapy/replacement medications. We also excluded those using muscle relaxers or supplements that influence fluid balance, such as potassium.
NHANES did not perform BIS on those who were older than 49 years; had amputations, artificial joints, pins, plates, or other types of metal objects in the body; had a pacemaker or automatic defibrillator; or had a coronary stent or metal suture material in the heart. DXA was not performed on persons who weighed over 300 pounds (limitation for the examination table), or were pregnant.
Bioimpedance Spectroscopy
BIS data were collected with a HYDRA ECF/ICF Bio-Impedance Spectrum Analyzer (Model 4200; Xitron Technologies, Inc., San Diego, California). This device uses a whole-body, tetrapolar, technique where an individual lies supine on a nonconductive surface, and electrodes are placed on their right hand, wrist, foot, and ankle. A detailed description of the method used with this BIS device is given elsewhere.8 This particular device is no longer commercially available, but more up – to – date spectroscopy devices that include technological and methodological refinements are commercially available.15,22
This device estimates the Cole model terms CM, RI, and RE by fitting impedance measurements taken at 50 frequencies between 5 kilohertz (kHz) and 1000 kHz.7 The model fit for each individual’s BIS data was labeled as excellent, good, marginal, questionable, bad, or unable to fit. For this study, we excluded individuals that were coded as “bad fit” or “unable to fit.” Further discussion of the Cole parameter derivation is beyond the scope of this paper, but several publications are dedicated to the Cole model and parameters.8,9,23
Cardiometabolic Disease Risk Group Classifications
Clinical measures were analyzed as continuous variables and included systolic and diastolic blood pressure (mmHg), fasting triglycerides (mg/dl), high-density lipoprotein (HDL) cholesterol (mg/dl), c-reactive protein (μg/mL), fasting glucose (mg/dl), fasting insulin (uIU/ml), and homeostatic model assessment of IR (HOMA-IR) calculated by the equation: fasting insulin (uIU/ml) x fasting glucose (mg/dl)/22.5.4
MetS was defined using the National Cholesterol Education Program Adult Treatment Program Three (NCEP ATP III) guidelines as having three or more of the following five conditions: abdominal obesity: waist circumference over 101 centimeters (men) or 88 centimeters (women); hypertension: blood pressure over 130/85 mmHg; hypertriglyceridemia: fasting triglyceride level over 150 mg/dl; dyslipidemia: fasting HDL level less than 40 mg/dl (men) or 50 mg/dl (women); and hyperglycemia: fasting glucose 100 mg/dl and above. 1 IR was defined as having HOMA-IR greater than 2.6.4
Participants were assigned to one of four groups based on their cardiometabolic disease risk:
Low/No Risk: Neither IR nor MetS.
MetS Only (MetS): Three or more MetS criteria and HOMA-IR ≤ 2.6.
IR Only: HOMA-IR > 2.6 with two or fewer MetS criteria.
IR and MetS (IR + MetS): Both IR and MetS.
Other variables
Anthropometric variables included height (cm), weight (kg), waist circumference (WC; cm), and body mass index (BMI; kg/m2). Body composition measures included total and regional measures of fat mass, lean mass, and percent fat from DXA (Hologic QDR 4500A; Software version 8.26:a3; Hologic, Inc., Bedford, Massachusetts). Behavioral factors were assessed as binary variables based on self-reported use of alcohol, tobacco, and prescription medications within the 30 days before testing. Serum measures of sodium (Na+), potassium (K+), and chloride (Cl-) in millimoles per liter (mmol/L) were measured by the UniCel® DxC800 Synchron Clinical System (Beckman Coulter, Inc; Brea, California). Serum osmolality in milliosmoles/kg of water (mOsm/kg) was estimated by the equation: Osmolality = 2 x (sodium + glucose + urea), with all in mmol/L.
Statistical Analysis
Descriptive statistics were calculated by sex and CMD risk group. The characteristics of each high/at risk group (i.e., MetS, IR, and IR+MetS) were compared to the low/normal-risk group using the Rao-Scott chi-square test for categorical variables and analysis of variance (ANOVA) for continuous variables. Continuous variables were expressed as the unweighted mean and standard deviation. Categorical/dichotomous variables were expressed as the unweighted percentage. The normality of variable distributions was checked by the Kolmogorov Smirnov or the Shapiro Wilk Tests. DXA fat mass, DXA lean mass, and HOMA-IR were log-transformed to achieve normality, but untransformed values are reported.
First, we performed ANOVA on CM (dependent) by CMD risk group (independent). Analyses showed a significant interaction term indicating that the effects of CMD group on CM differed by sex. Therefore, we conducted all analyses within sex groups.
To compare CM between those with low/no risk and those in high/at risk groups, two analysis of covariance (ANCOVA) models were performed on CM by CMD risk group. Model 1 adjusted for height2 and RI to account for their relationships with CM and to avoid using ratios (i.e., CM/RI or CM/ht2) in the analyses.15 Model 2 adjusted for height2, RI, age, African American race/ethnicity, Mexican American race/ethnicity DXA fat mass, DXA lean mass, smoking, alcohol, and medication use to account for other potential confounders. Additionally, we substituted BMI and DXA percent fat for height2, DXA fat mass, and DXA lean mass, but the substitution did not change the model fit or significance. Least squared (LS) means adjustments were made to account for unbalanced groups. The Tukey-Kramer post-hoc test was used to account for multiple comparisons.
To evaluate the association of CM with each clinical measure, we performed two multiple linear regression analyses for each measure, adjusting for the same covariates in model 1 and model 2, as described above. Normality and residuals were verified by visual inspection. A variance inflation factor (VIF) below ten was used to indicate an acceptable level of multicollinearity in the regression analyses.
We evaluated CM as a potential predictor of IR and MetS by analyzing the area under the curve (AUC) from receiver operating characteristic (ROC) curves. The optimal cut-off value of CM was located by finding the highest vertical axis point and the furthest left horizontal axis point, then maximizing the Youden Index [(sensitivity + specificity) −1] as previously described.24
All analyses were two-sided and were checked to conform to the appropriate test-dependent assumptions. Statistical significance was established at p < 0.05. Our analyses accounted for the complex NHANES design by 1) calculating 6-year sampling weights for the fasting subsample; 2) using NHANES-generated sampling statistical strata, clusters, and weights; and 3) performing all analyses using PROC SURVEY procedures (e.g., FREQ, REG, and MEANS) in SAS (version 9.4, SAS Institute, Inc; Cary, NC).25
Results
Sample Characteristics
The sample characteristics by sex and CMD risk group are presented in Table 1. Of the total sample (N = 2,191), 60.4% (N = 1,323) were classified as low/no CMD risk, 5.4% (N= 118) MetS, 21.9% (N= 480) IR, and 12.3% (N = 270) IR + MetS. Overall, the sample was 43.3% female, 44.2% (N = 968) Non-Hispanic White, 23.1% (N = 506) African American, 32.7% (N = 717) Mexican American, and the weighted mean age was 30.3 ± 9.8 years. The mean number of MetS criteria for the total sample was 1.4 ± 1.1: 0.9 ± 0.7 in the low/no CMD risk group, 3.2 ± 0.4 in the MetS group, 1.3 ± 0.8 in the IR group, and 3.4 ± 0.6 in the IR + MetS group. The mean HOMA-IR score was 2.3 ± 0.4 for the total sample: 1.5 ± 0.5 in the low/no group, 1.9 ± 0.5 in the MetS group, 3.9 ± 1.1 in the IR group, and 4.6 ± 1.6 in the IR+MetS group.
Table 1.
Comparison of Sample Characteristics by Sex and CMD Risk Group
| Characteristics | Low/No Risk (n=1,323) | High/At Risk Groups |
||||||
|---|---|---|---|---|---|---|---|---|
| Metabolic Syndrome Only (n=118) | Insulin Resistance Only (n=480) | Insulin Resistance + Metabolic Syndrome (n=270) | ||||||
| Women (n=592) | Men (n=731) | Women (n=42) | Men (n=76) | Women (n=212) | Men (n=268) | Women (n= 103) | Men (n=167) | |
| Demographics | ||||||||
| Age, years | 29.72 (9.47) | 29.79 (9.66) | 37.19 (7.88) B | 35.24 (8.82) C | 26.45 (9.11) A | 28.66 (9.69) | 33.01 (10.04) B | 35.93 (9.74) C |
| Non – Hispanic white, % | 55.41 | 44.87 | 38.10 | 55.26 | 25.00 C | 34.70 | 36.89 A | 41.92 |
| Non – Hispanic black, % | 19.43 | 26.16 | 21.43 | 11.84 B | 29.25 B | 23.13 | 30.10 | 16.17 |
| Mexican – American, % | 25.17 | 29.00 | 40.48 | 32.89 | 45.75 B | 42.16 B | 33.01 | 41.92 |
| Anthropometrics | ||||||||
| Weight, kg | 64.88 (13.34) | 75.34 (12.93) | 79.95 (12.14) C | 91.33 (14.69) C | 76.47 (18.28) C | 84.79 (15.67) C | 86.17 (15.56) C | 94.91 (14.47) C |
| Height, cm | 163.02 (7.09) | 175.85 (7.78) | 161.40 (5.76) | 176.29 (7.61) | 162.48 (6.89) | 175.26 (8.23) | 162.81 (6.26) | 175.20 (7.40) |
| Body Mass Index, kg/m2 | 24.38 (8.49) | 24.30 (3.48) | 30.68 (4.34) C | 27.37 (4.30) C | 28.89 (6.31) C | 27.55 (4.50) C | 32.40 (5.01) C | 30.87 (4.02) C |
| DXA Body Composition | ||||||||
| Total Fat Mass, kg | 35.29 (5.99) | 17.47 (6.51) | 34.17 (8.08) C | 27.31 (7.76) C | 31.59 (11.38) C | 24.16 (8.81) C | 37.04 (9.70) C | 29.77 (7.99) C |
| Total Fat Mass, % | 23.68 (8.49) | 22.43 (5.40) | 42.03(4.19) C | 29.23 (4.68) C | 39.90 (5.96) C | 27.49 (6.04) C | 42.10 (4.57) C | 30.70 (4.62) C |
| Total Lean Mass, kg | 39.70 (5.92) | 55.94 (8.06) | 44.17 (4.98) B | 62.04 (8.41) C | 43.40 (7.62) C | 58.78 (8.52) C | 47.57 (6.86) C | 63.23 (8.09) C |
| Clinical Measures | ||||||||
| HOMA – IR | 1.55 (0.56) | 1.52 (0.52) | 1.88 (0.51) C | 1.86 (0.50) C | 3.84 (1.13) C | 3.84 (1.16) C | 4.70 (1.64) C | 4.54 (1.59) C |
| Insulin, fasting, uU/ml | 7.13 (2.52) | 6.68 (2.25) | 8.22 9 (2.23) B | 7.76 (2.23) C | 16.91 (4.94) C | 16.40 (5.09) B | 19.62 (7.15) C | 18.16 (6.45) C |
| Glucose, fasting, mg/dl | 88.10 (6.39) | 91.94 (7.39) | 93.09 (8.57) C | 94.04 (8.44) C | 92.05 (6.34) B | 95.55 (8.25) C | 97.77 (8.46) C | 101.94 (8.85) C |
| Waist Circumference, cm | 82.30 (10.86) | 86.24 (10.31) | 98.48 (7.72) C | 102.76 (10.63) C | 92.38 (14.78) C | 95.51 (12.60) C | 102.47 (10.23) C | 105.61 (10.06) C |
| Systolic BP, mmHg | 108.15 (10.65) | 116.01 (11.10) | 122.40 (19.51) B | 124.71 (13.26) C | 110.58 (9.26) B | 117.58 (9.55) B | 118.69 (8.31) C | 125.13 (14.82) B |
| Diastolic BP, mmHg | 67.15 (9.20) | 68.91 (11.12) | 75.17 (12.93) A | 76.07 (10.60) C | 67.94 (9.25) | 70.86 (11.55) C | 72.81 (10.92) B | 77.51 (12.90) C |
| Triglycerides, fasting, mg/dl | 91.24 (81.48) | 95.92 (50.29) | 172.88 (98.25) B | 208.91 (223.03) B | 99.67 (62.47) | 139.56 (98.37) C | 181.32 (80.31) C | 237.14 (159.18) C |
| HDL-C,fasting, mg/dl | 58.12 (15.04) | 50.75 (12.52) | 48.24 (11.88) C | 42.70 (11.39) A | 51.29 (10.76) C | 45.52 (10.93) C | 42.59 (9.34) C | 38.68 (8.75) C |
| Serum Measures | ||||||||
| Potassium, serum, mmol/L | 4.04 (0.04) | 4.16 (0.01) | 4.05 (0.04) | 4.10 (0.04) | 4.09 (0.03) A | 4.13 (0.02) | 4.10 (0.04) A | 4.11 (0.03) |
| Sodium, serum, mmol/L | 139.13 (0.13) | 139.41 (0.16) | 139.13 (0.34) | 139.19 (0.26) | 139.61 (0.23) | 139.48 (0.19) | 138.59 (0.23) | 139.44 (0.14) |
| Chloride, serum, mmol/L | 103.83 (0.15) | 102.70 (0.18) | 104.97 (0.49) | 103.42 (0.37) | 104.08 (0.28) | 103.09 (0.26 | 104.26 (0.30) | 103.16 (0.25) |
| Osmolality, serum, mOsm/L | 275.51 (0.25) | 277.91 (0.31) | 276.65 (0.66) | 277.59 (0.54) | 275.73 (0.46) | 278.40 (0.37) | 275.50 (0.38) | 278.52 (0.27) |
| BIS Parameters | ||||||||
| CM, nF | 1.48 (0.34) | 2.37 (0.52) | 1.55 (0.24) B | 2.57 (0.51) B | 1.76 (0.26) C | 2.69 (0.49) B | 1.92 (0.36) C | 2.80 (0.53) C |
| RI, ohms | 1576.68 (270.05) | 1118.16 (186.85) | 1381.86 (192.09) C | 1051.58 (191.54) | 1460.58 (275.99) C | 1108.32 (192.46) | 1308.21 (186.06) C | 1108.32 (192.46) C |
| RE, ohms | 720.74 (85.94) | 610.27 (70.19) | 648.60 (66.16) C | 576.63 (68.47) A | 675.70 (96.09) B | 605.51 (71.87) | 634.16 (65.49) C | 605.51 (71.87) C |
| Medication Use | ||||||||
| Dyslipidemia Meds, % | 0.17 | 0.55 | 2.38 | 2.63 | 1.42 | 1.49 | 3.88 | 4.19 |
| Hypertension Meds, % | 0.17 | 0.14 | 4.76 | 1.32 | 0.00 | 0.75 | 1.94 | 1.20 |
| Health Behaviors | ||||||||
| Smoking, % | 28.89 | 38.99 | 47.62 | 43.42 | 17.92 | 32.84 | 36.89 | 47.71 |
| Alcohol Use, % | 53.21 | 64.57 | 64.29 | 81.58 | 34.91 A | 59.33 | 40.78 A | 13.59 A |
Data are given as mean (SD) value or percentage of participants unless otherwise specified.
Abbreviations: DXA, dual-x-ray-absorptiometry; HOMA-IR, homeostasis model assessment of insulin resistance; cm, centimeters; HDL-C, High density lipoprotein cholesterol; BIS, bioimpedance spectroscopy; CM, membrane capacitance; RI, resistance of intracellular fluid; RE, resistance of extracellular fluid.
Superscripts denote significance in ANOVA (continuous) or χ2 (categorical) comparisons:
p < 0.05
p < 0.01
p < .0001
Comparison of CM Between the High/At Risk and Low/No Risk Groups
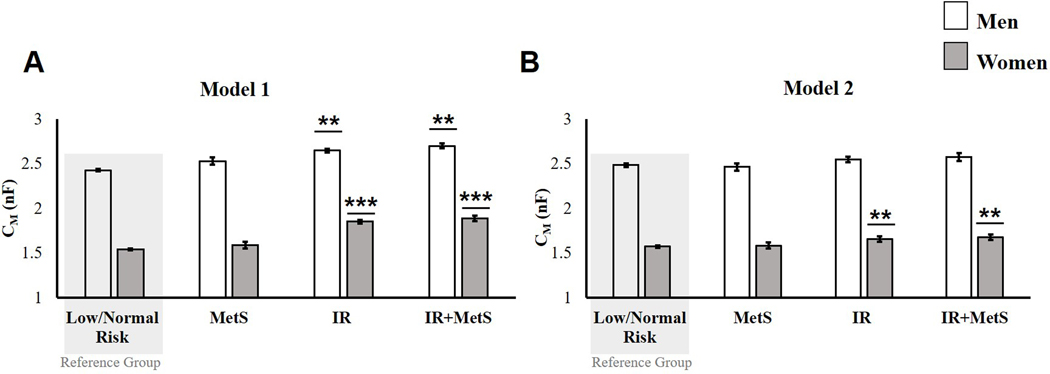
In model 1 (Figure 1A), CM was significantly higher among women in the IR (1.85 ± 0.02 nF, p = 0.0007) and IR + MetS (1.89 ± 0.05 nF, p = 0.0012) groups, compared to women in the low/no risk group (1.53 ± 0.01 nF). CM was also significantly higher among men in the IR (2.65 ± 0.02 nF, p =0.0002) and IR + MetS (2.70 ± 0.03, p <0.0001) groups, compared to men in the low/no-risk group (2.43 ± 0.02 nF). Among, women (1.67 ± 0.05 nF, p = 0.4526) and men (2.53 ± 0.04 nF, p = 0.1126) in the MetS groups, CM was not significantly different from those with low/no risk. In model 2 (fully adjusted) (Figure 1B), CM was significantly higher for women in the IR (1.66 ± 0.03, p = 0.0142) and IR + MetS (1.68± 0.03 nF, p = 0.0336) groups, compared to women with low/no risk (1.57 ± 0.03 nF). For men, CMD risk group was not a significant predictor of CM after adjusting for covariates [F (3,44) = 1.54, p=0.2173]. There were no significant differences between men in the low/no risk group (2.48 ± 0.02 nF) and higher risk groups (MetS: 2.46 ± 0.08 nF, p = 0.9842; IR: 2.46 ± 0.03 nF, p = 0.2170; or MetS + IR: 2.55 ± 0.04, p = 0.2915).
Figure 1.
ANCOVA comparing the membrane capacitance (CM) of women and men with low/no CMD risk (reference groups) to those in groups with increased CMD risk: metabolic syndrome (MetS), insulin resistance (IR), and insulin resistance and metabolic syndrome (IR + MetS). A) Model 1 adjusted for height2and intracellular resistance (RI); and B) Model 2 adjusted for height2, RI, age, African-American race/ethnicity, Mexican American race/ethnicity, DXA lean massǂ, DXA fat massǂ, medication use, smoking, and alcohol use.
Data are expressed as the least-squares adjusted mean ± standard error.
**p < 0.001 versus the low/no-risk group; *** p < 0.0001 versus the low/no-risk
ǂ variable is log-transformed.
Abbreviations: nF, nanoFarads; DXA, Dual-x-ray-absorptiometry.
Associations of CM with Clinical Measures Related to CMD Risk
In model 1, (Table 2) among women, CM was associated with HOMA-IR (β = 0.15, p < 0.0001), fasting insulin (β = 0.14, p < 0.0001), fasting glucose (β = 0.10, p < 0.0001), waist circumference (β = 0.18, p < 0.0001), and diastolic blood pressure (β = 0.05, p = 0.0406). Among men, CM was associated with HOMA-IR (β = 0.12, p < 0.0001) fasting insulin (β = 0.12, p < 0.0001), fasting glucose (β = 0.06, p = 0.0022), waist circumference (β = 0.18, p < 0.0001), diastolic blood pressure (β = 0.08, p = 0.0406), triglycerides (β = 0.07, p < 0.0001), and HDL-C (β = −0.03, p = 0.0427). In model 2 (fully adjusted), (Table 2) among women, CM was associated with HOMA-IR (β = 0.11, p = 0.0007), fasting insulin (β = 0.10, p = 0.0020), fasting glucose (β = 0.07, p = 0.0026), and waist circumference (β = 0.13, p = 0.0289). Among men, CM was associated with HOMA-IR (β = 0.06, p = 0.0381), fasting insulin (β = 0.05, p = 0.0385), and waist circumference (β = 0.17, p = 0.0102).
Table 2.
Multiple Linear Regression Results For Membrane Capacitance (CM)
| Model 1 Standardized β (p-value) |
Model 2 Standardized β (p-value) |
|||
|---|---|---|---|---|
| Clinical Measures | Women | Men | Women | Men |
| HOMA-IR ǂ | 0.15 (<0.0001) | 0.12 (<0.0001) | 0.11 (0.0007) | 0.06 (0.0381) |
| Fasting Insulin, uU/ml ǂ | 0.14 (<0.0001) | 0.12 (<0.0001) | 0.10 (0.0020) | 0.05 (0.0385) |
| Fasting Glucose, mg/dl | 0.10 (<0.0001) | 0.06 ( 0.0022) | 0.07 (0.0026) | 0.02 (0.2684) |
| Waist Circumference, cm | 0.18 (<0.0001) | 0.18 (<0.0001) | 0.13 (0.0289) | 0.17 (0.0102) |
| Systolic Blood Pressure, mmHg | 0.02 (0.2936) | 0.04 (0.0624) | −0.04 (0.1117) | −0.00 (0.8509) |
| Diastolic Blood Pressure, mmHg | 0.05 (0.0406) | 0.07 (<0.0001) | 0.00 (0.6792) | 0.00 (0.1839) |
| Fasting Triglycerides, mg/dl ǂ | 0.05 (0.0546) | 0.08 (<0.0001) | 0.05 (0.0528) | 0.03 (0.0574) |
| HDL-C, mg/dl | −0.03 (0.1946) | −0.03 (0.0427) | 0.00 (0.9473) | 0.00 (0.8354) |
Model 1 is adjusted for height2 and intracellular resistance (RI).
Model 2 is adjusted for height2, RI, age, African-American race/ethnicity, Mexican American race/ethnicity, DXA lean massǂ, DXA fat massǂ, medication use, smoking, and alcohol.
indicates that variable is log-transformed.
Bold values indicate statistical significance, p < 0.05.
Abbreviations: HOMA-IR, homeostasis model assessment of insulin resistance; HDL-C, High density lipoprotein cholesterol; DXA, dual-x-ray-absorptiometry.
CM as a predictor of IR and MetS
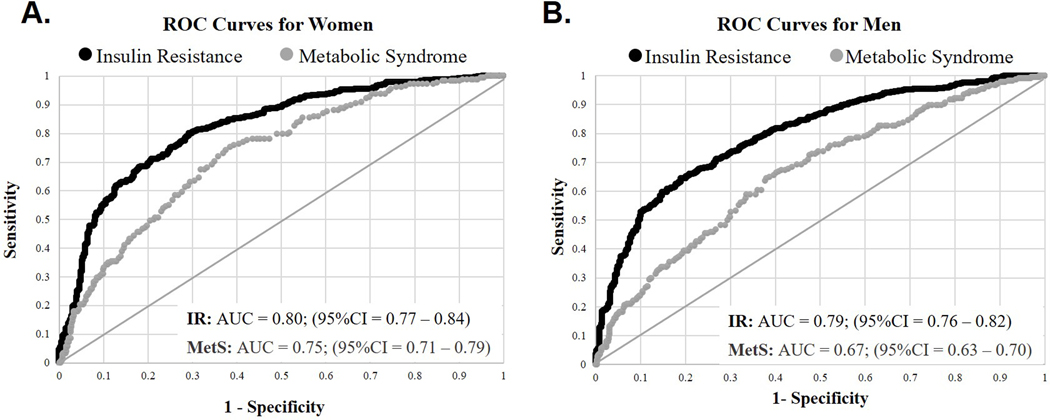
For women, CM was a significant predictor of IR [AUC = 0.80; (95% CI = 0.77 – 0.84)] and MetS [AUC = 0.75; (95% CI = 0.71 – 0.79)] (Figure 2A). For men, CM was also a significant predictor of IR [AUC = 0.79; (95% CI = 0.76 – 0.82)], and MetS [AUC = 0.67; (95% CI = 0.62 – 0.70)] (Figure 2B). The optimal cut-off value for CM as a predictor of IR was calculated as 1.65 nF for women and 2.67nF for men.
Figure 2.
Receiver operating characteristic (ROC) curves for membrane capacitance (CM) as a predictor of insulin resistance (black lines) and metabolic syndrome (grey lines). ROC models are adjusted for height2 and intracellular resistance (RI). The area under the curves (AUC) are shown for A) women [IR: AUC = 0.80 (95%CI = 0.77 – 0.84); MetS: AUC = 0.75 (95%CI = 0.71 – 0.79)] and B) men [IR: AUC = 0.79 (95%CI = 0.76 – 0.82); MetS: AUC = 0.67 (95%CI = 0.63 – 0.70)].
Discussion
The main finding of this study was that higher CM is associated with IR. We found that, compared to those with low/no disease risk, women and men with IR regardless of MetS status had higher CM. We also found that higher CM was present in women with IR independent of tested confounders. In contrast, for men with IR, DXA fat and lean mass accounted for the observed higher CM. We determined that women and men with low/no disease risk had an approximate CM of 1.53 nF and 2.43 nF, respectively, and that the optimal cut-off for IR within this cohort was 1.65 nF for women and 2.67 nF for men. Reference values for CM have not been reported in women, but our findings are consistent with the previously reported CM of 2.41 nF for the average healthy man.15 To the best of our knowledge, this is the first study to investigate the association of CM with IR. Nevertheless, our findings align with studies of the phase angle at 50 kHz, which is widely investigated and reported to reflect CM.15,26 Such studies indirectly show that higher CM is associated with IR in women with obesity.16,27 However, such associations have not been previously reported for men. Hence, the results of this study add to the existing literature that IR is associated with higher CM in apparently healthy women and men and that determinants of the association may be sex-specific. The results of this study provide evidence that with further investigation, higher CM might provide a non-invasive indicator of IR in relatively healthy women and men.
The second finding of this study was that higher CM was not associated with MetS, as it is clinically diagnosed by the NCEP: ATP III, in the absence of IR. We found that compared to those with low/no disease risk, CM was not statistically different among women or men with MetS in the absence of IR. We found that CM was positively associated with three of five clinical measures used to screen for MetS and associated with all five measures among men. However, after fully adjusting for covariates, only the association with waist circumference in both sexes and glucose among women remained significant. Several studies have reported an association between MetS and the phase angle, and MetS is linked to disturbances of the cell membrane and its surrounding milieu.28,29 One explanation for the difference in our study is that our MetS groups were relatively small. As a result, we may have been underpowered to detect a difference in CM, relative to the low/no risk groups. An alternative and more likely explanation is that previous bioimpedance studies might have detected IR, rather than MetS. This explanation is plausible because IR is the principal cause of cellular disturbances in MetS.1,2 Furthermore, most individuals with MetS generally also have IR.3 Taken together, the results of this study suggest that the association between CM and MetS is explained by other factors such as IR.
The association between CM and IR may be related, at least in part, to the underlying disruptions to the cell membrane that occur in IR. CM is a reflection of the health and function of cell membranes (e.g., membrane polarization, porosity, thickness, and integrity), which are essential regulators of insulin action and an important site of insulin resistance.11,12,27 IR is characterized by lower membrane fluidity, higher saturation, increased cell volume/surface area (skeletal muscle and adipose tissue), higher resting membrane potential, and lower hydration status.17–21,30 Therefore, it is plausible that these factors would lead to higher CM in those with IR.31–33 Some conditions that are co-morbid to IR, such as those of MetS may exert similar effects on the membrane.28,29 Therefore, future studies are needed to examine the specific mechanisms by which IR would influence CM and determine the sensitivity and specificity of the association between CM and IR.
The interpretation of this study’s findings should be made considering some limitations. First, this is a cross-sectional analysis of national survey data; therefore, causality could not be determined. Second, this study’s results may not be generalizable to persons with advanced age or higher body weight because NHANES only collected BIS data for adults less than 50 years old (range: 18–49) and under 300 lbs. (BMI range:15 to 49 kg/m2). Third, our analyses selected participants from the NHANES fasting subsample; however, some BIS data may not have been collected under fasting conditions. It is unclear to what extent this may have influenced results. Fourth, we were unable to account for the menstrual cycle or birth control usage among women, which may have influenced BIS measurements.34 Lastly, the dataset did not account for supplemental use of calcium, chloride, magnesium, and sodium and several other water-soluble vitamins (e.g., vitamin e) that could affect hydration status and electrolyte balance.
Our study has several strengths, despite its limitations. This study investigated an innovative and novel application for BIS, which is an existing technology that is affordable, easily accessible, and clinically-accepted. This study stringently tested hypotheses in a sample of relatively healthy adults that included an appropriate representation across sex and racial/ethnic groups. Lastly, our analyses rigorously adjusted for potential confounders and considered evidence from literature from electrophysiology and pathophysiology to support our inferences about CM.
With further investigation, measuring CM with BIS could be a cost-effective, non-invasive, and potentially high-throughput method for IR screening. Currently, IR screening is mostly not performed in patient-care settings due to a lack of clinically suitable methods.35,36 The results of this study suggest that a bioimpedance approach may detect bioelectrical variation in apparently healthy adults with IR. In this study, we found that the association between CM and IR, as reflected in the area under the ROC curves (0.80 for women; 0.79 for men) is similar to that observed between IR and more invasive surrogate measures such as fasting insulin and triglycerides.3 Collectively, the results of this study indicate that further research into CM is warranted.
This study should be followed up with further rigorous studies to inform on the reproducibility and validity of the association between CM and IR. Therefore, future studies should 1) evaluate CM against a more accurate measure of insulin sensitivity, such as would be given by the euglycemic clamp; 2) quantify the effects of hydration status and total body water taken by rigorous methods on CM 3) verify clinical cut-offs and explore the need for race/ethnicity- and age-specific cut-offs; 4) evaluate the reliability of the association between CM and IR with longitudinal studies and currently available BIS instruments.
Altogether, we determined that higher CM is significantly associated with IR in relatively healthy men and women. CM was not elevated in those with MetS by its clinical diagnostic criteria when isolated from IR, indicating that higher CM is specific to IR. The results of this study provide the first evidence that with further studies, CM has the potential to be used in the future as a non-invasive indicator of IR in men and women without chronic disease.
Study Importance.
- What is already known about this subject?
- Bioelectrical impedance spectroscopy (BIS) can be used to investigate the cellular health and fluid status of cells and tissues by non-invasively passing alternating current through the body.
- Membrane capacitance (CM) is formally the ratio of charge to potential across a cell membrane. CM measures the ability for cell membranes within the path of an applied current to store electrical charge and reflects the membrane’s structural integrity and substrate/electrolyte transport.
- What does your study add?
- Higher CM is associated with insulin resistance (IR), defined as a homeostatic model assessment of insulin resistance (HOMA-IR) > 2.6) in relatively healthy men and women.
- CM is linearly associated with HOMA-IR, waist circumference, and fasting insulin. These associations are independent of age, sex, race/ethnicity, height, fat mass, and lean mass.
- How might your results change the direction of research or the focus of clinical practice?
- The results of this study indicate that further investigation of the association between CM and insulin resistance is warranted. Measuring CM could be a potential approach to detect cell membrane dysfunction associated with IR. However, given that this is the first study to investigate this association, further rigorous studies must be performed to guide clinical applications.
Acknowledgments
The authors gratefully acknowledge the Division of Health and Nutrition Examination Surveys at the National Center for Health Statistics (NCHS) for providing the data used in this study. This work was supported by the National Institutes of Health [T32HL105349, P30DK056336, P30DK079626, and 4R25GM086256-08].
Funding: Material resources, in the form of data, were provided by the Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, [1999–2004].
Footnotes
Disclosure: The authors have nothing to disclose.
References
- 1.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5–6):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139(10):802–809. [DOI] [PubMed] [Google Scholar]
- 4.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15–E26. [DOI] [PubMed] [Google Scholar]
- 5.Cefalu WT, Petersen MP, Ratner RE. The Alarming and Rising Costs of Diabetes and Prediabetes: A Call for Action! Diabetes Care. 2014;37(12):3137–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulasi U, Kuchnia AJ, Cole AJ, Earthman CP. Bioimpedance at the bedside: current applications, limitations, and opportunities. Nutr Clin Pract. 2015;30(2):180–193. [DOI] [PubMed] [Google Scholar]
- 7.Martinsen OG, Grimnes S. Bioimpedance and bioelectricity basics. Academic press; 2011. [Google Scholar]
- 8.Jodal L Electrical theory behind the measurement of body fluids with bioimpedance spectroscopy (BIS). Lecture notes. 2008. [Google Scholar]
- 9.De Lorenzo A, Andreoli A, Matthie J, Withers P. Predicting body cell mass with bioimpedance by using theoretical methods: a technological review. J Appl Physiol (1985). 1997;82(5):1542–1558. [DOI] [PubMed] [Google Scholar]
- 10.Bera TK. Bioelectrical Impedance Methods for Noninvasive Health Monitoring: A Review. J Med Eng. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brantlov S, Jødal L, Frydensbjerg Andersen R, Lange A, Rittig S, Ward LC. Bioimpedance Resistance Indices and Cell Membrane Capacitance Used to Assess Disease Status and Cell Membrane Integrity in Children with Nephrotic Syndrome. Sci World J. 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malecka-Massalska T, Mlak R, Smolen A, Brzozowska A, Surtel W, Morshed K. Capacitance of Membrane As a Prognostic Indicator of Survival in Head and Neck Cancer. PLoS One. 2016;11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dittmar M, Reber H, Kahaly GJ. Bioimpedance phase angle indicates catabolism in Type 2 diabetes. Diabet Med. 2015;32(9):1177–1185. [DOI] [PubMed] [Google Scholar]
- 14.Jun H, Kim S, Ku M-B, et al. Glucose-independent segmental phase angles from multi-frequency bioimpedance analysis to discriminate diabetes mellitus. Sci Rep. 2018;8(1):648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthie JR. Bioimpedance measurements of human body composition: critical analysis and outlook. Expert Rev Med Devices. 2008;5:239–261. [DOI] [PubMed] [Google Scholar]
- 16.De Luis D, Aller R, Romero E, Dueñas A, Perez Castrillon J. Relation of phase angle tertiles with blood adipocytokines levels, insulin resistance and cardiovascular risk factors in obese women patients. Eur Rev Med Pharmacol Sci. 2010;14(6):521–526. [PubMed] [Google Scholar]
- 17.Weijers R NM Lipid Composition of Cell Membranes and Its Relevance in Type 2 Diabetes Mellitus. Curr Diabetes Rev. 2012;8(5):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai AJ, Miller LJ. Changes in the plasma membrane in metabolic disease: impact of the membrane environment on G protein-coupled receptor structure and function. Br J Pharmacol. 2018;175(21):4009–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritter O, Jelenik T, Roden M. Lipid-mediated muscle insulin resistance: different fat, different pathways? J Mol Med (Berl). 2015;93(8):831–843. [DOI] [PubMed] [Google Scholar]
- 20.Pilon M Revisiting the membrane-centric view of diabetes. Lipids Health Dis. 2016;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perona JS. Membrane lipid alterations in the metabolic syndrome and the role of dietary oils. BBA-Biomembranes. 2017;1859(9):1690–1703. [DOI] [PubMed] [Google Scholar]
- 22.Lukaski H Evolution of bioimpedance: a circuitous journey from estimation of physiological function to assessment of body composition and a return to clinical research. Eur J Clin Pharmacol. 2013;67(S1):S2. [DOI] [PubMed] [Google Scholar]
- 23.Cornish B, Ward L. Data analysis in multiple-frequency bioelectrical impedance analysis. Physiol Meas. 1998;19(2):275. [DOI] [PubMed] [Google Scholar]
- 24.Abbasi F, Silvers A, Viren J, Reaven GM. Relationship between several surrogate estimates of insulin resistance and a direct measure of insulin-mediated glucose disposal: Comparison of fasting versus post-glucose load measurements. Diabetes Res Clin Pract. 2018;136:108–115. [DOI] [PubMed] [Google Scholar]
- 25.Johnson CL, Paulose-Ram R, Ogden CL, et al. National health and nutrition examination survey. Analytic guidelines, 1999–2010. 2013. [PubMed] [Google Scholar]
- 26.Gonzalez MC, Barbosa-Silva TG, Bielemann RM, Gallagher D, Heymsfield SB. Phase angle and its determinants in healthy subjects: influence of body composition. Am J Clin Nutr. 2016;103(3):712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartels EM, Sørensen ER, Harrison AP. Multi-frequency bioimpedance in human muscle assessment. Physiol Rep. 2015;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozhan H, Alemdar R, Caglar O, et al. Performance of Bioelectrical Impedance Analysis in the Diagnosis of Metabolic Syndrome. J Invest Med. 2012;60(3):587–591. [DOI] [PubMed] [Google Scholar]
- 29.Enomoto M, Adachi H, Fukami A, et al. a Useful Tool as a Medical checkup in a general Population—Bioelectrical impedance analysis. Front Cardiovasc Med 2017;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buscemi S, Blunda G, Maneri R, Verga S. Bioelectrical characteristics of type 1 and type 2 diabetic subjects with reference to body water compartments. Acta Diabetologica. 1998;35(4):220–223. [DOI] [PubMed] [Google Scholar]
- 31.Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr. 2004;23(5):1226–1243. [DOI] [PubMed] [Google Scholar]
- 32.Jaffrin MY, Morel H. Body fluid volumes measurements by impedance: A review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods. Medical engineering & physics. 2008;30(10):1257–1269. [DOI] [PubMed] [Google Scholar]
- 33.Chumlea WC, Guo SS, Cockram DB, Siervogel RM. Mechanical and physiologic modifiers and bioelectrical impedance spectrum determinants of body composition. Am J Clin Nutr. 1996;64(3):413S–422S. [DOI] [PubMed] [Google Scholar]
- 34.Gleichauf CN RD. The menstrual cycle’s effect on the reliability of bioimpedance measurements for assessing body composition. Am J Clin Nutr. 1989;50(5):903–907. [DOI] [PubMed] [Google Scholar]
- 35.Borai A, Livingstone C, Kaddam I, Ferns G. Selection of the appropriate method for the assessment of insulin resistance. BMC medical research methodology. 2011;11(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAuley KA, Williams SM, Mann JI, et al. Diagnosing insulin resistance in the general population. Diabetes care. 2001;24(3):460–464. [DOI] [PubMed] [Google Scholar]