Abstract
Background:
The effectiveness of severely reduced left ventricular ejection fraction (LVEF <35%) as a predictor of sudden cardiac death (SCD) has diminished, and improvements in risk stratification await discovery of novel markers. Right ventricular (RV) abnormalities can be observed in conditions such as chronic obstructive pulmonary disease and sleep apnea, which have been linked to SCD.
Objective:
We evaluated whether RV abnormalities were associated with SCD after accounting for LVEF and other patient characteristics.
Methods:
In a large, prospective ongoing community-based study of SCD in the Portland OR metro area, SCD cases (age ≥18, 2002–2014) were compared to controls with coronary artery disease but no SCD. Using a novel archive of digital echocardiograms, a standardized approach was employed for evaluation of basal RV diameter (RVBD), RV end-diastolic area (RVEDA), and RV fractional area change (RVFAC).
Results:
A total of 350 subjects were studied, including SCD cases (n=81, 68.7 ± 13.6 years, 73% male) and controls (n=269, 66.5 ± 10.2 years, 69% male). In multivariate analysis, RVFAC was significantly associated with SCD (OR: 1.14 for each 5% decrease; 95% CI: 1.03–1.25; p=0.01). When modeled with LVEF ≤35%, RVFAC ≤35% was significantly associated with increased risk of SCD. Individuals with both LV and RV dysfunction had a three times higher odds of SCD than those with neither (OR: 3.19; 95% CI: 1.33–7.68; p=0.01).
Conclusion:
RV dysfunction was associated with a significantly increased risk of SCD independent of LVEF, and when combined with LVEF, had additive effects on SCD risk.
Keywords: cardiac arrest, sudden death, right ventricle, risk prediction, echocardiogram
Introduction
Sudden cardiac death (SCD) remains a significant public health problem worldwide with an annual incidence in the United States of approximately 350,000.1 In the landmark primary prevention trials, implantable cardioverter defibrillators (ICD) were shown to reduce mortality from SCD by delivering timely shock therapies.2,3 Indications for ICDs, however, remain narrow and reliant primarily on a reduced left ventricular ejection fraction (LVEF) of less than 35%.4 Recent studies have indicated that LVEF <35% is likely to be inadequate as the sole risk stratification criterion for SCD especially since it only accounts for approximately one third of cases.5–9 In a recent meta-analysis performed from the ARIC (Atherosclerosis Risk in Communities) and CHS (Cardiovascular Health Study) cohorts, Konety and colleagues reported associations between SCD and multiple echocardiographic predictors of SCD including LVEF, mitral annular calcification, increased left ventricular mass, increased left atrial diameter, and abnormal LV geometry.10 With these findings, we know that the echocardiogram is likely to provide more SCD predictors than the LVEF alone. Recently, conditions such as chronic obstructive pulmonary disease11 and obstructive sleep apnea12 have been associated with increased SCD risk. Both of these conditions are known to be causal in pulmonary hypertension13 and have detrimental long-term effects on the function of the right ventricle (RV).14–16 To date, there have been few studies evaluating the impact of RV dysfunction on the risk of SCD and what the best echocardiographic assessment for this purpose may be. We therefore sought to determine whether standard measures of right ventricular function including fractional area change (RVFAC), end-diastolic area (RVEDA), and basal diameter (RVBD) could be utilized as novel SCD predictors.
Methods
Study Population:
Case subjects included in this analysis were drawn from the ongoing Oregon Sudden Unexpected Death Study (Oregon SUDS), from SCD cases that occurred between February 1, 2002 and January 31, 2015 in the Portland, Oregon metropolitan area (population of ~1 million). As previously published from Oregon SUDS,17 SCD cases were identified via multiple sources including fire department, ambulance services, local hospital emergency rooms, and the county medical examiner’s office. SCD was defined as a sudden and unexpected pulseless condition of likely cardiac etiology if witnessed, and a sudden death within 24 hours of last having been seen in usual state of health if unwitnessed. All identifiable non-cardiac causes of death including trauma, drug overdose, pulmonary embolism, cerebrovascular accident, or chronic terminal illness were excluded. Survivors of SCD were included as cases. All cases of SCD were adjudicated via a three-physician review panel with access to all available medical records and autopsy reports. Control subjects were obtained from multiple sources including chest pain patients attended by emergency medical services, outpatient clinics, patients undergoing angiography, and patients from a large health maintenance organization in the Portland metro area. Control subjects were selected to be enriched for coronary artery disease, and 91% of controls included in the analysis had coronary artery disease, defined by ≥50% stenosis of a major coronary artery, history of myocardial infarction, or history of coronary artery bypass grafting or percutaneous coronary intervention. Controls with a prior history of ventricular arrhythmia or cardiac arrest were excluded. For this analysis we included all subjects age ≥18 with an available digital echocardiogram in electronic medical records. For SCD cases, the digital echocardiogram closest and prior to the SCD event was used for analysis. Digital echocardiogram files of cases and controls were de-identified and stored on a password protected digital archive in a core lab. This study was approved by the Institutional Review Boards of Cedars-Sinai Medical Center, Oregon Health and Science University, and all participating hospitals and health systems. All survivors of cardiac arrest provided informed consent; for non-survivors this requirement was waived.
Echocardiographic Assessment:
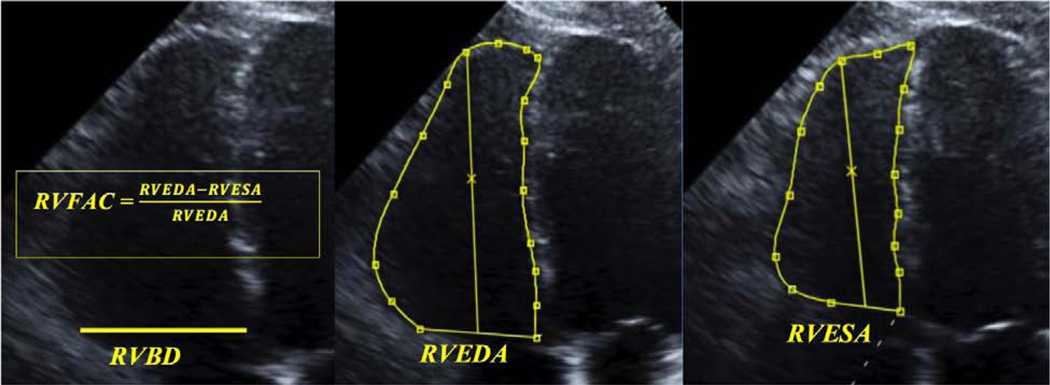
Using a three-physician blinded and standardized reading protocol, direct measurements were made from the digital echocardiograms of SCD cases and controls. The primary reader (SP) made all quantitative measurements of echocardiograms using ScImage PICOM 365 software (ScImage, Los Altos, CA). All measurements were then over-read by a second reader (TN), a cardiologist with special expertise in echocardiography. In case of disagreement, a second cardiologist with special expertise in echocardiography (TS) reviewed the echocardiogram and the majority vote determined the evaluation. LVEF was measured using the standard Simpson’s biplane method in the four chamber and two chamber views. To determine the presence of left ventricular hypertrophy (LVH), measurements were made in the parasternal long axis of the left ventricular internal diameter in diastole (LVIDD), posterior wall thickness in diastole (PWTD), and interventricular septal thickness in diastole (IVSTD). Using the formula recommended by the American Society of Echocardiography, LV mass index was then calculated as (0.8 × (1.04 [(LVIDD + PWTD + IVSTD)3− (LVIDD)3]) + 0.6) g divided by the body surface area in m2. LVH was defined as an LV mass index greater than 134 g/m2 for men and 110 g/m2 for women.18 Using the four-chamber view, measurements were made of RVEDA, RV end-systolic area (RVESA), and RVBD (Fig. 1) as per the 2010 ASE guidelines.19 RVFAC was calculated as the difference between RVEDA and RVESA, divided by RVEDA, and multiplied by 100%. Other measures of RV function such as tricuspid annular plane systolic excursion (TAPSE), RV index of myocardial performance (RIMP), tricuspid annular S′ wave velocity, RV strain, and RV systolic pressure were not consistently measurable in the majority of digital echocardiograms and thus were not included for comparison in this study.
Figure 1:
Measures of RV Function. RVBD: RV basal diameter. RVEDA: RV end-diastolic area. RVESA: RV end-systolic area. RVFAC: RV fractional area change
Statistical Analysis:
Baseline characteristics of SCD cases and controls including age, sex, and the presence of hypertension, diabetes mellitus, obesity (BMI ≥30), sleep apnea, and chronic obstructive pulmonary disease were compared using independent-samples t-tests with statistical significance set at a 2-tailed P value of ≤0.05. Additionally, the presence of LVH, mean LVEF, and proportion with LVEF ≤ 35% were also compared using available data from the digital echocardiograms. Similarly, the measured RV parameters of RVBD, RVEDA, and RVFAC were also compared.
Correlations were evaluated between RV measures of function and based on this a multivariable logistic regression analysis was used to calculate an odds ratio and 95% confidence interval for standard unit changes in RVEDA and RVFAC. An additional multivariable analysis was performed to evaluate SCD risk in subjects with only LVEF ≤ 35% or RVFAC ≤ 35%, and those with both LVEF ≤ 35% and RVFAC ≤ 35%. A joint distribution chi-squared analysis was also performed between these groups and the reference group of neither LVEF ≤ 35% nor RVFAC ≤ 35%. Both logistic regression models were adjusted for age, sex, diabetes mellitus, LVH, and LVEF. All analyses were performed using SAS version 9.3 (SAS Institute Inc, Cary, NC).
Results
Baseline Characteristics:
Digital echocardiograms were available for a total of 81 SCD cases and 269 controls. For cases, 50 of the 81 (62%) had echocardiograms performed within one year of arrest. Controls had echocardiograms performed within one year of ascertainment for 210 of 269 (78%). As shown in Table 1, there was no significant difference between the groups by age or sex, with a male predominance in both groups. There was no difference in the prevalence of most comorbid conditions including hypertension, obesity, chronic obstructive pulmonary disease (COPD), and sleep apnea. However, the prevalence of diabetes mellitus was higher in SCD cases than controls (59% vs 40%, p= 0.001), as was prevalence of left ventricular hypertrophy (LVH) (38% vs 17%, p <0.001). Mean LVEF was lower in SCD cases (0.44 ± 0.14 vs 0.47 ± 0.12, p = 0.04) and the proportion with LVEF ≤35% was higher in SCD cases (32% vs 19%, p=0.01).
Table 1:
Baseline Clinical Characteristics of SCD cases vs controls
| Total (n=350) | SCD Cases (n=81) | Controls (n=269) | p-value |
|---|---|---|---|
| Age, y | 68.7 ± 13.6 | 66.5 ± 10.2 | 0.17 |
| Male sex | 59 (73%) | 186 (69%) | 0.52 |
| Hypertension | 69 (85%) | 216 (81%) | 0.35 |
| Diabetes Mellitus | 48 (59%) | 104 (39%) | 0.001 |
| Obese (BMI ≥30) | 33 (45%) | 116 (44%) | 0.92 |
| Sleep Apnea | 16 (20%) | 34 (13%) | 0.11 |
| COPD | 19 (23%) | 44 (16%) | 0.15 |
| LVH | 28 (38%) | 45 (17%) | <0.001 |
| Mean LVEF | 0.44 ± 0.14 | 0.47 ± 0.12 | 0.04 |
| LVEF ≤ 35% | 25 (31%) | 47 (17%) | 0.009 |
Diabetes, hypertension, COPD, and sleep apnea history missing for 1 control; obesity missing for 7 cases and 5 controls; LVH missing for 7 cases and 9 controls.
Measures of RV Function:
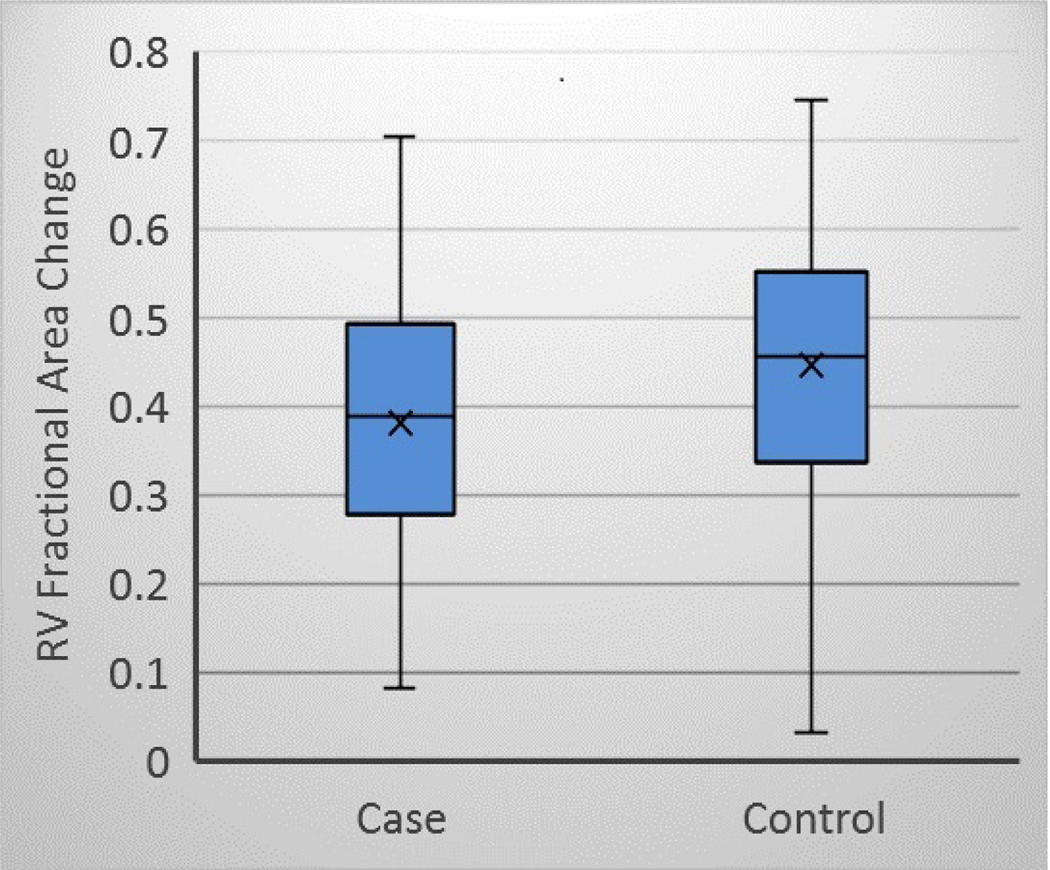
Differences were observed between SCD cases and controls for the mean values of each measure of RV function (Table 2). RVBD was higher in SCD cases (45.2 ± 9.9 vs 42.2 ± 8.7, p = 0.008). RVEDA was higher in SCD cases (24.7 ± 8.5 vs 22.4 ± 7.1, p = 0.03). RVFAC was lower in SCD cases (0.38 ± 0.14 vs 0.45 ± 0.14, p <0.001). The proportion of subjects with RVFAC <35% was also somewhat higher in SCD cases (38% vs 28% ± 0.12, p = 0.07). A boxplot distribution of RVFAC is shown in Figure 2.
Table 2:
Measures of RV function in cases vs controls
| Cases (n=81) | Controls (n=269) | p-value | |
|---|---|---|---|
| RVBD (mean ± SD) | 45.2 ± 9.9 | 42.2 ± 8.7 | 0.008 |
| RVEDA (mean ± SD) | 24.7 ± 8.5 | 22.4 ± 7.1 | 0.03 |
| RVFAC (mean ± SD) | 0.38 ± 0.14 | 0.45 ± 0.14 | <0.001 |
| RVFAC ≤35% | 31 (38%) | 75 (28%) | 0.07 |
RVBD: Basal RV diameter; RVEDA: RV end-diastolic area; RVFAC: RV fractional area change
Figure 2:
RVFAC in SCD cases and controls. Cases RVFAC mean 0.38 ± 0.14 and controls 0.45 ± 0.14, p<0.001.
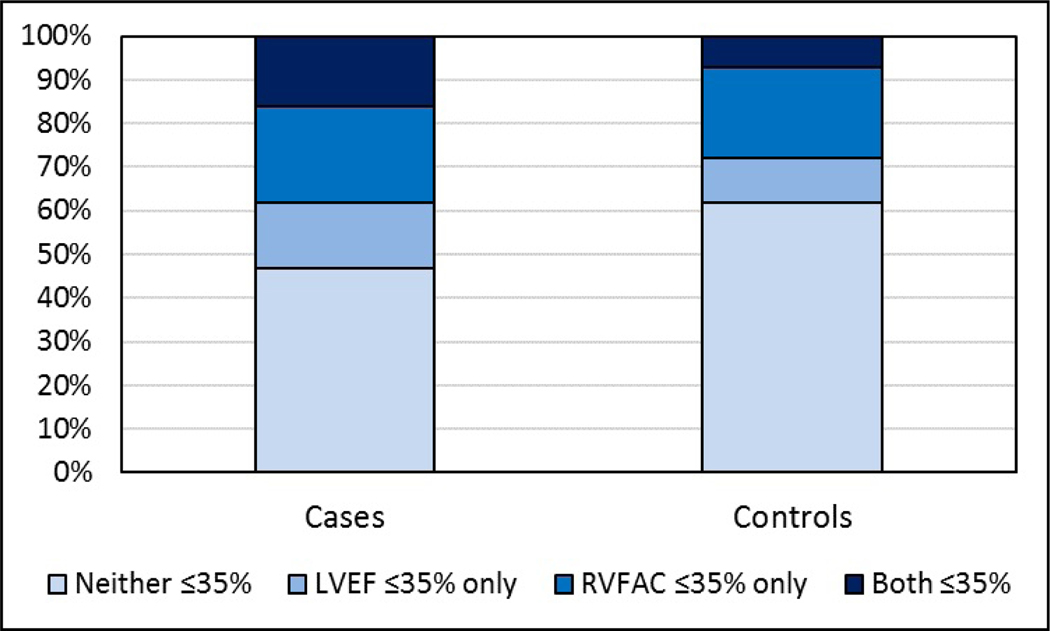
Before simultaneous modeling of RV and other measures, we examined correlations between RV variables. RVBD and RVEDA were strongly positively correlated (r=0.75, p<0.001), while RVFAC was less strongly and negatively correlated with both RVBD and RVEDA (r=−0.20, p<0.001 and r=−0.22, p<0.001 respectively). Because RVBD is a component of RVEDA, we included only RVEDA in the combined model with RVFAC. As detailed in Table 3, in a multivariable analysis including both RVFAC and RVEDA, RVFAC was significantly associated with SCD (Odds Ratio [OR]: 1.14 for each 5% decrease; 95% confidence interval [CI]: 1.03–1.25; p=0.01). In this model however, RVEDA did not significantly affect the risk of SCD (OR: 1.10 for each 5 cm2 increase; 95% CI: 0.92–1.32; p=0.29). As shown in Table 4, a separate multivariable analysis was also performed to determine the additive effect of RVFAC to LVEF in SCD risk prediction by modeling them together. For either those with only LVEF ≤35% or RVFAC ≤35% there was no significant increase in the risk of SCD. However, in those with both LVEF ≤35% and RVFAC ≤35%, there was a significant increase in the risk of SCD (OR: 3.19; 95% CI: 1.33–7.68; p=0.01). The joint distribution for subjects with and without LV or RV dysfunction is demonstrated in Figure 3 showing 16% of SCD cases vs 7% of controls had both LVEF ≤35% and RVFAC ≤35% (chi-squared p=0.03).
Table 3:
Multivariable analysis for measures of RV function as predictors of SCD
| Odds Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|
| 5cm2 increase in RVEDA | 1.10 | 0.92–1.32 | 0.29 |
| 5% decrease in RVFAC | 1.14 | 1.03–1.25 | 0.01 |
Model adjusted for age, sex, diabetes, LVH, and LVEF including 74 cases and 259 controls with complete data.
Model C statistic: 0.702
Model C statistic without RV measures (including only age, sex, diabetes, LVH, and LVEF): 0.677
Table 4:
Additive effects of RV and LV function as predictors of SCD
| Odds Ratio* | 95% Confidence Interval | p-value | |
|---|---|---|---|
| LVEF ≤35% only | 1.99 | 0.89 – 4.48 | 0.10 |
| RVFAC ≤35% only | 1.33 | 0.66 – 2.70 | 0.42 |
| Both LVEF and RVFAC ≤35% | 3.19 | 1.33 – 7.68 | 0.01 |
Reference category: Neither LVEF nor RVFAC ≤35%. Model adjusted for age, sex, diabetes, and LVH Model includes 74 cases and 259 controls with complete data. Model C statistic: 0.699
Figure 3:
Joint Distribution for LV and RV dysfunction. The proportion of subjects with both LVEF and RVFAC ≤35% was 16% of cases vs 7% of controls. Chi-square p-value=0.03
Discussion
To our knowledge, this is the first population-based study to report the association between reduced RV function and increased risk of SCD. We evaluated several potential quantitative measures of RV function to determine which could be reliably obtained in clinically acquired echocardiograms. Incremental changes in RVFAC were significantly associated with SCD in a multivariable analysis, independent of LVEF. In addition, our analysis demonstrated that when combined with LVEF ≤35%, an RVFAC ≤35% had an additive effect on prediction of SCD. This indicates that RVFAC has potential to enhance the current approach to SCD risk stratification beyond the LVEF.
Right ventricular dysfunction is emerging as a novel marker for risk stratification in SCD. Although RVFAC is a standard measure for echocardiographic assessment of right ventricular function,20 it is not clear at this time which measure of RV function would be the most suitable for risk stratification. Prior work has identified RV dysfunction as a potential predictor of SCD risk, though it has not been shown for quantitative measures in clinically acquired echocardiograms. Aktas et al. reported that severe RV dysfunction as subjectively determined by the reader of a 2D echocardiogram was independently associated with a combined endpoint of ICD therapy or death in a population that received ICDs for primary prevention of SCD.21 More recently, Makami et al. prospectively used RV ejection fraction as measured by cardiac MRI, the gold-standard for determining RV function, and were able to demonstrate that a reduced RVEF was a strong, independent predictor of arrhythmic events in a population with known systolic dysfunction by LVEF of ≤54%.22 Risum et al. found that RV free wall strain as measured by 2D chocardiographic analysis was significantly associated with ventricular arrhythmias/SCD and superior as a predictor when compared to TAPSE in an acute myocardial infarction population.23 One of the largest studies to date, by Naksuk et al. with 5463 subjects who had all been admitted to the coronary care unit at Mayo Clinic, Rochester, MN found that moderate to severe RV dysfunction as determined jointly by TAPSE, RV index of myocardial performance (RIMP), and tricuspid annular S′ wave velocity, was an incremental predictor of SCD in both patients with LVEF ≤ 35 % or LVEF >35%.24 These studies all indicate that RV dysfunction by various means of assessment can be predictive of SCD risk. In a recent meta-analysis by Lee et al., RVFAC was compared with TAPSE in its ability to correlate with RVEF by cardiac MRI.25 RVFAC was found to be superior in this regard, likely due to it being a two dimensional measure which allows it to account for regional differences in RV function.
Right ventricular failure can result from many different etiologies but most notably those that cause pulmonary hypertension by chronic hypoxemia.14–16 In 2015, Oregon SUDS demonstrated a link between COPD and SCD using 728 adjudicated cases.11 COPD was significantly associated with SCD (OR 2.2) independent of LVEF, medications, clinical markers, and ECG markers using a propensity score matched analysis. This relationship was found to be even stronger in subjects who had COPD and used short acting beta agonists but no beta blockers (OR 3.3). Obstructive sleep apnea (OSA) has also been linked to SCD as shown by Gami et. al who prospectively ascertained 142 cases of SCD in a cohort of over 10,000 patients undergoing routine polysomnography.12 They demonstrated that OSA along with its multiple parameters of severity were significantly predictive of SCD.
The mechanistic link between SCD and chronic hypoxic conditions such as COPD and sleep apnea requires further investigation, but there are several factors that can potentially be implicated. In individuals with heart disease, chronic hypoxemia would be expected to have a detrimental effect due to a reduced myocardial oxygen supply, especially during times of activity.26 Chronic hypoxia is also known to cause right ventricular remodeling that may over time increase the arrythmogenicity of the right ventricle by substrate modification.14 QTc intervals have been demonstrated to have increased duration and dispersion in both COPD and sleep apnea.27–29 These changes over time along with the increased sympathetic tone during hypoxic episodes30 can increase the potential for ventricular ectopy and subsequent deadly arrythmias. This is further supported in Oregon SUDS by the protective effect against SCD that cardio selective beta blockers seemed to have in COPD patients on short acting beta agonists.11
At this time, there is significant evidence that the LVEF is inadequate as the primary risk stratification tool for SCD.5–9 In order to improve risk stratification, other novel markers need to be identified and studied for their additive benefit to the LVEF in risk prediction.31 LVH has been previously demonstrated to predict risk of SCD (OR 1.8) independent of severely reduced LVEF (OR 1.9) in the Oregon SUDS,32 with LVH and severely reduced LVEF having an additive effect on SCD risk (OR 3.5). Our study similarly demonstrates that RV dysfunction was independently associated with an increased risk of SCD and had an additive effect when combined with the LVEF. When RVFAC ≤35% was combined with LVEF ≤35%, SCD risk prediction improved with OR 3.19. Thus, this novel marker may have a significant prognostic value in predicting SCD and improving risk stratification strategies. Given the inherent limitations of a case-control design, these results are not yet definitive and larger prospective studies of RVFAC in comparison with other measures of RV function are warranted.
Limitations:
Given that SCD occurs relatively infrequently in the general population (approximately 50/100,000 residents), we used a population-based case-control design to accrue feasible numbers for analysis. There are inherent limitations in community-based studies compared to cohort studies including missing information for patients that may not have seen a cardiologist and therefore did not receive an echocardiogram prior to their SCD event. Our results may be generalizable to individuals who have undergone clinically-indicated echocardiograms, a potentially important intermediate-risk population. Further, a digital echocardiogram file from each subject was required to perform a standardized reading of echocardiograms, which also reduced sample size. With this also comes the possibility that the selected cases may not be perfectly representative of the parent population. However, the comorbidity profile (obesity, hypertension, COPD, sleep apnea) was not significantly different comparing individuals with available digital echocardiograms to individuals who had echocardiogram results reported in clinical records but for whom no digital image was retrieved. Limiting the analysis to individuals with digital files available allowed standardized reading of all digital echocardiograms. Patients in our study did not have data on severity of pulmonary disease. We were able to assess pulmonary hypertension (by TR velocity) and loading (by diameter of the inferior vena cava) in a subset of echocardiograms, but each variable was missing for approximately 40% of subjects included in this analysis. When TR velocity and IVC diameter were included in a multivariable model in the subset with available data, the association of RVFAC with SCD was consistent though somewhat attenuated (from OR 1.14 per 5% decrease to OR 1.11 per 5% decrease). Future prospective studies would be well supplemented by also including data on loading, pulmonary artery pressures and severity of co-morbid pulmonary disease such as FEV1 for COPD and apnea-hypopnea-index for sleep apnea.
Conclusion:
In this population, RVFAC was independently associated with risk of SCD using a novel digital echocardiogram archive with a standardized reading protocol. Furthermore, when combined with LVEF, RVFAC had additive effects on SCD risk. These findings have potential implications for SCD risk stratification and warrant further prospective evaluation in larger populations.
Acknowledgements:
The authors would like to acknowledge the significant contribution of American Medical Response, Portland/Gresham Fire Departments, and the Oregon State Medical Examiner’s office.
Funding:
This was work was funded in part by National Heart, Lung, and Blood Institute grants R01HL122492 and R01HL126938 to Dr Chugh. Dr Chugh holds the Pauline and Harold Price Chair in Cardiac Electrophysiology at Cedars Sinai, Los Angeles.
Funding sources:
Funded by National Institutes of Health, National Heart Lung and Blood Institute (NHLBI) grants R01HL122492 and R01HL126938 to Dr Chugh. Dr Chugh holds the Pauline and Harold Price Chair in Cardiac Electrophysiology at Cedars-Sinai, Los Angeles. The funders played no role in study design, data collection, analysis or interpretation of data, or manuscript preparation.
Disclosures:
Dr. Nichols received unrelated research funding from Boehringer Ingelheim and Bristol-Myers Squibb. The other authors have no disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–83. [DOI] [PubMed] [Google Scholar]
- 4.Epstein AE, Dimarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for device-based therapy of cardiac rhythm abnormalities. Heart Rhythm 2008;5:e1–62. [DOI] [PubMed] [Google Scholar]
- 5.Chugh SS, Reinier K, Teodorescu C, et al. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis 2008;51:213–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chugh SS, Uy-Evanado A, Teodorescu C, et al. Women have a lower prevalence of structural heart disease as a precursor to sudden cardiac arrest: The Ore-SUDS (Oregon Sudden Unexpected Death Study). J Am Coll Cardiol 2009;54:2006–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabbag A, Suleiman M, Laish-Farkash A, et al. Contemporary rates of appropriate shock therapy in patients who receive implantable device therapy in a real-world setting: From the Israeli ICD Registry. Heart Rhythm 2015;12:2426–33. [DOI] [PubMed] [Google Scholar]
- 8.Stecker EC, Chugh SS. Prediction of sudden cardiac death: next steps in pursuit of effective methodology. J Interv Card Electrophysiol 2011;31:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stecker EC, Vickers C, Waltz J, et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol 2006;47:1161–6. [DOI] [PubMed] [Google Scholar]
- 10.Konety SH, Koene RJ, Norby FL, et al. Echocardiographic Predictors of Sudden Cardiac Death: The Atherosclerosis Risk in Communities Study and Cardiovascular Health Study. Circ Cardiovasc Imaging 2016;9:pii: e004431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narayanan K, Reinier K, Uy-Evanado A, et al. Chronic Obstructive Pulmonary Disease and Risk of Sudden Cardiac Death JACC Clinical Electrophysiology 2015;1:381–7. [DOI] [PubMed] [Google Scholar]
- 12.Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol 2013;62:610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin LJ, American College of Chest Physicians. Diagnosis and management of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest 2004;126:7S–10S. [DOI] [PubMed] [Google Scholar]
- 14.Zangiabadi A, De Pasquale CG, Sajkov D. Pulmonary hypertension and right heart dysfunction in chronic lung disease. Biomed Res Int 2014;2014:739674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuttica MJ, Kalhan R, Shlobin OA, et al. Categorization and impact of pulmonary hypertension in patients with advanced COPD. Respir Med 2010;104:1877–82. [DOI] [PubMed] [Google Scholar]
- 16.Minai OA, Ricaurte B, Kaw R, et al. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol 2009;104:1300–6. [DOI] [PubMed] [Google Scholar]
- 17.Chugh SS, Jui J, Gunson K, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol 2004;44:1268–75. [DOI] [PubMed] [Google Scholar]
- 18.Devereux RB, Lutas EM, Casale PN, et al. Standardization of M-mode echocardiographic left ventricular anatomic measurements. J Am Coll Cardiol 1984;4:1222–30. [DOI] [PubMed] [Google Scholar]
- 19.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. [DOI] [PubMed] [Google Scholar]
- 20.Dutta T, Aronow WS. Echocardiographic evaluation of the right ventricle: Clinical implications. Clin Cardiol 2017;40:542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aktas MK, Kim DD, McNitt S, et al. Right ventricular dysfunction and the incidence of implantable cardioverter-defibrillator therapies. Pacing Clin Electrophysiol 2009;32:1501–8. [DOI] [PubMed] [Google Scholar]
- 22.Mikami Y, Jolly U, Heydari B, et al. Right ventricular ejection fraction is incremental to left ventricular ejection fraction for the prediction of future arrhythmic events in patients with systolic dysfunction. Circ Arrhythm Electrophysiol 2017;10:. pii: e004067 [DOI] [PubMed] [Google Scholar]
- 23.Risum N, Valeur N, Søgaard P, Hassager C, Køber L, Ersbøll M. Right ventricular function assessed by 2D strain analysis predicts ventricular arrhythmias and sudden cardiac death in patients after acute myocardial infarction. Eur Heart J Cardiovasc Imaging 2018;19:800–807. [DOI] [PubMed] [Google Scholar]
- 24.Naksuk N, Tan N, Padmanabhan D, et al. Right ventricular dysfunction and long-term risk of sudden cardiac death in patients with and without severe left ventricular dysfunction. Circ Arrhythm Electrophysiol 2018;11:e006091. [DOI] [PubMed] [Google Scholar]
- 25.Lee JZ, Low SW, Pasha AK, Howe CL, Lee KS, Suryanarayana PG. Comparison of tricuspid annular plane systolic excursion with fractional area change for the evaluation of right ventricular systolic function: a meta-analysis. Open Heart 2018;5:e000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamori S, Onishi K, Ishida M, et al. Myocardial perfusion reserve is impaired in patients with chronic obstructive pulmonary disease: a comparison to current smokers. Eur Heart J Cardiovasc Imaging 2014;15:180–8. [DOI] [PubMed] [Google Scholar]
- 27.Sievi NA, Clarenbach CF, Camen G, Rossi VA, van Gestel AJ, Kohler M. High prevalence of altered cardiac repolarization in patients with COPD. BMC Pulm Med 2014;14:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zulli R, Donati P, Nicosia F, et al. Increased QT dispersion: a negative prognostic finding in chronic obstructive pulmonary disease. Intern Emerg Med 2006;1:279–86. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura T, Chin K, Hosokawa R, et al. Corrected QT dispersion and cardiac sympathetic function in patients with obstructive sleep apnea-hypopnea syndrome. Chest 2004;125:2107–2114. [DOI] [PubMed] [Google Scholar]
- 30.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995;96:1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chugh SS, Uy-Evanado A. Improved prediction of sudden cardiac death risk: Staying within the echocardiogram but extending beyond the ejection fraction. Circ Cardiovasc Imaging 2016;9: pii: e005133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reinier K, Dervan C, Singh T, et al. Increased left ventricular mass and decreased left ventricular systolic function have independent pathways to ventricular arrhythmogenesis in coronary artery disease. Heart Rhythm 2011;8:1177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]