Abstract
Objective
To determine the seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in healthcare workers (HCWs) based on risk of exposure to COVID-19 patients.
Method
This was a SARS-CoV-2 seroprevalence cross-sectional study in risk-stratified HCWs randomly selected from three main district hospitals in Oman.
Results
1078 HCWs were included, with an overall SARS-CoV-2 seroprevalence of 21%. The seropositivity rates in low-, variable-, and high-risk groups were 29%, 18%, and 17%, respectively (p-value < 0.001). The study found higher positivity in males (crude odds ratio [COR] 1.71, 95% confidence interval [CI] 1.28–2.3), and workers residing in high-prevalence areas (COR 2.09, 95% CI 1.42–3.07). Compared with doctors, workers from supporting services, administration staff, and nurses were more likely to test positive for SARS-CoV-2 antibodies (COR 9.81, 95% CI 5.26–18.27; 2.37, 95% CI 1.23–4.58; 2.08 95% CI 1.14–3.81). The overall rate of previously undetected infection was 12%, with higher values in low-risk HCWs. High district prevalence was a driving factor for seropositivity in the low-risk group (adjusted odds ratio [AOR] 2.36, 95% CI 1.0–5.59).
Conclusion
Low-risk supporting services workers can drive SARS-CoV-2 transmission in hospitals. More attention and innovation within this area will enhance the safety of health care during epidemics/pandemics.
Keywords: SARS-CoV-2, COVID-19, Healthcare workers, Community, Serosurvey, Infection, Oman
Introduction
The emergence of SARS-CoV-2 (causing COVID-19) in December 2019 rapidly evolved into a pandemic, with cumulative numbers of more than 83 million confirmed cases and 1.8 million deaths globally according to WHO (2021a). During the lengthy course of this pandemic, The Lancet (2020) reported that workers within healthcare facilities had been working at maximum capacity for many hours and over many shifts, and in some settings with limited protection. Being a frontline healthcare worker was found to be one of the risk factors for acquiring COVID-19, as shown in many serological studies, such as that by Galanis et al. in 2021. However, exposure in the community in the early phases of local spread has also been shown to be the cause of COVID-19 in a substantial proportion of HCWs, even before cases had been admitted to their hospital (Kluytmans-van den Bergh et al., 2020). The incidence of SARS-CoV-2 infection in HCWs has varied across different studies depending on the disease epidemiology, target healthcare professions, and diagnostic tools. Overall, studies of SARS-CoV-2 in HCWs using polymerase chain reaction (PCR) testing have shown infection incidence to be in the range of 0.4–49.6%, and seropositivity in the range of 1.6–31.6% (WHO, 2020a).
Oman is located on the southeast coast of the Middle East region, and has a population of around 4.6 million (Government of Oman, 2021). In each district (or governorate) of Oman is a primary Ministry of Health hospital, which is used as a referral center for all the healthcare institutions within the governorate, including the private sector. Each of these referral hospitals is set up as a COVID-19 center for admitting and managing moderate-to-severe cases. Aggressive measures aimed at protecting HCWs have been implemented, including administrative and engineering initiatives, and the provision of personal protection, as per the national guidelines (Ministry of Health, Oman, 2020). The total number of positive reported cases in Oman up to the end of December 2020 was 128 867, with 1499 deaths (WHO, 2021b).
The nature and durability of the humoral immune response to SARS-CoV-2 infection and the clinical utility of serological investigation are still debatable, but serology has been a great tool for assessing the disease spread in the community and in healthcare settings (Deeks et al., 2020, Tripathi et al., 2020, WHO, 2020b). Our point prevalence study of SARS-CoV-2 antibodies was conducted before the start of the vaccination program for COVID-19 in order to determine the disease epidemiology and risk factors within healthcare settings, stratified according to exposure risks.
Study methodology and design
Study setting
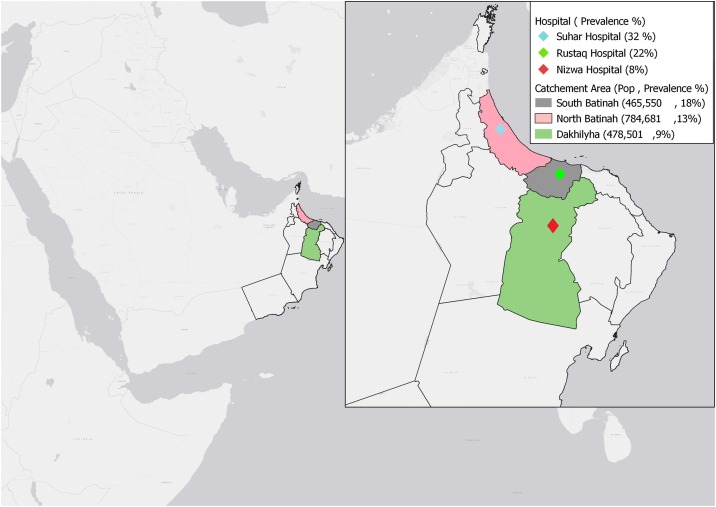
This study was conducted in three district COVID-19 management referral hospitals — Nizwa, Sohar, and Rustaq. These are the referral secondary care facilities for Al Dakhilia, North Batinah, and South Batinah governorates, respectively. Each hospital caters for the population of the governorate in which it is located, and almost all HCWs reside in the same governorate as where they work. The three centers have similar structures and levels of care, with inpatient capacity ranging from 300 to 400 beds. The locations of the hospitals and the served populations are shown in Figure 1 .
Figure 1.
The served populations1 and locations of the hospitals included in this SARS-CoV-2 seroprevalence study within Oman.
1Government of Oman. Main page. 2021. https://www.ncsi.gov.om (accessed 12 December 2020).
The national SARS-CoV-2 serosurvey phase 2, during first 2 weeks of September 2020, showed community prevalences of 18%, 13%, and 9% for South Batinah, North Batinah, and Al Dakhilia governorates, respectively (manuscript in preparation).
Each of the three hospitals started early preparations for a pandemic following the national preparedness plan and infection control guidelines for SARS-CoV-2 (Ministry of Health, Oman, 2020). There were specific pathways for receiving, investigating, and management of suspected/confirmed patients with COVID-19 from community centers and in the emergency department. For inpatient care, each hospital created a COVID-19 ward and intensive care area, ensuring the implementation of proper isolation precautions. Every entrance to the hospital was provided with active symptomatic screening and temperature checks. The hospitals started receiving COVID-19 patients in March 2020, with total inpatients by the end of August 2020 reaching 751, 350, and 310 for Sohar, Rustaq, and Nizwa hospitals, respectively.
Study design
A cross-sectional seroprevalence study was conducted in the period September 14–28, involving workers in a healthcare setting from the three defined centers. The detailed process of randomization and sample selection from each hospital and for each risk category is provided as supplementary material.
Study population
The sample size was calculated after grouping all the workers within the three hospitals into three risk categories based on the potential for being exposed to a suspected or confirmed COVID-19 patient within the hospital:
-
iHigh exposure risk: HCWs from COVID-19 wards, intensive care units, emergency departments, and internal medicine departments, regardless of their professional category.
-
iiVariable exposure risk: HCWs from surgical wards, pediatric/obstetric departments, and laboratories/pharmacies. Workers in this category were not directly involved in the care of suspected or confirmed cases of COVID-19, but may have been if a patient was initially undiagnosed.
-
iiiLow exposure risk: HCWs working in administration, medical records, engineering, finance, kitchen, laundry, IT, and security who were not directly involved in the clinical care of suspected or confirmed cases of COVID-19 or other clinical areas.
-
i
Each hospital sent its enrolment data sheet — with individuals classified as indicated into the three risk categories — to the central study team 2 weeks prior to collecting serology samples. In total, 3665 HCWs were included in a list of populations from all participating hospitals. Each HCW was assigned with a unique code and risk group label, based on hospital and professional categories.
Sampling and randomization
The sample size for the three hospitals was calculated based on a 95% confidence level and 5% margin of error, and for an estimated prevalence of 1–2% for each risk category. Accordingly, the total sample was 400 HCWs per category. Multivariable stratified random sampling was conducted to select the enrolled HCWs in each risk category, weighted by professions, using Microsoft Excel version 2010 (Microsoft Corporation, Redmond WA, USA). An illustration of the sampling and enrolment process for the study is provided as Supplementary material.
The randomized list for each hospital was sent to the study focal point for participation consent, questionnaire administration, and blood testing. Each hospital team ensured that the enrolled staff continued to operate within the allocated risk categories. Exclusion criteria included suspected cases or those with symptoms consistent with COVID-19 infection at the time of the survey, and those who did not consent to completing the questionnaire or giving a blood sample for the serology study. HCWs who were on leave or covering work in other facilities were also excluded. An effort was made during the 2 weeks of the study to replace some of the unavailable or unconsenting staff, ensuring that they were from the same category and profession.
The questionnaire
Each enrolled HCW completed a questionnaire that included demographic information, risk assessment, symptoms, and disease history sections. Demography included age, sex, nationality, and profession. The exposure risk assessment included personal protective equipment (PPE) use, infection prevention and control (IPC) training, and contact with positive cases. Clinical data included symptoms, symptom onset, COVID-19 history, and the presence of any pre-existing comorbidities. The questionnaire was adapted with modification from the World Health Organization (WHO, 2020c) assessment of risk factors for COVID-19 in health workers. A web link to the questionnaire was provided to the enrolled HCWs after they had consented to the study. The participants then took an antibody test once the study team had confirmed submission of the completed questionnaire. The questionnaire is provided as Supplementary material.
Laboratory methodology
Using a gel separator tube, a serum sample (5–10 ml) was collected by the central study team from all participants who had consented and filled out the questionnaire. At the regional hospitals, samples were centrifuged at 1000–3000 RPM for 10 min. The separated sample was then transferred to a 5 ml plain tube without a preservative. Samples were transported to the Central Public Health Laboratory and tested using Diasorin Liaison® XL (DiaSorin, Saluggia, Italy) SARS-CoV-2 S1/S2 immunoglobulin (IgG) kit. This is a fully automated serology test that uses chemiluminescence immunoassay technology for the quantitative determination of anti-S1 and anti-S2 specific IgG antibodies to SARS-CoV-2. A sample is considered negative if the IgG is < 12 AU/ml, equivocal in the range 12–15 AU/ml, and positive at levels ≥ 15 AU/ml.
The results for each participant were uploaded to the hospital’s database, along with their hospital ID number, after authorization by a virologist in the Central Public Health Laboratory. A laboratory line list with results was then sent to the central study team for analysis. For the study analysis, results were either positive or negative. Therefore, the equivocal results were classed as negative.
Data analyses
Baseline characteristics were described as percentages of the total. Community prevalences of SARS-CoV-2 at the wilayat (county) level were extracted from the national serosurvey (manuscript in preparation). The wilayat of residence was classified as having low or high prevalence of SARS-CoV-2 according to the mean wilayat level for the entire data (calculated to be 11.4%). Univariate analysis was performed using logistic regression to investigate the relationship between the positivity of serology (response variable) and the different demographic and questionnaire responses (explanatory variables). Differences in the presence of recognized infections among HCWs were tabulated and investigated using chi-square analysis. Recognized infection was defined as an answer of ‘yes’ to a history of an earlier confirmed infection. To highlight the drivers of positive serology within each risk category, a multivariate logistic regression analysis was performed for each risk category using positive serology as a response variable and other studied factors as explanatory variables. All statistical analyses were performed using R software version 4.02 (The R Project, https://cran.r-project.org/).
Results
Out of 1200 targeted HCWs from the three enrolled hospitals, 1078 (90%) were included in this study. Based on the risk of exposure classification, of the 1078 subjects, 345 (32%) were workers classified as high risk, 373 (35%) as variable risk, and 360 (33%) as low risk. Of the participating HCWs, 55% were female, 49% were in the 30–39 years age category, and the majority were Omani nationals (68%). The distribution, based on hospital and professional categories, is shown in Table 1 . The majority of the HCWs (82%) were living in their family’s house, while the remaining were living on hospital campus or in private shared accommodation. With regard to community prevalence of the HCW living areas; 83% were from low-prevalence communities (mean prevalence = 11.4%).
Table 1.
Baseline characteristics, including frequency of positive SAR-CoV-2 antibodies and COR seropositivity for different factors.
| Characteristic | Included N (%) |
Seropositive N (% within rows) |
Crude OR (95% CI) | p-Value |
|---|---|---|---|---|
| Total | 1078 | 229 (21%) | ||
| Sex | ||||
| Female | 598 (55%) | 103 (17%) | Ref | |
| Male | 480 (45%) | 126 (26%) | 1.71 (1.28–2.3) | < 0.001 |
| Age category (years) | ||||
| 20–29 | 195 (18%) | 44 (23%) | Ref | |
| 30–39 | 529 (49%) | 117 (22%) | 0.98 (0.66–1.45) | 0.898 |
| 40–49 | 295 (27%) | 56 (19%) | 0.8 (0.52–1.25) | 0.336 |
| 50–59 | 53 (4.9%) | 12 (23%) | 1 (0.49–2.08) | 0.99 |
| Over 60 | 6 (0.6%) | 0 | 0 (0–0) | 0.97 |
| Omani | 730 (68%) | 150 (21%) | 0.88 (0.65–1.2) | 0.419 |
| Hospital | ||||
| Nizwa | 378 (35%) | 32 (8%) | Ref | |
| Rustaq | 257 (24%) | 56 (22%) | 3.01 (1.89–4.81) | < 0.001 |
| Sohar | 443 (41%) | 141 (32%) | 5.05 (3.34–7.64) | < 0.001 |
| Exposure risk category | ||||
| Low | 360 (33%) | 104 (29%) | Ref | |
| Variable | 373 (35%) | 66 (18%) | 0.53 (0.37–0.75) | < 0.001 |
| High | 345 (32%) | 59 (17%) | 0.51 (0.35–0.73) | < 0.001 |
| Profession category | ||||
| Doctor | 156 (14%) | 14 (9%) | Ref | |
| Admin | 190 (18%) | 36 (19%) | 2.37 (1.23–4.58) | 0.01 |
| Medical assistanta | 136 (13%) | 20 (15%) | 1.75 (0.85–3.61) | 0.131 |
| Nurse | 417 (39%) | 71 (17%) | 2.08 (1.14–3.81) | 0.018 |
| Support staffb | 179 (17%) | 88 (66%) | 9.81 (5.26–18.27) | < 0.001 |
| Residency | ||||
| Campus | 197 (18%) | 68 (34.5%) | Ref | |
| Family house | 881 (82%) | 161.0 (18.3%) | 0.42 (0.3–0.6) | < 0.001 |
| Community prevalencec | ||||
| Low | 896 (83%) | 162 (18%) | Ref | |
| High | 149 (14%) | 47 (31%) | 2.09 (1.42–3.07) | < 0.001 |
| Unknown | 33 (3%) | 20 (60%) | ||
| Previously confirmed COVID-19 | ||||
| Yes | 139 (13%) | 118 (85%) | 41.9 (25.3–69.4) | < 0.001 |
| No | 939 (87%) | 111 (12%) | Ref | |
| Recent IPC training | 190 (18%) | 44 (23%) | 1.15 (0.79–1.67) | 0.477 |
| Symptoms in past 3 monthsd | ||||
| Fever | 107 (9.9%) | 65.0 (61%) | 7.62 (4.99–11.62) | < 0.001 |
| Myalgia | 262 (24%) | 86 (32.8%) | 2.3 (1.68–3.15) | < 0.001 |
| Sore throat | 286 (27%) | 68 (23.8%) | 1.22 (0.89–1.69) | 0.222 |
| Cough | 212 (20%) | 64 (30.2%) | 1.84 (1.31–2.58) | < 0.001 |
| Other respiratorye | 326 (30%) | 74 (22.7%) | 1.13 (0.83–1.55) | 0.442 |
| GI symptoms | 243 (23%) | 63 (25.9%) | 1.23 (0.92–1.64) | 0.172 |
| Other symptomsf | 517 (48%) | 119 (23.0%) | 1.41 (1.01–1.97) | 0.043 |
Includes medical orderlies, technicians, physiotherapists, dieticians, and pharmacists.
Includes information technology, medical engineers, security, medical records.
Data from a national serosurvey conducted at the same time as our study, as provided by the surveillance department for the included governorate, using a median of 11% to define high and low prevalence.
Reference is the absence of symptoms.
Includes nasal congestion or runny nose, chest pain, difficulty breathing, wheezing.
Includes headache, fatigue, and all others not mentioned in the list reported as free text entries.
Self-reporting of recent IPC training was provided by 190 (18%). Of the enrolled cohort, 139 (13%) reported that they previously had a confirmed SARS-CoV-2 infection.
Overall, 229 of the 1078 HCWs (21%) tested positive for IgG antibodies against SARS-CoV-2. In the high-risk exposure category, 59/345 (17%) tested positive for IgG antibodies against SARS-CoV-2, 66/373 (18%) in the variable-risk category, and 104/360 (29%) in the low-risk category. Differences in the serology positivity between the risk categories were statistically significant (p-value < 0.001) with 0.51 COR (95% CI 0.35–0.73) for high risk and 0.53 COR (95% CI 0.37–0.75) for variable risk when compared with the low-risk category.
The COR (Table 1) showed significantly higher seropositivity in male HCWs (COR 1.71, 95% CI 1.28–2.3), and in workers residing in a high-prevalence area (COR 2.09, 95% CI 1.42–3.07). Among the professional categories the workers from supporting services, administration, and nursing were significantly more likely to test positive for SARS-CoV-2 antibodies compared with doctors, with COR values of 9.81 (95% CI 5.26–18.27), 2.37 (95% CI 1.23–4.58), and 2.08 (95% CI 1.14–3.81), respectively. Among the seropositive HCWs, 39% were not able to recall being symptomatic in the 3-month period prior to the sample being collected. However, those with memories of having fever, myalgia, and/or cough were significantly associated with a higher antibody positivity rate, with COR values of 7.62 (95% CI 4.99–11.62), 2.3 (95% CI 1.68–3.15), and 1.84 (95% CI 1.31–2.58), respectively. Lower seropositivity was noted in workers living with their family (COR 0.42, 95% CI 0.3–0.6).
Among the 1078 enrolled HCWs, there were 139 (13%) with previously confirmed COVID-19 via PCR test. Of these, 118 (85%) tested positive for SARS-CoV-2 antibodies, with a COR of 41.9 (95% CI 25.3–69.4), while 111 (12%) of those with no previously confirmed infection had positive serology (Table 1). The rates of previously undetected SARS-CoV-2 infection were 15/59 (25%), 36/66 (55%), and 60/104 (58%) for the high-, variable-, and low-risk groups, respectively (p-value < 0.001) (Table 2 ).
Table 2.
Prevalence of recognized and unrecognized COVID-19 infections among HCWs according to risk category.
| Risk category | Previous infection N (% within row) |
Total | p-Value | |
|---|---|---|---|---|
| Unrecognized | Recognized | |||
| High risk | 15 (25.4 %) | 44 (74.6%) | 59 | < 0.001 |
| Variable risk | 36 (54.5%) | 30 (45.5%) | 66 | |
| Low risk | 60 (57.7%) | 44 (42.3%) | 104 | |
| Total | 111 (48.5%) | 118 (51.5%) | 229 | |
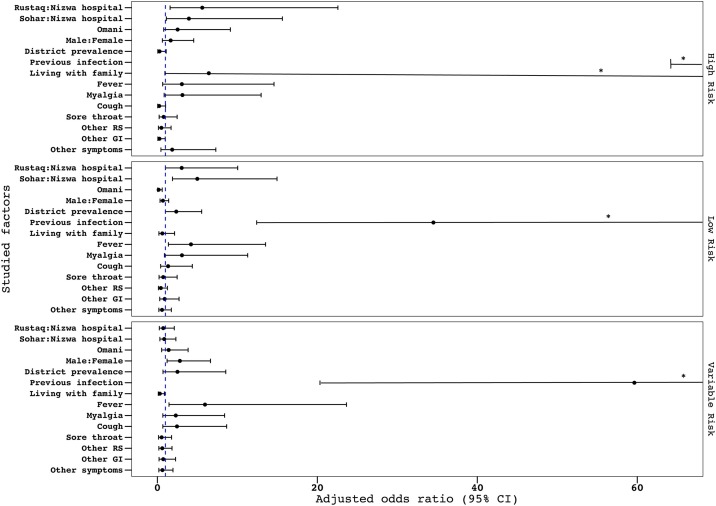
The adjusted odds ratios are shown in Figure 2 . Rustaq and Sohar hospitals showed higher seropositivity compared with Nizwa hospital only in the high- and low-risk groups. A high district prevalence was a statistically significant driving factor for seropositivity in the low-risk group (AOR 2.36, 95% CI 1.0–5.59) compared with the other groups. Living with family was protective in the variable-risk group (AOR 0.31, 95% CI 0.11–0.88), while it showed a tendency to drive seropositivity in the high-risk group, although this was not statistically significant (AOR 6.43, 95% CI 0.94–68.56).
Figure 2.
The AOR of having positive serology for SARS-CoV-2 for the selected factors, according to stepwise regression analysis and classification by risk categorization.
*The lower limit of the CI exceeds the graph’s x-axis limit.
Discussion
In this prospective cohort of 1078 HCWs from three different districts hospitals in Oman, our study found a 21% overall SARS-CoV-2 seroprevalence by the end of September 2020, with, interestingly, a significantly higher prevalence of 29% among the group of workers with a low exposure risk. The odds of having SARS-CoV-2 antibodies was significantly lower for both the workers with a high exposure risk (e.g., staff working in COVID-19 intensive care units and wards, and emergency departments) and a variable exposure risk (e.g., staff working in pediatric wards, obstetrics, and surgical units). This finding was contrary to what had been reported in earlier months of the pandemic, when seroprevalence was higher among HCWs working in COVID-19 units, especially in areas where there had been inadequate infection control measures and interrupted or shortage of PPE supply (Grant et al., 2021, Iversen et al., 2020, Rudberg et al., 2020). Many of the COVID-19 units later in the pandemic were more prepared and adherent to IPC measures, including the use of PPE among HCWs looking after suspected or confirmed COVID-19 patients in high-risk areas.
As community transmission increases, the risk of SARS-CoV-2 infection for HCWs outside healthcare settings becomes similar or even higher through their household, friends, or other unmitigated transmission encounters (Belingheri et al., 2020, Liu et al., 2020, Muhi et al., 2020). In later stages of the pandemic, the healthcare cluster is likely to be due to a lapse in early case detection in a worker or a patient, especially with COVID-19. This is because, they may have mild or atypical symptoms and there could be presymptomatic transmission. In line with our findings, seropositivity in the Grant et al. study was found to be lower among intensive care unit HCWs because of enhanced PPE, closed-circuit ventilation of intubated patients, and the admission of COVID-19 patients beyond day 10, when viral shedding is less (Grant et al., 2021, Bullard et al., 2020, Zhou et al., 2020). Throughout the pandemic, the healthcare setting remained a high-risk area, with the overall risk of infection in HCWs always higher than in the background population through a combination of community and healthcare sources.
Many studies have reported infection rates among HCWs utilizing SARS-CoV-2 antibody detection (Moscola et al., 2020, Paderno et al., 2020, Steensels et al., 2020, Stubblefield et al., 2020). It is not surprising to find a wide range of variation in the findings of seroprevalence studies, whether in community or healthcare settings, for several reasons, such as disease epidemiology, the included population, the type of antibody tests used, the design and quality of the study, and the different timing during the pandemic. A recent meta-analysis study found that, overall, 8.7% (95% CI 6.7–10.9%) of HCWs had higher seroprevalence of SARS-CoV-2 antibodies in studies from North America (12.7%) compared with those from Europe (8.5%), Africa (8.2), and Asia (4%) (Galanis et al., 2021). Prevalence had also been highly variable across different centers in the USA from March 23 to May 12, 2020, ranging from 1% to 6.9% (Havers et al., 2020). Previous studies with HCWs have produced mixed results, with significant heterogeneity, even when only frontline (high-risk) HCWs were included, but seroprevalence mostly correlated with community rates (Self et al., 2020). The high SARS-CoV-2 prevalence in our study may be explained by the inclusion of low-risk workers, the use of serology for diagnosis, and the study being carried out done 6 months after the epidemic had begun nationally, when disease spread was broader than it had been earlier.
The heterogeneity of studies was also reflected in the risk of infection based on profession or job title. While some studies have shown no differences in the infection rate based on profession or job (Steensels et al., 2020, Vahidy et al., 2020), others have reported high rates of infection or antibody positivity among nurses compared with doctors (Al Maskari et al., 2021, Barrett et al., 2020). Our findings showed the risk to be significantly lower in doctors compared with nurses and workers from other professional categories, which may be due to their awareness of risk or their lower level of contact and time of contact with patients compared with nurses. In some studies, however, clinicians had higher infection rates, while lower rates were found in support roles, such as janitorial staff (Eyre et al., 2020, Lombardi et al., 2020).
A study by Korth et al. (2020) evaluating IgG antibodies to SARS-CoV-2 among HCWs in Germany found the overall rate of unrecognized infection to be 1.6%. A recent serology study from the UK by Shields et al. (2020) found a 24.4% seroprevalence in HCWs, which was a much higher cumulative infection rate than determined in earlier studies using molecular testing. Comparing our serology study results with previously confirmed COVID-19 cases showed a 12% overall rate of unrecognized infection, with the majority of these in the low-risk worker category, despite the unified accessibility of testing, quarantine, and isolation procedures. This undetectable rate by molecular testing can be explained by existing data demonstrating the relative insensitivity of nasopharyngeal swabs in determining viral carriage (Hains et al., 2020, Wang et al., 2020).
The differences in undetectable infection rates within our study risk categories may also reflect insight into infection risks by the high- and variable-risk cohorts of HCWs, strict symptomatic screening procedures, and a sense of responsibility for not transmitting the infection to sick patients and/or colleagues in the clinical area. The rate of undetected SARS-CoV-2 infection may also indicate the percentage of mild or asymptomatic (SARS-CoV-2) infection among HCWs, which was in 39% in our study’s positive group. This compares with 17.1% in the study by Shields et al. (2020) and a staggering 44% of positive-testing HCWs who did not realize they were ill in a serological study conducted by the Influenza Vaccine Effectiveness in the Critically Ill (IVY) Network (Kuehn, 2020). This high rate of undetected earlier infection in individuals working within a healthcare setting, whether in direct contact with patients or not, could have driven the spread of the disease via direct or indirect contact with uninfected personnel and/or environmental contamination.
The factors driving antibody positivity within each risk category showed clear evidence that the hospital setting was important for the high- and variable-risk categories. This could be due to limited protection, especially during healthcare procedures, a reflection of presymptomatic transmission before universal masking at the national level became mandatory at end of May 2020, or non-strict adherence to preventive measures, especially among coworkers (Al Maskari et al., 2021, Heinzerling et al., 2020, Wei et al., 2020). The low-risk category was affected mostly by community disease prevalence. Living with family, compared with living on hospital campus or in private shared accommodation, increased positivity among high-risk workers, as this group is mostly protected in the working environment, but with family members close contact risk is hard to mitigate.
Our study’s strength was in the segregation and randomization of all individuals working within healthcare facilities based on their risk of being exposed to suspected or confirmed patients with COVID-19. This allowed a better understanding of healthcare-related transmission versus the impact of disease spread in the community. The use of quantitative measurement for the serology testing helped in capturing actual infection prevalence for at least the previous 6 months (Patel et al., 2020). Recall bias, incomplete filling of the questionnaire, inaccurate information, and/or wrong entries are some inherent limitations with surveys like this, although their impact in the outcome analysis was minimized by the exclusion of poor-quality information. Given the kinetics of antibody development against SARS-CoV-2, individuals tested shortly after infection may not have mounted an antibody response, while classifying those with equivocal results as negative may have, to a small extent, underestimated the prevalence of COVID-19 in HCWs.
Prevention of infection in the workplace requires a multipronged, integrated approach that includes IPC strategies, occupational health and safety measures, adherence to public health measures, and mitigation of social behavioral risk in the community. The findings of our study will influence future healthcare preparedness for infectious disease outbreaks, epidemics, and pandemics by encouraging more attention to, and innovations for, the control of community-driven spread into healthcare facilities, such as universal masking, symptom detection, carrier status identification, and environmental decontamination.
Conclusion
The epidemiology of SARS-CoV-2 in a healthcare setting is driven largely by disease prevalence in the community and workers from supporting services. The current infection control measures have succeeded in managing transmission in the high- and variable-risk categories; however, more attention is required for low-risk workers. Enforcing symptomatic screening, quarantine of exposed individuals, universal masking, and encouraging innovation in diagnostic and monitoring tools within the healthcare setting will enhance the safety of health care during epidemics and pandemics.
Author contributions
AM, AW, JS, BA, and ES handled the study conception and design, and the analysis and interpretation of data. ER, LA, KD, MS, SM, SB, AA, and AS were responsible for conducting the study in the included centers and communication with the study team centrally. EB, NZ, AQ, HK, KS, AJ, and SA participated in the study conception, design, and logistics management. All the authors drafted the article or revised it critically for important intellectual content, and provided final approval of the version for submission.
Ethical approval
Ethical approval for this study was issued from all the participating hospitals, and each included participant signed informed consent to be part of the study.
Funding
None.
Competing interests
None declared by any of the authors.
Acknowledgments
We are grateful to all the healthcare workers who are directly or indirectly involved in the prevention and management of SARS-CoV-2 infection during this pandemic. We thank all who participated in and supported the conduction of this study, from hospital administration staff, infection prevention and control teams, quality and patient safety teams, professional development and career guidance departments, nursing departments, laboratory departments, and all the healthcare workers who agreed to be investigated. Special thanks go to Dr Zawan Hamid Al Hasni and Ms Muna Rashid Al Hinai from Rustaq hospital, Ms Shiekha Al Maqbali, and Ms Mitha Al Jabri from Sohar hospital for their assistance to the study team.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2021.04.071.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Al Maskari Z., Al Blushi A., Khamis F., Al Tai A., Al Salmi I., Al Harthi H., et al. Characteristics of healthcare workers infected with COVID-19: a cross-sectional observational study. Int J Infect Dis. 2021;102:32–36. doi: 10.1016/j.ijid.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E.S., Horton D.B., Roy J., Gennaro M.L., Brooks A., Tischfield J., et al. Prevalence of SARS-CoV-2 infection in previously undiagnosed health care workers in New Jersey, at the onset of the U.S. COVID-19 pandemic. BMC Infect Dis. 2020;20(1):853. doi: 10.1186/s12879-020-05587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belingheri M., Paladino M.E., Riva M.A. Beyond the assistance: additional exposure situations to COVID-19 for healthcare workers. J Hosp Infect. 2020;105(2):353. doi: 10.1016/j.jhin.2020.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard J., Dust K., Funk D., Strong J.E., Alexander D., Garnett L., et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks J.J., Dinnes J., Takwoingi Y., Davenport C., Spijker R., Taylor-Phillips S., et al. COVID-19 Diagnostic Test Accuracy Group. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;6(6) doi: 10.1002/14651858.CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D.W., Lumley S.F., O’Donnell D., Campbell M., Sims E., Lawson E., et al. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. eLife. 2020;9 doi: 10.7554/eLife.60675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanis P., Vraka I., Fragkou D., Bilali A., Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120–134. doi: 10.1016/j.jhin.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government of Oman . 2021. Main page.https://www.ncsi.gov.om . [Accessed 12 December 2020] [Google Scholar]
- Grant J.J., Wilmore S.M.S., McCann N.S., Donnelly O., Lai R.W.L., Kinsella M.J., et al. Seroprevalence of SARS-CoV-2 antibodies in healthcare workers at a London NHS Trust. Infect Control Hosp Epidemiol. 2021;42(2):212–214. doi: 10.1017/ice.2020.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains D.S., Schwaderer A.L., Carroll A.E., Starr M.C., Wilson A.C., Amanat F., et al. Asymptomatic seroconversion of immunoglobulins to SARS-CoV-2 in a pediatric dialysis unit. JAMA. 2020;323(23):2424–2425. doi: 10.1001/jama.2020.8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havers F.P., Reed C., Lim T., Montgomery J.M., Klena J.D., Hall A.J., et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23–May 12, 2020. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzerling A., Stuckey M.J., Scheuer T., Xu K., Perkins K.M., Resseger H., et al. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient — Solano County, California, February 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):472–476. doi: 10.15585/mmwr.mm6915e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen K., Bundgaard H., Hasselbalch R.B., Kristensen J.H., Nielsen P.B., Pries-Heje M., et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020;20(12):1401–1408. doi: 10.1016/S1473-3099(20)30589-2. Erratum in: Lancet Infect Dis 2020;20(10):e250. PMID: 32758438; PMCID: PMC7398038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluytmans-van den Bergh M.F.Q., Buiting A.G.M., Pas S.D., Bentvelsen R.G., van den Bijllaardt W., van Oudheusden A.J.G., van Rijen M.M.L., et al. Prevalence and clinical presentation of health care workers with symptoms of coronavirus disease 2019 in 2 Dutch hospitals during an early phase of the pandemic. JAMA Netw Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth J., Wilde B., Dolff S., Anastasiou O.E., Krawczyk A., Jahn M., et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128 doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn B.M. Health care worker SARS-CoV-2 infection is flying under the radar. JAMA. 2020;324(16):1600. doi: 10.1001/jama.2020.19554. [DOI] [PubMed] [Google Scholar]
- Liu J., Ouyang L., Guo P., Wu H., Fu P., Chen Y., et al. Epidemiological, clinical characteristics and outcome of medical staff infected with COVID-19 in Wuhan, China: a retrospective case series analysis. medRxiv. 2020 03.09.2003311. [Google Scholar]
- Lombardi A., Consonni D., Carugno M., Bozzi G., Mangioni D., Muscatello A., et al. Characteristics of 1573 healthcare workers who underwent nasopharyngeal swab testing for SARS-CoV-2 in Milan, Lombardy, Italy. Clin Microbiol Infect. 2020;26(10) doi: 10.1016/j.cmi.2020.06.013. 1413.e9–1413.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health, Oman . 2020. Ministry of Health infection prevention and control guideline for COVID-19. 20 July 2020. https://www.moh.gov.om/documents/10194/3903020/IP%26C+guideline+version+7.pdf/03f03fd7-28b8-2d89-ef4f-3979abad7907. [Accessed 11 December 2020] [Google Scholar]
- Moscola J., Sembajwe G., Jarrett M., Farber B., Chang T., McGinn T., et al. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York city area. JAMA. 2020;324(9):893–895. doi: 10.1001/jama.2020.14765. Erratum in: JAMA 2020;324(22):2328. PMID: 32780804; PMCID: PMC7411936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhi S., Irving L.B., Buising K.L. COVID-19 in Australian health care workers: early experience of the Royal Melbourne Hospital emphasises the importance of community acquisition. Med J Aust. 2020;213(1):44–44.e1. doi: 10.5694/mja2.50664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paderno A., Fior M., Berretti G., Schreiber A., Grammatica A., Mattavelli D., et al. SARS-CoV-2 infection in health care workers: cross-sectional analysis of an otolaryngology unit. Otolaryngol Head Neck Surg. 2020;163(4):671–672. doi: 10.1177/0194599820932162. [DOI] [PubMed] [Google Scholar]
- Patel M.M., Thornburg N.J., Stubblefield W.B., Talbot H.K., Coughlin M.M., Feldstein L.R., et al. Change in antibodies to SARS-CoV-2 over 60 days among health care personnel in Nashville, Tennessee. JAMA. 2020;324(17):1781–1782. doi: 10.1001/jama.2020.18796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudberg A.S., Havervall S., Månberg A., Jernbom Falk A., Aguilera K., Ng H., Gabrielsson L., et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11(1):5064. doi: 10.1038/s41467-020-18848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self W.H., Tenforde M.W., Stubblefield W.B., Feldstein L.R., Steingrub J.S., Shapiro N.I., et al. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network — 13 Academic Medical Centers, April–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(35):1221–1226. doi: 10.15585/mmwr.mm6935e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A., Faustini S.E., Perez-Toledo M., Jossi S., Aldera E., Allen J.D., et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75(12):1089–1094. doi: 10.1136/thoraxjnl-2020-215414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensels D., Oris E., Coninx L., Nuyens D., Delforge M.L., Vermeersch P., et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324(2):195–197. doi: 10.1001/jama.2020.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubblefield W.B., Talbot H.K., Feldstein L., Tenforde M.W., Rasheed M.A.U., Mills L., et al. Seroprevalence of SARS-CoV-2 among frontline healthcare personnel during the first month of caring for COVID-19 patients — Nashville, Tennessee. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Lancet COVID-19: protecting health-care workers. Lancet. 2020;395(10228):922. doi: 10.1016/S0140-6736(20)30644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi S.C., Deshmukh V., Patil A., Tripathy J.P. COVID 19 diagnostic multiplicity and its role in community surveillance and control. Infez Med. 2020;28(suppl. 1):18–28. PMID: 32532934. [PubMed] [Google Scholar]
- Vahidy F.S., Bernard D.W., Boom M.L., Drews A.L., Christensen P., Finkelstein J., et al. Prevalence of SARS-CoV-2 infection among asymptomatic health care workers in the Greater Houston, Texas, area. JAMA Netw Open. 2020;3(7) doi: 10.1001/jamanetworkopen.2020.16451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W.E., Li Z., Chiew C.J., Yong S.E., Toh M.P., Lee V.J. Presymptomatic transmission of SARS-CoV-2 — Singapore, January 23–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):411–415. doi: 10.15585/mmwr.mm6914e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Interim guidance: Prevention, identification and management of health worker infection in the context of COVID-19. 30 October 2020. WHO/2019-nCoV/HW_infection/2020.1. [Google Scholar]
- WHO . 2020. Public health surveillance for COVID-19: interim guidance. Interim guidance 7 August 2020. COVID-19: Surveillance, case investigation and epidemiological protocols. WHO/2019-nCoV/SurveillanceGuidance/2020.7. [Google Scholar]
- WHO . World Health Organization; 2020. Risk assessment and management of exposure of health care workers in the context of COVID-19: interim guidance, 19 March 2020.https://apps.who.int/iris/handle/10665/331496 . License: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- WHO . 2021. Coronavirus disease (COVID-19): situation report — 5 January 2021.https://www.who.int/publications/m/item/weekly-epidemiological-update---5-january-2021.file:///C:/Users/user/Downloads/20210105_Weekly_Epi_Update_21.pdf . [Accessed 9 January 2021] [Google Scholar]
- WHO . 2021. WHO coronavirus disease (COVID-19) dashboard.https://covid19.who.int/region/emro/country/om . [Accessed 16 April 2021] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. Erratum in: Lancet 2020;395(10229):1038. PMID: 32171076; PMCID: PMC7270627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.