Abstract
The progressive and fatal outbreak of the newly emerged coronavirus, SARS-CoV-2, necessitates rigorous collaboration of all health care systems and researchers from all around the world to bring such a devastating pandemic under control. As there is so far no officially approved drug or ideal vaccine for this disease, investigations on this infectious disease are actively pursued. Chitin and chitosan have shown promising results against viral infections. In this review, we first delve into the problematic consequences of viral pandemics followed by an introduction on SARS-CoV-2 taxonomical classification. Then, we elaborate on the immunology of COVID-19. Common antiviral therapies and their related limitations are described and finally, the potential applicability of chitin and chitosan to fight this overwhelming viral pandemic is addressed.
Keywords: Chitin, Chitosan, COVID-19, SARS-CoV-2
1. The pandemics of viral infections and their economic and social consequences
Human history has experienced various viral pandemic and epidemic events. Viruses that have evolved from mutations and genetic variations of their parents can affect humans for months and even years. From influenza and measles to hepatitis, HIV, ebola, and zika virus, viral infections have troubled the world from the past to the present. Obviously, human beings have paid many costs to deal with infectious pandemics and epidemics including economic and social costs that have put a lot of pressure on communities. The recent pandemic of coronavirus is no exception from this rule and has brought problems, costs, and suffering with itself. Feasibly, the socio-economic consequences of microbial pandemics can be stratified into the following categories (Fig. 1 ).
Fig. 1.
Socio-economic consequences of microbial pandemics.
The infected person should be isolated from the community and exposed individuals should be retained in quarantine as long as they are contagious. The head of the household may pass away leaving economic consequences for the family [1]. Aggression, assault, and psychological tensions are issues for which preventative health institutions and campaigns must adopt strategies to cope with [2].
All of these issues and the management of crises such as infectious disease pandemics are very challenging for all governments, and especially the consequences of these crises for governments and health systems of low- and middle-income countries can be much more substantial [1].
2. The position of SARS-CoV-2 in taxonomical classification
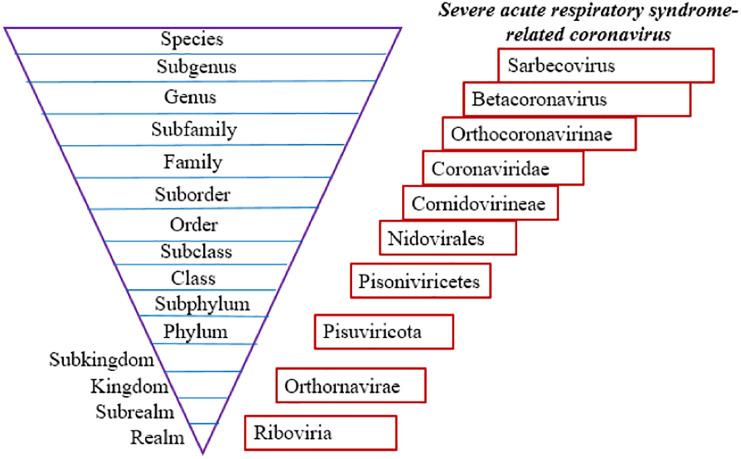
The International Committee on Taxonomy of Viruses (ICTV) system is responsible for virus nomenclature and designing virus classification guidelines [3]. In recent years, with the advancement of molecular technology, including next-generation sequencing, viruses are classified based on their evolutionary and ancestral relationships and the approaches like phylogenic and genomic characteristics provide the basis for their categorization. According to the Committee's nomenclature rules, viruses are classified as a hierarchical scheme of categories and a fifteen-degree classification hierarchy is currently used (Fig. 2 ) [4].
Fig. 2.
Fifteen-degree classification hierarchy of viruses and classification of the newly emerged SARS-CoV-2 virus in this taxonomic hierarchy.
The new coronavirus was temporarily named 2019-nCoV but was then renamed SARS-CoV-2, due to its genetic similarity to the SARS (Severe Acute Respiratory Syndrome) virus detected in 2003. SARS-CoV-2 is a single-stranded positive-sense RNA virus, which belongs to the subfamily Orthocoronavirinae in the family of Coronaviridae in the order Nidovirales. Orthocoronavirinae subfamily includes α-coronavirus, β-coronavirus, γ-coronavirus, and delta-coronavirus. The novel coronavirus is a β-coronavirus (Fig. 2) [4].
The World Health Organization (WHO), which is responsible for naming human diseases, introduced the name COVID-19 (coronavirus disease 2019) to denote the disease caused by SARS-CoV-2.
3. Immunology and immunopathology of COVID-19
As mentioned above, SARS-CoV-2 belongs to the coronavirus family, and its structure consists of the spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins. The spike or S glycoprotein of SARS-CoV-2 comprises of two domains of S1 and S2; the receptor-binding domain (RBD) in S1 cooperates with angiotensin-converting enzyme 2 (ACE2) on the human host cell surface [5]. So, the high expression of ACE2 in lung epithelial cells, particularly type II pneumocytes, justifies the tendency of the virus to enter these cells. Besides, the evidence of multi-organ failure in certain patients shows that ACE2 is expressed in the cells of other organs, too. ACE2 is also expressed on monocytes and macrophages, although at lower levels, so it appears that ACE2 may likewise serve as a possible entry gate into immune cells for SARS-CoV-2 [6]. However, based on the recent studies, the roles of other receptors such as CD147 (7)or the phenomenon of antibody-dependent enhancement (ADE) that occurs via phagocytosis of immune complexes associated with virus particles should not be spared [8].
3.1. Innate immune response
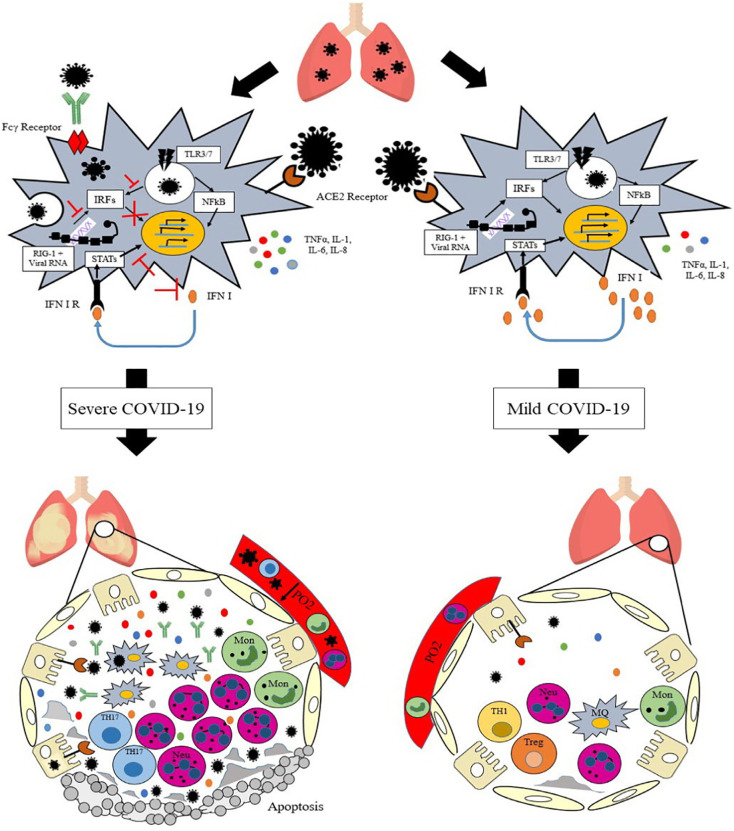
The immune response to this virus starts simultaneously with its attachment to pattern recognition receptors (PRRs) expressed by host cells. PRRs such as Toll-like receptor 3 and 7, retinoic acid-inducible gene I (RIG-I), and melanoma differentiation-associated protein 5 (MDA5) that recognize viral RNA in different parts of the cytoplasm have an important role in mediating response to SARS-CoV-1, SARS-CoV-2, and middle east respiratory syndrome coronavirus (MERS-CoV) [6,9]. The recognition of the virus by these PRRs prompts the expression of type I interferon (IFN) (through IRF3 pathway) and other cytokines such as interleukin (IL)-1, IL-6, and tumor necrosis factor-α (TNF-α) through the nuclear factor kappa B (NFκB) pathway. Therefore, these pathways constitute the first line of defense against the viral infection at the entry site (Fig. 3 ). Type I IFN, after attaching to its receptor, activates signal transducer and activator of transcription-1 (STAT-1) and STAT-2 that in addition to controlling the viral replication, stimulates an adequate adaptive immune response [6]. Antiviral defense in target cells and the induction of adaptive immune responses are improved by IL-1, IL-6, and TNF [10]. Moreover, neutrophils recruited to sites of infection by these cytokines can eliminate the virus via oxidative burst, defensin secretion, and neutrophil extracellular traps (NETs) formation [11].
Fig. 3.
The immunology and immunopathology of COVID-19.
3.2. Adaptive immune response
Alongside innate immune activity, approximately 7–14 days after infection, specific humoral and cellular immune responses, that are effective for viral clearance, are induced following viral antigens presentation. T helper type 1 (TH1) cells, via cytokine secretion, CD8+ T cells, with cytotoxic activity, and B cells, by antibody production against RBD of the viral S protein, generate specific anti-viral responses [12].
However, despite the effectiveness of these immune mechanisms to control infection in many cases, some of the patients with COVID-19 exhibit a moderate to severe form of the disease [13]. According to the observations, genetic defects in immune system components, a particular group of diseases, and/or immunosenescence may account for the greater severity of COVID-19 [7].
3.3. Immune evasion, cytokine storm, lung injury, and acute respiratory distress syndrome (ARDS)
In some infected people, SARS-CoV-2 via escaping innate immune responses can reproduce uncontrollably in primarily infected tissues thereby causing more severe disease and poorer prognosis. These patients show high blood plasma levels of IL-2, IL-6, IL-7, IL-10, IFNγ, granulocyte colony-stimulating factor (GCSF), IFN-γ-induced protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP1), macrophage inflammatory protein-1α (MIP1α), TNFα, and an overall picture of immunodeficiency caused by hyper-inflammation that are positively correlated with disease severity and death [5,10]. Further investigations have shown that besides the strength of the immune response, the time when the innate immune response engages is important for the control of coronavirus infection. It is speculated that SARS-CoV-2, similar to other coronaviruses, complicates early viral control by delaying the production of type I IFNs and counteracting type I IFN signaling through inhibition of STAT family activity. This suppression of innate immune mechanisms in infected cells prevents the induction of an effective antiviral response hence allowing further replication of the coronaviruses [14]. Thus, although a timely production of type I IFN serves to protect against viral infections, a delay in IFN production makes it difficult to control the viral replication, which subsequently causes cellular damage of airway epithelia and the lung parenchyma [6]. In general, uncontrolled viral replication due to lack of effective and timely immune response stimulates necrosis or pyroptosis in cells and this leads to pro-inflammatory cytokines expression, recruitment and activation of immune cells and a fatal inflammatory cytokine storm. In the cytokine storm, high serum levels of many innate cytokines and an increase in neutrophils and the monocyte-macrophages influx cause viral sepsis and inflammatory-induced lung injury [9]. Studies on the SARS-CoV-2 indicate that the downregulation of ACE2, as well as the induction of ACE2 shedding after a viral infection, are other possible mechanisms through which SARS-CoV-2 can induce inflammatory responses [9] as the absence of ACE2 on cells disrupts the renin-angiotensin system (RAS) which affects blood pressure and fluid/electrolyte balance as well as the control over inflammation and vascular permeability in the airways [8].
On the other hand, it was reported that patients with moderate to severe COVID-19 have a reduced number of some immune cells including natural killer (NK) cells, CD4 + T cells, and CD8 + cytotoxic T cells, memory T cells, and B cells which indicate a defective immune response against the virus. Further, some reports indicate that regulatory T (Treg) cells are reduced in severe COVID-19 cases further contributing to the development of COVID-19 lung immunopathology in these patients [12,15]. In this way, a study also reported augmented apoptosis, autophagy, and p53 pathways in peripheral blood mononuclear cells (PBMCs) of patients with COVID-19 compared to healthy controls [16]. Thus, it appears that lymphocytes become depleted in the inflammatory cytokine milieu by apoptosis during the development of a cytokine storm. Also, several studies have shown that in addition to lymphopenia in the severe cases of the disease, there are elevated exhaustion levels and decreased functional diversity in T cells because of the expression of programmed cell death protein-1 (PD-1), lymphocyte activation gene 3 (LAG3), and T cell immunoglobulin mucin-3 (Tim-3) which help to the progression of COVID-19 [12].
As a result of the expression of inflammatory cytokines and the presence of nuclear antigens due to the destruction of tissue cells, acquired immune cells also can be strongly stimulated further intensifying inflammation at later disease stages [6]. In this regard, one study has shown that the number of pathogenic TH1 and in particular T helper type 17 (TH17) cells are increased following high levels of IL-1β and TNF-α in a patient with severe COVID-19, underlying a TH17 type cytokine storm in this disease [17]. Antibody-dependent enhancement (ADE) that happens following the production of high titers of neutralizing antibodies against pathogens such as coronaviruses is another factor of adaptive immunity that leads to organ failure including acute respiratory distress syndrome (ARDS), and poor prognosis (Fig. 3). In this event, viruses that are trapped in immune complexes are not completely neutralized by antibodies which increases their exposure to the cells via antibody binding to Fcγ receptors (FcγR). Thus, ADE contributes to the survival, proliferation and pathogenesis of the virus in immune cells which leads to immune complex-mediated inflammatory responses. ADE can also induce the pro-inflammatory (M1) macrophages in the lungs which lead to lung injury [8]. Overall, an increase in the ratio of neutrophils to lymphocytes (NLR) and acute inflammatory mechanisms following a high viral load and cytokine storm, damage the alveolar-capillary barrier in the lungs exacerbating vascular leakage and alveolar edema (characteristics of ARDS) which result in shock, organ failure (especially in organs that have a high ACE2 expression) and finally death [15]. Therefore, early identification of such patients is crucial and an effective and timely immunomodulatory therapy is useful to downregulate the cytokine storm and mortality in the severe cases of COVID-19. Also, applying an antiviral agent, alongside an immunomodulatory agent, can be helpful.
At the present, there is no approved effective treatment, and/or vaccine for COVID-19. Hence, the introduction of new immunomodulatory and antiviral agents and also vaccines that induce a TH1 response and high titers of neutralizing antibodies are critical for successful control of SARS-CoV-2.
4. Common antiviral approaches and the related limitations
Viruses are microbial agents that, as obligate intracellular parasites, are capable of replicating in host cells by employing cell's protein/nucleic acid synthesis machinery. Therefore, treatment of viral infections is not as easy as bacteria and other pathogenic microorganisms. Antiviral drugs can also be toxic to host cells. In the last 30 years, more than 30 new viruses have emerged and caused epidemics [18]. So far, various antiviral drugs have been developed and used. Various drugs have different mechanisms of action. Some inhibit viral proteins (polymerases or enzymes), or virus genome synthesis, transcription, and metabolism, and some target host cellular proteins that are involved in virus activity. Some medications boost the immune system to counteract viruses such as IFNs, antibodies, and vaccines. Some agents, such as organic solutions, detergents, and UV light, kill the virus on the surfaces in one step [19]. Understanding the exact mechanisms of the life cycle and molecular biology of the viruses help to develop drugs and vaccines against them. However, viruses have the potential to develop drug resistance, and this is one of the limitations that researchers face [20]. Antiviral drugs should have the following characteristics [19]:
-
-
Being non-mutagenic, non-carcinogenic, and non-toxic,
-
-
Do not damage the host cell and specifically kill the virus,
-
-
The drug should be chemically and metabolically stable, well absorbed, and only enter the target organ and function there,
-
-
Be clinically effective and safe.
However, antiviral drugs have some limitations: The high rate of replication and, consequently, the high rate of mutations in the genome of viruses give rise to drug-resistant generations. Antiviral drugs often affect the active and replicating form of the virus and do not kill the latent viruses that hide inside the host cells. Virus escape is another limitation of antiviral compounds. One of the side effects of some antiviral drugs is the suppression of host immune responses, which weakens the immune system by inhibiting or reducing the proliferation of dividing immune cells [21]. Some drugs inhibit DNA synthesis and have a toxic and lethal effect on the host cell. Antiviral drugs are often selective and specific and thus inhibit a small range of viruses. Antiviral therapies should be started promptly and on time before the onset of tissue damage by the virus, and delay in using them will lead to no effective treatment. Drug resistance highlights the necessity of combined use of drugs that both increase the cost and have more side effects on the patient. Drug resistance of viruses forces researchers to develop new drugs against them. Due to these restrictions, other antiviral therapies instead of chemical drugs are under research; such as antisense technology, peptide aptamers, and ribozymes [19]. Drug research and development is a time-consuming and costly task. The help of advanced tools and techniques in molecular biology and modern medicine approaches can reduce time and costs. The design and production of drugs is a challenging process and requires both knowledge and appropriate research tools as well as a financial plan and budget. Detailed knowledge on life cycle, genetics, molecular biology, pathogenesis, and epidemiology of viruses, along with the development of genomics, proteomics, and immunoinformatics sciences, understanding the host-pathogen interactions and using systems biology approaches, improvement of rational drug design programs and algorithms are beneficial in developing and selecting appropriate antiviral interventions. Drug repurposing is another useful outlook that helps to the rapid discovery of medication for newly emerged and unknown viral infectious diseases, which affect public health [20]. Generally, these approaches have enormous potential and renovate researchers' attitudes to treating viral infections. Viruses with high epidemic potential and mortality are a priority for drug development. Bioavailability, appropriate drug dynamics, efficacy, and safety of drugs should be considered in drug design.
Effective treatment of the newly emerged coronavirus warrants extensive research in the field of antiviral drugs. In the absence of appropriate treatment and vaccine for SARS-CoV-2, it is necessary to find alternative solutions for controlling the spread and transmission of the virus, and until the development of a definitive drug or effective vaccine, traditional health practices such as quarantine, isolation, and social distancing should be observed.
5. Chitin and chitosan as tools to combat viral infections
5.1. Chitin and chitosan source and structure
Natural polymers are widespread and have wide applicability. Among the natural polymers, chitin and chitosan occupy a distinct position due to unique properties including nontoxicity, biocompatibility, biodegradability, antibacterial, antifungal, and also antiviral properties, etc. Chitin is a naturally occurring polysaccharide composed of N-acetyl-D-glucosamine and D-glucosamine units and found in the shells of living organisms such as lobsters, crabs, shrimps, and insects or it can be generated via fungal fermentation processes. Chitosan is a derivative of chitin. It is obtained by partial deacetylation of chitin using enzymatic hydrolysis or chemical methods [22].
5.2. Direct antiviral effects of chitin and chitosan
The antimicrobial properties of chitin and chitosan biopolymers and their derivatives have been extensively investigated [[23], [24], [25]]. They are capable of inhibiting the growth of a wide range of bacteria and fungi [26]. The protective effects of chitin or chitosan administration as direct antimicrobial agents have been examined against various pathogens [27]. Due to the presence of NH3+ groups, they exert antimicrobial mechanisms by interacting with the negative surfaces of the bacterial membranes. They can also cause transcriptional inhibition by binding to the DNA molecule in the nucleus, thereby inhibiting pathogen protein synthesis [26].
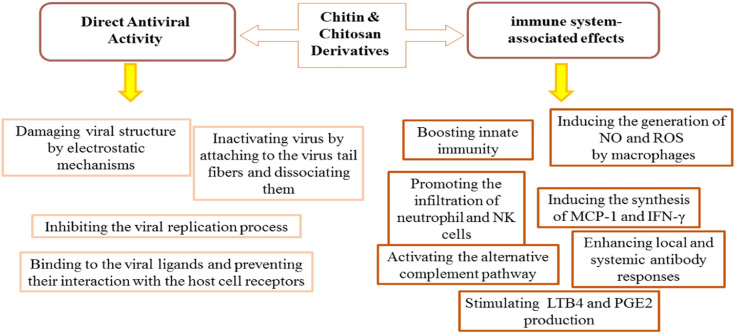
Also, studies have shown that chitin and chitosan are effective against viruses. It is suggested that chitin and chitosan polymers have the ability to fight against viral infections by two approaches: direct antiviral activity and inducing antiviral immune responses (Fig. 4 ).
Fig. 4.
Direct antiviral activities of chitin and chitosan derivatives and their immune-associated effects.
Observations have shown that chitosan inhibits viral infections in plants, animals, and microorganisms (phage infection) [28]. This polymer has the ability to induce resistance to viral infection in plant hosts and can prevent the spread of phages in microbial cultures. Various structural factors affect the anti-phage functions of chitosan. The degree of polymerization, the degree of de-acetylation, the positive charge, and the nature of the chemical changes of the molecules can all affect its function. Chitosan anionic derivatives such as sulfate and succinate sulfate have no anti-phage effects, so the presence of positive charges on the molecule is effective. Oligomers are more effective than polymeric precursors in inhibiting phages. The higher the degree of polymerization, the greater the effect of inhibition.
Antiviral effects of chitosan in plants, in addition to preventing viral spread, involve the induction of resistance genes expression as well as stimulation of a wide range of defense responses against the virus. Chitosan inhibits the replication of viruses in the infected tissues. The concentration, molecular weight, and poly-cationic nature of chitosan are effective in its ability to inhibit plant viral infection [28]. The formation of local necrosis by tobacco mosaic virus (TMV) was inhibited by low molecular weight chitosan. Davydova et al. showed that low molecular weight chitosan can exhibit antiviral activity. They pointed out that the chemical hydrolysis of high molecular weight chitosan increases the antiviral properties of these derivatives compared to its enzymatic hydrolysis due to the formation of oxidized groups and the distribution of acetate groups. The source of chitosan and the method used in its purification also affect the biological activity of the polymer [29].
The effects of chitosan on viral infections in animal cells have also been studied. Chitosan sulfate derivatives specifically inhibit human immunodeficiency virus-1 (HIV-1) replication. In cultured T cells, it prevents the interaction of viral gp120 with the CD4 receptor, thereby inhibiting virus entry into the cell. Also, it competitively inhibits the binding of reverse transcriptase enzyme to the poly-A template [30]. Chitosan anionic derivatives prevent virus fusion with cell membranes by binding electrostatically to the positive charges of the gp120 molecule. It makes sense that the position of sulfate groups on the glucosamine residues is effective on the mechanism of inhibitory action of chitosan [31]. Ishihara et al. used sulfated derivatives of chitin and chitosan microparticles against herpes simplex type-1 virus (HSV-1) and friend murine leukemia helper virus (F-MuLV) in vitro and in vivo. The results of this group showed that these compounds interfere with the uptake and penetration of the virus into the cells and inhibit the pathogenesis of the virus [32]. In the absence of broad-based antiviral medication, Pauls [33] and colleagues successfully displayed the antiviral activity of chitosan against adenovirus on NIH-3 T3 cells. Mori et al. studied the antiviral effects of silver nanoparticle/chitosan composites against the H1N1 influenza virus. In this study, chitosan was used as a polymer matrix to fix silver and prevent them from spreading into the environment and reduce nanoparticle toxicity. This composite reduced the median tissue culture infectious dose (TCID50) ratio of viral suspension, and the smaller the silver particle size, the stronger the antiviral property [34]. Hassan et al. [35] have shown antiviral activity of chitosan nanoparticles against Rift Valley fever (RVF) and Coxsackieviruses as RNA viruses and HSV-1 as a DNA virus by protecting Vero cells from cytopathic effects of these viruses.
Chitosan nanoparticles can also be used as a delivery system for antiviral drugs to both enhance the therapeutic efficacy of the drugs and reduce their dose. The use of these nanoparticles causes controlled drug release and improved antiviral activity of drugs. Loutfy et al. showed antiviral property of curcumin chitosan (CuCs) nanocomposite against hepatitis C virus genotype 4a (HCV-4a). They declared that in addition to curcumin, nanocomposites have synergistic antiviral activity, preventing the virus from entering and replicating in human hepatoma Huh7 cells [36]. Human noroviruses are enteric viruses that cause gastroenteritis. They are single-stranded, non-enveloped, positive-sense RNA viruses. Different molecular weight chitosans were effective against norovirus cultivable surrogates and caused a reduction in these viruses' titers [37].
Generally, it can be said that, in the context of direct antiviral activity, chitin and chitosan cause structural damages in the viruses through electrostatic interactions. Chitosan positive charges interact with the negative charges of capsid proteins facilitating the destruction of virus structure. Also, they inactivate viruses by attaching to the virus tail fibers and cause virus dissociation, or by interfering with the viral replication process (Fig. 4) [38]. Characteristics such as molecular weight, degree of de-acetylation, chitosan concentration, and environmental pH, affect their antiviral features.
5.3. Immune-boosting effects of chitin and chitosan
Chitin and chitosan are capable of augmenting antiviral immune responses. These polymers, by stimulating the innate immune cells (such as macrophages and NK cells); create protective responses against pathogenic challenges. It is shown that they can increase the number of phagocytes (blood polymorphonuclear leukocytes and macrophages) and the N-acetyl-glucosamine residues in chitosan can raise the production of reactive oxygen species (ROS), secretion of nitric oxide (NO), and myeloperoxidase activity in phagocytes. Furthermore, these polymers can promote the migration of neutrophils and the levels of systemic (IgG) and mucosal (IgA) humoral responses [39]. The phagocytosis of chitosan by macrophages and the production of ROS induce the synthesis of IFN-γ in spleen cells. IFN exerts its antiviral effect by inhibiting the translation of genomic RNA or viral mRNAs [40]. Chitosan causes neutrophil chemotaxis, and both chitin and chitosan activate the alternative pathway of the complement system [41]. Chitosan stimulates the production of leukotriene B4 and prostaglandin E2 in canine polymorphonuclear cells [41] (Fig. 4).
These polysaccharides depending on size (large/intermediate/small/super small), molecular purity, molecular weight (MW), morphology, degree of deacetylation (DD), viscosity, concentration, and anatomical site of encounter are of significant interest in the field of immunology. Chitin microparticles that have been reported to possess immunomodulatory effects are recognized by PRRs and then degraded in the vertebrate immune system. Thus, they can stimulate immune responses [22,[42], [43], [44], [45], [46], [47]].
Based on the studies performed on different murine models, it is specified that chitin microparticles (<40 μm) at proper concentrations, in addition to inducing TH1 cell-mediated immunity, stimulate IL-10 production from macrophages through a pathway dependent on dectin-1 and Syk kinase, which downregulate pro-inflammatory cytokine production [48]. So, it seems that these fragments can induce controlled innate and adaptive immune responses that are beneficial against viral infections. To examine the effects of chitin microparticles as adjuvant against HIV infection, Hamajima and his colleagues [49] administered a DNA vaccine formulation containing env and rev genes of HIV-1IIIB (pCMV160IIIB and pcREV) complexed with chitin microparticles in BALB/c mice via the intranasal route and reported that the combination of DNA/chitin microparticles elevated HIV-specific antibodies and the numbers of virus-specific T cells compared to DNA alone indicating that chitin can improve adaptive immune responses to the virus. Further, it has been reported that inactivated hemagglutinin (HA) of H1N1 influenza virus [50] and recombinant HA protein of H5N1 influenza virus [51], when co-administrated intranasally with chitin microparticles (≤20 μm) in female BALB/c mice, can stimulate humoral immune responses (both mucosal and systemic) and induce protection against viral challenge after immunization. In Hasegawa et al. study, it was demonstrated that the production of IgG2a following the use of chitin microparticles as adjuvant caused a TH1 immune response profile as an effective immunity against viruses [50]. The influenza virus, due to inducing the production of pro-inflammatory cytokines and chemokines in large quantities, is highly pathogenic. In this line, Ichinohe and his colleagues [52] administrated chitin microparticles (10 μm) through intranasal route in mice before viral challenge (with H5N1 and H1N1) and observed elevated numbers of NK cells expressing tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in the local lymphoid tissues. Also, viral burden and overproduction of cytokines were suppressed following intranasal administration of chitin microparticles resulting in the reduction of clinical symptoms and an increased survival rate following H5N1 and H1N1 virus infection. Another study showed that treatment with chitin microparticles with a diameter of 3.72 μm via the intranasal route before infection, can enhance the recruitment of immune cells and provide enhanced antiviral activity against the influenza virus. Also, the increase of dendritic cells (DCs) following treatment of mice with chitin microparticles increased T cells stimulation to fight the virus in the airways 14 days after infection [53]. Overall, these results indicate that chitin microparticles are highly effective as mucosal adjuvants when used with viral vaccines or ideal prophylactic drugs because they stimulate the innate immune system to diminish viral load and also induce adaptive immunity following infection.
Chitosan, as a natural cationic polysaccharide, is the deacetylated form of chitin that has drawn a lot of attention due to its anti-microbial, anti-oxidative, and immunomodulatory effects. Some studies have reported that chitosan, by inducing mitochondrial stress and cGAS-STING pathway, can result in type I IFN production which promotes DCs maturation [54]. On the other hand, it has also been shown that chitosan by inducing the production of ROS, the efflux of K+, and lysosomal rupture activates the NLRP3 inflammasome in a phagocytosis-dependent manner thereby increasing the release of pro-inflammatory factors [54,55]. Thus, this polysaccharide, through both of the above-mentioned pathways, induces cellular TH1 type responses and IgG2c isotype switching as effective immune responses against viral infections. Based on physical and chemical properties, there are several types of chitosan polymers including solutions, powders, micro/nanoparticles, and hydrogels. It has been reported application of these polymers as antigen carriers and/or adjuvants in mucosal vaccines induces both cellular and humoral immune responses. Ghendon et al. [56] found that the use of chitosan in the form of a solution in combination with inactivated influenza vaccines can increase the immunogenicity of these vaccines and stimulate humoral immune responses against different variants of the influenza virus. Sui and his colleagues also demonstrated that intranasal administration of chitosan solution along with influenza matrix protein 1 and 2 (M1 and M2), which are highly conserved in all influenza A strains, can induce the production of IgG and IgA antibodies via increasing the immunogenicity of M1 and M2 proteins [57,58]. Chitosan solution efficiency against live attenuated influenza vaccine has been confirmed in Wang et al. study, too [59]. They presented that increased antigen-specific antibodies and enhanced population of IFN-γ-secreting T cells following the use of chitosan provide a good protection against influenza viruses. Trimethylated chitosans (TMC), a water-soluble derivative of chitosan, has a greater positive charge than chitosan, a property causing it to more efficiently deviate immune responses toward TH1 responses and production of antibody [60]. In several studies, administration of whole inactivated influenza virus (WIV) [61,62] and H3N2 subunit antigen [63] with TMC via intranasal route resulted in increased mucosal absorption of antigen through the nasal epithelium leading to systemic and local immune responses with significantly higher influenza-specific antibody levels. Moreover, numerous studies have reported that the use of chitosan nanoparticles besides viral antigens such as hepatitis B surface antigen (HBsAg) [64], recombinant influenza A virus H1N1 [65], avian infectious bronchitis virus (IBV) [66], and recombinant influenza HA antigen [67] not only leads to elevated humoral (both systemic and mucosal) immune responses but also can stimulate cell-mediated immune responses and IFNγ production upon immunization. Albeit some studies have represented that nasal immunization using chitosan nanoparticle-based formulations results in the induction of a balanced TH1 and TH2 responses [65,68,69]. Respiratory syncytial virus (RSV) is another serious virus of the lower respiratory system which causes intensive disease in newborn children, elderly people, and immunocompromised individuals. Studies [70,71] have shown that intranasal immunization with chitosan and DNA vaccines which code for RSV epitopes can induce high titers of serum IgG and mucosal IgA antibodies, increase the number of IFN-γ-secreting T cells and effective cytotoxic T lymphocyte (CTL) responses, associated with a significant reduction of viral load. Hence, the use of chitosan with DNA can significantly diminish inflammation of lung tissue and enhance antiviral responses.
In conclusion, these data show that chitosan, as a mucosal adjuvant is capable of inducing effective immune protection against various types of viruses, especially respiratory viruses.
5.4. Chitosan as a drug delivery vehicle for the treatment of viral infections
As a cationic biological polysaccharide, chitosan has been regarded as a promising nanomaterial with broad medical applications [72]. Over the past decade, new nanomaterials have successfully been developed from chitosan for targeted drug delivery purposes based on the following properties: 1- biocompatibility and capability of serving as reaction sites for other bioactive compounds, 2- protecting unstable drug molecules against blood flow responses and strong gastric acids 3- colon-targeted administration and 4- able to adhere to mucosal tissues for improved absorption of specific drugs [73]. Also, chitosan is generally recognized as safe, which along with its low cost and abundance, make it a particularly interesting material to be used in the pharmaceutical field [74]. At marginally acidic pH values, chitosan glucosamine residues carry positive charges making chitosan a polycationic biopolymer able to easily interact with polyanionic molecules including phospholipids, DNA, and many proteins. Also, the presence of both amino and hydroxyl groups in this material provides multiple options for chemical modification [75]. These modifications offer novel polymeric materials with a variety of physicochemical and biopharmaceutical features, e.g., pH sensitivity, solubility, adsorption, and thermos-responsive features with diverse functionalities [76].
Chitosan-based drug delivery is influenced by several factors: deacetylation and substitution degree of polysaccharide, the molecular weight of the polymer, porous structure, nanoparticle size, and compression force. The conformation and length of chitosan polysaccharide chains affect the accessibility of its functional groups by drug molecules that is critical to creating electrostatic interactions for subsequent incorporation of drug molecules into such systems [77].
Drugs administered by injection or orally invariably cause systemic side effects. Both of these administration routes are suboptimal when the biological target of the drug is localized in a certain organ. But the therapeutic potential of a drug would be more effective when it is possible to direct it to a certain location, for example directing a drug to the lungs via an aerosol. Drug delivery to the lungs possesses several advantages including high efficacy, rapid and sustained drug delivery, no first-pass metabolism, and that both local and systemic effects could be achieved [78]. Drug delivery to the lungs can be accomplished through chitosan-based micro and nanoparticles. The positive charge of chitosan biopolymer provides it with mucoadhesive properties increasing the potential for drug absorption. Also, this positive charge opens the tight junctions resulting in an increased uptake of chitosan micro/nanoparticles [79]. In a study employing chitosan as a polymer, a nanoparticle dry powder inhalation of rifampicin, which is an anti-tubercular drug, was formulated. This formulation showed sustained drug release up to 24 h and had no toxic effects on both cells and organs [80]. Jafarinejad et al. applied a strategy for encapsulation of itraconazole into chitosan-based nanoparticles by a modified ionic gelation method and also fabricated inhalable microparticles using spray drying technique. Itraconazole, as a highly hydrophobic drug, has some limitations when administered orally owing to its low solubility [81]. Debnath and colleagues administered prothionamide (an anti-tubercular drug) via the pulmonary route in the form of nanoparticles that were coated with chitosan [82].
Nanotechnology is gaining increasing popularity in the field of respiratory drug delivery and is becoming the core of focus in today's research. Nanochitosan can be applied as targeted delivery of anti-COVID-19 therapeutics.
5.5. Chitin and chitosan potential antiviral effects for SARS-CoV-2
As a contagious disease, COVID-19 has a high transmission rate. An infected individual can produce many aerosol particles (containing SARS-CoV-2 viruses) through breathing or talking and this poses a threat of infection if these particles are inhaled by adjacent persons near the COVID-19 patient [83]. In addition, there exists a contact hazard as these particles may settle on surfaces and remain infective [84]. Also, the particles can be too small to settle and instead remain dispersed in the air thus posing an infective threat even at far distances [83]. These issues underscore the critical importance of providing people, and especially health care providers, with adequate personal protective clothing and equipment when taking care of such patients. Since SARS-CoV-2 is regarded as a particle, and specifically a nanoparticle, it shares similar properties with all the nanoparticles such as possessing a zeta potential which is mostly assumed to be positive primarily owing to its capsid [85]. Besides, the spike protein of SARS-CoV-2 contains polybasic arginine-rich motifs which impart a positive zeta potential at the physiological pH and this appears to be a distinguishing unique property of this novel virus when comparing with some previous SARS-related sequences. Of note, these sequences have been reported to take key parts in SARS-CoV-2 entrance into the host cells along with implications in virus infectivity [86]. On such a basis, an interesting opinion aiming at conferring protection against this pandemic virus has been suggested involving the exploitation of positively charged polymers like chitosan for preparing nanofibers capable of being incorporated in the clothes of health care providers. This way, electrostatic repulsive forces between the cloth surface and SARS-CoV-2 particles could reduce the viral load around these individuals and hence result in lower transmissibility of the virus [87].
Milewska et al. have developed and described chitosan-based anti-coronavirus compounds able to effectively inhibit infection of all low pathogenic human coronaviruses both in vitro and ex vivo. Indeed, interaction analysis of this chitosan derivative with the recombinant ectodomain of the S protein confirmed binding, resulting in the formation of polymer-protein complexes. They showed that the polymer interacts with the coronavirus spike protein and inhibits its interaction with the cellular receptor [88]. The same group in 2020 used cell culture models mimicking the fully differentiated layer that lines the conductive airways, as well as the site of coronavirus replication. This culture system was infected with a given virus in the presence of the chitosan derivative. The analysis demonstrated that the polymer could efficiently inhibit SARS-CoV-2 and MERS-CoV in this model, too [89].
The use of chitosan with DNA can significantly diminish inflammation of lung tissue and enhance antiviral responses. Raghuwanshi et al. [90] used a DNA vaccine expressing SARS CoV nucleocapsid protein together with chitosan nanoparticles intranasally to increase the efficacy of this DNA vaccine by targeting nasal-resident DCs that present antigens to T cells and activate them. The group's findings showed that this strategy leads to increased titers of systemic IgG and nasal IgA antibodies which are specific for the N protein of the virus compared to the control group, and boosts the immune system against SARS-CoV.
Nanochitosan application as vaccine adjuvant or vehicles for delivery of drugs, siRNA, and peptides via the intranasal route has been reported. It can be a very promising approach to fight against SARS-CoV-2 [91]. Chitosan due to stimulation of the immune system, the ability to penetrate through tight junctions between the mucosal epithelial cells, and the possibility of making physicochemical changes to the antigen molecules to improve their immunogenicity, can be considered as a proper option against COVID-19. Biotinylated chitosan nanoparticles were functionalized by a fusion protein vector for selective targeting of DCs and delivering the plasmid DNA encoding SARS-CoV N protein which led to elevated mucosal IgA concentration as well as enhanced systemic IgG against the N protein after intranasal administration [90]. In 2020, Bioavanta-Bosti announced the potential of its chitosan nanoparticle technology for formulating aerosol anti-COVID-19 drugs. Aerosol formulations of Novochizol™ nanoparticles can be applied to confine and deliver any potential anti-COVID-19 drug to the lungs of severely ill patients. The source material for the production of Novochizol™ is chitosan. Novochizol™ technology generates chitosan spherical chitosan nanoparticles with unique, customizable physicochemical characteristics. Novochizol™ nanoparticles are simply formulated as an aerosol capable of being administered at all disease stages for example in ventilators. In the respiratory tract, Novochizol™ nanoparticles adhere to mucosal membranes, and the encapsulated active material is gradually released from immobilized Novochizol™ nanoparticles because of diffusion and slow chitosan degradation. Therefore, this localized pharmacokinetics ensures a high local concentration of the drug without systemic distribution [92].
6. Conclusion
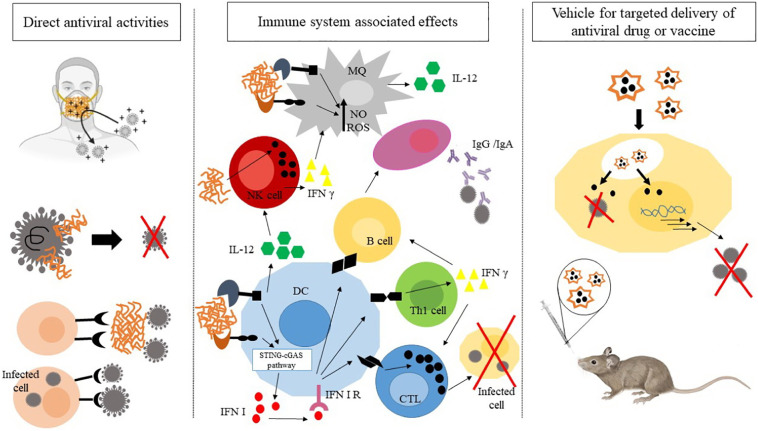
Increasing global transmission of the new coronavirus, SARS-CoV-2, is tremendously affecting human society from different aspects. In addition to its life-threatening impact on human health, SARS-CoV-2 has resulted in several socioeconomic problems further increasing the global burden of COVID-19. As there is no licensed drug or mucosal vaccine till date, conducting further research in this field is hoped to envision a more bright future in this regard. Nanotechnology-based strategies have illustrated a dynamic field incorporating prophylactic, diagnostic, therapeutic, and prognostic perspectives to fight a variety of diseases including infectious ones. In this regard, chitin, chitosan, and their derivatives have held great promises in terms of coping with viral infections. These polymers and their derivatives can be exploited in several ways against infectious agents. In this review paper, we addressed some of the previously published reports on the potential application of such materials in terms of their direct antiviral activities, the immune system associated effects as well as vehicles for targeted delivery of antiviral drugs. Fig. 5 depicts this “triple approach”. Some studies have tried to decipher the ability of chitosan nanoparticle technology to hinder coronaviral infections. However, to achieve a fully verified strategy based on the application of these nanoparticles and the mentioned polymers against COVID-19, further detailed studies involving animal studies and clinical trials are required. Future studies are deemed to concentrate on the different aspects of the applicability of these micro-and nano-structures against this pandemic infection. Chitin, chitosan, and their derivatives hold substantial promises to be clinically employed against COVID-19 in near future.
Fig. 5.
The “triple approach” envisaged for chitin, chitosan and the related derivatives in the fight against COVID-19.
References
- 1.Cojocaru F.D., Botezat D., Gardikiotis I., Uritu C.M., Dodi G., Trandafir L., et al. Nanomaterials designed for antiviral drug delivery transport across biological barriers. Pharmaceutics. 2020;12(2) doi: 10.3390/pharmaceutics12020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMichael A.J. Environmental and social influences on emerging infectious diseases: past, present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004;359(1447):1049–1058. doi: 10.1098/rstb.2004.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhn J.H., Institutes N., States U. 2020. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19. The COVID-19 resource centre is hosted on Elsevier Connect, the company’s public news and information. (January) [Google Scholar]
- 4.Gorbalenya A.E., Krupovic M., Mushegian A., Kropinski A.M., Siddell S.G., Varsani A., et al. The new scope of virus taxonomy: partitioning the virosphere into 15 hierarchical ranks. Nat. Microbiol. 2020;5(5):668–674. doi: 10.1038/s41564-020-0709-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol.. 2020;20(6):363–74. [DOI] [PMC free article] [PubMed]
- 6.Felsenstein S., Herbert J.A., PS McNamara, Hedrich C.M. COVID-19: immunology and treatment options. Clin. Immunol. 2020:215. doi: 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yazdanpanah F., Hamblin M.R., Rezaei N. The immune system and COVID-19: friend or foe? Life Sci. 2020;256 doi: 10.1016/j.lfs.2020.117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol. Sin. 2020 doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikolich-Zugich J., Knox K.S., Rios C.T., Natt B., Bhattacharya D., Fain M.J. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. GeroScience. 2020;42(2):505–514. doi: 10.1007/s11357-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jamilloux Y., Henry T., Belot A., Viel S., Fauter M., El Jammal T., et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun. Rev. 2020;19(7) doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., et al. Immunology of COVID-19: current state of the science. Immunity. 2020;1 doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ong E.Z., Chan Y.F.Z., Leong W.Y., Lee N.M.Y., Kalimuddin S., Haja Mohideen S.M., et al. A dynamic immune response shapes COVID-19 progression. Cell Host Microbe. 2020;27(6):879–882.e2. doi: 10.1016/j.chom.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Z., Ren L., Zhang L., Zhong J., Xiao Y., Jia Z., et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27(6):883–890. doi: 10.1016/j.chom.2020.04.017. (e2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020;2019:4–10. doi: 10.1093/cid/ciaa248. (Xx Xxxx) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong Y, Liu Y, Cao L, Wang D, Guo M, Jiang A, et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect.. 2020;9(1):761–70. [DOI] [PMC free article] [PubMed]
- 17.Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020;53(3):368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization . WHO Regional Office for South-East Asia; New Delhi PP - New Delhi: 2005. Combating Emerging Infectious Diseases in the South-East Asia Region. [Google Scholar]
- 19.Saxena S.K., Saxena S., Saxena R., Arvinda Swamy M., Gupta A., Nair M.P. Emerging trends, challenges and prospects in antiviral therapeutics and drug development for infectious diseases. Electron. J. Biol. 2010;6(2):26–31. [Google Scholar]
- 20.Asboe D., Pozniak A. Antiviral therapy. Infect. Dis. Third Ed. 2010;2:1033–1042. [Google Scholar]
- 21.Bean B. Antiviral therapy: current concepts and practices. Clin. Microbiol. Rev. 1992;5(2):146–182. doi: 10.1128/cmr.5.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haji Molla Hoseini M., Sadeghi S., Azizi M., Pouriran R. In: Handbook of Chitin and Chitosan. Thomas S., Pius A., Gopi S., editors. India; Susan Dennis: 2020. Immunomodulatory activities of chitin and chitosan microparticles; pp. 609–639. [Google Scholar]
- 23.Kong M., Guang X., Xing K., Jin H. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int. J. Food Microbiol. 2010;144(1):51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Raafat D., Sahl H.G. Chitosan and its antimicrobial potential - a critical literature survey. Microb. Biotechnol. 2009;2:186–201. doi: 10.1111/j.1751-7915.2008.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vin J, Vav E. Chitosan derivatives with antimicrobial, antitumour and antioxidant activities - a review. 2011;3596–607. [DOI] [PubMed]
- 26.Hadwiger L.A., Kendra D.F., Fristensky B.W., Wagoner W. 1986. Chitosan both activates genes in plants and inhibits RNA synthesis in fungi; pp. 209–210. (Table 1) [Google Scholar]
- 27.Okawa Y., Kobayashi M., Suzuki S., Suzuki M. Comparative study of protective effects of chitin, chitosan, and N-acetyl chitohexaose against Pseudomonas aeruginosa and Listeria monocytogenes infections in mice. Biol. Pharm. Bull. 2003;26(6):902–904. doi: 10.1248/bpb.26.902. [DOI] [PubMed] [Google Scholar]
- 28.Chirkov S.N. The antiviral activity of chitosan (review) Prikl. Biokhim. Mikrobiol. 2002;38(1):12–13. [PubMed] [Google Scholar]
- 29.Davydova V.N., Nagorskaya V.P., Gorbach V.I., Kalitnik A.A., Reunov A.V. Chitosan antiviral activity: dependence on structure and depolymerization method. Appl. Biochem. Microbiol. 2011;47(1):103–108. [PubMed] [Google Scholar]
- 30.Sosa M.A., Fazely F., Koch J.A., Vercellotti S.V., Ruprecht R.M. N-carboxymethylchitosan-N,O-sulfate as an anti-HIV-1 agent. Biochem. Biophys. Res. Commun. 1991;174(2):489–496. doi: 10.1016/0006-291x(91)91443-g. Jan. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura S.I., Kai H., Shinada K., Yoshida T., Tokura S., Kurita K., et al. Regioselective syntheses of sulfated polysaccharides: specific anti-HIV-1 activity of novel chitin sulfates. Carbohydr. Res. 1998 Jan;306(3):427–433. doi: 10.1016/s0008-6215(97)10081-7. [DOI] [PubMed] [Google Scholar]
- 32.Ishihara C., Yoshimatsu K., Tsuji M., Arikawa J., Saiki I., Tokura S., et al. Anti-viral activity of sulfated chitin derivatives against friend murine leukaemia and herpes simplex type-1 viruses. Vaccine. 1993;11(6):670–674. doi: 10.1016/0264-410x(93)90315-o. [DOI] [PubMed] [Google Scholar]
- 33.Pauls T. 2016. Chitosan as an Antiviral. [Google Scholar]
- 34.Mori Y., Ono T., Miyahira Y., Nguyen V.Q., Matsui T., Ishihara M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013;8(1):93. doi: 10.1186/1556-276X-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassan M.I., Mohamed A.L.Y.F., Taher F.A. Antimicrobial activities of chitosan nanoparticles prepared from lucilia cuprina maggots (Diptera: Calliphoridae). J. Egypt. Soc. Parasitol. 2016;46(3):563–570. [PubMed] [Google Scholar]
- 36.Loutfy S.A., Elberry M.H., Farroh K.Y., Mohamed H.T., Mohamed A.A., Mohamed E.B., et al. Antiviral activity of chitosan nanoparticles encapsulating curcumin against hepatitis C virus genotype 4a in human hepatoma cell lines. Int. J. Nanomedicine. 2020;15:2699–2715. doi: 10.2147/IJN.S241702. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Davis R., Zivanovic S. Effectiveness of chitosan on the inactivation of enteric viral surrogates. Food Microbiol. 2012;32(1):57–62. doi: 10.1016/j.fm.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Kochkina ZM, Surgucheva NA, Chirkov SN. [Coliphages inactivation using chitosan derivatives]. Mikrobiologiia. 2000;69(2):261–5. [PubMed]
- 39.Bacon A., Makin J., Sizer P.J., Jabbal-Gill I., Hinchcliffe M., Illum L., et al. Carbohydrate biopolymers enhance antibody responses to mucosally delivered vaccine antigens. Infect. Immun. 2000;68(10):5764–5770. doi: 10.1128/iai.68.10.5764-5770.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibata Y., Foster L.A., Metzger W.J., Myrvik Q.N. Alveolar macrophage priming by intravenous administration of chitin particles, polymers of N-acetyl-D-glucosamine, in mice. Infect. Immun. 1997;65(5):1734–1741. doi: 10.1128/iai.65.5.1734-1741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ichiro Tanioka S., Minami S., Matsuhashi A. Migration of canine neutrophils to chitin and chitosan. J. Vet. Med. Sci. 1994;56(6):1215–1216. doi: 10.1292/jvms.56.1215. [DOI] [PubMed] [Google Scholar]
- 42.Komi D., Sharma L., Dela Cruz CS. Chitin and its effects on inflammatory and immune responses. Clin. Rev. Allergy Immunol. 2017;54(2):1–11. doi: 10.1007/s12016-017-8600-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azizi M., Yousefi R., Yeganeh F., Mami S., Haji Molla Hoseini M. Co-administration of chitin micro-particle and Leishmania antigen proposed a new immune adjuvant against experimental leishmaniasis. Parasite Immunol. 2019;41(12) doi: 10.1111/pim.12676. [DOI] [PubMed] [Google Scholar]
- 44.Dehghani F., Haji Molla Hoseini M., Memarnejadian A., Yeganeh F., Rezaie A.M., Khaze V., et al. Immunomodulatory activities of chitin microparticles on Leishmania major-infected murine macrophages. Arch. Med. Res. 2011;42(7):572–576. doi: 10.1016/j.arcmed.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Hoseini M.H.M., Moradi M., Alimohammadian M.H., Shahgoli V.K., Darabi H., Rostami A. Immunotherapeutic effects of chitin in comparison with chitosan against Leishmania major infection. Parasitol. Int. 2016;65(2):99–104. doi: 10.1016/j.parint.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Mami S., Yeganeh F., akbar Salari A., Anissian A., Azizi M., Hajimollahoseini M. Oral chitin treatment improved demyelination in murine autoimmune encephalomyelitis model by inhibition of inflammatory responses. Int. Immunopharmacol. 2020;84 doi: 10.1016/j.intimp.2020.106536. [DOI] [PubMed] [Google Scholar]
- 47.Mami S., Yeganeh F., Farahani E., Anissian A., MHM Hoseini. Chitin micro particles regulate splenocytes immune response in experimental autoimmune encephalomyelitis. Iran. J. Allergy Asthma Immunol. 2019;18(2):190–199. [PubMed] [Google Scholar]
- 48.Lee C.G., Da Silva C.A., Lee J.Y., Hartl D., Elias J.A. Chitin regulation of immune responses: an old molecule with new roles. Curr. Opin. Immunol. 2008;20(6):684–689. doi: 10.1016/j.coi.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamajima K., Kojima Y., Matsui K., Toda Y., Jounai N., Ozaki T., et al. Chitin micro-particles (CMP): a useful adjuvant for inducing viral specific immunity when delivered intranasally with an HIV-DNA vaccine. Viral Immunol. 2003;16(4):541–547. doi: 10.1089/088282403771926355. [DOI] [PubMed] [Google Scholar]
- 50.Hasegawa H., Ichinohe T., Strong P., Watanabe I., Ito S., Tamura S.I., et al. Protection against influenza virus infection by intranasal administration of hemagglutinin vaccine with chitin microparticles as an adjuvant. J. Med. Virol. 2005;75(1):130–136. doi: 10.1002/jmv.20247. [DOI] [PubMed] [Google Scholar]
- 51.Asahi-Ozaki Y., Itamura S., Ichinohe T., Strong P., ichi Tamura S., Takahashi H., et al. Intranasal administration of adjuvant-combined recombinant influenza virus HA vaccine protects mice from the lethal H5N1 virus infection. Microbes Infect. 2006;8(12−13):2706–2714. doi: 10.1016/j.micinf.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 52.T. I, N. N, P. S, S.-I. T, H. T, A. N, et al. Prophylactic effects of chitin microparticles on highly pathogenic H5N1 influenza virus. J. Med. Virol.. 2007;79(6):811–9. Available from: 10.1002/jmv.20837. [DOI] [PubMed]
- 53.BJG Baaten, Clarke B., Strong P., Hou S. Nasal mucosal administration of chitin microparticles boosts innate immunity against influenza A virus in the local pulmonary tissue. Vaccine. 2010;28(25):4130–4137. doi: 10.1016/j.vaccine.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 54.HBT Moran, Turley J.L., Andersson M., Lavelle E.C. Immunomodulatory properties of chitosan polymers. Biomaterials. 2018;184:1–9. doi: 10.1016/j.biomaterials.2018.08.054. [DOI] [PubMed] [Google Scholar]
- 55.Bueter C.L., Lee C.K., Wang J.P., Ostroff G.R., Specht C.A., Levitz S.M. Spectrum and mechanisms of inflammasome activation by chitosan. J. Immunol. 2014;192(12):5943–5951. doi: 10.4049/jimmunol.1301695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghendon Y., Markushin S., Krivtsov G., Akopova I. Chitosan as an adjuvant for parenterally administered inactivated influenza vaccines. Arch. Virol. 2008;153(5):831–837. doi: 10.1007/s00705-008-0047-4. [DOI] [PubMed] [Google Scholar]
- 57.Sui Z., Chen Q., Fang F., Zheng M., Chen Z. Cross-protection against influenza virus infection by intranasal administration of M1-based vaccine with chitosan as an adjuvant. Vaccine. 2010;28(48):7690–7698. doi: 10.1016/j.vaccine.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 58.Sui Z., Chen Q., Wu R., Zhang H., Zheng M., Wang H., et al. Cross-protection against influenza virus infection by intranasal administration of M2-based vaccine with chitosan as an adjuvant. Arch. Virol. 2010;155(4):535–544. doi: 10.1007/s00705-010-0621-4. [DOI] [PubMed] [Google Scholar]
- 59.Wang X., Zhang W., Liu F., Zheng M., Zheng D., Zhang T., et al. Intranasal immunization with live attenuated influenza vaccine plus chitosan as an adjuvant protects mice against homologous and heterologous virus challenge. Arch. Virol. 2012;157(8):1451–1461. doi: 10.1007/s00705-012-1318-7. [DOI] [PubMed] [Google Scholar]
- 60.Sayin B., Somavarapu S., Li X.W., Thanou M., Sesardic D., Alpar H.O., et al. Mono-N-carboxymethyl chitosan (MCC) and N-trimethyl chitosan (TMC) nanoparticles for non-invasive vaccine delivery. Int. J. Pharm. 2008;363(1–2):139–148. doi: 10.1016/j.ijpharm.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 61.Hagenaars N., Mania M., de Jong P., Que I., Nieuwland R., Slütter B., et al. Role of trimethylated chitosan (TMC) in nasal residence time, local distribution and toxicity of an intranasal influenza vaccine. J. Control. Release. 2010;144(1):17–24. doi: 10.1016/j.jconrel.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 62.Hagenaars N., Verheul R.J., Mooren I., de Jong P.H.J.L.F., Mastrobattista E., Glansbeek H.L., et al. Relationship between structure and adjuvanticity of N,N,N-trimethyl chitosan (TMC) structural variants in a nasal influenza vaccine. J. Control. Release. 2009;140(2):126–133. doi: 10.1016/j.jconrel.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 63.Amidi M., Romeijn S.G., Verhoef J.C., Junginger H.E., Bungener L., Huckriede A., et al. N-Trimethyl chitosan (TMC) nanoparticles loaded with influenza subunit antigen for intranasal vaccination: biological properties and immunogenicity in a mouse model. Vaccine. 2007;25(1):144–153. doi: 10.1016/j.vaccine.2006.06.086. [DOI] [PubMed] [Google Scholar]
- 64.Borges O., Tavares J., de Sousa A., Borchard G., Junginger H.E., Cordeiro-da-Silva A. Evaluation of the immune response following a short oral vaccination schedule with hepatitis B antigen encapsulated into alginate-coated chitosan nanoparticles. Eur. J. Pharm. Sci. 2007;32(4–5):278–290. doi: 10.1016/j.ejps.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 65.Gupta N.K., Tomar P., Sharma V., Dixit V.K. Development and characterization of chitosan coated poly-(e{open}-caprolactone) nanoparticulate system for effective immunization against influenza. Vaccine. 2011;29(48):9026–9037. doi: 10.1016/j.vaccine.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 66.Lopes P.D., Okino C.H., Fernando F.S., Pavani C., Casagrande V.M., Lopez R.F.V., et al. Inactivated infectious bronchitis virus vaccine encapsulated in chitosan nanoparticles induces mucosal immune responses and effective protection against challenge. Vaccine. 2018;36(19):2630–2636. doi: 10.1016/j.vaccine.2018.03.065. [DOI] [PubMed] [Google Scholar]
- 67.Moon H.J., Lee J.S., Talactac M.R., MYE Chowdhury, Kim J.H., Park M.E., et al. Mucosal immunization with recombinant influenza hemagglutinin protein and poly gamma-glutamate/chitosan nanoparticles induces protection against highly pathogenic influenza A virus. Vet. Microbiol. 2012;160(3–4):277–289. doi: 10.1016/j.vetmic.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 68.Pawar D., Mangal S., Goswami R., Jaganathan K.S. Development and characterization of surface modified PLGA nanoparticles for nasal vaccine delivery: effect of mucoadhesive coating on antigen uptake and immune adjuvant activity. Eur. J. Pharm. Biopharm. 2013;85(3 PART A):550–559. doi: 10.1016/j.ejpb.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 69.Vicente S., Peleteiro M., Díaz-Freitas B., Sanchez A., González-Fernández Á., Alonso M.J. Co-delivery of viral proteins and a TLR7 agonist from polysaccharide nanocapsules: a needle-free vaccination strategy. J. Control. Release. 2013;172(3):773–781. doi: 10.1016/j.jconrel.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 70.Kumar M., Behera A.K., Lockey R.F., Zhang J., Bhullar G., Perez de la Cruz C., et al. Intranasal gene transfer by chitosan-DNA nanospheres protects BALB/c mice against acute respiratory syncytial virus infection. Hum. Gene Ther. 2002;13(12):1415–1425. doi: 10.1089/10430340260185058. [DOI] [PubMed] [Google Scholar]
- 71.Iqbal M., Lin W., Jabbal-Gill I., Davis S.S., Steward M.W., Illum L. Nasal delivery of chitosan-DNA plasmid expressing epitopes of respiratory syncytial virus (RSV) induces protective CTL responses in BALB/c mice. Vaccine. 2003;21(13–14):1478–1485. doi: 10.1016/s0264-410x(02)00662-x. [DOI] [PubMed] [Google Scholar]
- 72.Şenel S., SJ McClure. Potential applications of chitosan in veterinary medicine. Adv. Drug Deliv. Rev. 2004;56(10):1467–1480. doi: 10.1016/j.addr.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 73.Mao S., Sun W., Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug Deliv. Rev. 2010;62(1):12–27. doi: 10.1016/j.addr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 74.Sinha V.R., Singla A.K., Wadhawan S., Kaushik R., Kumria R., Bansal K., et al. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004;274(1–2):1–33. doi: 10.1016/j.ijpharm.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 75.Zhang J., Xia W., Liu P., Cheng Q., Tahirou T., Gu W., et al. Chitosan modification and pharmaceutical/biomedical applications. Mar. Drugs. 2010;8(7):1962–1987. doi: 10.3390/md8071962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prabaharan M. Review paper: chitosan derivatives as promising materials for controlled drug delivery. J. Biomater. Appl. 2008;23(1):5–36. doi: 10.1177/0885328208091562. [DOI] [PubMed] [Google Scholar]
- 77.Lucio D., Martínez-Ohárriz M.C. Biol Act Appl Mar Polysaccharides. 2017. Chitosan: strategies to increase and modulate drug release rate. [Google Scholar]
- 78.Ruge C.C., Kirch J., Lehr C.M. Pulmonary drug delivery: from generating aerosols to overcoming biological barriers-therapeutic possibilities and technological challenges. Lancet Respir. Med. 2013;1(5):402–413. doi: 10.1016/S2213-2600(13)70072-9. [DOI] [PubMed] [Google Scholar]
- 79.Islam N., Ferro V. Recent advances in chitosan-based nanoparticulate pulmonary drug delivery. Nanoscale. 2016;8(30):14341–14358. doi: 10.1039/c6nr03256g. [DOI] [PubMed] [Google Scholar]
- 80.Rawal T., Parmar R., Tyagi R.K., Butani S. Rifampicin loaded chitosan nanoparticle dry powder presents: an improved therapeutic approach for alveolar tuberculosis. Colloids Surf. B Biointerfaces. 2017;154:321–330. doi: 10.1016/j.colsurfb.2017.03.044. [DOI] [PubMed] [Google Scholar]
- 81.Jafarinejad S., Gilani K., Moazeni E., Ghazi-Khansari M., Najafabadi A.R., Mohajel N. Development of chitosan-based nanoparticles for pulmonary delivery of itraconazole as dry powder formulation. Powder Technol. 2012;222:65–70. [Google Scholar]
- 82.Debnath S.K., Saisivam S., Debanth M., Omri A. Development and evaluation of chitosan nanoparticles based dry powder inhalation formulations of Prothionamide. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0190976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meselson M. Droplets and aerosols in the transmission of SARS-CoV-2. N. Engl. J. Med. 2020;382(21):2063. doi: 10.1056/NEJMc2009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Forrey C., Muthukumar M. Electrostatics of capsid-induced viral RNA organization. J. Chem. Phys. 2009;131(10) [Google Scholar]
- 86.Jaimes J.A., Millet J.K., Whittaker G.R. Proteolytic cleavage of the SARS-CoV-2 spike protein and the role of the novel S1/S2 site. iScience. 2020;23(6) doi: 10.1016/j.isci.2020.101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hathout R.M., Kassem D.H. Positively charged electroceutical spun chitosan nanofibers can protect health care providers from COVID-19 infection: an opinion. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Milewska A., Ciejka J., Kaminski K., Karewicz A., Bielska D., Zeglen S., et al. Novel polymeric inhibitors of HCoV-NL63. Antivir. Res. 2013;97(2):112–121. doi: 10.1016/j.antiviral.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Milewska A., Chi Y., Szczepanski A., Barreto-Duran E., Liu K., Liu D., et al. Vol. 5. 2020. HTCC as a highly effective polymeric inhibitor of SARS-CoV-2 and MERS-CoV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Raghuwanshi D., Mishra V., Das D., Kaur K., Suresh M.R. Dendritic cell targeted chitosan nanoparticles for nasal DNA immunization against SARS CoV nucleocapsid protein. Mol. Pharm. 2012;9(4):946–956. doi: 10.1021/mp200553x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Itani R., Tobaiqy M., Al Faraj A. Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patients. Theranostics. 2020;10(13):5932–5942. doi: 10.7150/thno.46691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Immediate BA, Of A, Chitosan ITS . 2020. PRESS RELEASE 26 March 2020. (March) [Google Scholar]