Abstract
During the COVID-19 pandemic, frontline healthcare workers have been exposed to very stressful conditions. Measuring hair cortisol concentrations (HCCs), which reflect the integrated long-term cortisol levels, may elucidate the impact of COVID-19 related stress on healthcare professionals. In the current study, we investigated experienced stress in 693 healthcare workers, with hair samples for cortisol analysis collected from a subset of 67 female nurses. The HCCs in two 3 cm hair segments corresponding to periods before and during the peak of the first wave of COVID-19 were compared. To evaluate the effect of working in the first line, the sample was divided into two groups based on the COVID-19 risk estimated by the nurses. Covariates in the model included perceived stress (PSS), perceived social support (MSPSS), and quality of sleep (PSQI) measured via an online questionnaire. The data showed that more than 75% of healthcare workers agreed that COVID-19 led to increased stress at their workplace. The hair cortisol analysis showed higher HCCs in the hair segments corresponding to the time of the pandemic compared to hair corresponding to an earlier period (partial η290%CI = 0.123–0.397); in the same model, higher HCCs were also found in nurses from high-risk environments compared to low-risk ones (partial η290%CI = 0.002–0.176). None of the subjective questionnaire measures were significant predictors of HCCs. In conclusion, these data showed that HCCs reflect the increased stress among nurses during the COVID-19 pandemic as well as the difference in nurses between high- and low-risk environments.
Keywords: Cortisol, Hair, Stress, Nurses, COVID-19
1. Introduction
The spread of the new coronavirus disease (COVID-19) represents not only a serious physical health risk but is also a potential mental health problem (Kannampallil et al., 2020, Rajkumar, 2020). Roughly one-third to half of the general population reported experiencing COVID-19 related psychological distress in the first months of the pandemic (Wang et al., 2020, Qiu et al., 2020, Moccia et al., 2020).
The situation is exceptionally hard for frontline healthcare workers who are directly responsible for the treatment and care of patients with COVID-19 (Krystal and McNeil, 2020). Facing this novel situation – which is characterized by increased pressure, high time demands, a risk of infection, and inadequate protection (Kang et al., 2020), as well as unpredictability and low control – leads to the experience of stress and the increased risk of mental health problems such as anxiety, depression, insomnia, burnout, and posttraumatic stress disorder (Kannampallil et al., 2020, Spoorthy et al., 2020, Shechter et al., 2020). A survey done by Lai et al. (2020) in affected regions in China showed that more than 70% of healthcare professionals reported suffering from distress, 50% reported depressive symptoms, and 44% reported increased anxiety during the beginning of the COVID-19 outbreak. According to their results, the most severe symptoms were present in nurses, in women, and in professionals who were working in the first line of treatment with infected patients.
These findings are similar to earlier studies on the mental health of medical staff done during the SARS outbreak (Styra et al., 2008) which highlighted that work in high-risk units, a high perception of personal risk, the experienced impact on one’s work, and higher depressive affect are all risk factors for healthcare workers. On the other hand, social support from family and friends, institutional support from the government (Cai et al., 2020), and adequate coping strategies and personality traits, such as resilience (Barzilay et al., 2020), serve as protective factors.
In this study, we looked at the long-term psychological stress in healthcare professionals in Slovakia during the first wave of the COVID-19 pandemic, measuring their hair cortisol concentrations (HCCs) as the neuroendocrine marker of stress in addition to self-report measures. HCCs reflect the long-term cumulative cortisol secretion during the period of hair growth, which can be analyzed retrospectively for several months (Russell et al., 2012, Stalder and Kirschbaum, 2012). According to a meta-analysis by Stalder et al. (2017), groups exposed to long-term stress (e.g., work stress) were estimated to have their HCC level elevated by approximately 22%, with an even stronger effect if the chronic stress was still ongoing at the time of measurement. In particular, increased HCCs were found in caregivers of patients with dementia (Stalder et al., 2014) and people suffering from burnout (Penz et al., 2018). Based on the available data from studies, we consider HCCs to be a relevant biological marker to assess the levels of long-term stress in healthcare workers during the COVID-19 pandemic.
Using questionnaires administered online, we aimed to explore the extent of experienced stress related to work in healthcare professionals during the COVID-19 pandemic, as well as their sleep quality and perceived social support. In more detail, we investigated a subset of female nurses, which were previously considered to be a high-risk group (e.g., Lai et al., 2020), via collecting their hair samples for cortisol analysis. We hypothesized that HCCs from the period corresponding to the peak of the COVID-19 pandemic would be higher, compared to HCCs reflecting an earlier period. Secondly, as the effects of stress were previously found to be more pronounced in healthcare professionals who worked directly with infected patients, a comparison of nurses from high-risk environments for COVID-19 with those who worked in lower-risk environments was undertaken; expecting higher HCCs among those participants who faced more risk of being exposed to COVID-19 in the workplace. Finally, we aimed to assess whether subjectively reported perceived stress, perceived social support, and sleep quality relate to HCC levels or interact with the above-mentioned factors of time and estimated risk in their effect on HCCs.
2. Material and methods
2.1. Participants & procedure: online survey
The distribution of the online survey took place between May 11 and June 5, 2020. The survey was posted in Facebook groups for healthcare professionals and was distributed via the mailing list of the Slovak Association of Nurses and Midwives. A total of 693 participants filled in the survey during the given period. Of this sample, 658 were female and 35 were male; 633 worked as nurses, 22 were physicians, and 38 were in other positions in the healthcare services. Volunteers were recruited from all regions throughout Slovakia.
The survey included an informed consent form with detailed information on the research aims and all following procedures as well as the analysis and sharing of anonymized data. At the end of the survey, participants also provided consent with sending us hair samples and contact information for further communication and sampling instructions. Participants were informed that the reward for completing the entire protocol, including providing the hair samples, was 20 euros in the form of gift vouchers. The research protocol was approved by the Ethics Committee of the Faculty of Arts of Comenius University in Bratislava and was given the number EK/07/2020.
2.2. Participants & procedure: hair samples collection
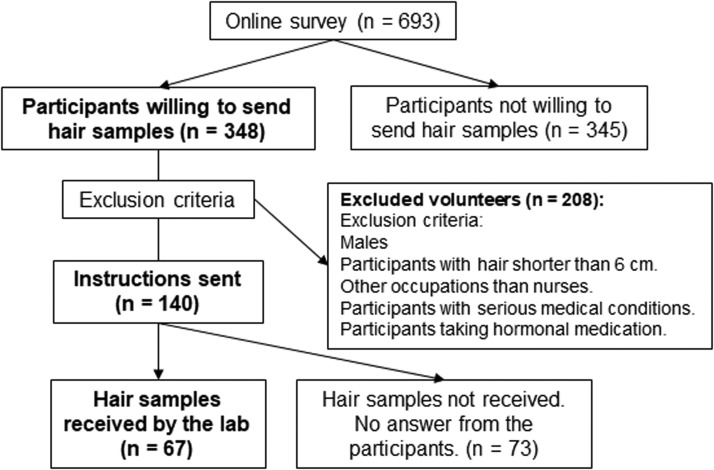
Of the whole sample, 348 participants were willing to provide hair samples for analysis and included their contact information (for an overview of the participant recruitment, see Fig. 1). The selection of nurses for the hair cortisol study was done based on the answers to the control questions in the survey. First, only female nurses were selected. Second, the necessary hair length for participation was 6 cm (to enable an analysis of two 3 cm segments). Third, the exclusion criteria also included chronic health conditions – including metabolic, endocrine, and psychiatric conditions – as well as hormonal medication (e.g., oral contraceptives, corticosteroids, and thyroid hormones).
Fig. 1.
Flowchart showing the steps in recruiting participants, selection and hair sampling.
After deactivating the survey, 140 female nurses eligible for hair sampling were contacted via email with detailed instructions for the hair sampling. Participants were instructed to collect their hair samples at home with the help of a second person. Samples were to be taken from the occipital region of the scalp. Before hair collection, hair in this region was to be combed into a straight line from which two strands of hair were isolated using a packthread loop before being cut as close to the scalp as possible. Hair samples were then stored in an aluminum envelope. The instructions for hair sampling were adapted to the Slovak language in a text & picture format (as well as in a video format) from the instructions available at the website of the Biopsychology Laboratory of the Technical University in Dresden, where the samples were later analyzed. Email and chat support to the participants was also provided. The hair sample collection took place between June 8 and July 8. In the case of no reply to our instructions, participants were prompted once via the same email; if there was no reply, they were no longer contacted by the research team.
In total, samples from 67 participants were collected, 62 of which were sent to us via the postal service. In five cases, participants preferred to have their hair samples taken by the research team at the Department of Psychology, Faculty of Arts of Comenius University. The professional sample collection followed the same procedure as the hair collection by the participants.
2.3. Hair analysis
Two hair samples of a minimum thickness of 3 mm and length of 6 cm were collected from each of the nurses. Samples were shipped to the Biopsychology Laboratory of the Technical University in Dresden, where they were analyzed for HCCs.
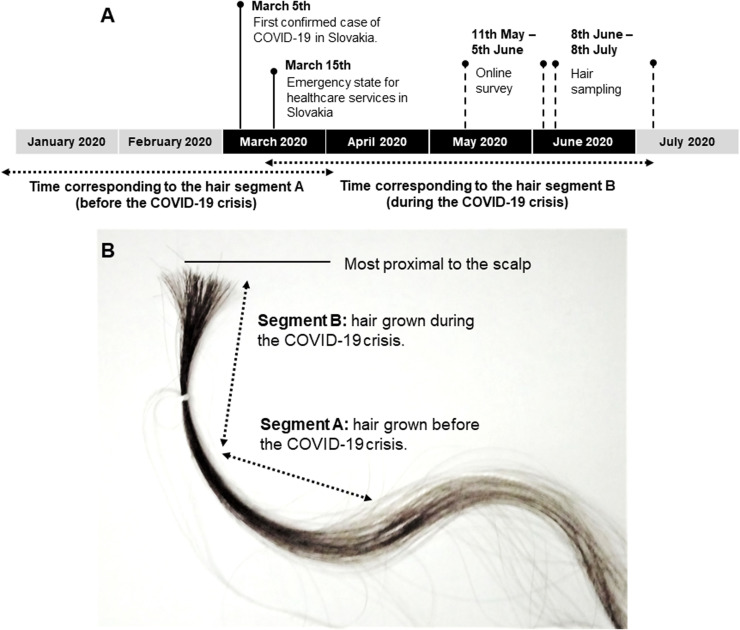
From each participant, two 3 cm segments of hair (7.5 mg each) were analyzed separately to determine the cortisol concentrations in the corresponding periods retrospectively. Assuming an average hair growth of approximately 1 cm monthly (Stalder and Kirschbaum, 2012), each segment should represent an approximately three-month-long period: segment A – a period of hair growth approximately from January to the first half of March before the start of the first wave of the COVID-19 pandemic in Slovakia; and segment B – reflecting the hair growth from the second half of March to roughly the end of June, which was the peak of the pandemic in Slovakia (for a full timeline, see Fig. 2A. Fig. 2B illustrates the analyzed hair segments on a hair sample).
Fig. 2.
A: Timeline of the data collection regarding epidemiological situation in Slovakia; B: Illustrative picture of collected hair sample, with two 3 cm hair segments highlighted.
The samples were washed twice with 2.5 ml of isopropanol, and the extraction process was conducted with 1.5 ml of methanol. The HCCs were determined via a commercially available immunoassay with chemiluminescence detection (CLIA, IBL-Hamburg, Germany). The inter- and intra-assay variation coefficients were below 8% (Kirschbaum et al., 2009, Gao et al., 2013).
2.4. Questionnaires
The online survey consisted of general questions about participants’ gender, work position, and the number of years in healthcare services, followed by a question of whether they worked with infected patients or had a high probability of getting in contact with infected patients at their workplace. Based on the answers, participants were distributed into high-risk (whole sample: n = 345; subset with hair samples: n = 33) and low-risk (348; 34) groups. Secondly, the survey included items regarding commonly mentioned stress factors and changes in participants’ work with a 7-point scale with answers from “very strong disagreement” to “very strong agreement” (for individual items, see Table 1). These were followed by the following questionnaires: a 10-item Perceived Stress Scale (PSS) (Cohen et al., 1994), the Multidimensional Scale of Perceived Social Support (MSPSS) (Zimet et al., 1988), and the Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989). A brief COPE questionnaire was also administered; however, it is not analyzed in this article.
Table 1.
Agreement with COVID-19 related statements in all survey respondents (n = 693).
| All (% of agreement) | Low risk group (% of agreement) | High risk group (% of agreement) | |
|---|---|---|---|
| Increase in overall stress at the workplace during COVID-19 pandemic | 75.9% | 71.6% | 80.2% |
| Significant increase in working hours | 34.3% | 26.4% | 42.2% |
| Overload by the number of patients | 44.0% | 38.6% | 49.4% |
| Stress due to insufficient or restricting safety measures | 80.2% | 74.8% | 85.6% |
| Fear of contracting COVID-19 | 54.3% | 51.6% | 56.9% |
| Feelings of exhaustion | 61.6% | 53.3% | 69.8% |
| Worsening of the relationships at the workplace during the crisis | 39.1% | 31.9% | 46.3% |
| No time for breaks (even for personal hygiene) | 28.8% | 24.1% | 33.6% |
| Fear of harming the patients | 39.4% | 34.2% | 44.5% |
2.5. Statistical analysis
The statistical analysis of the data was performed in JASP (JASP Team, 2020). In this article, we present only a part of the results, mainly descriptive statistics from the whole survey dataset. The answers on a 7-point scale were further visualized in terms of % of agreement, with answers from 1 to 3 representing degrees of disagreement with the statement and answers from 5 to 7 representing degrees of agreement. In the statistical comparisons, a paired-samples Wilcoxon test was used on data from the original 7-point scale.
The main sample of interest was the 67 female nurses who provided hair samples for HCC analysis; all further analyses were performed on this sample. Regarding hair cortisol, data were analyzed via two repeated measures models with HCCs as an outcome measure. In the first model, two hair segments were analyzed as a within-subject measure and a high/low COVID-19 risk as a between-subject group factor. The second model also included covariates of perceived stress (PSS score), perceived social support (MSPSS score), and sleep quality (PSQI score). Confidence intervals for the partial eta squared were calculated in the R package MBESS (Kelley, 2020). Plots were drawn in Graph Pad Prism 8.0 for Windows.
Hair data and questionnaire answers from the sample of 67 participants are available at https://doi.org/10.17605/OSF.IO/BPS7N.
3. Results
3.1. Online survey data (n = 693)
From the whole sample of healthcare workers who filled in the survey in May 2020 (n = 693), 75.9% agreed (either very strongly, strongly, or slightly) that overall stress at their workplace had increased during the first wave of the COVID-19 pandemic. When comparing the groups from the high-risk and low-risk environments, agreement with the statement of overall increased stress was higher in the high-risk environment. Table 1 summarizes COVID-19 related statements and expresses agreement in % for both high and low-risk groups. All group differences were statistically significant at α = 0.05.
3.2. Hair data from nurses (n = 67)
Table 2 shows the general characteristics of the final sample of nurses. All participants in the sample were female, all were healthy, and none took regular medication besides nutritional supplements. The table also shows the means and standard deviations of HCCs in both segments and self-report questionnaire scores in both groups working in high-risk and low-risk environments.
Table 2.
Descriptive statistics in sample of nurses with both hair samples and questionnaire data (n = 67).
| Whole sample (n = 67) | Low risk group (n = 34) | High risk group (n = 33) | Questionnaire normal range | Internal consistency (α) | |
|---|---|---|---|---|---|
| General | |||||
| Age (years), (M, SD) | 39.22 ± 9.93 | 39.94 ± 9.5 | 38.45 ± 10.48 | ||
| Years of practice (M, SD) | 16.35 ± 10.9 | 17.35 ± 11.76 | 15.32 ± 10.0 | ||
| Hair treatment(a) (n, %) | 47 (70.15%) | 23 (67.65%) | 24 (72.73%) | ||
| Hair cortisol concentrations (pg/mg) | |||||
| Before COVID-19 crisis (M, SD) | 5.47 ± 3.98 | 4.49 ± 2.27 | 6.49 ± 5.03 | ||
| During COVID-19 crisis (M, SD) | 6.67 ± 4.13 | 5.89 ± 2.78 | 7.69 ± 5.05 | ||
| Self-report questionnaires | |||||
| PSS score (M, SD) | 22.39 ± 5.36 | 22.41 ± 4.32 | 22.33 ± 6.32 | 15.70 ± 7.51(b) | 0.856 |
| PSQI score (M, SD) | 8.91 ± 4.16 | 9.24 ± 3.24 | 8.56 ± 4.96 | 2.67 ± 1.70(c) | |
| MSPSS score (M, SD) | 5.34 ± 1.18 | 5.37 ± 1.10 | 5.30 ± 1.28 | 5.80 ± 0.86(d) | 0.952 |
Hair treatment included conditions such as hair dying, bleaching etc.
Reference range from: Cohen and Janicki‐Deverts (2012), data from 1704 participants.
Reference range from: Buysse et al., 1989.
Reference range from: Zimet et al., 1988.
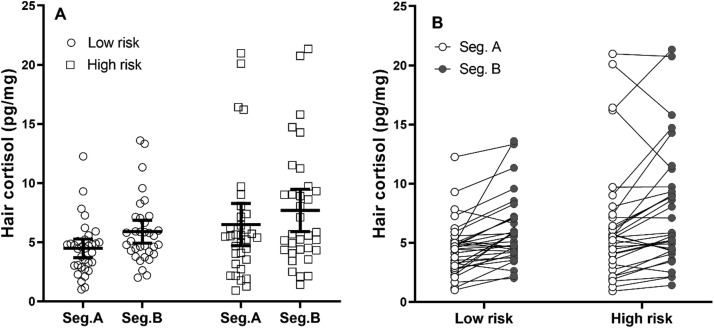
Overall, HCCs in the two segments correlated strongly r = 0.857, 95%CI [0.791, 1.0], showing the stability of HCCs between the two hair segments. In the two-way ANOVA with a within-subject factor of hair segment and a between-subject factor of high/low-risk group, the data showed both the main effect of hair segment F(1, 65) = 23.683, p < 0.001, = 0.267, 90%CI [0.123, 0.397] with higher HCCs in segment B (hair grown during the first wave of the pandemic), as well as the main effect of COVID-19 risk group F(1, 65) = 4.152, p = 0.046, = 0.06, 90%CI [0.002, 0.176] with higher HCCs in nurses from high-risk facilities.
The interaction between both factors was not significant: p = 0.699, = 0.002, 90%CI [0, 0.053]. Both within- and between-subject effects in this model are illustrated in Fig. 3. For a post-hoc comparison, an independent samples Welch t-test was conducted to compare low- and high-risk groups only using HCCs from segment B resulting in non-significant effect t(75.629) = − 1.852, p = 0.068, with 95%CI for the difference being − 3.729 to 0.136 pg/mg.
Fig. 3.
A: Means and 95% confidence intervals for both hair segments in high and low risk groups. Jittered dots represent individual measured HCC values. B: Paired differences in HCC between the two hair segments in each participant (shown separately for low- and high-risk group).
In the second repeated ANOVA model, the covariates of the perceived stress score, the perceived social support score, and the quality of sleep score were added. The two main factors – hair segment: F(1, 60) = 4.031, p = 0.049, = 0.063, 90%CI [0.001, 0.167]; and risk group: F(1, 60) = 4.434, p = 0.039, = 0.069, 90%CI [0.002, 0.175] also remain significant predictors of HCCs in this second model. Regarding the covariates, the null hypothesis could not be rejected for either main effect of PSS: F(1, 60) = 0.038, p = 0.846, < 0.001, 90%CI [0, 0.032]; PSQI: F(1, 60) = 0.729, p = 0.397, = 0.012, 90%CI [0, 0.085]; or any of the interactions with factors of group or hair segment (data not shown). MSPSS was also not a significant predictor, although a small effect might be present: F(1, 60) = 3.190, p = 0.079, = 0.05, 90%CI [0, 0.150].
4. Discussion
The first wave of the COVID-19 pandemic represented a significant stress factor in the lives of healthcare workers. Over 75% of those who filled in the survey agreed that COVID-19 had led to increased stress at their workplaces. Data from the questionnaires also showed higher perceived stress as well as worse sleep quality among healthcare workers during the first wave of the COVID-19 pandemic when compared to the population norms for these questionnaires (Buysse et al., 1989, Cohen and Janicki‐Deverts, 2012, Zimet et al., 1988). This is similar to the bulk of recent questionnaire research on stress in healthcare workers during the pandemic (Kannampallil et al., 2020, Lai et al., 2020, Shechter et al., 2020, Spoorthy et al., 2020). We also found that healthcare workers who reported having a high chance of working with COVID-19 patients experienced more stress than those who reported a lower chance of working with infected patients.
The main aim of this study was to investigate cumulative HCCs in female nurses from hair samples corresponding to the time before and during the COVID-19 pandemic. The situation of frontline medical workers during the pandemic could be seen as a model of a stressful situation due to its high unpredictability, their low control, and high personal risk; therefore, we believe hair cortisol findings from this specific situation are relevant to other fields of psychosocial stress as well.
Overall, the data showed higher HCCs in the sample reflecting the three months of the pandemic compared to data from an earlier period. In the same model, there were higher HCCs in nurses who reported having a high risk of working with patients infected with COVID-19. These findings can be interpreted in light of the relationship between long-term stress and HCCs. It is hypothesized that in chronically stressed individuals, the frequent number of cortisol stress responses and the dysregulation of daily cortisol rhythm are reflected in increased HCCs (Stalder et al., 2017, Staufenbiel et al., 2013). The evidence of higher HCCs in chronic stress has been shown in various populations, for example, among shift workers (Manenschijn et al., 2011), the unemployed (Dettenborn et al., 2010), caregivers of patients with dementia (Stalder et al., 2014), and people suffering from burnout (Penz et al., 2018). Other steroids relevant to stress and neuroinflammatory pathways, such as low serum 25-hydroxyvitamin D, were recently shown to predict the extent of psychological distress during the COVID-19 pandemic in a sample of depressed patients (Di Nicola et al., 2020). On the other hand, higher stress and anxiety, as well as higher serum cortisol, might also be predictors of worse clinical outcomes for patients infected with COVID-19 (Ramezani et al., 2020). However, at this point, more data on this subject are needed.
In the second part of this study, we also looked at self-report measures of perceived stress, perceived social support, and sleep quality as potential predictors of HCCs. These factors represent important self-report variables linked to stress; however, the main effects or the interactions of these variables with the above-mentioned factors of hair segment and COVID-19 risk were not large enough to be reliably differentiated from random variation. While social support is a measure previously linked to lower cortisol stress responses (e.g., Heinrichs et al., 2003), there is a lack of evidence of HCCs being related to perceived social support measured via questionnaire (Stalder et al., 2017). The relationship between HCCs and perceived stress has been investigated repeatedly, with no consistent correlation found between these measures (Stalder et al., 2017). The PSS score is a general stress measure; it might not capture differences in specific situations, such as the difference between high-risk and low-risk groups among healthcare workers. Moreover, PSS is not designed to measure stress retrospectively in periods longer than a few weeks (Cohen et al., 1983); therefore, it is understandable that it does not reflect HCCs that correspond to a different period. The same problem applies to the subjective assessment of sleep quality using PSQI, which is also hard to interpret retrospectively for longer periods.
An interesting issue is a relationship between the experienced stress among nurses and the objective severity of the situation. During the first wave of the COVID-19 pandemic, Slovakia was not so severely hit, as the daily number of confirmed cases peaked at 114 in April 2020 (WHO, 2021), which is much lower compared to later developments of the pandemic. However, based on the data presented here, the initial phase of the pandemic led to high subjectively reported stress as well as increased HCCs in nurses. We believe that the novelty of this dangerous virus, low predictability, and low control over the situation, which are common attributes of psychosocial stressors (Koolhaas et al., 2011), could explain the observed effects better than the objective situation at the time.
Regarding the limitations of this study, several issues are related to the interpretation of the difference in HCCs between the two hair segments First, because newer hair was compared to older hair, cortisol washout effects could have contributed to the difference. Although this effect cannot be ruled out based on the present data, existing studies (e.g., Dettenborn et al., 2010; Kirschbaum et al., 2009) show that hair cortisol is relatively stable in hair for up to 6 months and can be analyzed retrospectively. Second, the fact that participants collected hair themselves could be seen as a limitation; however, a recent study by Enge et al. (2020) concluded that there are no significant differences in HCCs between self- and professionally collected hair samples, with the only difference being a slightly larger sample loss. Third, the majority of participants (70%) have had some hair treatment, and the differences in frequency of hair washing between participants were not measured. At this point, the importance of these factors is not clear, as the majority of studies have found null effects or only small effects regarding hair treatment and/or hair washing (Gonzalez et al., 2019, Stalder et al., 2017). Other limitations include the use of self-administered questionnaires, the data from which could be biased. Questionnaires were adapted to Slovak from their English version; however, they were not standardized on the Slovak population. A potential limitation is also the online survey invitation; some groups could have been neglected, thus making the determination of the survey’s participation rate impossible. Finally, small differences in the timeline for individual participants, as the hair samples were collected in a timeframe of one month, could have added to the variability in the data.
In conclusion, more than 75% of the healthcare professionals who answered the online survey agreed that work-related stress had significantly increased during the first wave of the COVID-19 pandemic. As a neuroendocrine measure of long-term stress, we looked at HCCs in nurses and found differences between samples reflecting the period before and during the COVID-19 related state of emergency, with higher HCCs during the three months when the first wave of the pandemic took place in Slovakia. At the same time, differences in HCCs were found between nurses from high- and low-risk medical facilities concerning COVID-19, again with higher HCCs in high-risk (and potentially more stressful) environments. The subjectively reported measures of general perceived stress, social support, and sleep quality were not shown to be reliable predictors of HCCs.
Funding
The study was supported by a grant from the Slovak Research and Development Agency under contract APVV-17-0451, and by Research Grant Agency of the Slovak Ministry of Education, Science, Research and Sport (VEGA) under contract 1/0739/17 and 1/0757/19.
Conflict of interest
We declare no conflict of interest.
Acknowledgment
We would like to thank the Slovak Association of Nurses and Midwives (SK SaPA).
Data files
References
- Barzilay R., Moore T.M., Greenberg D.M., DiDomenico G.E., Brown L.A., White L.K., Gur R.C., Gur R.E. Resilience, COVID-19-related stress, anxiety and depression during the pandemic in a large population enriched for healthcare providers. Transl. Psychiatry. 2020;10(1):291. doi: 10.1038/s41398-020-00982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse D.J., Reynolds C.F., Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cai H., Tu B., Ma J., Chen L., Fu L., Jiang Y., Zhuang Q. Psychological impact and coping strategies of frontline medical staff in hunan between january and march 2020 during the outbreak of coronavirus disease 2019 (COVID‑19) in Hubei, China. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020;26 doi: 10.12659/MSM.924171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Janicki‐Deverts D. Who’s stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 2009 1. J. Appl. Soc. Psychol. 2012;42(6):1320–1334. doi: 10.1111/j.1559-1816.2012.00900.x. [DOI] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Cohen, S., Kamarck. T., Mermelstein, R., 1994, Perceived stress scale. Measuring Stress: A Guide for Health and Social Scientists 10, 1–2.
- Dettenborn L., Tietze A., Bruckner F., Kirschbaum C. Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology. 2010;35(9):1404–1409. doi: 10.1016/j.psyneuen.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Di Nicola M., Dattoli L., Moccia L., Pepe M., Janiri D., Fiorillo A., Sani G. Serum 25-hydroxyvitamin D levels and psychological distress symptoms in patients with affective disorders during the COVID-19 pandemic. Psychoneuroendocrinology. 2020;122 doi: 10.1016/j.psyneuen.2020.104869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enge S., Fleischhauer M., Hadj-Abo A., Butt F., Kirschbaum C., Schmidt K., Miller R. Comparison of hair cortisol concentrations between self- and professionally-collected hair samples and the role of five-factor personality traits as potential moderators. Psychoneuroendocrinology. 2020;122 doi: 10.1016/j.psyneuen.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Stalder T., Foley P., Rauh M., Deng H., Kirschbaum C. Quantitative analysis of steroid hormones in human hair using a column-switching LC–APCI–MS/MS assay. J. Chromatogr. B. 2013;928:1–8. doi: 10.1016/j.jchromb.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Gonzalez D., Jacobsen D., Ibar C., Pavan C., Monti J., Fernandez Machulsky N., Balbi A., Fritzler A., Jamardo J., Repetto E.M., Berg G., Fabre B. Hair cortisol measurement by an automated method. Sci. Rep. 2019;9(1):8213. doi: 10.1038/s41598-019-44693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M., Baumgartner T., Kirschbaum C., Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry. 2003;54(12):1389–1398. doi: 10.1016/S0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- JASP Team , 2020. JASP (Version 0.13.1)[Computer software].
- Kang L., Li Y., Hu S., Chen M., Yang C., Yang B.X., Wang Y., Hu J., Lai J., Ma X., Chen J., Guan L., Wang G., Ma H., Liu Z. The mental health of medical workers in Wuhan, China dealing with the 2019 novel coronavirus. Lancet Psychiatry. 2020;7(3):14. doi: 10.1016/S2215-0366(20)30047-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannampallil T.G., Goss C.W., Evanoff B.A., Strickland J.R., McAlister R.P., Duncan J. Exposure to COVID-19 patients increases physician trainee stress and burnout. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley K. , 2020. The MBESS R Package. Retrieved from: 〈https://cran.r-project.org/web/packages/MBESS/index.html〉.
- Kirschbaum C., Tietze A., Skoluda N., Dettenborn L. Hair as a retrospective calendar of cortisol production—Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34(1):32–37. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Koolhaas J.M., Bartolomucci A., Buwalda B., de Boer S.F., Flügge G., Korte S.M., Fuchs E. Stress revisited: a critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011;35(5):1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Krystal J.H., McNeil R.L. Responding to the hidden pandemic for healthcare workers: stress. Nat. Med. 2020;26(5):639. doi: 10.1038/s41591-020-0878-4. [DOI] [PubMed] [Google Scholar]
- Lai J., Ma S., Wang Y., Cai Z., Hu J., Wei N., Wu J., Du H., Chen T., Li R., Tan H., Kang L., Yao L., Huang M., Wang H., Wang G., Liu Z., Hu S. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw. Open. 2020;3(3) doi: 10.1001/jamanetworkopen.2020.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manenschijn L., van Kruysbergen R.G., de Jong F.H., Koper J.W., van Rossum E.F. Shift work at young age is associated with elevated long-term cortisol levels and body mass index. J. Clin. Endocrinol. Metab. 2011;96(11):E1862–E1865. doi: 10.1210/jc.2011-1551. [DOI] [PubMed] [Google Scholar]
- Moccia L., Janiri D., Pepe M., Dattoli L., Molinaro M., De Martin V., Chieffo D., Janiri L., Fiorillo A., Sani G., Di Nicola M. Affective temperament, attachment style, and the psychological impact of the COVID-19 outbreak: an early report on the Italian general population. Brain Behav. Immun. 2020;87:75–79. doi: 10.1016/j.bbi.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penz M., Stalder T., Miller R., Ludwig V.M., Kanthak M.K., Kirschbaum C. Hair cortisol as a biological marker for burnout symptomatology. Psychoneuroendocrinology. 2018;87:218–221. doi: 10.1016/j.psyneuen.2017.07.485. [DOI] [PubMed] [Google Scholar]
- Qiu J., Shen B., Zhao M., Wang Z., Xie B., Xu Y. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: implications and policy recommendations. Gen. Psychiatry. 2020;33(2) doi: 10.1136/gpsych-2020-100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar R.P. COVID-19 and mental health: a review of the existing literature. Asian J. Psychiatry. 2020;52 doi: 10.1016/j.ajp.2020.102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani M., Simani L., Karimialavijeh E., Rezaei O., Hajiesmaeili M., Pakdaman H. The role of anxiety and cortisol in outcomes of patients with Covid-19. Basic Clin. Neurosci. 2020;11(2):179–184. doi: 10.32598/bcn.11.covid19.1168.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E., Koren G., Rieder M., Van Uum S. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology. 2012;37(5):589–601. doi: 10.1016/j.psyneuen.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Shechter A., Diaz F., Moise N., Anstey D.E., Ye S., Agarwal S., Birk J.L., Brodie D., Cannone D.E., Chang B., Claassen J., Cornelius T., Derby L., Dong M., Givens R.C., Hochman B., Homma S., Kronish I.M., Lee S.A.J., Abdalla M. Psychological distress, coping behaviors, and preferences for support among New York healthcare workers during the COVID-19 pandemic. Gen. Hosp. Psychiatry. 2020;66:1–8. doi: 10.1016/j.genhosppsych.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoorthy M.S., Pratapa S.K., Mahant S. Mental health problems faced by healthcare workers due to the COVID-19 pandemic–a review. Asian J. Psychiatry. 2020;51 doi: 10.1016/j.ajp.2020.102119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T., Kirschbaum C. Analysis of cortisol in hair–state of the art and future directions. Brain Behav. Immun. 2012;26(7):1019–1029. doi: 10.1016/j.bbi.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Stalder T., Tietze A., Steudte S., Alexander N., Dettenborn L., Kirschbaum C. Elevated hair cortisol levels in chronically stressed dementia caregivers. Psychoneuroendocrinology. 2014;47:26–30. doi: 10.1016/j.psyneuen.2014.04.021. [DOI] [PubMed] [Google Scholar]
- Stalder T., Steudte-Schmiedgen S., Alexander N., Klucken T., Vater A., Wichmann S., Kirschbaum C., Miller R. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology. 2017;77:261–274. doi: 10.1016/j.psyneuen.2016.12.017. [DOI] [PubMed] [Google Scholar]
- Staufenbiel S.M., Penninx B.W.J.H., Spijker A.T., Elzinga B.M., van Rossum E.F.C. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology. 2013;38(8):1220–1235. doi: 10.1016/j.psyneuen.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Styra R., Hawryluck L., Robinson S., Kasapinovic S., Fones C., Gold W.L. Impact on health care workers employed in high-risk areas during the Toronto SARS outbreak. J. Psychosom. Res. 2008;64(2):177–183. doi: 10.1016/j.jpsychores.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Pan R., Wan X., Tan Y., Xu L., Ho C.S., Ho R.C. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int. J. Environ. Res. Public Health. 2020;17(5):1729. doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, (2021, January 18). 〈https://covid19.who.int/table〉.
- Zimet G.D., Dahlem N.W., Zimet S.G., Farley G.K. The multidimensional scale of perceived social support. J. Personal. Assess. 1988;52(1):30–41. doi: 10.1207/s15327752jpa5201_2. [DOI] [Google Scholar]