Abstract
Background
The impact of COVID-19 on the diagnosis and management of tuberculosis (TB) patients is unknown.
Methods
Participating centres completed a structured web-based survey regarding changes to TB patient management during the COVID-19 pandemic. The study also included data from participating centres on patients aged ≥18 diagnosed with TB in 2 periods: March 15 to June 30, 2020 and March 15 to June 30, 2019. Clinical variables and information about patient household contacts were retrospectively collected.
Results
A total of 7 (70%) TB units reported changes in their usual TB team operations. Across both periods of study, 169 patients were diagnosed with active TB (90 in 2019, 79 in 2020). Patients diagnosed in 2020 showed more frequent bilateral lesions in chest X-ray than patients diagnosed in 2019 (P = 0.004). There was a higher percentage of latent TB infection and active TB among children in households of patients diagnosed in 2020, compared with 2019 (P = 0.001).
Conclusions
The COVID-19 pandemic has caused substantial changes in TB care. TB patients diagnosed during the COVID-19 pandemic showed more extended pulmonary forms. The increase in latent TB infection and active TB in children of patient households could reflect increased household transmission due to anti-COVID-19 measures.
Keywords: Tuberculosis, COVID-19, Impact, Household contact screening, Pandemic, Transmission
Introduction
On 31 December 2020, China first reported a group of cases with atypical pneumonia caused by the SARS-CoV-2 (Lu et al., 2020). As of 8 December 2020, more than 68.5 million people were infected with the virus, and >1.5 million have died as a result of it (World Health Organization, 2020). In Spain, to date, >1.5 million people have been diagnosed with COVID-19 infection, and 47 624 people have died from the disease (Spanish Government, 2020). To reduce the risk of transmission, governments have launched urgent measures that include widespread use of facemasks, closure of public spaces and personal mobility restrictions. Health services have reduced to a minimum the number of daily outpatient visits to decrease the chance of nosocomial transmission. These strategies have allowed control of the first wave of SARS-CoV-2 infection; however, there is concern that we have paid a large toll in the control of other diseases (Saunders and Evans, 2020).
Tuberculosis (TB) is one of the top 10 causes of death globally and, since 2015, is the most frequent cause of death due to an infectious aetiology. In 2019, TB was responsible for an estimated 1.2 million deaths among people not infected with HIV and 251 000 deaths among HIV-positive patients (World Health Organization, 2019). TB has decreased significantly in high-income countries in recent decades; it is mainly diagnosed in immigrants from high incidence TB countries or immunosuppressed patients (Sánchez-Montalvá et al., 2018). Despite the global trend towards a progressive decrease in cases in recent years, recent research has concluded that new cases may increase by 6.3 million and deaths by 1.4 million over the next 5 years as measures taken to control SARS-CoV-2 infection prevent national TB programs from maintaining a minimum set of actions (Hogan et al., 2020). One of the measures adopted at the beginning of the pandemic is that anyone with mild symptoms related to COVID-19 (cough, fever) self-isolated for 14 days or longer if symptoms persisted. Symptoms and signs of pulmonary TB are similar to those related to respiratory viral infections, including COVID-19, which may cause delays in the diagnosis of TB. Moreover, close coexistence is a risk factor for transmitting TB among household contacts (Pienaar et al., 2010, Velen et al., 2020). Therefore, the control measures implemented during the peak of the first wave may have increased the number of TB infections. Additionally, difficulty accessing healthcare assistance may have delayed TB diagnosis, increasing transmission. Conversely, mask use and movement restrictions may positively affect TB transmission. We do not yet know the impact of COVID-19 on the diagnosis and management of TB patients.
Methods
Spanish healthcare providers with TB patient management programs were invited to participate in the study. A TB centre was defined as any healthcare facility with regular management of TB patients. A total of 90 TB centres were invited. Participating centres were asked to complete a structured web-based survey regarding the main changes in the management of TB patients during the COVID-19 pandemic. In addition, they were asked to provide data on all patients aged ≥18 years diagnosed with TB from March 15 to June 30, 2020 (pandemic case group) and the same period in 2019 (pre-pandemic control group). The study protocol was approved by the Vall d’Hebron Research Institute Ethics Committee, PR(AG)354/2020, Barcelona, Spain and local ethics committees as required. Informed consent was not considered necessary.
Data collection
The survey regarding changes at the organizational level and in management of TB patients was conducted using an electronic web-based tool (Google Form). The questionnaire consisted of 23 questions divided into 7 different sections: (1) modifications of the healthcare team working in the TB unit; (2) COVID-19 surveillance in TB patients; (3) modifications of the hospital wards where TB patients attended; (4) modifications in diagnostic tests for TB patients; (5) modifications in the follow-up of TB patients; (6) modifications in the treatment of TB patients; and (7) modifications in the household contacts screening program. The full version of the questionnaire is available as Online Appendix 1.
The patient data collection form included sociodemographic characteristics, comorbidities, radiological and microbiological and treatment variables. All data were retrospectively captured. Smear grade was defined by number of acid-fast bacilli (AFB) observed: 1+, 1–9 AFB/100 fields; 2+, 10-99 AFB/100 fields; 3+, 1–10 AFB/field; 4+, >10 AFB/field. Chest X-ray (CXR) was classified as normal if no parenchymal lesion was observed, unilateral lesions when only a lung field was affected, and bilateral lesions when both lung fields were affected. Presence of cavities was also collected. Time to diagnosis was defined as the number of days between symptoms onset and diagnosis date. Time to treatment initiation was defined as the number of days between symptoms onset and the date of first anti-TB treatment dose. Need for hospital admission, number and type of follow-up visits, adherence to treatment and smear conversion during the first month of treatment were also assessed. Information on number of household contacts assessed and diagnosis of these contacts was collected and classified as: not assessed, not infected, latent TB infection (LTBI), TB disease, and lost to follow-up. Not assessed was defined as a participant not undergoing symptoms evaluation, tuberculin skin test, or interferon gamma release assay and CXR. LTBI is defined as a participant without symptoms of active TB, CXR result not showing active signs of TB disease and positive tuberculin skin or interferon gamma release assay.
Data analysis
Anonymized patient data was entered into a database with a coding system and centralized at the lead hospital for the study, where database merge and cleaning was also carried out. Descriptive statistics are presented as number (percent) and median (interquartile range, [IQR]) or mean (SD) depending on variable normality. Smear grade and CXR classification was performed only in patients with a pulmonary form. To compare variables between patients diagnosed in 2019 and 2020, we used chi-square tests or Fisher’s exact tests for categorical variables and t-test or Mann–Whitney U test for continuous variables.
Description of TB cases was summarized temporally by months and fortnights. The yearly incidence rate (IR) was calculated. Incidence was calculated over the total population covered by the hospital, considering the whole hospital-covered population had the same risk of acquiring TB disease. The incidence rate ratio (IRR) was used to compare if the number of cases had increased or decreased. Additionally, the IR for the lockdown (15 March to 30 April) and the post-lockdown period (1 May to 30 June) were calculated for 2019 and 2020 using the average of total cumulative cases per period to make the comparison. We used the IRR between the lockdowns in 2019 and 2020 and the post-lockdown periods in 2019 and 2020. We did not perform age or gender adjustment for the calculation of IRs. A P value <0.05 was considered significant. All analyses were performed using SPSS statistical software (IBM SPSS version 23, Armonk, N.Y.).
Results
Changes in the management of TB patients
A total of 13 Spanish centres answered the questionnaire regarding changes at organizational level and in the management of TB patients; 10 had a specialized TB unit. The median (IQR) number of healthcare workers in the TB units was 3.5 (3–6.25). The number of healthcare workers was reduced due to the COVID-19 pandemic in 3/10 (30%) of the TB units and 7 (70%) reported changes in the usual operations of the TB team, mainly due to cancellation of meetings or substitution of face-to-face meetings for online/telephone meetings. SARS-CoV-2 screening was systematically performed in all TB patients in 3/13 (23.1%) centres, exclusively in patients with symptoms suggesting SARS-CoV-2 infection in 5 (38.5%), and not performed in 5 (38.5%).
Hospitalization wards where TB patients were admitted changed during the COVID-19 pandemic in 12 (92.3%) centres. The admission ward was determined based on whether the patient was concomitantly infected by SARS-CoV-2. However, the healthcare team attending TB patients was the same as before the pandemic with minimum modifications in 12 (92.3%) centres. Delays in performance of laboratory tests were reported by 3 (23.1%) centres and 11 (84.6%) reported difficulties in accessing other complementary exams, such as radiology exams or minimally invasive procedures (cancellation in 1, less test availability in 7, both in 3).
Follow up visits were either cancelled or delayed in 10 (76.9%) centres due to the pandemic. Changes in household contacts screening programs were described by 7 (53.8%) centres; screening was not performed in 1, delayed in 1 and both delayed and prioritized for vulnerable populations, such as the paediatric population and immunosuppressed contacts, in 5 (38.5%). None of the centres reported problems in drug supply.
Comparisons between patients diagnosed with TB 15 March to 30 June 2019 and 15 March to 30 June 2020
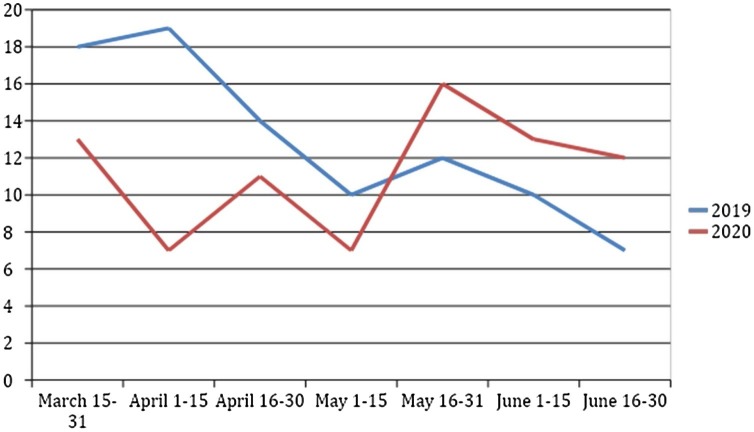
A total of 11 TB centres shared aggregated data on patients. The population covered by each TB centre is provided in Online Appendix 2. Across both study periods 169 patients were diagnosed with active TB (90 in 2019, 79 in 2020). The IR was 10.25 and 9.31 per 100 000 person-years in 2019 and 2020, respectively, IRR = 1.14 (95% CI (0.84–1.54); P = 0.442). The number of patients with active TB diagnosed per fortnight in both periods is represented in Figure 1 . For the lockdown periods, the IR was 12.9 per 100 000 person-years in 2019 and 8.57 per 100 000 person-years in 2020 (IRR 1.61 (1.03–2.52); P = 0.04). In the post-lockdown period, we observed an IR of 10.43 and 9.86 in 2019 and 2020, respectively, with an IRR of 1.2 (95% CI (0.79–1.83); P = 0.46).
Figure 1.
Number of patients with active TB diagnosed along both periods.
There were no significant differences in demographics between cohorts. In both years, approximately 40% of the patients were Spanish born. The World Health Organization regions of Europe and the Eastern Mediterranean accounted for approximately two-thirds of the cases. In 2019, 48/90 patients (53.3%) had a relevant comorbidity, in 2020, this was 48/79 patients (60.8%); the distribution did not differ significantly between the cohorts. Extrapulmonary disease was frequent in both years, in 42/90 (46.7%) and 33/79 (41.8%) patients in 2019 and 2020, respectively. The most frequent extrapulmonary forms, in decreasing prevalence, were adenopathic, pleural and disseminated disease. Patients diagnosed in 2020 showed more frequent bilateral lesions in CXR compared with patients diagnosed in 2019 (56.5% vs 27.1%, P = 0.004).
Demographics, clinical and microbiological characteristics of patients diagnosed in both periods are summarized in Table 1 .
Table 1.
Demographics, clinical and microbiological characteristics of tuberculosis patients in both periods.
| 2019 Cohort | 2020 Cohort | P value | |
|---|---|---|---|
| N = 90 | N = 79 | ||
| Sex, female | 30 (33.3%) | 27 (34.2%) | 0.908 |
| Age, years (mean, SD) | 41.0 (31–59) | 47.5 (34.5–65.7) | 0.595 |
| Country of birth | |||
| Spain | 35 (38.9%) | 31 (39.2) | 0.963 |
| WHO region of origin | |||
| Africa | 6 (6.7%) | 6 (7.6%) | 0.628 |
| America | 8 (8.9%) | 12 (15.2%) | |
| South-East Asia | 7 (7.8%) | 4 (5.1%) | |
| Europe | 43 (47.8%) | 38 (48.1%) | |
| Eastern Mediterranean | 20 (22.2%) | 17 (21.5%) | |
| Western Pacific | 6 (6.7%) | 2 (2.5%) | |
| Comorbidities | 48 (53.3%) | 48 (60.8%) | 0.609 |
| Chronic pulmonary disease | 13 (14.4%) | 15 (19.0%) | 0.133 |
| Cardiomyopathy | 8 (8.9%) | 8 (10.1%) | 0.867 |
| Liver disease | 10 (11.1%) | 5 (6.3%) | 0.216 |
| Renal disease | 3 (3.3%) | 2 (2.5%) | 0.389 |
| Diabetes | 16 (17.8%) | 6 (7.6%) | 0.050 |
| Hypertension | 16 (17.8%) | 13 (16.5%) | 0.971 |
| Psychiatric disease | 10 (11.2%) | 7 (8.9%) | 0.348 |
| HIV infection | 5 (5.6%) | 6 (7.6%) | 0.845 |
| Hepatitis B infection | 1 (1.1%) | 2 (2.5%) | 0.770 |
| Hepatitis C infection | 4 (4.4%) | 5 (6.3%) | 0.857 |
| Solid organ transplant | 2 (2.2%) | 2 (2.5%) | 1.000 |
| Immunosuppressive treatment | 6 (6.7%) | 6 (8.9%) | 0.564 |
| Current smoker | 40 (44.4%) | 23 (29.1%) | 0.021 |
| Alcohol use | 19 (21.1%) | 11 (13.9%) | 0.066 |
| Illegal drug use | 6 (6.7%) | 5 (6.3%) | 0.639 |
| Homeless | 4 (4.4%) | 3 (3.8%) | 1.000 |
| Pulmonary TB | 48 (53.3%) | 46 (58.2%) | 0.523 |
| Non-pulmonary TB | 42 (46.7%) | 33 (41.8%) | |
| Adenitis | 12 (28.6%) | 12 (36.4%) | |
| Pleural | 10 (23.8%) | 10 (30.3%) | |
| Abdominal | 1 (2.4%) | 2 (6.1%) | |
| Urinary | 0 | 1 (3.0%) | |
| Bone | 3 (7.1%) | 1 (3.0%) | |
| Skin | 1 (2.4%) | 0 | |
| Pericardic | 1 (2.4%) | 0 | |
| Disseminated | 11 (26.2%) | 6 (18.2%) | |
| CNS | 1 (2.4%) | 0 | |
| Radiological findings (CXR) | |||
| Normal CXR | 2/48 (4.2%) | 0/46 | 0.495 |
| Unilateral lesions | 33/48 (68.8%) | 20/46 (43.5%) | 0.014 |
| Bilateral lesions | 13/48 (27.1%) | 26/46 (56.5%) | 0.004 |
| Cavitation | 14/48 (29.2%) | 19/46 (41.3%) | 0.218 |
| AFB smear positive | 29/48 (60.4%) | 29/46 (63.0%) | 0.611 |
| Grade sputum smear | |||
| 1-9 AFB per 100 fields | 11/29 (37.9%) | 11/29 (37.9%) | 0.891 |
| 10-99 AFB per 100 fields | 4/29 (13.8%) | 6/29 (20.7%) | |
| 1-10 AFB per field | 4/29 (13.8%) | 4/29 (13.8%) | |
| >10 AFB per field | 10/29 (34.5%) | 8/29 (27.6%) | |
| NAAT positive in sputum | 25/48 (52.1%) | 29/46 (63.0%) | 0.421 |
| Culture positive for TB | 65 (72.2%) | 68 (86.1%) | 0.078 |
| RMP resistance | 1 (1.1%) | 0 | |
| INH resistance | 1 (1.1%) | 4 (5.3%) | |
| PZA resistance | 1 (1.1%) | 1 (1.3%) | |
| ETB resistance | 0 | 0 | |
| SM resistance | 1 (1.1%) | 4 (5.1%) | |
| Days to diagnosisa, median (IQR) | 59 (22–134) | 63.4 (29–116) | 0.546 |
| Days to treatment, median (IQR) | 60 (21·2–134) | 63 (25–116) | 0.865 |
SD = standard deviation; WHO = World Health Organization; TB = tuberculosis; CNS = central nervous system; CXR = chest X-ray; AFB = acid fast bacilli, NAAT = nucleic acid amplification test; RMP = rifampin; INH = isoniazid; PZA = pyrazinamide; ETB = ethambutol; SM = streptomycin.
Time from symptoms onset to diagnosis.
In the 2020 cohort 59/79 patients were tested for SARS-CoV-2; none of the tests were positive, however, 19 (24.1%) patients were isolated at home due to suspicion of infection. The number of follow-up visits in 2020 was lower than in 2019 (median of 2 vs 1 follow-up visits, P = 0.002) and the type of follow-up visits differed between both periods, with more follow-ups by telephone in 2020. This information is summarized in Table 2 .
Table 2.
Management and follow-up of patients with tuberculosis.
| 2019 Cohort | 2020 Cohort | P value | |
|---|---|---|---|
| N = 90 | N = 79 | ||
| More than one consultation previous to diagnosis | 44 (48.9%) | 39 (49.4%) | 0.982 |
| Tested for SARS-CoV-2 | |||
| Home isolation due to SARS-CoV-2 suspicion | – | 59 (74.7%) | – |
| Need for hospital admission during diagnosis | – | 19 (24.1%) | – |
| Need for hospital admission during Follow-upa | |||
| Initial treatment regimen | 53 (59.6%) | 52 (66.7%) | 0.422 |
| Rifampin based treatment | |||
| Rifabutin based treatment | 12 (13.3%) | 11 (13.9%) | 0.412 |
| Quinolones supplemented treatment | |||
| AFB conversion time (days), median (IQR) | |||
| Treatment adherence (≥75%) | 88 (97.7%) | 73 (92.4%) | 0.488 |
| Adverse effects | 0 | 1 (1.2%) | |
| Treatment interruption | 2 (2.2%) | 4 (5.1%) | |
| 33·5 (21.7–45.7) | 34 (14–45) | 0.306 | |
| 83 (92.2%) | 75 (94.9%) | 0.509 | |
| 24 (26.7%) | 13 (16.5%) | 0·212 | |
| 9 (10.0%) | 5 (6.3%) | 0.083 | |
| Number (median, SD) of FU visitsb | 2 (1–3) | 1 (1–2) | 0.002 |
| Type of FU visits | |||
| Face-to-face visit | 58/71 (81.7%) | 40/59 (67.8%) | 0.006 |
| Telephone | 0 | 11/59 (18.6%) | |
| Both (face-to-face and telephone) | 9/71 (12.7%) | 4/59 (6.8%) |
RMP = rifampin; INH = isoniazid; PZA = pyrazinamide; ETB = ethambutol; Rb = rifabutin; Mx = moxifloxacin; Lx = levofloxacin; AK = amikacin.
Patients who were admitted for diagnosis but could not be discharged during the first month of follow-up were also included.
Patients who needed hospital admission during diagnosis were excluded.
Information from the household contacts screening program was available for 68 (75.6%) and 51 (64.6%) TB patients diagnosed in 2019 and 2020, respectively. More adult contacts were screened in 2020 than in 2019 (84.8% vs 61.6%, P < 0.001). We observed a higher percentage of LTBI and active TB among children who were household contacts of patients diagnosed in 2020 compared with patients diagnosed in 2019 (57.7% vs 5.3%, P < 0.001). See Table 3 .
Table 3.
Household contacts with latent tuberculosis infection or active tuberculosis.
| 2019 Cohort | 2020 Cohort | P value | |
|---|---|---|---|
| N = 68 | N = 51 | ||
| Total adult contacts | 159 | 105 | |
| Adult contacts screened | 98/159 (61.6%) | 89/105 (84.8%) | <0.001 |
| LTBI among adult contacts | 32/98 (32.7%) | 33/89 (37.1%) | 0.526 |
| Active TB among adult contacts | 2/98 (2.0%) | 3/89 (3.4%) | 0.670 |
| LTBI or active TB adult contact | 34/98 (34.7%) | 36/89 (40.4%) | 0.417 |
| Total child contacts | 24 | 31 | |
| Child contacts screened | 19/24 (79.2%) | 26/31 (83.9%) | 0.654 |
| LTBI among children contacts | 1/19 (5.3%) | 7/26 (26.9%) | 0.061 |
| Active TB among child contacts | 0 | 8/26 (30.8%) | 0.014 |
| LTBI or active TB child contact | 1/19 (5.3%) | 15/26 (57.7%) | <0.001 |
LTBI = latent tuberculosis infection; TB = tuberculosis.
Bold values are statistically significant values (<0.005).
Discussion
To our knowledge, this is the first study describing changes in TB management and comparing the clinical variables of patients diagnosed with TB during the COVID-19 pandemic with the same period in 2019. We observed substantial changes in the clinical care of patients with TB during the COVID-19 pandemic. Moreover, we observed that patients diagnosed during 2020 showed more frequently bilateral lesions in CXR, and their children household contacts were more frequently diagnosed with LTBI or active TB.
During the COVID-19 pandemic both human and economic health resources have been re-allocated due to the high priority of this disease, causing disruption in the diagnosis and treatment of several health conditions (Raymond et al., 2020) and cancelling many outpatient activities and elective procedures (Ng et al., 2020). In addition, all community-based disease prevention and health promotion programs have been dramatically affected in many countries (Abdela et al., 2020). In our study, we observed that the number of healthcare workers usually taking care of TB patients was reduced due to the COVID-19 pandemic and most of the TB units reported organizational and operational changes. Moreover, most centres in the study reported difficulties in access to complementary tests, either cancellation or delay in follow-up visits, and changes in the household contact screening program. The impact of COVID-19 in the care of infectious, mainly pulmonary diseases might have been higher than in other disease areas, as the teams involved in their care were frequently mobilized to be part of the COVID-19 teams, often composed of infectious diseases or pneumology physicians, and specialized nurses. Global disruptions of TB services have been previously described (Migliori et al., 2020, Nikolayevskyy et al., 2020).
The restrictions imposed by governments (e.g., stay-at-home orders) to decrease the transmission of SARS-CoV-2 has changed the delivery of patient care, impacting negatively on the health of the patients through delayed care of acute emergencies, exacerbation of chronic diseases and psychological distress (Hamadani et al., 2020, Woolf et al., 2020). Across the United States a decrease in overall outpatient visits was observed during the first period of the pandemic and a rebound during the last months (Mehrotra et al., 2020). Similarly, we also observed a lower number of new TB diagnoses during the first months of the lockdown period, and a rebound during the last months. However, the overall number of patients with a diagnosis of TB has been lower in 2020 compared with the same period in 2019, as has been reported in other countries (Lai and Yu, 2020, Kwak et al., 2020). The decrease in IR may be partially explained by the long-term trend of gradual TB incidence reduction in Spain. However, we also observed an upturn in diagnosed cases after the lockdown period, so the IR reduction may not be due to the historical trend in TB IR reduction but rather an effect of the lockdown itself. Notification of new TB cases during the next months will help to clarify this point.
The similarities in TB and COVID-19 symptoms may hinder the detection of TB, leading to a misdiagnosis impacting on community transmission. In our study, more than 24% of patients with active TB were isolated at home due to suspicion of SARS-CoV-2 infection. This home isolation may have increased household transmission. One of the most important strategies to fight against TB is early diagnosis, isolation and treatment of active TB patients so that they rapidly become non-infectious and secondary cases are avoided. Community TB transmission may have been reduced through population-wide wearing of face masks and social distancing measures. Studies on TB incidence during the next months and years will help us to better know the long-term epidemiological impact of COVID-19 measures.
The disruption of TB programs directed to highly vulnerable populations (e.g., economically disadvantaged, racial and ethnic minorities, homeless, people living with HIV, undocumented migrants) and restrictions to personal mobility combined with diagnosis delay may have negatively impacted vulnerable populations and household transmission, respectively (Amimo et al., 2020). In our study, patients diagnosed with TB during the COVID-19 pandemic showed more frequent bilateral lesions in chest radiographies, suggesting more advanced disease. We cannot be sure that there are no other variables involved in this finding and we did not observe a longer duration of symptoms in 2020 than in 2019. However, a relationship is likely to changes in health-seeking behaviours and delays in healthcare attention caused by difficulties in accessing healthcare facilities, overworked microbiology and other complementary laboratories, and patients’ fears of interacting with other people and being infected (Jones et al., 2020). Moreover, during the pandemic, the only way to request an appointment with a medical practitioner was by phone or online. It is well known that TB usually affects people from low socioeconomic settings to whom remote medical consultation may be less suited, thereby increasing the barriers to healthcare assistance (Jones et al., 2020).
Our study did not observe differences between the cohorts in need for hospital admission or days to sputum smear conversion, indicating that TB units have been able to continue to manage TB patients with a high level of care during the pandemic. Reflecting on this finding may bring opportunities to implement new approaches to ensure TB programs remain successful and apply lessons learned from this emergency. Rapid restoration of TB services is essential to prevent long-term negative impacts (Cilloni et al., 2020), but we can also scale up successful initiatives such as the use of digital tools to cope with a TB epidemic (Chiang et al., 2020, Hopewell et al., 2021, Togun et al., 2020).
The participating TB units reported changes in TB household contact screening programs. Despite reported changes, the percentage of household contacts screened was higher than during the previous period; however, we also observed an alarming rise in child household contacts with either LTBI or active TB. One possible explanation is that during 2020 high-risk contacts had been prioritized. It is easy to understand that delays in diagnosis combined with stay-at-home orders are a dangerous combination in the household environment for airborne transmitted diseases such as TB. Preventing active TB disease through prompt screening and treating LTBI is a critical component of the World Health Organization’s End TB Strategy (Uplekar et al., 2015) and one of the priority interventions required for countries with a low TB incidence to progress towards elimination of TB (Lönnroth et al., 2015).
Our study has some limitations. First, all variables were collected retrospectively with the inherent limitations of this study type. Second, we did not have data on household contacts for some patients. However, these were mainly patients with extrapulmonary TB in which contact studies aim to diagnose missing index cases. Third, our study cannot establish a causative effect between COVID-19 measures and delays in TB diagnosis and increased household transmission. However, it seems reasonable to conclude that the COVID-19 pandemic may have unknown consequences for the TB dynamic. Fourth, only 12% (effective response rate) of the centres returned the survey, and raw IRs were calculated without age or gender adjustment, so external validity could be compromised. However, the total population covered by the participating TB centres is >3 million people.
In conclusion, our study shows that the COVID-19 pandemic has caused substantial changes in TB care. TB patients diagnosed during the COVID-19 pandemic showed more extended pulmonary forms. The increase in LTBI infection and active TB in children who were household contacts of patients reflects increased household transmission due to anti-COVID-19 measures. More studies assessing the impact of the COVID-19 pandemic on TB dynamics both globally and locally are urgently needed. The situation may be an opportunity to implement lessons learned to ensure TB programs remain successful.
Ethical approval
The study protocol was approved by the Vall d’Hebron Research Institute Ethics Committee, PR(AG)354/2020, Barcelona, Spain and local ethics committees when needed.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors
Conflicts of interest
None.
Acknowledgment
MLA was supported by a postdoctoral grant “Rio Hortega” and ASM was supported by a postdoctoral grant “Juan Rodés” (JE18/00022) from the Instituto de Salud Carlos III through the Spanish Ministry of economy and competitiveness.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2021.04.075.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Abdela S.G., van Griensven J., Seife F., Enbiale W. Neglecting the effect of COVID-19 on neglected tropical diseases: the Ethiopian perspective. Trans R Soc Trop Med Hyg. 2020;114:730–732. doi: 10.1093/trstmh/traa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amimo F., Lambert B., Magit A. What does the COVID-19 pandemic mean for HIV, tuberculosis, and malaria control? Trop Med Health. 2020;48:32. doi: 10.1186/s41182-020-00219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C.Y., Islam T., Xu C., Chinnayah T., Garfin A.M.C., Rahevar K. The impact of COVID-19 and the restoration of tuberculosis services in the Western Pacific Region. Eur Respir J. 2020;56 doi: 10.1183/13993003.03054-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilloni L., Fu H., Vesga J.F., Dowdy D., Pretorius C., Ahmedov S. The potential impact of the COVID-19 pandemic on the tuberculosis epidemic a modelling analysis. EClinicalMedicine. 2020;28 doi: 10.1016/j.eclinm.2020.100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamadani J.D., Hasan M.I., Baldi A.J., Hossain S.J., Shiraji S., Bhuiyan M.S.A. Immediate impact of stay-at-home orders to control COVID-19 transmission on socioeconomic conditions, food insecurity, mental health, and intimate partner violence in Bangladeshi women and their families: an interrupted time series. Lancet Glob Health. 2020;8:E1380–89. doi: 10.1016/S2214-109X(20)30366-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan A.B., Jewell B.L., Sherrard-Smith E., Vesga J.F., Watson O.J., Whittaker C. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8:E1132–E1141. doi: 10.1016/S2214-109X(20)30288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopewell P.C., Reichman L.B., Castro K.G. Parallels and mutual lessons in tuberculosis and COVID-19 transmission, prevention, and control. Emerg Infect Dis. 2021;27:3. doi: 10.3201/eid2703.203456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D., Neal R.D., Duffy S.R.G., Scott S.E., Whitaker K.L., Brain K. Impact of the COVID-19 pandemic on the symptomatic diagnosis of cancer: the view from primary care. Lancet Oncol. 2020;6:748–750. doi: 10.1016/S1470-2045(20)30242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak N., Hwang S.S., Yima A.J. Effect of COVID-19 on tuberculosis notification, South Korea. Emerg Infect Dis. 2020;26:2506–2508. doi: 10.3201/eid2610.202782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.C., Yu W.L. The COVID-19 pandemic and tuberculosis in Taiwan. J Infect. 2020;81:E159–E161. doi: 10.1016/j.jinf.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnroth K., Migliori G.B., Abubakar I., D’Ambrosio L., de Vries G., Diel R. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J. 2015;45:928–952. doi: 10.1183/09031936.00214014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra A., Chernew M., Linetsky D., Hatch H., Cutler D. Commonw Fund; 2020. The impact of the COVID-19 pandemic on outpatient visits: practices are adapting to the new normal. [Google Scholar]
- Migliori G.B., Thong P.M., Akkerman O., Alffenaar J.W., Álvarez-Navascués F., Assao-Neino M.M. Worldwide effects of coronavirus disease pandemic on tuberculosis services, January–April 2020. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2611.203163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J.J., Ho P., Dharmaraj R.B., Wong J.C.L., Choong A.M.T.L. The global impact of COVID-19 on vascular surgical services. J Vasc Surg. 2020;71:2182–2183. doi: 10.1016/j.jvs.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolayevskyy V., Holicka Y., van Soolingen D., van der Werf M.J., Ködmön C., Surkova E. Impact of COVID-19 pandemic on tuberculosis laboratory services in Europe. Eur Respir J. 2020 doi: 10.1183/13993003.03890-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienaar E., Fluitt A.M., Whitney S.E., Freifeld A.G., Viljoen H.J. A model of tuberculosis transmission and intervention strategies in an urban residential area. Comput Biol Chem. 2010;34:86–96. doi: 10.1016/j.compbiolchem.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond E., Thieblemont C., Alran S., Faivre S. Impact of the COVID-19 outbreak on the management of patients with cancer. Targeted Oncol. 2020;15:249–259. doi: 10.1007/s11523-020-00721-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Montalvá A., Salvador F., Molina-Morant D., Molina I. Tuberculosis e inmigración. Enferm Infecc Microbiol Clin. 2018;36:446–455. doi: 10.1016/j.eimc.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Saunders M.J., Evans C.A. COVID-19, tuberculosis and poverty: preventing a perfect storm. Eur Respir J. 2020;56 doi: 10.1183/13993003.01348-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togun T., Kampmann B., Stoker N.G., Lipman M. Anticipating the impact of the COVID-19 pandemic on TB patients and TB control programmes. Ann Clin Microbiol Antimicrob. 2020;19:21. doi: 10.1186/s12941-020-00363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uplekar M., Weil D., Lonnroth K., Jaramillo E., Lienhardt C., Dias H.M. WHO’s new end TB strategy. Lancet. 2015;385:1799–1801. doi: 10.1016/S0140-6736(15)60570-0. [DOI] [PubMed] [Google Scholar]
- Velen K., Nhung N.V., Anh N.T., Cuong P.D., Hoa N.B., Cuong N.K. Risk factors for TB among household contacts of patients with smear-positive TB in eight provinces of Vietnam: a nested case-control study. Clin Infect Dis. 2020;(November) doi: 10.1093/cid/ciaa1742. [DOI] [PubMed] [Google Scholar]
- Woolf S.H., Chapman D.A., Sabo R.T., Weinberger D.M., Hill L. Excess deaths from COVID-19 and other causes, March-April 2020. JAMA. 2020;324:510–530. doi: 10.1001/jama.2020.11787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2019. Global tuberculosis report 2019. [Google Scholar]
- World Health Organization . WHO; 2020. Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.